1. Introduction
Epithelial-mesenchymal transition (EMT) is a biological process where epithelial cells lose their adhesive properties and gain invasive, metastatic, and mesenchymal properties.(1) EMT plays an important role during embryo development and has been characterized in tumor formation and the metastatic process. Growing evidence suggests that EMT is not a simple one-directional process wherein epithelial (E) cells become mesenchymal (M) cells but is a dynamic process that involves multiple stages of differentiation and adaptation. In addition, many of these intermediate stages reveal a high degree of plasticity.
EMT involves multiple steps, which starts with: 1. the loss of apical-basal cell polarity; 2. disruption of cell-to-cell interaction; 3. extracellular matrix (ECM) disassembly, resulting in degradation of the basal membrane; 4. ECM reorganization, followed by 5. reorganization of the actin cytoskeleton.(1) Several transcription factors including genes in the SNAIL, TWIST, and Zeb families have been described as the hallmarks of mesenchymal transformation(2); however, they may not play a role in the early stages of cytoskeletal remodeling, loss of apical-basal polarity, cell-cell adhesion weakening and loss of integrity of the basement membrane. The characteristics of these early EMT cells, their importance in tumor progression, and the key regulators in the tumor microenvironment that support this phenotype are still poorly understood.
Ovarian cancer (OC) is the fifth leading cause of cancer deaths among women in the United States with approximately 22,000 women diagnosed and 14,000 women dying from the disease annually. Four out of five women with OC are diagnosed in advanced stages with a precipitous drop in 5-year survival from 95% to between 35-60% in advanced stage disease.(3) Unfortunately, the biological components of early stages of malignant transformation and metastatic process in ovarian cancer has not been elucidated.
The origin of the High Grade Serous Ovarian Cancers (HGSOC) is widely accepted to be the fallopian tubes where early lesions have been identified and are known as serous tubal intraepithelial carcinoma (STIC) or serous tubal intraepithelial lesions (STILs). The main characteristic of these lesions is the presence of a “p53” signature determined by immunocytochemistry. (4, 5) It is thought that the early stages of EMT occurs at the STIC or STIL, generating mesenchymal cells that migrate into the ovaries during ovulation (6) and the peritoneal cavity.(7) These findings suggest that the early events associated with EMT may occur within the fallopian tube epithelium.(4) Thus, identification of this “trigger/s”, its downstream targets and therapeutic targeting is essential in efforts to substantively improve the survival of women with OC.
Connective tissue growth factor (CTGF) is a secreted extracellular protein encoded by the CCN2 gene. It belongs to the CCN family of extracellular matrix associated heparin binding proteins and has been implicated in the control of a number of biological processes including cell proliferation, differentiation and adhesion.(8, 9) Secreted CTGF can bind to several cell surface receptors including integrins, heparin sulfate proteoglycans, lipoprotein receptor related proteins and tyrosine kinase receptors functioning as a bridge between the ECM and the epithelial structure of the cells.(10) The interaction between the epithelial cell and the extra-cellular matrix components including laminin, collagen and integrins have been shown to be important in regulating EMT.(11, 12)
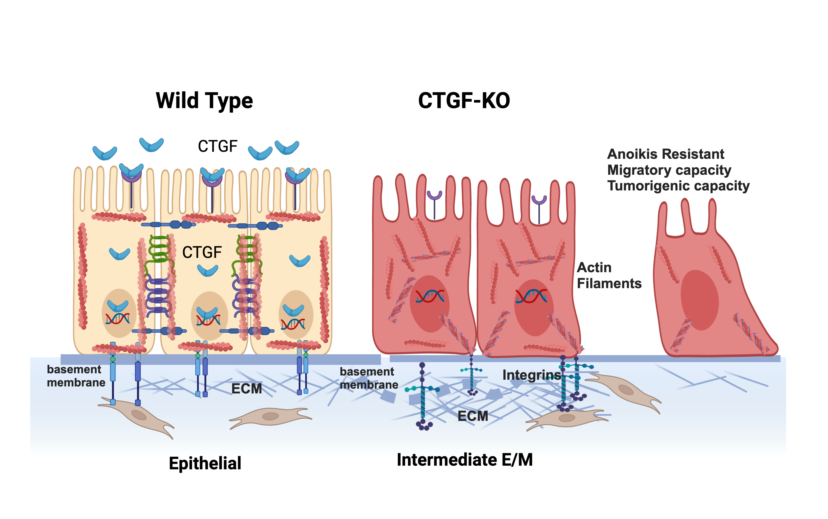
Using an in-vitro model of ovarian cancer EMT we identified CTGF as one of the genes differentially expressed during the early stages of epithelial differentiation towards a mesenchymal phenotype. We hypothesize that CTGF, by maintaining the interaction between the epithelium and the ECM, preserves their epithelial phenotype. The overall objective of this study was to better understand the molecular changes associated with the early transition between the epithelial and mesenchymal phenotype in OC and more specifically to elucidate the role of CTGF in this transition. Using in vitro and in vivo studies, we demonstrate that the loss of CTGF expression is associated with cytoskeleton reorganization, ECM remodeling and acquirement of early mesenchymal properties.
2. Materials and Methods
Cell lines and culture conditions: Samples were obtained with patient consent and approved by the Human Investigation committee of Yale University School of Medicine. Ovarian cancer cells were isolated from patients with stage III or IV high-grade serous carcinoma. These in-house derived ovarian cancer cells (R182, R2615, MR182, MR2615) have been previously described (13-16). Ovarian cancer cell lines (OVCAR3, OVCAR432, OVCAR433, SKOV3 and A2780) were purchased from ATCC(Manassas, Virginia). Cell lines were cultured in RPMI1640 (ThermoFisher Scientific) supplemented with 10% FBS, and 1% each of Na pyruvate, HEPES, MEM-NEAA and penicillin-streptomycin under standard cell culture conditions. All cell lines were tested regularly for mycoplasma by PCR and authenticated once a year by STR profiling and used within 6 passages between experiments.
Antibodies and reagents: Antibodies used were listed in Supplementary Table 1. Phalloidin iFluor488 (Abcam, Boston, MA) was used for f-actin staining. Recombinant human CTGF (rCTGF) were purchased from PeproTech(Cranbury, NJ).
Anoikis Assay: The anoikis assay was performed in a 6-or 96- well Costar cell culture plate with Ultra-low attachment surface(Corning, Kennebunk, ME). To determine cell viability, in 96 well plate, 10,000 cells were plated/well in 200ul growth media. At 0, 24, 48 and 72 hour of culture 40 ul of Cell Titer 96 AqueousOne Solution(Promega, Madison, WI ) was added to triplicate wells(including wells with media only), incubated for 4 hour @ 37oC and then absorbance readings taken at 450nm. Data is presented as relative cell viability(1=cell viability at Time=0)
Conditioned media protocol: R182 and R182 CTGFKO cells were plated into 6 well TC plates at 4 x 105/well in growth media. When cells are completely confluent(24 hour), media is exchanged for Optimem media(Gibco), 4ml/well. After 24 hours, media is collected from cells(=conditioned media), centrifuged at 1500rpm, 10 min, 4oC and supernatant transferred to new tube. Conditioned media is either frozen immediately at -80oC or concentrated. Conditioned media was concentrated using Amicon Ultra4-Centrifugal Filter (Millipore, Billerica, MA), 30kD cutoff. 4ml conditioned media added to filter, centrifuged at 4000g, 15 min at RT. Concentrate collected and volume measured. Concentrating resulted in a 60 – 88 fold concentrate.
CRISPR: Knockout of CTGF was done using the CTGF sgRNA CRISPR/CAS9 All-in-One Lentivector set(Human)(Applied Biological Materials Inc., Richmond, BC, CANADA), which includes 3 different guides. Selection for knockout was with puromycin. CTGF knockout was confirmed with Sanger sequencing. Genomic DNA was extracted from the CTGF k-o cells with Qiagen DNAeasy Blood & Tissue kit. PCR with CTGF primers was done and the pcr product sent to Genewiz LLC (South Plainfield, NJ) for sequencing.
IC50 assay chemosensitivity. IC50 were calculated as described previously. (17)
Invasion assay: Invasion assays were performed in 96-well plates. Briefly, 3000 cells were plated in 50% Cultrex-BME, RGF (R&D Systems,Inc., Minneapolis,MN)/RPMI1640 cell culture media/well of 96 well plate and then placed in a humidified (37*C, 5% Co2) BioTek BioSpa live cell analysis system (BioTek, Santa Clara CA). Cells were monitored and imaged every 4 hours for a total of 6 days using a Cytation 5 cell imaging system (BioTek, Santa Clara CA) and confluence was determined.
