1. Introduction
2.Natural history of Placuna placenta
3.Exploitation and benefits derived from bivalves
3.1. Historical perspective of exploitation
3.2. Modern economic exploitation
3.3. Nutritional benefits and challenges of bivalves
3.4. Pathogens, allergens and contaminants in bivalves
4. Potentials/processing possibilities for windowpane oyster by-products
4.1. Bioactive compounds in marine invertebrates
4.1.1. Peptides
4.1.2. Shell proteins and polysaccharides
4.1.3. Glycosaminoglycans in marine invertebrates
4.2. Windowpane oyster by-products and waste
5. Conclusion
1. Introduction
Marine and freshwater organisms provide a range of essential foodstuffs and materials. Thus, the harvest of aquatic animals was estimated at 178 million tonnes in 2020, capture fisheries contributing 51% (USD 141 billion) and aquaculture 49% (USD 265 billion), with a total value of USD 406 billion [
1]. While most is destined for human consumption, there are nevertheless 20 million tonnes destined for non-food use. Not surprisingly given its geography, fisheries are an important part of the Philippines’ economy. For example, in 2019, it ranked 8
th among the top producing countries and 11
th in the world for aquaculture fisheries production with 2.07% and 1.01% share in total fishery and in aquaculture production, respectively, with the latter being valued at ~ USD 2.05 billion of global aquaculture production [
2]. The windowpane oyster (
Placuna placenta) is one of the most important fishery products of the Philippines. It is a bivalve marine mollusc, which is known locally as “kapis”, capiz, “pios” or “lamperong”. Unusually for fisheries it is the shell which is the major economic value. This is well known to be a very versatile material for making aesthetic handicrafts such as lamp shades, flower vases, chandeliers and can also be used for producing animal glue, chalk, shellack, soldering lead, and paint. The shell craft products are exported widely to economically richer countries [
3]. Windowpane oyster fisheries in the Philippines face important challenges, yet at the same time, local and small-scale endeavours demonstrate routes to overcoming these challenges. In this review we use the natural history of the oyster, and past and current practice in the fisheries, to identify the challenges faced by the industry. We document the evidence for successful remedial action that could be implemented at a provincial and national level, the opportunities to harness economically valuable by-products, and identify new avenues for research that may yield to further valuable by-products and so the introduction of a more circular economy for this oyster fishery. Alongside we identify some of the benefits of the oysters as proxy monitors for coastal water quality and physical stability.
2. Natural History of Placuna placenta
Marine bivalves are common in large numbers in coastal waters. Bivalve molluscs are thought to have originated in warm shallow euhaline coastal waters and gradually invaded estuaries and brackish systems, as well as all the reaches of the world ocean [
4]. In these systems, phytoplankton are consumed from tidal waters sweeping over dense beds of bivalves, mainly clams, cockles, mussels, oysters, and scallops. The dominant bivalves in these systems are adapted to changing temperatures and salinity, and some are adapted to exposure and wave action in the intertidal zone.
Adult forms of the majority of these animals are benthic or bottom dwelling, and therefore found burying within burrows in unconsolidated soft sediments; attaching byssal threads to pebbles or cemented to shells or rocks; and as semi-mobile members of the epibenthos [
5].
Placuna placenta is the most common of the genus
Placuna (established by Solander in 1786) belonging to the Family
Placunidae, which is closely related to Family
Anomiidae [
6]. It is the latter author who undertook and published the pioneering work on
Placuna placenta [
6]. Bivalves belonging to the Families
Anomiidae and
Placunidae have organs which exhibit a very high degree of asymmetry and distinctly inequivalve shells. Valves show a pronounced tendency to assume an orbicular outline and are very much flattened or compressed laterally with the right bulb being almost flat and the left, weakly convex. In younger specimens, the shell is thin, mica-like and more or less translucent, becoming opaque with age and secured by a V-shaped ligament [
7]. Pigmentation of the adult shell is rare, though it is common among young forms to show a beautiful satiny pink or yellowish lustre. Despite common characteristics,
Anomiidae and
Placunidae have highly developed specializations:
Anomiidae has calcified byssus, and
Placunidae a single genital aperture, and the foot develops into a very long and trumpet-shaped organ [
6]. The specific gravity of water in
Placuna beds ranges from 1.019 g/mL to 1.015 g/mL at temperatures ~30.5 °C to 32 °C. Unusually large influxes of fresh water during an exceptionally wet season entails the destruction of the oysters [
6].
The valves of
Placuna placenta seldom bear any parasitic growth of the type common in true pearl oysters [
6]. It prefers brackish water and thrives best in areas with bluish-soft mud (fine silt, clays, organic material and iron sulfide) or slightly sandy-muddy substratum in bays, coves, lagoons and in general shallow areas up to 100 m deep, and can reach a diameter of up to 150 mm [
8]. Unlike other bivalves, they cannot anchor themselves to substrate, but rather rest on the sea floor. The bivalve is also a filter feeder, eating primarily plankton and organic waste [
3]
The windowpane oyster is highly prolific and spawns periodically [
3]. The sexes are separate, as in the true pearl oysters, distinguished by the colour of their gonads with the genital glands opening into the kidneys [
6]. Maturity of the bivalve starts at shell diameters of 70- 100 mm, though gonads can be observed at sizes 50-80 mm. Reproduction is sexual, fertilization happening externally in open water, a small number are hermaphrodites [
3,
9]. The oyster larvae gradually develop translucent shells and float with the current for approximately 14 days before settling on the bottom. As the oysters are predominantly immobile once they have settled on the sea floor, the species disperses and repopulate other areas by passive transport of the larvae through wave action and currents [
9]. The distribution of the windowpane oyster is from the shallows of the Gulf of Aden around India and the Malay Peninsula to the Southern coast of China and along the north coast of Borneo to the Philippines [
8].
In general, bivalves exhibit changes in filtration rate, preferential filtration, and pre-ingestion sorting based on particle size, shape, surface chemistry, and filtrate composition. Rejected particles encapsulated in mucus are expelled as pseudofeces before ingestion while less nutritious items are rapidly egestion as feces. Concurrently, feces and pseudofeces repackage nutrients as biodeposits [
5]. However, some of these aspects of oyster biology remain to be established for the windowpane oyster.