RNA sequencing: RNA sequencing of CTGF knockout (KO) and wildtype (WT) R182 cells were performed by GENEWIZ LLC (South Plainfield, New Jersey, USA) as described previously(18). Differential gene expression analysis comparing R182-CTGFKO cells to WT R182-WT cells was performed by GENEWIZ LLC using the DESeq2 package. (19) Simply, a generalized linear model based on the two groups being compared was fitted to the gene expression data with Wald’s test performed to compute fold changes and corresponding p-values for each gene.(19) Genes were considered differentially expressed if the corresponding FDR-adjusted p-value was < 0.05 and absolute log2 fold change > 0.6. Gene Ontology analysis was performed using the iPathwayGuide (20-22)software from AdvaitaBio (Advaita Corporation, Ann Arbor, Michigan, USA). Gene Ontology biological process terms were considered significantly enriched if the respective FDR-adjusted p-values was < 0.05. The enriched biological processes as well as volcano plot of differentially expressed genes were graphed using the ggplot2 package in R.(23)
q-PCR: Total RNA was extracted using the RNeasy Mini Kits (Qiagen, Austin, TX, USA) according to manufacturer’s instructions. cDNA was synthesized using iScript cDNA kit (Bio-Rad, Hercules, CA, USA). Quantitative PCR was performed followed by detection with the CFX96TM PCR detection system (Bio-Rad, Hercules, CA, USA). GAPDH was used as the reference control. Relative expression was calculated using the comparative ΔΔCT method. All experiments were repeated in triplicate. Primer sequences are included in Supplementary material.
Protein extraction and quantification: Protein extraction and quantification using BCA assay as previously described. (24)
SDS polyacrylamide gel electrophoresis and western blot analysis: Protein samples were denatured in sample buffer and underwent electrophoresis on a 12% SDS-polyacrylamide gel as previously described. The blots were imaged using GE ImageQuant LAS 500 chemiluminescence (Cytiva Life Sciences, Marlborough, MA)
Immunofluorescence and Microscopy: For immunofluorescence, cells were grown and treated in 4-chamber cell culture slides (Lab-Tek,ThermoFisher, Waltham,MA). Cells were fixed with 4% paraformaldehyde and permeabilized with 0.3% TritonX-100/PBS. After washing with PBS, cells were incubated overnight@4oC with 1o antibody(in 3%BSA/PBS), washed with PBS, then incubated with 2o antibody(in 3%BSA/PBS), 1 hour at room temperature. After washing with PBS, chambers were removed from slide and coverslip was mounted with SlowFade Diamond Antifade Mountant with DAPI (Invitrogen,Eugene, OR). Slides were then imaged on a fluorescent microscope(Nikon Eclipse 90i, NikonUSA, Melville, NY) with pictures taken (NIS Elements AR, NikonUSA, Melville, NY).
Stiffness Assay: The mechanical properties of the cells were quantified by a confocal Brillouin microscope. The detail of the instrumentation is reported before.(25) In brief, the instrument consists of a laser source (660 nm, Laser Quantum), a standard microscope (IX83, Olympus), a home-built Brillouin spectrometer, and optical components that were used to guide laser beam into the microscope and collect backward scattered Brillouin signal. The measured Brillouin frequency shift is related to the elastic longitudinal modulus by the equation , where is laser wavelength, and are refractive index and density of the material. For cells in different physiological conditions, the ratio can be approximated as constant. Therefore, we here can use Brillouin shift to estimate the cellular mechanical properties.
Tumorigenic potential in vivo: All of the in vivo studies described herein have been approved by Wayne State University Animal Care and Use Committee. To assess tumorigenic potential 5 million or 20 million R182 WT or R182 CTGF KO cells were injected into NCG mice (Charles River, NCG strain 572, NOD-Prkdcem26Cd52Il2rgem26Cd22/NjuCrl) in a 50:50 mixture of Hydrogel (TheWell Bioscience TWG001) and cell suspension in 1x PBS. Cells in Hydrogel were injected into the right flank of each mouse in a volume of 100uL. Subcutaneous volume was assessed every 2-3 days by caliper and subcutaneous volume was calculated as {(W x (H2)}/2 mm3. Animals were sacrificed once a timepoint of 46 days was reached.
Statistical Analysis: The unpaired two-tailed t-test was used for comparison between different groups. Data from each group were collected with at least 3 biological repeats. P values of less than 0.05 were considered statistically significant. All statistical analysis were performed using Prism 9 software (GraphPad Software, San Diego, CA). Illustrations were performed using BiorenderTM software.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1.1. Differential expression of CTGF during early EMT
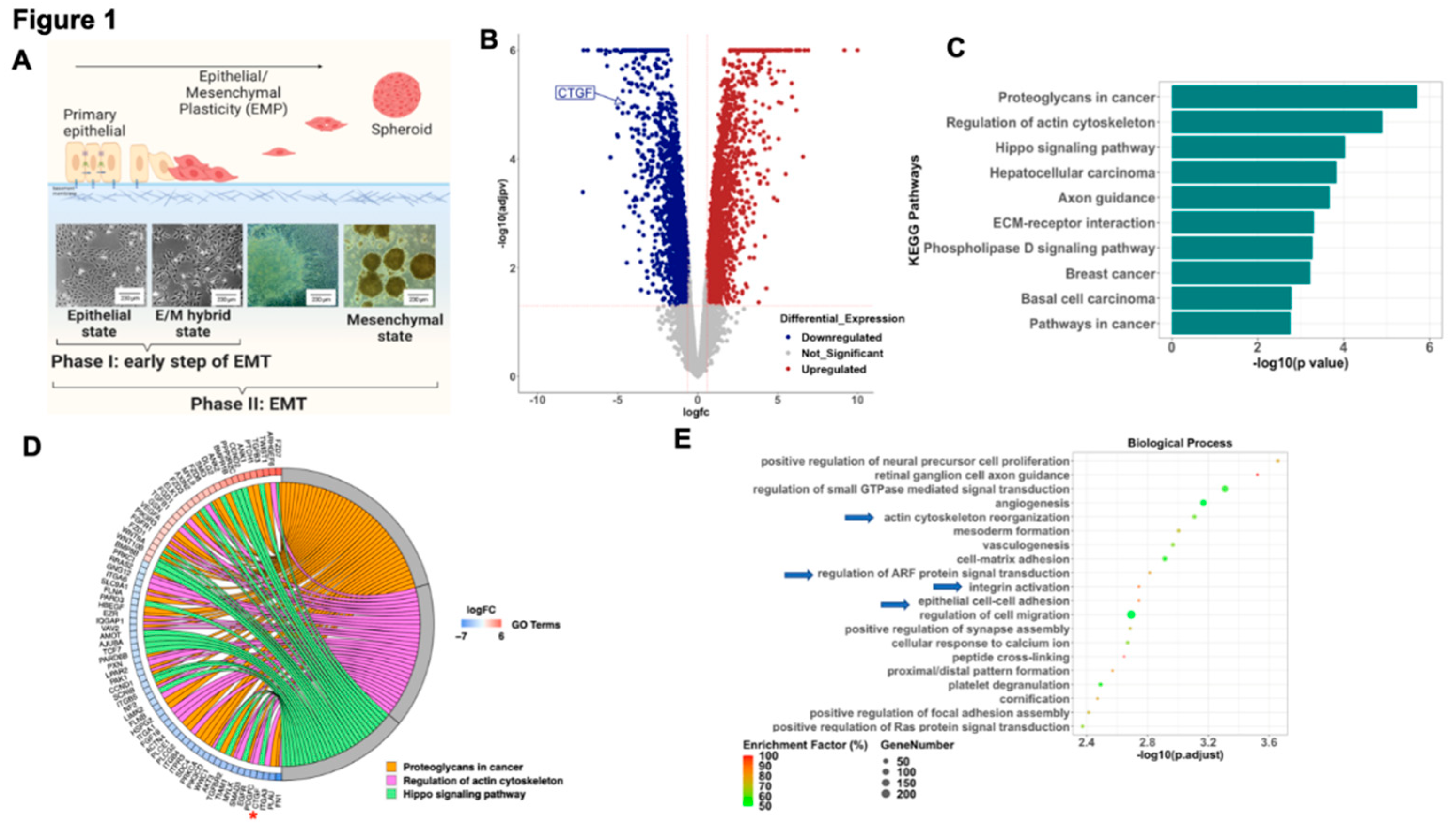
To better understand the early modifications associated with the process of EMT we analyzed the transcriptome of epithelial ovarian cancer cells (EOCC) undergoing mesenchymal transformation using a previously reported in
vitro model of EMT.(15) In short, the EOCCs (clones R182 and R2615, anoikis sensitive, non-invasive, and non-tumorigenic) cultured in high confluence in low-serum conditions for 12 days undergo morphological changes characteristic of EMT.(15) In this model we can define three stages of differentiation based on cellular morphology: 1) epithelial state; 2) E/M hybrid state (at 48 hours); and 3) spheroid or mesenchymal state (9-12 days) (
Figure 1A). To characterize the molecular signature of cells in the E/M hybrid state, we performed bulk RNA sequencing and compared epithelial R182 EOCCs (CK18+/β-catenin+/ Slug-/Twist1-)(26) with their corresponding E/M hybrid state of differentiation.(15, 17, 27) RNA sequencing data was analyzed utilizing the iPathwayGuide
TM software. We identified a total of 3,860 DEGs (
Figure 1B) at an absolute log2- fold change threshold of 0.6 and FDR-adjusted p value threshold of 0.05. The top 10 differentially regulated pathways are shown in
Figure 1C and
Figure 1D shows Chord diagram with DEGs for the top 3 differentially regulated pathways (Proteoglycans in cancer, Regulation of actin cytoskeleton, and Hippo signaling pathway). Further analysis of the differentially regulated biological processes during this early stage of differentiation revealed processes associated with remodeling of cytoskeleton, integrin activation, ARF signal transduction and cell-cell adhesion (
Figure 1E). Interestingly, the top KEGG pathways and GO biological process terms differentially impacted in the early stages of EMT are regulation of the actin cytoskeleton and HIPPO signaling pathway. Taken together this data suggests that a characteristic of the early stage of EMT is associated with the regulation of the cytoskeleton and the interaction with the surrounding ECM.