3. Exploitation and Benefits Derived from Bivalves
Humans have long exploited bivalves as sources of food and products used in materials/building, concrete, fertilizer, and cultured pearls. Indirect benefits include shoreline stabilization and nutrient mitigation [
5]. Other indirect benefits accrue from the fact they are filter feeders, and accumulate microorganisms and heavy metals [
10,
11]. These properties may mitigate pollution, but render the meat dangerous, however, they mean that bivlaves such as the windowpane oyster can be useful proxies for monitoring the quality of coastal waters. More recently, molluscs have been studied as a source of novel bio-active molecules.
3.1. Historical Perspective of Exploitation
Windowpane oyster is the name given by travellers in Southern China, which is derived from the use of the shell. Windowpane oyster shells were extensively used in the Portuguese settlements in 1675 in India, because of the scarcity and cost of window glass. At the start of the 20
th Century, window glazing is seen in the Dutch Indies, in the Philippines and in Canton and other districts of Southern China. The Chinese were the first to utilize the shell, and dissemination of this use is credited to the Portuguese [
6] , though without further historical evidence, this may reflect a European colonial perspective, since the extensive trading empires in Asia, e.g., the Austronesian [
12] and later Indian maritime traders [
13] may well have spread this practice.
Windowpane oyster meat is edible, and while pearls of inferior quality are yielded in some quantity, it is the translucent shell that is nowadays commercially and economically important. However, this was not always the case.
Placuna placenta provided a fishing industry of local importance in four widely separated localities in eastern seas. Shells for glazing were half-grown (about 18 months old). These were cleaned and polished by soaking, tossing and shaking several times until dirt and roughness were removed and a translucent mica-like appearance was obtained [
6]. Interestingly, there was a seed pearl fishery in North Bornean waters, which was less well-known, and therefore able to provide for sustainable exploitation by the Badjao divers (Malay inhabitants who, in present time, are one of the cultural minorities in the Philippines currently known as sea gypsies, and scattered along the coastal areas of Mindanao) [
14]. No shell was allowed to be fished under 10 cm in diameter and a license was required before shells could be harvested. Badjao women and children shucked the shells, and slowly heated and purified the flesh for 3 days. After thorough cleaning, seed pearls obtained by the Badjaos were sold to Chinese dealers and exported to China where the bulk was used in preparation of quaint medicines for treatment of eye diseases and syphilis. Similarly small pearls from Ceylon were also brought to India for use as components in native medicines or as a cosmetic, while bigger pearls were sold separately.
Placuna placenta pearls have low value because of their small size, poor luster, irregular shape and lack of hardness compared to gem pearl and the fanciful beliefs and traditions regarding medical uses of the pearls diminished with the progression of evidence-based medicine. However, the implementation of rules to govern windowpane oyster harvesting provides inspiration for the present day.
Philippine history traces the popularity of the shells to the 1860 edition of “Vocabolario de la lengua Tagala”, the first dictionary of the Tagalog language [
15]. Within it, the entry for capiz reads la Ventana (window). Pre-colonially, seashells were used widely in building weapons, decorating clothing, and trading goods [
16]. During Spanish colonization, churches and homes were built using capiz shells as a substitute for glass. Thus, windowpane oyster was the most common type of window material used in the Philippines between 1755 and 1960. The thin translucent shells were individually squared and then set like glass panes into wooden lattice frames to be used as window shutters, a unique feature of Philippine architecture from the Spanish colonial period (
Figure 1). This includes the sliding windows of the 19th century. Such windows are also found in Goa in India. Nowadays, the shells, are used in the manufacture of decorative items such as chandeliers, Christmas decorations, windowpanes, and many more [
17] and from 1960 the shell served as raw material of a lucrative export-oriented shell craft industry (
Figure 2). Thus, windowpane oyster shell products are among the Philippines’ most important fishery exports.
3.2. Modern Economic Exploitation
The windowpane oyster was once very abundant along the Philippine coastline. Before World War II windowpane oyster processing was a lucrative industry in the province of Bataan. It is one of the indigenous seashells originally found in Samal and in the nearby municipalities of Abucay, Balanga, Pilar, Orion, and as far as Limay. However, the population of these bivalves has decreased. Contributing factors include the mechanized boats used by fishermen in collecting small pieces of stones or chips of sea shells locally known as “gasang”, which destroys the natural habitat of the bivalve and destructive methods of fishing and gathering such as trawling, using mechanical rakes and dredges, dynamite fishing and compressor diving [
9]. Thus, the high demand for this bivalve, both locally and internationally, has resulted in over-harvesting and habitat degradation [
16]. In addition, the increase in prawn hatcheries may also contribute to the decline in windowpane oyster harvest. In the late 1980s, prawn hatcheries flushed water laced with antibiotics back to the sea and the contamination may have killed windowpane oysters [
18]. The result is a documented decline in harvest. Locally in Bataan, based on the records of the Bataan provincial Agriculturist’ Office in 2016, 248 tonnes of windowpane oysters were harvested and there was a reduction in harvest of the shellfish to 154 tonnes in 2017 and to 138 tonnes in 2018. The same scenario is repeated across all regions of the Philippines where windowpane oysters are harvested. Thus, export of shells and by-products for the country as a whole showed a substantial decline from 3260 tonnes in 1994 to 1765 tonnes in 1999 [
9]. Consequently, the export of windowpane oyster products, which had an important economic impact in the Philippines, ranking fifth among the major fishery exports in 1991, and generating USD 33.5 million from 1989 to 1991 in shell crafts [
19,
19,
19], declined to USD 7.15 million (900 tonnes) in 1994 [
20], then to USD 4.45 million (498 tonnes) in 1996 [
21]. In 2021, the share of shells and by-products to export of fish and fishery products was only 731 tonnes contributing about USD 1.085 million to Philippine export revenue [
2]. There are fisheries regulations to control harvesting to protect the oysters, however, the high market demand for the shells and a lack of education and enforcement results in gatherers continuing to collect shells with sizes less than 80 mm and more than 100 mm, and natural resources continue to be depleted [
18]. Thus, the export of windowpane oyster shell has become of lesser importance to Philippine fishery export since 2002.
Some remedial measures have been taken, though these have been local, rather than across the entire historical range of the windowpane oyster. Researchers of the Bureau of Fisheries and Aquatic Resources of the province of Bataan, Philippines, demonstrated that stock enhancement of windowpane oyster breeders can be made possible by enclosing a certain area using bamboo fence with nylon nets, and letting the oyster breed naturally. They recommended that strict implementation of fishery laws, rules and regulations could increase the production of the bivalve in the province [
22]. In another study, it was found that transplantation of
Placuna placenta was feasible. Survival after three months of the oysters transplanted during the rainy season was 35-48% and 57-60% for those transplanted in the in the dry season, though larger ones grew only during the dry season. Gonad sizes also increased in weight over the three months, but more during the dry season. Among the larger individuals, gonads matured 3-4 months after transplantation and 60% of the animals spawned in June [
23].