To better understand the gene(s) responsible for these changes we analyzed DEGs within the HIPPO pathway during early EMT and identified CTGF as significantly downregulated in the early mesenchymal stage compared to the epithelial stage (4-fold) (
Figure 1D, asterisk). Given that CTGF is a validated downstream target of the HIPPO pathway and is known to play a role in cell differentiation (28) we focused on the validation and characterization of the role of CTGF during early EMT.
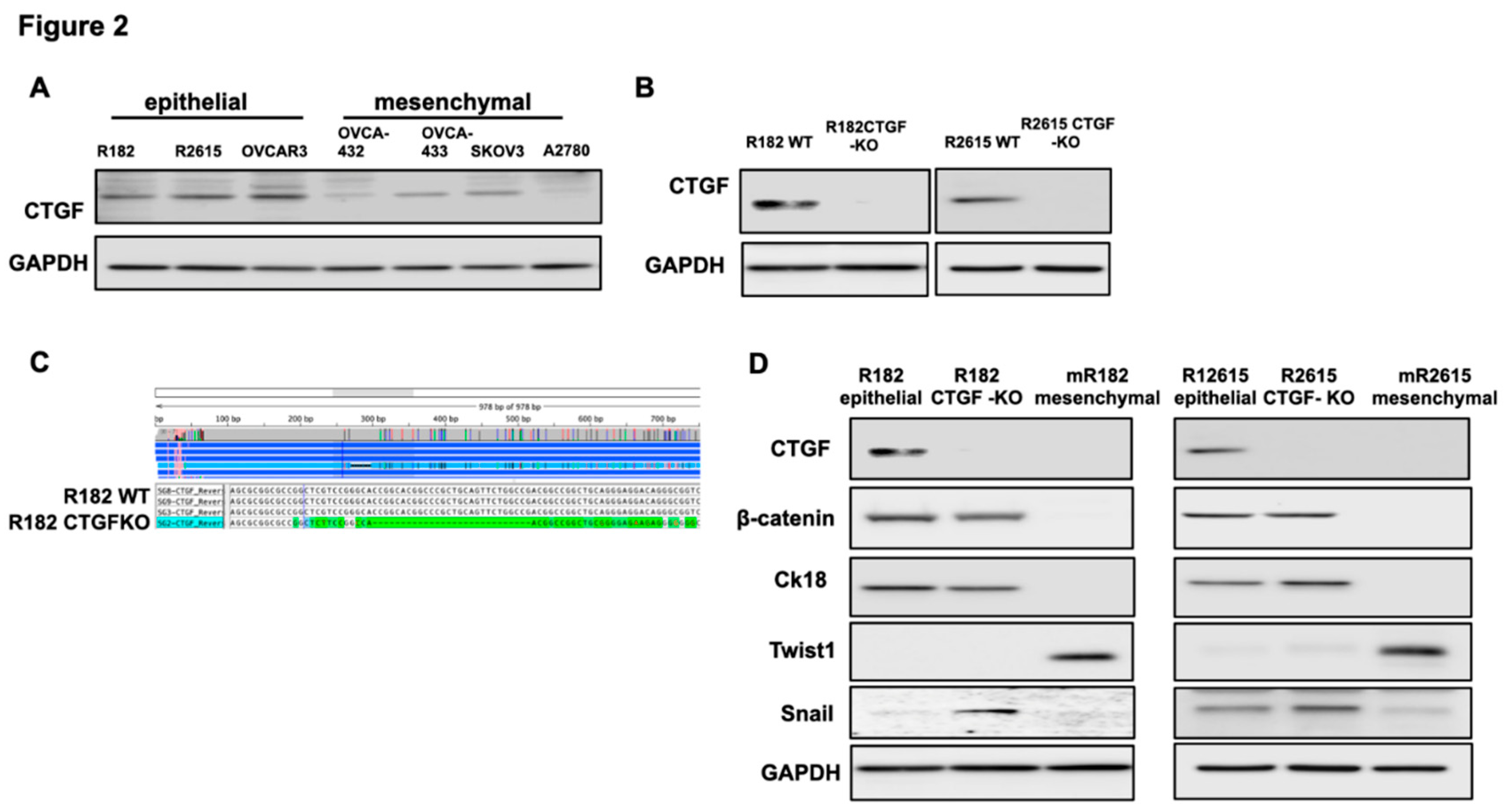
To validate the findings from the RNA seq we screened the panel of OCC at different stages of differentiation. Clones R182, R2615 and OVCAR3 have epithelial characteristics(29-31) while OVCAR432, OVCA433, SKOv3 and A2780 have mesenchymal characteristics.(29, 30) As shown in
Figure 2A, CTGF is expressed in OCC with epithelial characteristics and downregulated or not detectable in OCC with mesenchymal phenotype (Fig. 2A).
3.1.2. Loss of CTGF reprograms ECM in ovarian cancer cells.
To better understand CTGF function, we knocked-out CTGF in epithelial OCC clones R182 and R2615 using the CRISPR/Cas9 system.
Figure 2B shows successful KO of CTGF in the two cell lines using Western blot and
Figure 2C verified the KO using Sanger sequencing.
Our next objective was to determine the biological changes associated with CTGF deletion in the EOCCs. We first characterized the expression of known epithelial and mesenchymal markers in the wild type and CTGF-KO cell lines. CTGF-KO cells maintained the epithelial markers β-catenin and CK18, gained (R182 CTGF-KO) or upregulated (R2615 CTGF-KO) the mesenchymal marker Snail and remained Twist1- (Fig. 2D). The gain in Snail expression in CTGF-KO cells is interesting given that Snail is associated with the early stages of EMT(32, 33). This suggests that the lack of CTGF may be related to a transitional stage between epithelial and mesenchymal phenotype.
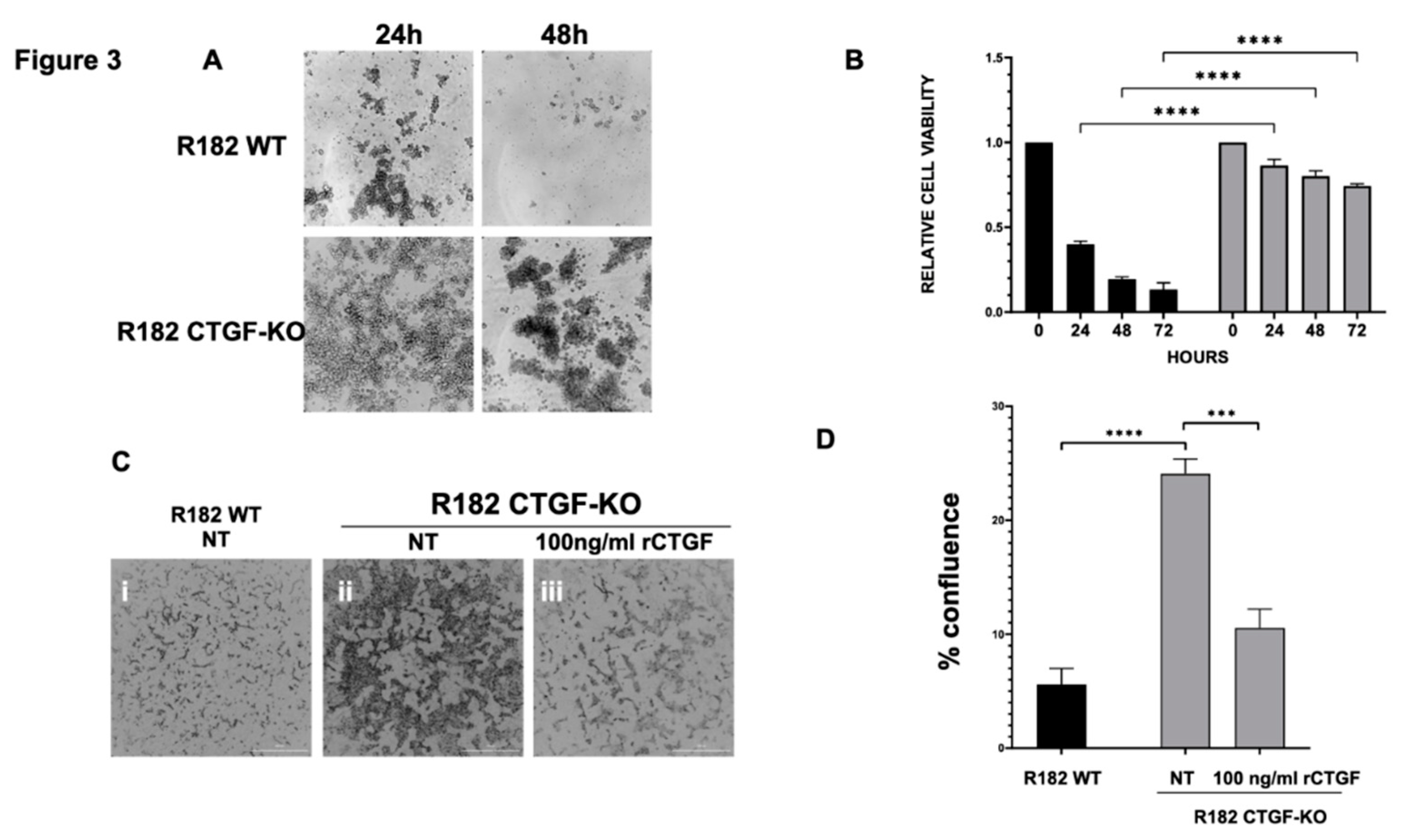
One of the early adaptation changes observed during EMT is the acquisition of Anoikis resistance. Anoikis resistance or the ability of a cell to resist apoptosis and survive in unattached conditions is an essential property of mesenchymal cells. We hypothesized that the loss of CTGF expression may affect Anoikis resistance. To test this hypothesis, clones R182/R2615 CTGF-KO cells and R182/R2615 WT cells were grown in ultra-low attachment plates. While WT epithelial cells undergo cell death when cultured in low attachment plates, loss of CTGF permitted OC cells to survive in detached conditions for up to 72 hours (p<0.01) (
Figure 3A, 3B). Taken together, our findings so far demonstrate that CTGF is necessary to maintain an epithelial phenotype and that its loss confers Anoikis resistance.
Another phenotype acquired during EMT is the ability to invade. To determine if the loss of CTGF can confer invasion capacity, R182WT and R182 CTGF-KO cells were seeded in 50% Matrigel and invasion was monitored by live imaging and quantified by measuring cell culture confluence. We observed a significant increase in invasion capacity in R182 CTG-KO cells compared to R182 WT EOCCs by day 6 (p=0.02; Fig. 3C,D). Interestingly, this was abrogated with the addition of recombinant CTGF (Fig. 3C,D) further proving that CTGF negatively regulates invasion.
Next, we evaluated whether the lack of CTGF could affect the response of cancer cells to chemotherapy; however, we did not find any significant difference between the WT and CTGF-KO cells in their response to Cisplatin (data not shown). Taken together, our data demonstrate that the loss of CTGF in OC cells leads to acquisition of Anoikis resistance and increased invasiveness but did not impact their response to chemotherapy, which are properties supportive of early changes associated with epithelial mesenchymal plasticity.
3.1.3. Inhibition of CTGF expression is associated with changes on the transcriptome of OCC.
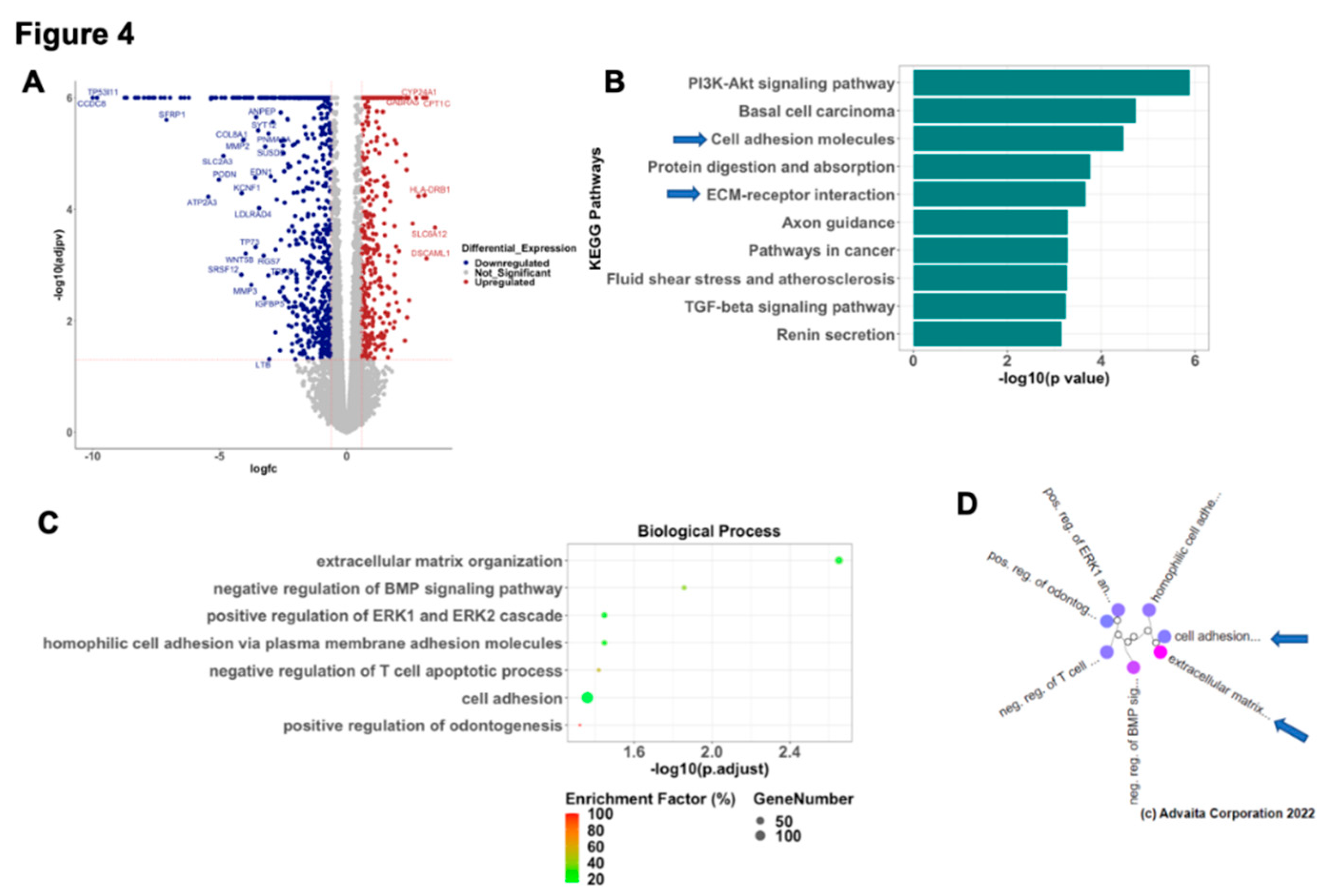
To better understand the molecular mechanism associated with the CTGF regulation of epithelial phenotype, we performed RNA sequencing in WT and R182 CTGF-KO OC cells and compared their transcriptome. We found that the loss of CTGF in EOCC is associated with significant changes on their transcriptome. Out of 14,054 genes with measured expression, we observed 1106 differentially expressed genes (DEGs; p<0.05 and absolute log2-fold-change >0.6) in the CTGF-KO OC cells. Of these DEGs, 385 were upregulated and 721 were downregulated (
Figure 4A). Pathway Enrichment Analysis showed 47 significantly impacted pathways including PI3K-Akt pathway, Basal cell carcinoma, cell adhesion molecules, protein digestion and absorption and ECM- receptor interaction being the top 5 differentially regulated pathways (
Figure 4B). Gene ontology analysis identified extracellular matrix organization as the most significantly regulated biological process associated with loss of CTGF (
Figure 4C). Gene Ontology analysis with high-specificity pruning identified extracellular matrix organization, negative regulation of BMP signaling pathway, positive regulation of ERK1 and ERK2 cascade, homophilic cell adhesion via plasma membrane adhesion molecules and negative regulation of T cell apoptotic process as top 5 differentially regulated biological processes (
Figure 4D). These findings further support the initial observation that early EMT involves cytoskeleton and ECM remodeling and CTGF is a central regulator of these changes.