Placuna placenta with an average shell length of 10 cm were successfully spawned by raising the water temperature to 29±0.5 °C. Three water treatment schemes were tested for larval rearing: chlorination, ultraviolet irradiation, and filtration (control). Larvae survived to the umbo veliger stage (180 μm, day 10) in chlorinated sea water, whereas mass mortality occurred at the straight-hinge stage in both UV-treated and filtered sea water. Eggs measured 45 μm on average, and fecundity was 5,000–10,000 per female. Larvae were reared on a combination of the microalgae
Isochrysis galbana, Tetraselmis sp., and
Chaetoceros calcitrans, maintained at a density of 100,000 cells/ml [
23]. It appeared that the combination of the diatom
I. galbana and the green alga
T. tetrahele enhanced gonad development in
P. placenta more than using single algal species [
24]. The Tigbauan coast, Iloilo, Philippines had been depleted of the natural population of the windowpane oysters, but rretained the conditions necessary for their growth and development. Benthic organisms associated with plankton species needed by the animals for food were still abundant and, therefore, oysters could naturally repopulate the area. To achieve this, stocking was carried out during the first and last quarters of the year to avoid the rainy season, which could affect the restocking [
9,
25].
3.3. Nutritional Benefits of Bivalves
Fish and seafood such as molluscs play a key role in human nutrition. Edible molluscs like mussels, clams, scallops, and oysters are naturally low in carbohydrate, e.g., raw scallops, oysters, and mussels have been reported to contain between 3% and 5% carbohydrate [
26], and lipids, but have a relatively higher content of unsaturated fatty acids. Moreover, they can be excellent sources of omega-3 fatty acids, vitamin B12, choline and minerals such as iron, selenium, and zinc, and in these context have a similar or better nutrient value than some shellfish and land-based protein sources [
26] [
27]. Marine bivalves are considered nutritious because they have high quality protein, containing all the essential amino acids [
28,
29], as summarized by the proximate analysis of a number of bivalves (
Table 1).
Calcium is an essential component of the calcium carbonate shells of molluscan shellfish and it is recruited from seawater and deposited at fairly high concentrations in their tissues [
26]. Thus, levels of calcium in shellfish are 2- to 10-fold higher than those found in beef, chicken, or pork. United States Department of Agriculture National Nutrient Database shows that raw oysters and mussels are an excellent source of iron with clams and scallops containing less iron. Thus, while scallops contain less than half the levels of iron of beef, chicken, and pork, oysters, mussels and clams contain several times the amount for land-based meats [
26] (
Table 2). The high levels of minerals found in molluscs are also reflected in
Placuna placenta. Because of the high iron content, a three-ounce serving of windowpane oyster provides 44 % of the daily recommended intake. Although iron is important for red blood cell count, this mineral may cause hemochromatosis or over-absorption of iron in the digestive tract [
26]. However, a significant proportion of the population is moderately to highly iron deficient, and in the Philippines, zinc deficient too, the former problem being most common in women of reproductive age [
33]. A number of essential vitamins are also present (
Table 2). Of particular note is vitamin A, as deficiency in this vitamin can cause blindness, a problem that is prevalent in rice-based diets such as found in the Philippines [
34]. This highlights the potential of the windowpane oyster to address this issue at least in part.
Bivalve lipids have appreciable proportions of omega-3 long-chain polyunsaturated fatty acids (PUFAs), particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Generally, the contents of PUFA are higher than those of saturated fatty acids, and monounsaturated fatty acids (MUFAs) [
37]. The contents of EPA and DHA in shellfish usually range between 300 and 500 mg% in raw muscle, and are generally lower than those of oily finfish such as Atlantic mackerel, salmon, and sardine [
29,
38] (
Table 3).
3.4. Pathogens, Allergens and Contaminants in Bivalves
It is common to consume marine bivalves raw, steamed, or lightly processed [
40], consequently they can act as vectors of a variety of pathogens (
Table 4). This issue is compounded by the fact that bivalves are filter feeders and pathogen pollution is increasingly common in coastal waters. Moreover, bivalves contain allergens that can have major effects on susceptible people. Finally, the high content of certain nutrients can itself cause health issues.
The presence of microbial pathogens and parasites because of the pollution of coastal waters can result in contamination of bivalve shellfish by a variety of microorganisms [
29,
43], a problem compounded by the fact that bivlaves are filter feeders and so can accumulate these pathogens in their tissues or organs [
42]. Resistance of some of these pathogenic microorganisms to antibiotics and the tolerance of bivlaves to heavy metals such as copper, lead, and cadmium can further add further risk. The habit of consuming raw or lightly cooked bivalves increases pathogen-associated risks. Food-associated parasites are recognized as a threat to food safety and human health. Increasing globalization of the food supply, the trend of consuming food raw, and general ignorance about parasites add to this hazard [
29].
The main helminth parasites of bivalves are trematodes, cestodes and nematodes. Trematodes larvae are more important as bivalve pathogens than cestodes and nematodes. Infection in bivalves is most common in tropical and subtropical waters, where elasmobranchs constitute an important proportion of the vertebrate fauna [
41].
Giardia,
Cryptosporidium and
Toxoplasma which are major parasites of humans and animals may retain their infectivity in raw or undercooked molluscs. These are transmitted by contaminated water and food and can cause human gastroenteritis.
Some parasites are not pathogenic to humans, but may reduce the visual appeal of oysters like pea crabs. However, no serious parasites have been detected in stocks of commercially edible
P. placenta in the Philippines. None of the parasites in
Table 4 appear to be a problem due to either low infestation level or low pathological effect [
44].
In addition to the possibility of hemochromatosis alluded to above, oysters may cause stomach problems because of their zinc content. Eighty five g of oysters can contain 67 milligrams of zinc, which is enough to trigger gastrointestinal reactions, because it is more than the tolerable upper intake level of 40 milligrams per day. These reactions include vomiting, diarrhea and abdominal cramps. The problems caused by the zinc in oysters generally arise within three to ten hours of consumption and fade quickly after zinc levels return to normal [
26].