3.1.4. Loss of CTGF alters Extracellular Matrix
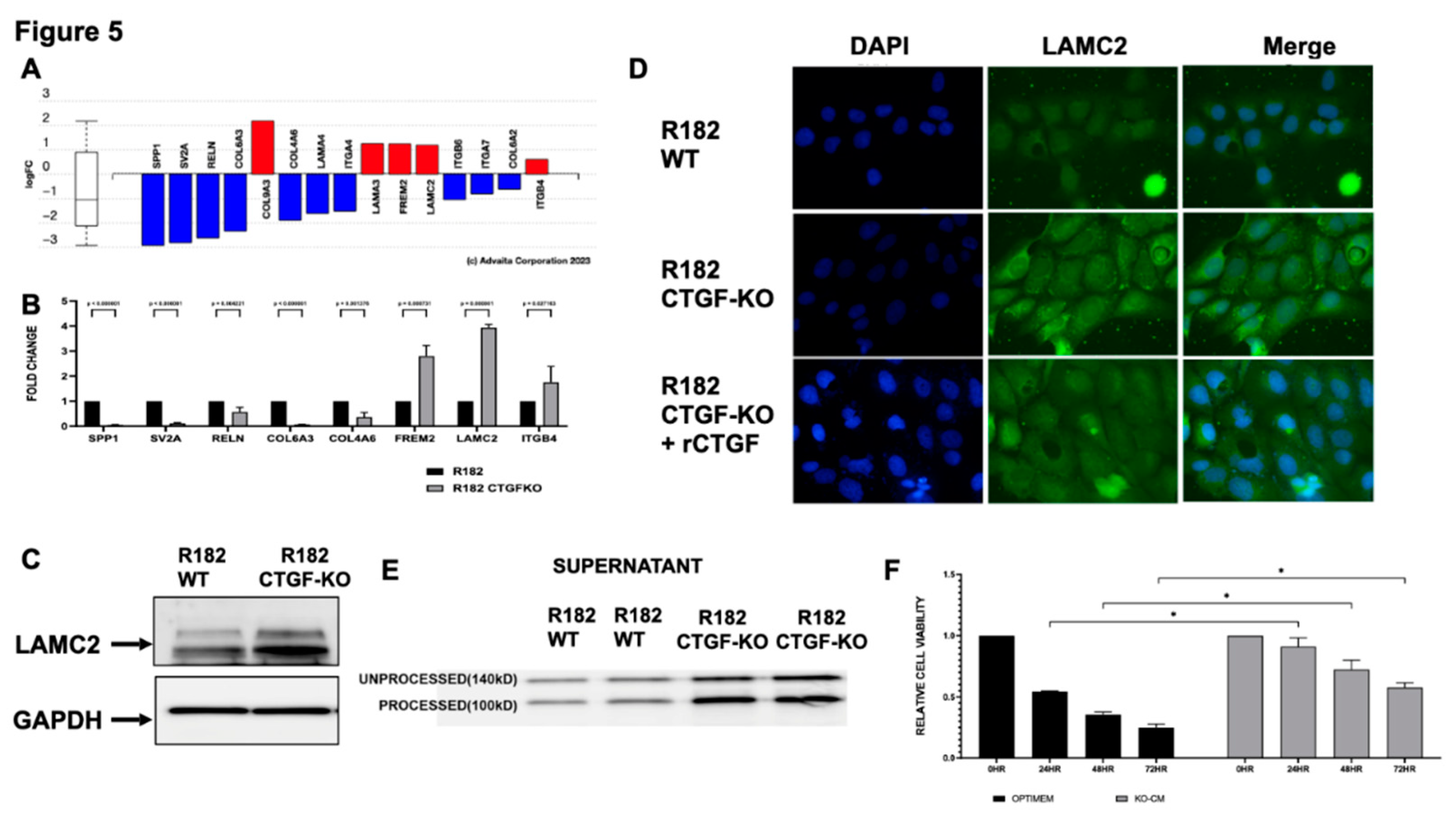
Given the significant representation of extracellular matrix re-organization in the CTGF KO cells (
Figure 5A) we validated several DEG in the ECM receptor interaction pathway by qPCR (
Figure 5B). Similar to the findings in the RNA seq, mRNA expression for SPP1, SV2A, RELN, COL6A3 and COL4A6 were significantly down regulated in the CTGF KO cells while FREM2, LAMC2 and ITGB4 were upregulated in the CTGF KO cells (
Figure 5B). Interestingly, mRNA expression levels for LAMC2, a gene encoding the gamma subunit of Laminin332 of extracellular matrix proteins, was significantly upregulated (4X) in cells lacking CTGF (
Figure 5B). To confirm that the changes in the mRNA are translated into protein we evaluated LAMC2 protein expression by western blot analysis and observed increased expression of LAMC2 in CTGF KO cells compared to WT (
Figure 5C). Similarly, Immunofluorescence (IF) for LAMC2 showed enhanced staining in R182 CTGF-KO compared to R182 WT cells (
Figure 5D). To confirm that the differential expression in LAMC2 is due to the absence of CTGF, we treated CTGF-KO cells with rCTGF and observed a decrease in LAMC2 expression to levels similar to those observed in the R182 WT cells (
Figure 5D). To further validate the observed regulation of LAMC2 by CTGF, we analyzed the presence of secreted LAMC2 in the conditioned media from WT and CTGF KO cells. LAMC2 is a secreted protein during the process of ECM remodeling, and it is subjected to cleavage into a mature 100KD form. As shown in
Figure 5E, we observe high expression of the processed LAMC2 (100 Kd) in the conditioned media from CTGF-KO cells, further supporting the role of CTGF on the regulation of ECM remodeling.
To determine the functional significance of gaining LAMC2, we exposed WT R182 cells (Anoikis sensitive) to conditioned media containing secreted LAMC2 collected from CTGF KO cells. As shown above, WT R182 cells are Anoikis sensitive undergoing 75% cell death within 72 hours of culturing in unattached conditions. The addition of conditioned media from CTGF KO cells to WT R182 cells was able to rescue Anoikis as demonstrated by a significant increase in viability at 24, 48 and 72 hours (p
<0.05 respectively) (
Figure 5F). The addition of CTGF KO conditioned media had no effect on the invasion capacity of WT cells (Data not shown). Taken together, these data demonstrate that CTGF negatively regulates LAMC2 expression and promotes sensitivity to Anoikis.
3.1.5. Loss of CTGF is associated with cytoskeleton remodeling.
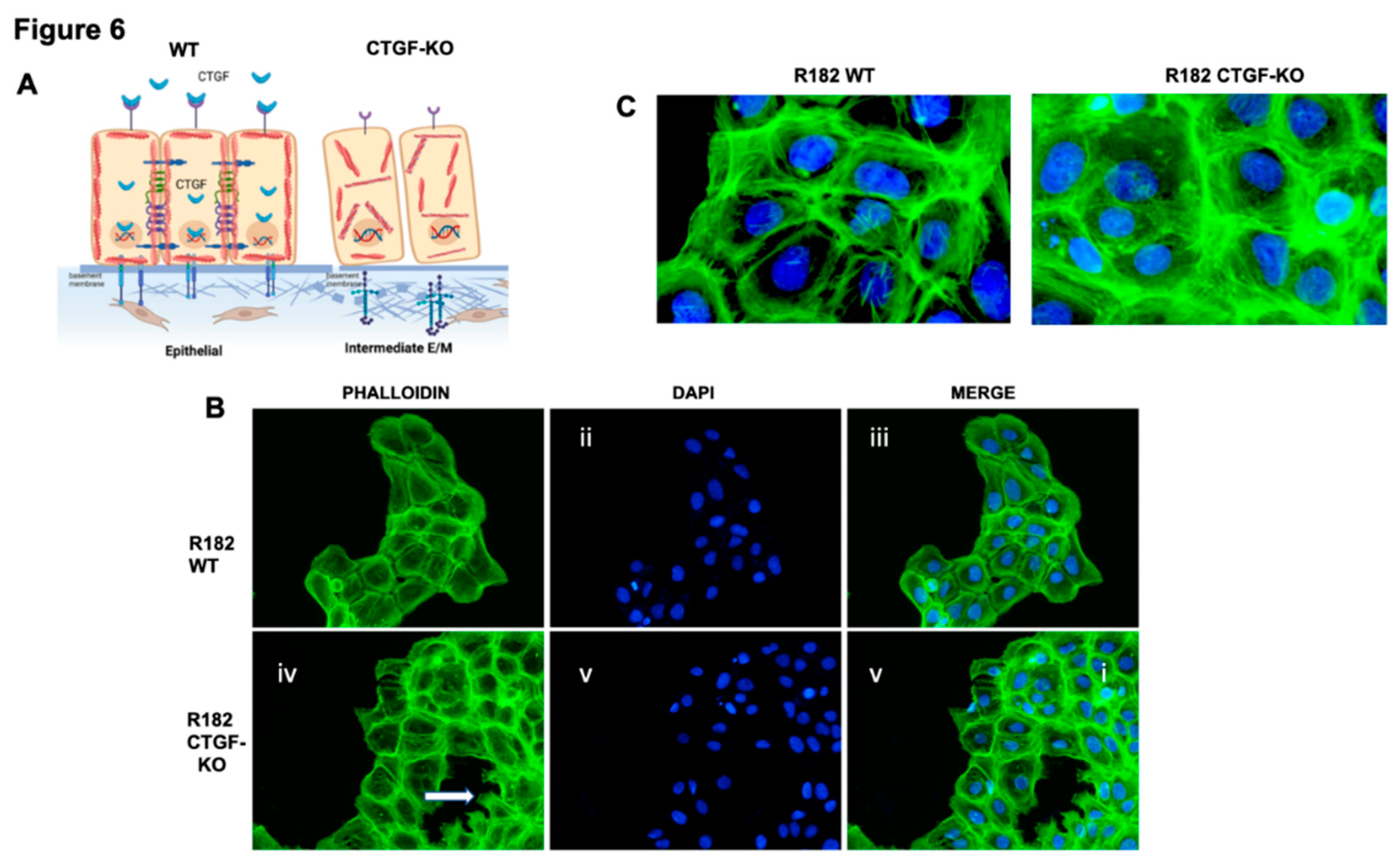
Cytoskeleton remodeling is a critical step during the early EMT process; and is one of the main pathways altered the CTGF KO cells (
Figure 6A). To determine whether CTGF expression is associated with cytoskeleton remodeling we evaluated F-actin expression in R182 WT and CTGF-KO cells by IF. F-actin are the linear polymers of the G-actin subunit and make up the microfilaments in the cellular cytoskeleton.(34) Epithelial R182 cells show a uniform pattern of F-actin expression, mainly localized in the periphery and adjacent to the cell membrane (
Figure 6B i-iii and 6C). In R182-CTGF-KO cells, we observed higher intensity in F-actin staining, which is mostly cytoplasmic (
Figure 6B iv-vi and 6C). We also observed strong F-actin staining in the lamellipodia present only in R182-CTGF-KO cells, which usually indicates cell migration (
Figure 6B iv). These data further demonstrate a role for CTGF in maintaining an epithelial phenotype by regulation of cytoskeleton remodeling as shown in the model in
Figure 6A.