Molluscan shellfish are also recognized as important food allergens. The prevalence of molluscan shellfish allergy is largely unknown, but may have parallel consumption patterns, with higher frequency in areas of frequent consumption[
45]. The major allergen of molluscan shellfish is their tropomyosin, a muscle protein which elicits IgE binding in the sera of half or more of patients with shellfish allergies. It was first identified as the major allergen from shrimp and later recognized as a pan-allergen among invertebrate species including crustaceans and molluscan shellfish, e.g., squid, octopus, cuttlefish, mussel, scallop, and oyster. Although bivalves are likely to be the most frequently ingested class of molluscan shellfish, the existence of allergic reactions to bivalves is rather poorly documented in the medical literature. The prevalence of allergies to oyster, clam, mussels, scallops and cockles have been reported in different countries, but these were based mainly on surveys without any diagnostic follow-up. However, evidence for the existence of mussel allergy is reasonably strong because of sensitivity to mussels by the presence of mussel-specific IgE in patient blood serum[
46]. Arginine kinase (AK) has more recently been identified as a novel allergen in
Crassostrea angulata. After cloning to produce recombinant AK (rAK), it was found that native AK and rAK had similar IgG/IgE-binding activity and physicochemical properties, and exhibited cross-reactivity among oysters, shrimps, and crabs after serological analysis of oyster-sensitive individuals [
47].
The accumulation of metals and human pathogens by bivalves also provides an opportunity because bivalves tolerate these contaminants. Their immobility and wide distribution allows bivalves to be used for monitoring the concentration of such pollutants in coastal areas [
11]. They also remove contaminants by being hyperaccumulators of Cu (
Crassostrea virginica [2013 mg Cu kg
−1 ]), Pb (
Mytilus edulis [506 mg Pb kg
−1 ]), Cd (
Pinctada albina albina [108 mg Cd kg
−1 ]) and Al (
Crassostrea rhizophorae [2240 mg Al kg
−1 ]), and approached this status for Zn (
Crassostrea virginica [9077 mg Zn kg
−1]) [
10]. However, there is no information yet regarding hyperaccumulation of heavy metals by windowpane oyster. Similarly, with respect to human pathogens, allergens and contaminants, there are no systematic data on the windowpane oyster. The above considerations of other molluscs indicates that this is an area ripe for investigation.
4. Potentials/Processing Possibilities for Windowpane Oyster by-Products
Marine animals such as bivalves are a rich source of bio-active compounds (briefly summarised in
Section 4.1), though the potential of the windowpane oyster in this respect is unknown. There have been local initiatives to develop products based on the windowpane oyster meat, but as described in
Section 4.2, these have not been followed up. Moreover, while there are a considerable number of studies that have assessed the nutritional value of oysters and other molluscs, only one analysis has been performed on
Placuna placenta, reported in the Food and Nutrition Research Institute published Philippine Food Composition Table (PhilFCT), which has nutrition facts of different Filipino foods (
Table 1,
Table 2 and
Table 3). How robust these data are and how variable they may be in terms of location and season is not known. Thus, although consumers and researchers interested in windowpane oyster meat can consult the Philippine Food Composition Table as reference, the information listed in the Tables is limited to proximate composition, some minerals, vitamin content and fatty acid composition. A more thorough investigation of the biochemical characteristics of this bivalve needs to be conducted to determine the processing potential and safety of its by-product meat for human consumption.
4.1. Bioactive Compounds in Marine Invertebrates
4.1.1. Peptides
Marine organisms significantly contribute to economic and research development because these are a major part of the human diet, and many contain bio active compounds. Their complex habitats and exposure to extreme conditions, such as salinity, pressure, temperature, and illumination result in marine bio-active peptides differing in terms of their sequences and properties from those derived from land animals. It has been suggested that because marine organisms are in close contact with microbes they consequently are a substantial source of antimicrobial peptides [
48]. Thus, in recent years, researchers have isolated antimicrobial peptides from a variety marine organisms including oysters, and these peptides are safe, natural, inexpensive, and exhibit high bio-activity.
4.1.2. Shell Proteins and Polysaccharides
The shells of molluscs can be considered to be a specialised extracellular matrix; CaCO
3 crystals are embedded in a matrix of protein and polysaccharides, which control the shell mineral deposition and many of the proteins have been characterised [
49,
50]. However, since the windowpane oyster shell in itself is on high value, use of its proteins and polysaccharides would only be feasible for the waste from the shell processing industry and would require the identification of a high value protein or polysaccharides such ones with therapeutic activity. Anecdotal data indicate that the windowpane oyster contains a large amount of mucus, commonly referred to as ‘slime’, to the extent that traditional processing of the meat starts with extensive washing to remove this. Thus, there may be high value compounds in the shell waste, the meat and in the mucus. Although to date nothing is known about the proteins and polysaccharides in the windowpane oyster, examination of what has been found in other marine organisms supports the contention that this is an area ripe for investigation.
4.1.3. Glycosaminoglycans in Marine Invertebrates
Glycosaminoglycans in molluscs and other marine organisms have received considerable attention as a potential alternative source of heparin, a World Health Organisation essential drug, following the 2008 heparin contamination scandal, where batches of anticoagulant heparin were contaminated with over sulfated chondroitin sulfate, resulting in the deaths of patients [
51]. Glycosaminoglycans are characteristic of all multicellular organisms [
52] and genes likely to encode their biosynthetic enzymes [
53] have been identified in choanoflagellates, which are considered to be the descendants of the last common unicellular ancestor of multicellular organisms. Importantly, the glysocaminoglycan chondroitin sulfate has been isolated from a choanoflagellate and demonstrated to regulate sexual reproduction, one of the functions of GAGs found in multicellular organisms [
54]. It is, therefore, not surprising that molluscs contain glycosaminoglycans.
The GAGs in marine organisms may be even more structurally diverse than those in land vertebrates (reviewed [
55]). A unique HS from bivalve
Nodipecten nodosus has disaccharide units chain composed of nonsulfated, 2-O-sulfated or 3-O-sulfated GlcA as well as 2-N-sulfated and/or 6-O-sulfated GlcN, and the anticoagulant activity of the mollusc HS was 5-fold lower than that of porcine heparin [
55] The GAGs of the marine clam
Scapharca inaequivalvis are enriched in dermatan sulfate, 58% of total GAG content, with CS and HS contributing 16 % and 26 %, respectively [
55]. The bivalve
Mytilus galloprovincialis produces considerable amounts of hyaluronic acid of average size 200 kDa; HA from other sources is used as a filler for wrinkles in the cosmetic industry [
55]. The fresh water bivalve,
Anodonta (
Anodonta)
cygnea, has been shown to contain a heparin, that is structurally analogous to that from bovine lung heparin, with an anticoagulant activity similar to that of porcine intestinal heparin [
56].
The considerable work done on shrimp GAGs provides further support for research on marine sources of GAGs. The shrimp
Litopenaeus vannamei has emerged as a promising reservoir of bioactive glycans. One such compound, resembling heparin in structure and function, has demonstrated remarkable anti-inflammatory effects [
57]. This shrimp heparin-like compound not only reduced inflammatory cell migration to injury sites in acute inflammation models, but also exhibited the ability to mitigate matrix metalloproteinase (MMP) activity, which is crucial for tissue remodelling. Impressively, its anti-inflammatory potential surpassed that of mammalian heparin while displaying minimal anticoagulant activity. Similarly, a novel heparin/heparan sulfate-like compound from the shrimp's head has been identified. Although distinct from conventional heparin or heparan sulfate, this compound displayed shared structural features, including
N- and 6-
O-sulfation, as well as comparable glucuronic acid content [
58]. Notably, this unique compound exhibited significant anticoagulant activity in aPTT and Factor-Xa inhibition assays, consistent with its ability to stabilise antithrombin III [
59]. This shrimp compound's dual attributes of potent anticoagulation and minimal haemorrhagic effects sets it apart as a potential alternative to mammalian heparin.
The exploration of marine-derived glycans extends beyond their anti-inflammatory and anticoagulant activities to encompass their role in angiogenic processes. Heparin and heparan sulfate’s ability to modulate angiogenic factors and cytokines has prompted the investigation into the potential of a shrimp-derived heparin-like compound in this realm. An unusual heparin-like molecule containing significantly high amounts of glucosamine 3-O-sulfation [
60] was found to interact with growth factors crucial to angiogenesis, such as fibroblast growth factor-2, epidermal growth factor family members, and vascular endothelial growth factor, leading to the inhibition of endothelial cell proliferation. Furthermore, its impact extended to modulating the architecture of capillary-like structures and reducing choroidal neovascularisation (CNV) area [
61].
The multifaceted properties of marine-derived glycans, particularly the shrimp heparin-like compounds, showcase their potential as valuable candidates for addressing diseases including neovascular age-related macular degeneration and other antiproliferative disorders, offering a fresh avenue for therapeutic development based on natural sources. The above work in shrimp-derived heparin/heparan sulfate, alongside that done on some bivalves and extensive studies on tunicate-derived glycosaminoglycans (reviewed [
62,
63]) highlights that there is ample justification for exploring the activities of GAGs from bivalves such as the windowpane oyster, both as sources of existing drugs such as anticoagulant heparin and novel ones. Moreover, since most of these compounds are derived from by-products generated during their commercial exploitation (such as shrimp heads), this would exemplify a successful implementation of the circular economy paradigm. This endeavour not only aids the food industry, but also holds the promise of deriving potential pharmaceutical resources from waste materials. This, in turn, could have far-reaching effects on waste management practices and enhance the environmental sustainability of these products, contributing to their "green premium."
4.2. Windowpane Oyster By-Products and Waste
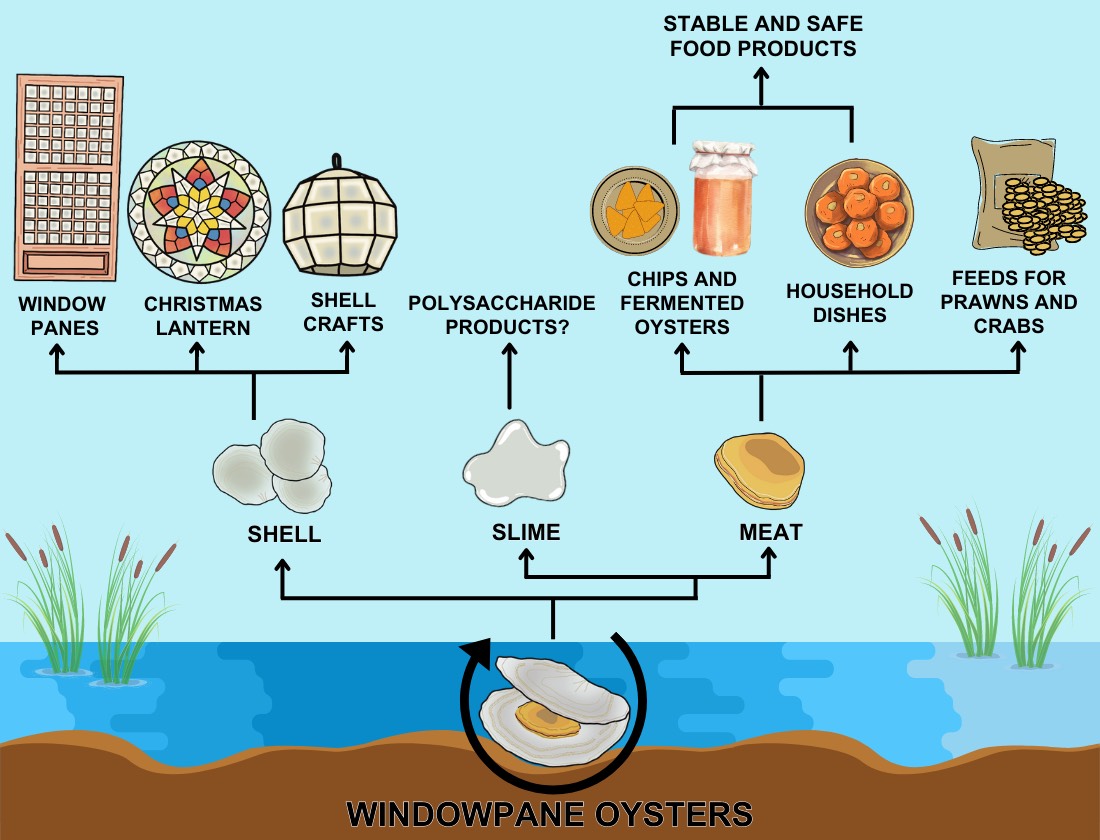
Fish and other aquatic organisms are processed into various food and non-food products. During processing of the windowpane oyster large amounts of by-products are under-utilized if not wasted. Harvesting of windowpane oysters provides local fishermen not only income from selling translucent and durable shells for shell craft industry, but also cheap and nutritious protein sources for their families [
3] (
Figure 3), though as noted above, over reliance on this food source may causes issues. A more efficient utilization of by-products of windowpane oyster processing would reduce the problem of disposal and subsequent environmental pollution. Furthermore, the shellfish will be valorized, which would elevate the nutritional and economic status of the fishing community wherein windowpane oyster processing is one of the primary industries.