3.1.6. Loss of CTGF alters cell stiffness.
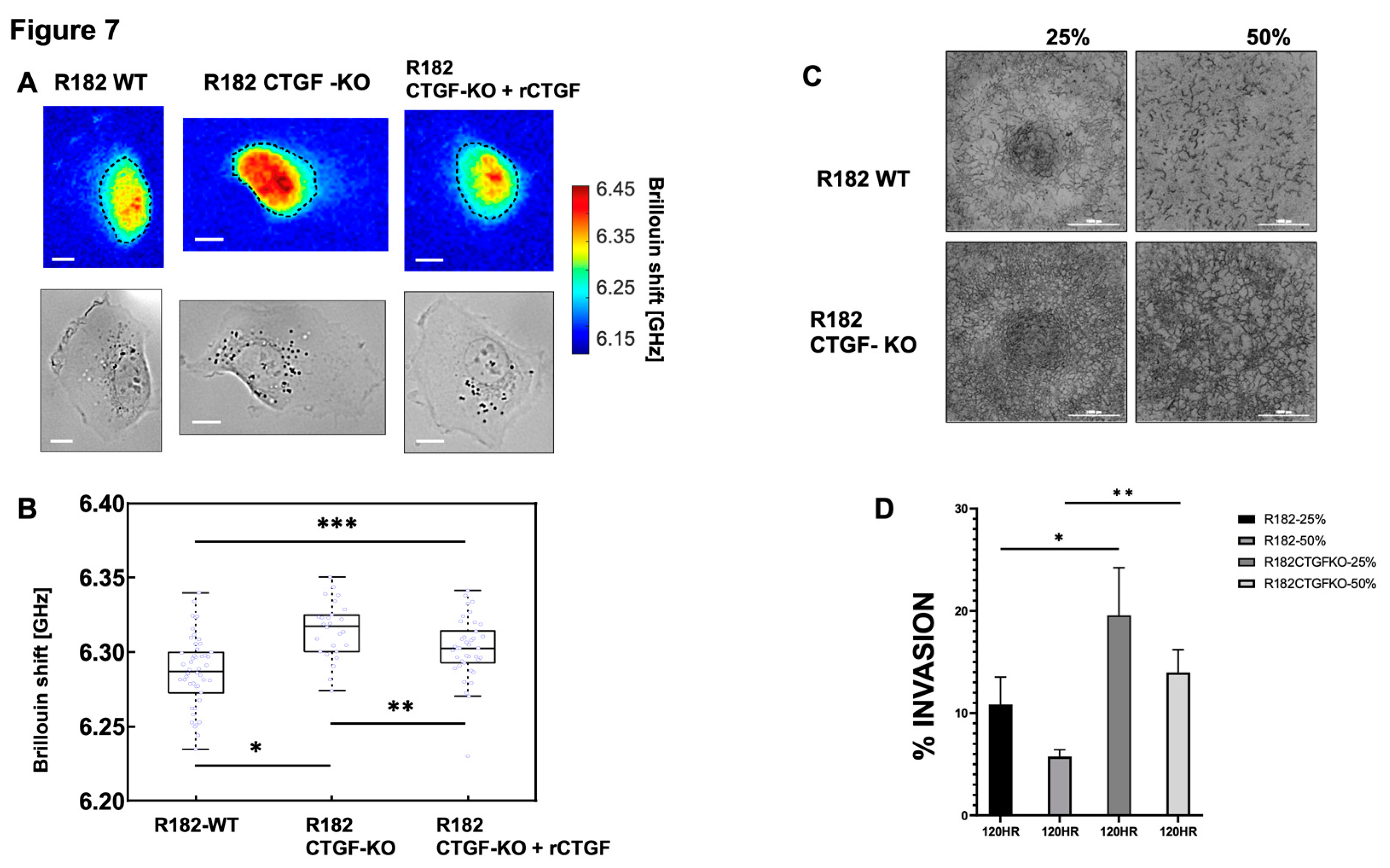
Cell stiffness is a mechanical property of a cell that is often related to adhesion, motility, differentiation, and invasiveness (35) and measured by the gold standard of atomic force microscopy. More recently optical Brillouin microscopy has been used to measure cell membrane stiffness.(36, 37) This technique is an all-optical method and evaluates the mechanical properties of a cell utilizing Brillouin light scatter.(37, 38) The Brillouin technology has been shown to be well correlated with the gold standard of atomic force microscopy in OC.(39) Cell stiffness is partly determined by the types of actin filaments and their organization. Because changes in extracellular matrix may be associated with changes in cell stiffness, we measured the mechanical properties of R182 WT and R182 CTGF-KO cells utilizing Brillouin microscopy. The representative Brillouin images and the co-registered bright-field images of cells are shown in
Figure 7A. We then used the averaged Brillouin shift of cell body to represent the mechanical property of each cell. R182-CTGF KO cells demonstrated increased cell stiffness compared to R182 WT cells (p<0.01). The addition of rCTGF (100ng/ml) abrogated the increase in stiffness and returned the levels close to those of the WT cells (
Figure 7B). We then determined the ability of R182 CTGF-KO cells to invade through matrix with increasing stiffness by culturing them in matrices with increasing concentrations of Matrigel (25, 50%). We find again that at baseline, R182 CTGF- KO cells are more invasive that R182 WT cells and that they are able to maintain this invasive phenotype even with increasing stiffness of the matrix (
Figure 7C, 7D).Taken together with the IF data demonstrating increased F-actin staining in CTGF-KO cells, the observed increase in cell stiffness suggests that cytoskeletal remodeling is an important outcome of CTGF loss in OC cells.
3.1.7. Lack of CTFG provides tumorigenic capacity.
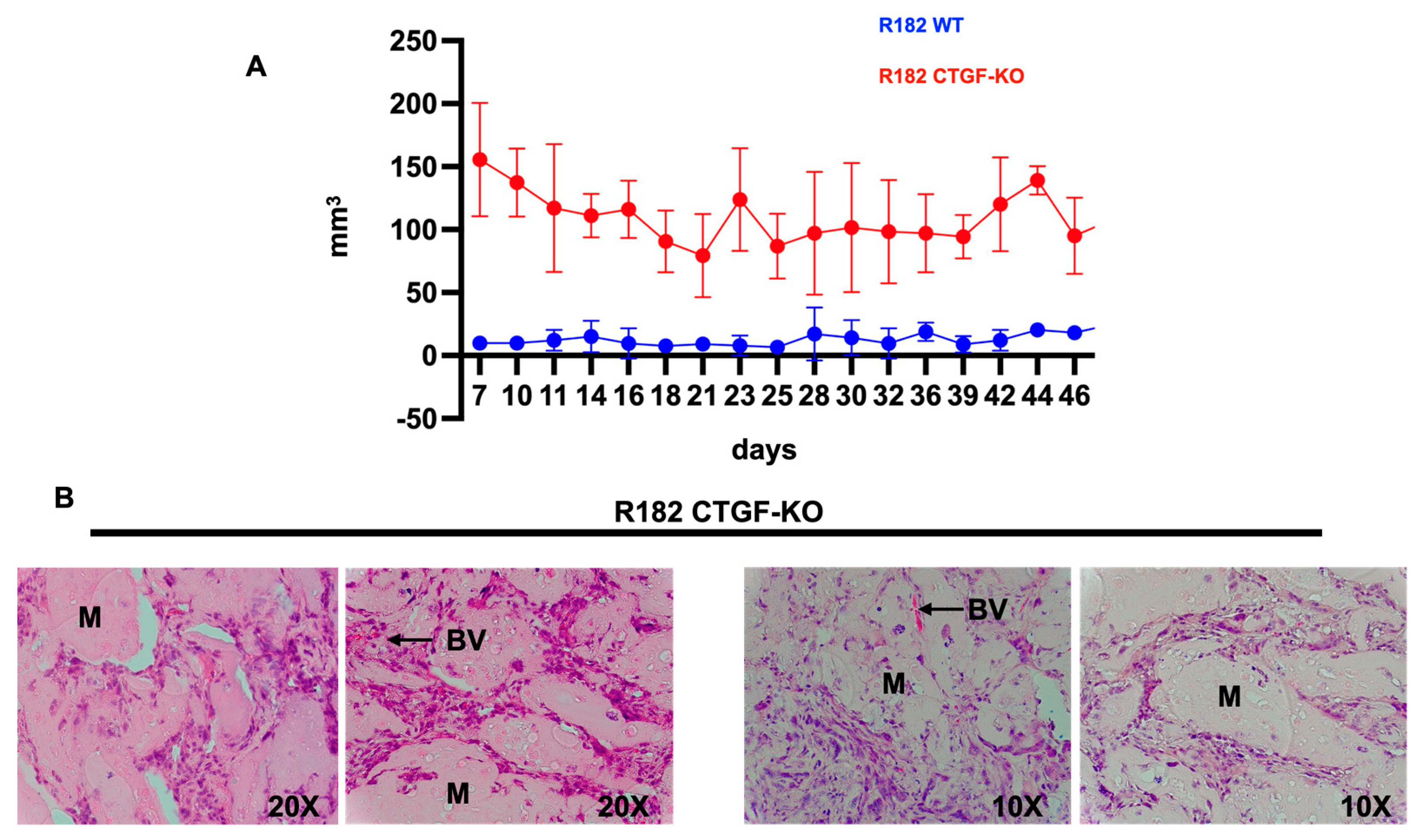
Finally, we determined whether the characteristics observed
in vitro following CTGF inhibition would provide cancer cells with tumor formation capacity. Thus, 20 million R182 CTGF-KO or R182 WT OC cells were injected in the flank of NSG mice in 50% Hydrogel as described in the materials and methods section. Tumor growth was monitored by caliper measurement beginning 7 days post injection of cells. Mice were euthanized at 55 days post-injection and histological analysis was performed to determine the presence of cancer cells. We observed consistently higher tumor volume in mice injected with R182 CTG-KO cells compared to mice injected with R182 WT cells which had no palpable tumor by the end of the study (
Figure 8A). Histological analysis showed the presence of cancer cells within the Matrigel as well as the presence of new blood vessels in animals injected with R182 CTG-KO cells (
Figure 8B). No tumors or presence of cancer cells was detected in the sites injected with R182 WT cells (data not shown).