Notable amongst the local initiatives to develop the windowpane oyster meat, is the work of the Chairman of the Kaliwanag Livelihood Association (KLA) of Samal, Bataan, who enthused that they hope to make windowpane oyster a major source of income for town residents, and if every member of the organization got involved, the benefits would increase and the oysters’ shells and meat would become distinctive products of their town. Thus, the Mayor was of the opinion that the molluscs should be one of the prime commodities of the town, claiming they harvest about 250 tons per year, which are then made into various products [
64].
Thus, members of the Kaliwanag Rural Improvement Club of Samal, Bataan, engaged in the development of windowpane oyster-based products, utilized windowpane oyster meat in processing chips and fermented oysters [
22], and standardized recipes of dishes with windowpane oyster meat as the main ingredient [
65]. It has been claimed that although “kapis” chips are smaller than mussel chips, the product is tastier and very rich in protein [
65]. However, these claims are not as yet supported by any systematic and controlled analysis regarding nutritional value (which can alter during processing), sensory evaluation, shelf life and so on. In part due to the lack of such scientific underpinning,
the efforts of advocates of windowpane oyster meat processing and their efforts in the transfer of knowledge to develop the industry has not been sustained, and the oyster has not entered the Philippine national food system. Thus, although windowpane oyster meat is edible and has potential as a raw material for producing low cost nutritious products, it still has very low value and its use is confined to local dishes and consumed by families in the fishing community and so does not provide extra income to the gatherers and shuckers. Thus, a rigorous development of windowpane oyster meat products and their analysis will be important to promote its wider consumption, contributing to more balanced nutrition. Importantly, the technology of processing the meat should not be limited to making chips and cracklings. Since windowpane oyster meat is already used as primary ingredient in cooking dishes locally, studies of such dishes should be conducted to make these dishes more acceptable, stable, and guarantee their safety to the consumer. The latter would include rigorous quality control to exclude dead oysters and any harvested during bloom of toxic algae (colloquially known as ‘red tides’). Consequently, these products have yet to enter the higher value urban food system and these ambitions have not been firmly established locally, let alone nationally or been supported by systematic analysis of the quality and safety of the products, to support marketing of products.
Which glycosaminoglycans are present in windowpane oyster meat and their properties is unknown. Another source of polysaccharides and likely other bioactive compounds is the extensive mucus slime, which is currently discarded. However, nothing is known about its molecular composition or biological activities. Given the number of potentially useful bioactivities discovered in other molluscs, which range from anticoagulant heparins to antimicrobial peptides, it is likely that analysis of the mucus would provide products and active structures that could be synthesised in clinically useful quantities.
5. Conclusion
Exploitation of the windowpane oyster Placuna placenta has considerable potential economic and social benefits. To achieve these will require research programmes ranging from developing food products that can enter the food system, to generate additional income to the oyster harvesting communities, to analysis of the mucus slime to identify bioactive compounds. In parallel, given the extensive destruction of the oyster beds through over harvesting, often by inappropriate means, an effort to regenerate the oyster beds and regulate harvesting effectively is required. While the means to do so are established, their implementation at scale may require the incentive of increasing the economic value of the windowpane oyster though exploitation of the meat and other by-products. The need for quality control of food and medicinal products would, in turn, provide a proxy monitoring of the quality of coastal waters. In addition, restoration of the oyster beds would contribute to the physical stabilisation of the coasts, increasingly important in the face of global warming and increased numbers and strengths of typhoons experienced by the Philippines. A multidisciplinary research programme to harness developments in these different areas and promote appropriate development of the windowpane oyster industry into new avenues would thus be timely.
References
- FAO. The State of World Fisheries and Aquaculture 2022; Toward Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, 2022. [Google Scholar]
- PHILFIS Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources, 2021.
- Adan, R.I.Y. SEAFDEC Asian Aquaculture. August 2000, p. 23,31.
- Dame, R.F.; Kenneth, M.J. Ecology of Marine Bivalves An Ecosystem Approach; Oven, A., Ed.; Marine Science; Second.; CRC Press Taylor and Francis Group: Boca Raton Florida. USA, 2012; ISBN 9781439839096.
- Vaughn, C.C.; Hoellein, T.J. Bivalve Impacts in Freshwater and Marine Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2018, Vol. 49, 183–208. [Google Scholar] [CrossRef]
- Hornell, J. Marine Zoology of Okhamandal India; Biodiversity Heritage library, 1909.
- Jiratipayabood, P. Windowpane Oysters (Placuna Placenta) in Siit Bay; 2017; pp. 1–11.
- Yonge, M. Form and Evolution in the Anomiacea (Mollusca: Bivalvia)-Pododesmus, Anomia, Patro, Enigmonia (Anomiidae): Placunanomia, Placuna (Placunidae Fam. Nov.). Philos. Trans. R. Soc. B 1977, 276, 453–523. [Google Scholar] [CrossRef]
- Madrones-Ladja, J.A. Salinity Effect on the Embryonic Development, Larval Growth and Survival at Metamorphosis of Placuna Placenta Linnaeus (1758). Aquaculture 2002, 214, 411–418. [Google Scholar] [CrossRef]
- Gifford, S.; O’Connor, W.; Koller, C.E.; MacFarlane, G.R.; Dunstan, R.H. Aquatic Zooremediation: Deploying Animals to Remediate Contaminated Aquatic Environments. Trends Biotechnol. 2007, 25, 60–65. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.P. National Distribution of Chemical Concentrations in Mussels and Oysters in the USA. Mar. Environ. Res. 2002, 53, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, W. The Dispersal of Austronesian Boat Forms in the Indian Ocean". In Blench, Roger; Spriggs, Matthew (Eds.). In One World Archaeology; Archaeology and Language III: Artefacts languages, and texts.; Routledge: London and New York, 1999; Vol. 34, pp. 144–179 ISBN 978-0-415-10054-0.
- Bellina, B. Southeast Asia and the Early Maritime Silk Road. In Lost Kingdoms: Hindu-Buddhist Sculpture of Early Southeast Asia; Guy, J., Ed.; Yale University Press: New York, USA, 2014; pp. 22–25. ISBN 978-1-58839-524-5. [Google Scholar]
- National Commission on Indigenous peoples Badjao. In The Indigenous Peoples of the Philippines; Rex Bookstore Inc., 2007; pp. 3–5 ISBN 978-971-23-4670-5.
- Noceda, J.J. de; Sanlucar, P. de Vocabulario de la lengua tagala: compuesto por varios religiosos doctos y graves, y coordinado; Ramirez y Giraudier, 1860.
- Bautista, N. What You Need to Know about Capiz Shell Jewelry 2023.
- Floren, A.S. The Philippine Shell Industry with Special Focus on Mactan Cebu; Department of Environment and Natural Resources, 2003; pp. 1–50.
- Park, M. Capiz Shells and Their Uses; 2009.
- PHILFIS Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources, 1991.
- Philippine Fisheries Profile 1994.
- PHILFIS Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources, 1996.
- Hermoso, R.S. Kapis Chips: A Nutritious Finger Food from the Sea; Bureau of Agricultural Research, 2018.
- Garibay, S.S.; Golez, S.N.; Unggui, A.S. Transplantation, Hatchery, and Grow-out of Window-Pane Oyster Placuna Placenta in Guimaras and Iloilo. In Research Output of the Fisheries Sector Program; Bagarinao, T.U., Ed.; Reports on Fisheries and Aquaculture; SEAFDEC/AQD-Department of Agriculture (DA) - Philippines: Quezon City, Philippines: Bureau of Agricultural Research, Department of Agriculture, 2007; Vol. 2, pp. 119–122 ISBN 971-8511-77-6.
- Gallardo, W.G.; De Castro, Ma.T.R.; Buensuceso, R.T.; Espegadera, C.C.; Baylon, C.C. Gonad Development of Placuna Placenta Linnaeus Fed Isochrysis Galbana Parke, Tetraselmis Tetrahele (G.S. West) Butch, or Their Combination. Aquaculture 1992, 102, 357–361. [Google Scholar] [CrossRef]
- Gallardo, W.G.; Siar, S.V.; EncenaI, V. Exploitation of the Window-Pane Shell Placuna Placenta in the Philippines. Biol. Conserv. 1995, 73, 33–38. [Google Scholar] [CrossRef]
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional Value and Food Safety of Bivalve Molluscan Shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- FDA CFR - Code of Federal Regulations Title 21 2023.
- Miletic, I.; Miric, M.; Lalic, Z.; Sobajic, S. Composition of Lipids and Proteins of Several Species of Molluscs, Marine and Terrestrial, from the Adriatic Sea and Serbia. Elsevier 1991, 41, 303–308. [Google Scholar] [CrossRef]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef]
- Asha, K.K.; Anandan, R.; Mathew, S.; Lakshmanan, P.T. Biochemical Profile of Oyster Crassostrea Madrasensis and Its Nutritional Attributes. Egypt. J. Aquat. Res. 2014, 40, 35–41. [Google Scholar] [CrossRef]
- Food and Nutrition Research Institute Philippine Food Composition Table Online Database (PhilFCT); 2019.
- Naidu, K.S.; Botta, J.R. Taste Panel Assessment and Proximate Composition of Cultured and Wild Sea Scallops, Placopecten Magellanicus (Gmelin). Aquaculture 1978, 15, 243–247. [Google Scholar] [CrossRef]
- Palanog, A.D.; Calayugan, M.I.C.; Descalsota-Empleo, G.I.; Amparado, A.; Inabangan-Asilo, M.A.; Arocena, E.C.; Sta. Cruz, P.C.; Borromeo, T.H.; Lalusin, A.; Hernandez, J.E.; et al. Zinc and Iron Nutrition Status in the Philippines Population and Local Soils. Front. Nutr. 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Solon, F.S.; Popkin, B.M.; Fernandez, T.L.; Latham, M.C. Vitamin A Deficiency in the Philippines: A Study of Xerophthalmia in Cebu. Am. J. Clin. Nutr. 1978, 31, 360–368. [Google Scholar] [CrossRef]
- Chakraborty, K.; Selsa, J. Chakkalakal,; Deepu Joseph; Asokan, P.K.; Vijayan, K.K. Nutritional and Antioxidative Attributes of Green Mussel (Perna Viridis L.) from the Southwestern Coast of India. J. Aquat. Food Prod. Technol. 2016, 25, 968–985. [Google Scholar] [CrossRef]
- FISHER, R.A.; RIPPEN, T.E.; DUPAUL, W.D. Nutritional, Proximate, and Microbial Characteristics of Phosphate Processed Sea Scallops (Placopecten Magellanicus). J. Muscle Foods 1996, 7, 73–92. [Google Scholar] [CrossRef]
- Bergé, J.-P.; Barnathan, G. Fatty Acids from Lipids of Marine Organisms: Molecular Biodiversity, Roles as Biomarkers, Biologically Active Compounds, and Economical Aspects. In Advances in Biochemical Engineering/Biotechnology; Springer,: Berlin, Heidelberg, 2005; Volume 96, pp. 49–125. ISBN 978-3-540-31549-0. [Google Scholar]
- Hossain, Z.; Takahashi, K. Health Benefits of Bio-Functional Marine Lipids. In Second International Congress on Seafood Technology on Sustainable Innovative and Healthy Seafood: FAO/The University of Alaska, 10 - 13 May 2010, Anchorage, the United States of America; Ryder, J., Ed.; FAO fisheries and aquaculture proceedings; Food and Agriculture Organization of the United Nations: Rome, 2012 ISBN 978-92-5-107108-3.
- Napolitano, G.E.; MacDonald, B.A.; Thompson, R.J.; Ackman, R.G. Lipid Composition of Eggs and Adductor Muscle in Giant Scallops (Placopecten Magellanicus) from Different Habitats. Mar. Biol. 1992, 113, 71–76. [Google Scholar] [CrossRef]
- Lattos, A.; Chaligiannis, I.; Papadopoulos, D.; Giantsis, I.A.; Petridou, E.I.; Baker, G.L.; Staikou, A.; Michaelidis, B. How Safe to Eat Are Raw Bivalves? Host Pathogenic and Public Health Concern Microbes within Mussels, Oysters, and Clams in Greek Markets. Foods- MDPI 2021, 10. [Google Scholar] [CrossRef]
- Gosling, E. Marine Bivalve Mollusks; Second.; Wiley and Sons, 2015.