4. Discussion
We describe, for the first time, the novel role of CTGF in maintaining an epithelial phenotype by regulating cytoskeleton remodeling and ECM interaction. While still maintaining their epithelial phenotype, cells lacking CTGF undergo reorganization of the cytoskeleton, reprogramming of the ECM and acquisition of anoikis resistance, cell migration and tumor formation capacity.
The epithelial phenotype is characterized by stable epithelial cell–cell junctions, apical–basal polarity and interactions with basement membrane.(40) These junctions are linked to the cytoskeleton, including a circumferential F-actin belt as well as keratins.(41) The interaction between epithelial cells and the ECM is essential for their apical-basal polarity and maintenance of tissue homeostasis. The ECM is composed of a complex network of macromolecules that assemble into three-dimensional supramolecular structures that regulate cell growth, survival, motility, and differentiation by ligating specific receptors such as integrins, syndecans and discoidin receptors.(42) An important aspect of the ECM is its high degree of plasticity and remodeling capacity specifically tailored to the structure/function and composition of each organ, and its configuration changes to reflect the physiological state of the tissue.(12) In this study, we found that one of early events in the process of EMT is associated with ECM and cytoskeleton remodeling, two important cellular events controlling tumor formation.(43)
Epithelial–mesenchymal transition (EMT) is a cellular process by which epithelial cells gain mesenchymal characteristics by downregulation of their epithelial features.(1) For an epithelial cell to acquire a mesenchymal phenotype, it needs to be exposed to signals that are originated from their microenvironment.(43),(15) As the epithelial cells undergo EMT, there are changes in gene expression and posttranslational regulation mechanisms leading to the repression of these epithelial characteristics and the acquisition of mesenchymal characteristics.(15) EMT does not result in a single mesenchymal state, but rather results in multiple intermediate states with diverse levels of epithelial and mesenchymal features.(26). Recently we described the characterization of early stages of EMT and defined those cells as E/M hybrid state, which represents a stable state, with anoikis resistance and invasive capacity.(15) Using an in vitro model that recapitulates the acquisition of mesenchymal characteristics while maintaining the morphology and the expression of epithelial genes, we analyzed their transcriptome and identified CTGF as one of the genes that is differentially expressed between the epithelial and intermediate stage.
CTGF is a secreted matricellular protein with very complex biology, which modulates many signaling pathways primarily related to cell adhesion and migration, angiogenesis, myofibroblast activation, and extracellular matrix deposition and remodeling. (44) We found that the loss of CTGF in epithelial ovarian cancer cells is associated with modifications on the expression of several genes associated with the ECM, including LAMC2 SPP1, SV2A, RELN, COL6A3 and COL4A6. Changes in the ECM has been proposed to influence each of the classically defined and emerging hallmarks of cancer (43) including cell survival and metastasis. LAMC2 is a subunit of the heterotrimeric glycoprotein laminin-332, which is a fundamental component of epithelial basement membranes and regulates cell motility and adhesion.(41) High LAMC2 expression correlates with poorer survival in multiple cancers including thyroid, NSCLC and cholangiocarcinoma.(45, 46) Our data suggest that LAMC2 expression is directly regulated by CTGF, as shown by the significant increase in LAMC2 expression in cells lacking CTGF and downregulation of LAMC2 expression following exposure to rCTGF. Interestingly, our data indicates a connection between CTGF-LAMC2 and anoikis resistance. CTGF-KO cells secrete high levels of LAMC2, which if exposed to epithelial cells will confer to them a resistance to anoikis. Indeed, LAMC expression has been reported to be a characteristic of highly metastatic subpopulation of Tumor Initiating Cells (TIC).(47)
In ovarian cancer, molecular subgroups of HGSOC established by the TCGA identified a “mesenchymal” subgroup signature(48) which has been associated with overexpression of EMT markers, lowest rates of optimal surgical debulking and worse OS. (49, 50) LAMC2 expression was correlated with worse survival, lymph node metastasis, tumor-node-metastasis stages, and tumor status. (45)
The results of our RNA seq data identifying extracellular matrix remodeling as the most significantly associated biological process with loss of CTGF, suggests that one of the major mechanisms of CTGF function is its regulation of ECM remodeling. We postulate that secretion of CTGF maintains the ECM necessary for the epithelial phenotype and the regulation of epithelial function. On the other hand, loss of CTGF is associated with ECM remodeling towards a mesenchymal phenotype as demonstrated by the expression of LAMC2 and reorganization of the actin filaments, both early steps in the EMT process. The mechanism by which CTGF regulates LAMC2 is currently unknown. One possible model is that loss of CTGF releases the inhibition of the mesenchymal marker SNAIL/ ZEB promoting the remodeling of the extracellular matrix. Interestingly no expression of TWIST was seen in the CTGF KO cells suggesting that loss of CTGF is an early step in EMT but alone does not support a fully mesenchymal phenotype. This hypothesis is also supported by the maintenance but not growth or metastasis of viable tumor cells in our in vivo experiments.
Finally, we show that loss of CTGF increases the stiffness of epithelial R182 OC cells. Indeed, CTGF-KO cells are more invasive than WT cells even in a stiffer substrate. Consequently, it is plausible that inhibition of CTGF expression leading to remodeling of the ECM and the cytoskeleton modifies the stiffness of the cells conferring migratory capacity. Given that cell stiffness measurements reflect the polymerization of F-actin, it is possible that not only the amount of F-actin, but the angle of the actin cytoskeleton and the stiffness of the ECM may be integral to the process of invasion.
The mechanisms regulating CTGF expression during this early process is still under investigation. CTGF is a validated downstream target of YAP, a key regulator of cell surface and substrate stimuli including cell stiffness. Although we do not see an increase in YAP expression in CTGF KO cells, one possible hypothesis is that loss of CTGF increases the nuclear localization of YAP thus increasing cell stiffness. Experiments are currently ongoing to test this hypothesis.
5. Conclusions
In conclusion, we demonstrate that loss of CTGF is one of the early events leading to EMT. We demonstrate that following inhibition of CTGF expression, epithelial cells undergo a process of cytoskeleton remodeling and expression of a different type of ECM, one that will promote their differentiation into an intermediate stage, maintaining some of the epithelial phenotype but acquiring mesenchymal characteristic such as anoikis resistance tumor formation and migration capacity. Further studies evaluating the upstream regulators and downstream targets of CTGF may provide novel therapeutic targets that can curtail the metastatic process in ovarian cancer.
Author Contributions
“Conceptualization, R.G. A.A and G.M.; methodology, S.G.; A.F.; software, V.G.; H.C. and NA;; formal analysis, R.T.; investigation, C.S.;Y.Y. resources, G.M.; writing—original draft preparation, RG.; writing—review and editing, G.M.; supervision, R.G.; project administration, A.A.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded in part by the Janet Burros Memorial Foundation.
Institutional Review Board Statement
All the in vivo studies described herein have been approved by Wayne State University Animal Care and Use Committee. “Not applicable” for studies involving humans.
Informed Consent Statement
Not applicable
Data Availability Statement
Once the manuscript is accepted for publication all the data obtained from the RNA seq will be deposited and make available.
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial-mesenchymal transition and its transcription factors. Biosci Rep 2022, 42. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Cancer Facts and Figures 2019.