- Tedde, T.; Piras, G.; Salza, S.; Nives, R.M.; Sanna, G.; Tola, S.; Culurgioni, J.; Piras, C.; Merella, P.; Garippa, G.; et al. Investigation into Cryptosporidium and Giardia in Bivalve Mollusks Farmed in Sardinia Region and Destined for Human Consumption. Ital. J. Food Saf. 2013, 2, 91–93. [Google Scholar] [CrossRef]
- Brinkley, M. Raw Oysters and Food Poisoning Available online: https://www.livestrong.com/article/435577-side-effects-of-eating-oysters/.
- Pagador, E.G. Parasites of Window-Pane Oyster (Placuna Placenta Linnaeus, 1758) from Trapiche, Oton in West Central Philippines. Philipp. Agric. Sci. 2015, 98, 323–327. [Google Scholar]
- FARRP Allergenic Foods and Their Allergens: Molluscan Shellfish. Available online: https://farrp.unl.edu/informallmollshellfish (accessed on 23 August 2023).
- Taylor, S.L. Chapter 4 - Molluscan Shellfish Allergy. In Advances in Food and Nutrition Research; Elsevier, 2008; Vol. 54, pp. 139–177.
- Huan, F.; Han, T.-J.; Liu, M.; Li, M.-S.; Yang, Y.; Liu, Q.-M.; Lai, D.; Cao, M.-J.; Liu, G.-M. Identification and Characterization of Crassostrea Angulata Arginine Kinase, a Novel Allergen That Causes Cross-Reactivity among Shellfish. Food Funct. 2021, 12, 9866–9879. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. BioMed Res. Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. In Current Topics in Developmental Biology; 2007; Vol. 80, pp. 209–276.
- Latire, T.; Florence Legendre; Bigot, N.; Carduner, L.; Kellouche, S.; Mouloud Bouyoucef,; Serpentini, A.; Frederic Marin; Carreiras, F.; Jean-Marc Lebel; et al. Shell Extracts from the Marine Bivalve Pecten Maximus Regulate the Synthesis of Extracellular Matrix in Primary Cultured Human Skin Fibroblasts. PLOS ONE 2014, 9. [CrossRef]
- Hedlund, K.D.; Coyne, D.P.; Sanford, D.M.; Huddelson, J. The Heparin Recall of 2008. Perfusion 2012, 28, 61–65. [Google Scholar] [CrossRef]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. The Heparanome and Regulation of Cell Function: Structures, Functions and Challenges. Front Biosci 2008, 13, 4309–4338. [Google Scholar] [CrossRef] [PubMed]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. A Systems Biology Approach for the Investigation of the Heparin/Heparan Sulfate Interactome. J Biol Chem 2011, 286, 19892–19904. [Google Scholar] [CrossRef]
- Woznica, A.; Gerdt, J.P.; Hulett, R.E.; King, N. Mating in the Closest Living Relatives of Animals Is Induced by a Bacterial Chondroitinase. Cell 2017, 170, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K.; Ozbek, S. Evolution of Glycosaminoglycans: Comparative Biochemical Study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef]
- Volpi, N.; Maccari, F. Glycosaminoglycan Composition of the Large Freshwater Mollusc Bivalve Anodonta Anodonta. Biomacromolecules 2005, 6, 3174–3180. [Google Scholar] [CrossRef]
- Brito, A.S.; Arimatéia, D.S.; Souza, L.R.; Lima, M.A.; Santos, V.O.; Medeiros, V.P.; Ferreira, P.A.; Silva, R.A.; Ferreira, C.V.; Justo, G.Z.; et al. Anti-Inflammatory Properties of a Heparin-like Glycosaminoglycan with Reduced Anti-Coagulant Activity Isolated from a Marine Shrimp. Bioorg. Med. Chem. 2008, 16, 9588–9595. [Google Scholar] [CrossRef]
- Brito, A.S.; Cavalcante, R.S.; Palhares, L.C.G.F.; Hughes, A.J.; Andrade, G.P.V.; Yates, E.A.; Nader, H.B.; Lima, M.A.; Chavante, S.F. A Non-Hemorrhagic Hybrid Heparin/Heparan Sulfate with Anticoagulant Potential. Carbohydr. Polym. 2014, 99, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Hughes, A.J.; Veraldi, N.; Rudd, T.R.; Hussain, R.; Brito, A.S.; Chavante, S.F.; Tersariol, I.I.; Siligardi, G.; Nader, H.B.; et al. Antithrombin Stabilisation by Sulfated Carbohydrates Correlates with Anticoagulant Activity. MedChemComm 2013, 4, 870. [Google Scholar] [CrossRef]
- Chavante, S.F.; Brito, A.S.; Lima, M.; Yates, E.; Nader, H.; Guerrini, M.; Torri, G.; Bisio, A. A Heparin-like Glycosaminoglycan from Shrimp Containing High Levels of 3-O-Sulfated D-Glucosamine Groups in an Unusual Trisaccharide Sequence. Carbohydr. Res. 2014, 390, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.L.; Regatieri, C.V.; Lima, M.A.; Paredes-gamero, E.J.; Brito, A.S.; Chavante, S.F.; Belfort jr, R.; Farah, M.E.; Nader, H.B. A Heparin Mimetic Isolated from a Marine Shrimp Suppresses Neovascularization. J. Thromb. Haemost. 2010, 8, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.S.G. Glycosaminoglycans Analogs from Marine Invertebrates: Structure, Biological Effects, and Potential as New Therapeutics. Front. Cell. Infect. Microbiol. 2014, 4, 123. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, K.; Espinosa, D.C.R.; Fortuna-Costa, A.; Pavão, M.S.G. Biological Function of Unique Sulfated Glycosaminoglycans in Primitive Chordates. Glycoconj. J. 2017, 34, 277–283. [Google Scholar] [CrossRef]
- Gonzaga, R. After Jessica Sanchez, Samal’s Capiz Products Now in Limelight. Philipp. Dairy Inq. 2012.
- Brion, A.C. Kapis Chips: New Delicacy from the Sea. Press. Philipp. Star 2015.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).