- Shih, I.M.; Wang, Y.; Wang, T.L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am J Pathol 2021, 191, 26–39. [Google Scholar] [CrossRef]
- Soong, T.R.; Kolin, D.L.; Teschan, N.J.; Crum, C.P. Back to the Future? The Fallopian Tube, Precursor Escape and a Dualistic Model of High-Grade Serous Carcinogenesis. Cancers (Basel) 2018, 10. [Google Scholar] [CrossRef]
- Yang-Hartwich, Y.; Gurrea-Soteras, M.; Sumi, N.; Joo, W.D.; Holmberg, J.C.; Craveiro, V.; Alvero, A.B.; Mor, G. Ovulation and extra-ovarian origin of ovarian cancer. Scientific reports 2014, 4, 6116. [Google Scholar] [CrossRef]
- Alvero, A.B.; Kim, D.; Lima, E.; Sumi, N.J.; Lee, J.S.; Cardenas, C.; Pitruzzello, M.; Silasi, D.A.; Buza, N.; Fahmy, T.; et al. Novel approach for the detection of intraperitoneal micrometastasis using an ovarian cancer mouse model. Scientific reports 2017, 7, 40989. [Google Scholar] [CrossRef]
- Yeger, H.; Perbal, B. CCN family of proteins: critical modulators of the tumor cell microenvironment. J Cell Commun Signal 2016, 10, 229–240. [Google Scholar] [CrossRef]
- Yeger, H.; Perbal, B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal 2007, 1, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hendesi, H.; Barbe, M.F.; Safadi, F.F.; Monroy, M.A.; Popoff, S.N. Integrin mediated adhesion of osteoblasts to connective tissue growth factor (CTGF/CCN2) induces cytoskeleton reorganization and cell differentiation. PLoS One 2015, 10, e0115325. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, G.; Kavasi, R.M.; Voudouri, K.; Berdiaki, A.; Spyridaki, I.; Tsatsakis, A.; Nikitovic, D. Role of the extracellular matrix in cancer-associated epithelial to mesenchymal transition phenomenon. Dev Dyn 2018, 247, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.E.; Weinberg, S.H.; Lemmon, C.A. Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition. Front Cell Dev Biol 2019, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.; Montagna, M.K.; Pitruzzello, M.; Lima, E.; Mor, G.; Alvero, A.B. Adipocyte microenvironment promotes Bclxl expression and confers chemoresistance in ovarian cancer cells. Apoptosis 2017, 22, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Alvero, A.B.; Heaton, A.; Lima, E.; Pitruzzello, M.; Sumi, N.; Yang-Hartwich, Y.; Cardenas, C.; Steinmacher, S.; Silasi, D.A.; Brown, D.; et al. TRX-E-002-1 Induces c-Jun-Dependent Apoptosis in Ovarian Cancer Stem Cells and Prevents Recurrence In Vivo. Mol Cancer Ther 2016, 15, 1279–1290. [Google Scholar] [CrossRef]
- Tedja, R.; Alvero, A.B.; Fox, A.; Cardenas, C.; Pitruzzello, M.; Chehade, H.; Bawa, T.; Adzibolosu, N.; Gogoi, R.; Mor, G. Generation of Stable Epithelial-Mesenchymal Hybrid Cancer Cells with Tumorigenic Potential. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Li, J.; Alvero, A.B.; Nuti, S.; Tedja, R.; Roberts, C.M.; Pitruzzello, M.; Li, Y.; Xiao, Q.; Zhang, S.; Gan, Y.; et al. CBX7 binds the E-box to inhibit TWIST-1 function and inhibit tumorigenicity and metastatic potential. Oncogene. [CrossRef]
- Artymovich, K.; Appledorn, D.M. A multiplexed method for kinetic measurements of apoptosis and proliferation using live-content imaging. Methods Mol Biol 2015, 1219, 35–42. [Google Scholar] [CrossRef]
- Chehade, H.; Purandare, N.; Fox, A.; Adzibolosu, N.; Jayee, S.; Singh, A.; Tedja, R.; Gogoi, R.; Aras, S.; Grossman, L.I.; et al. MNRR1 is a driver of ovarian cancer progression. Transl Oncol 2023, 29, 101623. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014, 15, 550. [Google Scholar] [CrossRef]
- Ahsan, S.; Draghici, S. Identifying Significantly Impacted Pathways and Putative Mechanisms with iPathwayGuide. Curr Protoc Bioinformatics 2017, 57, 7–15. [Google Scholar] [CrossRef]
- Draghici, S.; Khatri, P.; Tarca, A.L.; Amin, K.; Done, A.; Voichita, C.; Georgescu, C.; Romero, R. A systems biology approach for pathway level analysis. Genome Res 2007, 17, 1537–1545. [Google Scholar] [CrossRef]
- Tarca, A.L.; Draghici, S.; Khatri, P.; Hassan, S.S.; Mittal, P.; Kim, J.S.; Kim, C.J.; Kusanovic, J.P.; Romero, R. A novel signaling pathway impact analysis. Bioinformatics 2009, 25, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag New York: 2016.
- Tedja, R.; Roberts, C.M.; Alvero, A.B.; Cardenas, C.; Yang-Hartwich, Y.; Spadinger, S.; Pitruzzello, M.; Yin, G.; Glackin, C.A.; Mor, G. Protein kinase Calpha-mediated phosphorylation of Twist1 at Ser-144 prevents Twist1 ubiquitination and stabilizes it. J Biol Chem 2019, 294, 5082–5093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Scarcelli, G. Mapping mechanical properties of biological materials via an add-on Brillouin module to confocal microscopes. Nat Protoc 2021, 16, 1251–1275. [Google Scholar] [CrossRef]
- Li, J.; Alvero, A.B.; Nuti, S.; Tedja, R.; Roberts, C.M.; Pitruzzello, M.; Li, Y.; Xiao, Q.; Zhang, S.; Gan, Y.; et al. CBX7 binds the E-box to inhibit TWIST-1 function and inhibit tumorigenicity and metastatic potential. Oncogene 2020, 39, 3965–3979. [Google Scholar] [CrossRef]
- Pan, L.H.; Beppu, T.; Kurose, A.; Yamauchi, K.; Sugawara, A.; Suzuki, M.; Ogawa, A.; Sawai, T. Neoplastic cells and proliferating endothelial cells express connective tissue growth factor (CTGF) in glioblastoma. Neurol Res 2002, 24, 677–683. [Google Scholar] [CrossRef]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol 2018, 68-69, 44–66. [Google Scholar] [CrossRef]
- Beaufort, C.M.; Helmijr, J.C.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van, I.W.F.; Heine, A.A.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One 2014, 9, e103988. [Google Scholar] [CrossRef]
- Hernandez, L.; Kim, M.K.; Lyle, L.T.; Bunch, K.P.; House, C.D.; Ning, F.; Noonan, A.M.; Annunziata, C.M. Characterization of ovarian cancer cell lines as in vivo models for preclinical studies. Gynecol Oncol 2016, 142, 332–340. [Google Scholar] [CrossRef]
- Liu, M.; Mor, G.; Cheng, H.; Xiang, X.; Hui, P.; Rutherford, T.; Yin, G.; Rimm, D.L.; Holmberg, J.; Alvero, A.; et al. High frequency of putative ovarian cancer stem cells with CD44/CK19 coexpression is associated with decreased progression-free intervals in patients with recurrent epithelial ovarian cancer. Reprod Sci 2013, 20, 605–615. [Google Scholar] [CrossRef]
- Taki, M.; Abiko, K.; Baba, T.; Hamanishi, J.; Yamaguchi, K.; Murakami, R.; Yamanoi, K.; Horikawa, N.; Hosoe, Y.; Nakamura, E.; et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nature communications 2018, 9, 1685. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, M.; Zielinska, W.; Halas-Wisniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim Biophys Acta 2016, 1860, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Polacheck, W.J.; Nia, H.T.; Patel, K.; Grodzinsky, A.J.; Kamm, R.D.; Yun, S.H. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat Methods 2015, 12, 1132–1134. [Google Scholar] [CrossRef]
- Zhang, J.; Nou, X.A.; Kim, H.; Scarcelli, G. Brillouin flow cytometry for label-free mechanical phenotyping of the nucleus. Lab Chip 2017, 17, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Prevedel, R.; Diz-Munoz, A.; Ruocco, G.; Antonacci, G. Brillouin microscopy: an emerging tool for mechanobiology. Nat Methods 2019, 16, 969–977. [Google Scholar] [CrossRef]
- Conrad, C.; Gray, K.M.; Stroka, K.M.; Rizvi, I.; Scarcelli, G. Mechanical Characterization of 3D Ovarian Cancer Nodules Using Brillouin Confocal Microscopy. Cell Mol Bioeng 2019, 12, 215–226. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: necessary or permissive for metastasis? Mol Oncol 2017, 11, 755–769. [Google Scholar] [CrossRef]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional diversity of laminins. Annu Rev Cell Dev Biol 2012, 28, 523–553. [Google Scholar] [CrossRef]
- Sala, M.; Ros, M.; Saltel, F. A Complex and Evolutive Character: Two Face Aspects of ECM in Tumor Progression. Front Oncol 2020, 10, 1620. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef]
- Fu, T.; Liu, J.X.; Xie, J.; Gao, Z.; Yang, Z. LAMC2 as a prognostic biomarker in human cancer: a systematic review and meta-analysis. BMJ Open 2022, 12, e063682. [Google Scholar] [CrossRef]
- Garg, M.; Kanojia, D.; Okamoto, R.; Jain, S.; Madan, V.; Chien, W.; Sampath, A.; Ding, L.W.; Xuan, M.; Said, J.W.; et al. Laminin-5gamma-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J Clin Endocrinol Metab 2014, 99, E62–72. [Google Scholar] [CrossRef]
- Cave, D.D.; Buonaiuto, S.; Sainz, B., Jr.; Fantuz, M.; Mangini, M.; Carrer, A.; Di Domenico, A.; Iavazzo, T.T.; Andolfi, G.; Cortina, C.; et al. LAMC2 marks a tumor-initiating cell population with an aggressive signature in pancreatic cancer. J Exp Clin Cancer Res 2022, 41, 315. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Konecny, G.E.; Wang, C.; Hamidi, H.; Winterhoff, B.; Kalli, K.R.; Dering, J.; Ginther, C.; Chen, H.W.; Dowdy, S.; Cliby, W.; et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst 2014, 106. [Google Scholar] [CrossRef]
- Wang, C.; Armasu, S.M.; Kalli, K.R.; Maurer, M.J.; Heinzen, E.P.; Keeney, G.L.; Cliby, W.A.; Oberg, A.L.; Kaufmann, S.H.; Goode, E.L. Pooled Clustering of High-Grade Serous Ovarian Cancer Gene Expression Leads to Novel Consensus Subtypes Associated with Survival and Surgical Outcomes. Clin Cancer Res 2017, 23, 4077–4085. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).