Submitted:
26 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
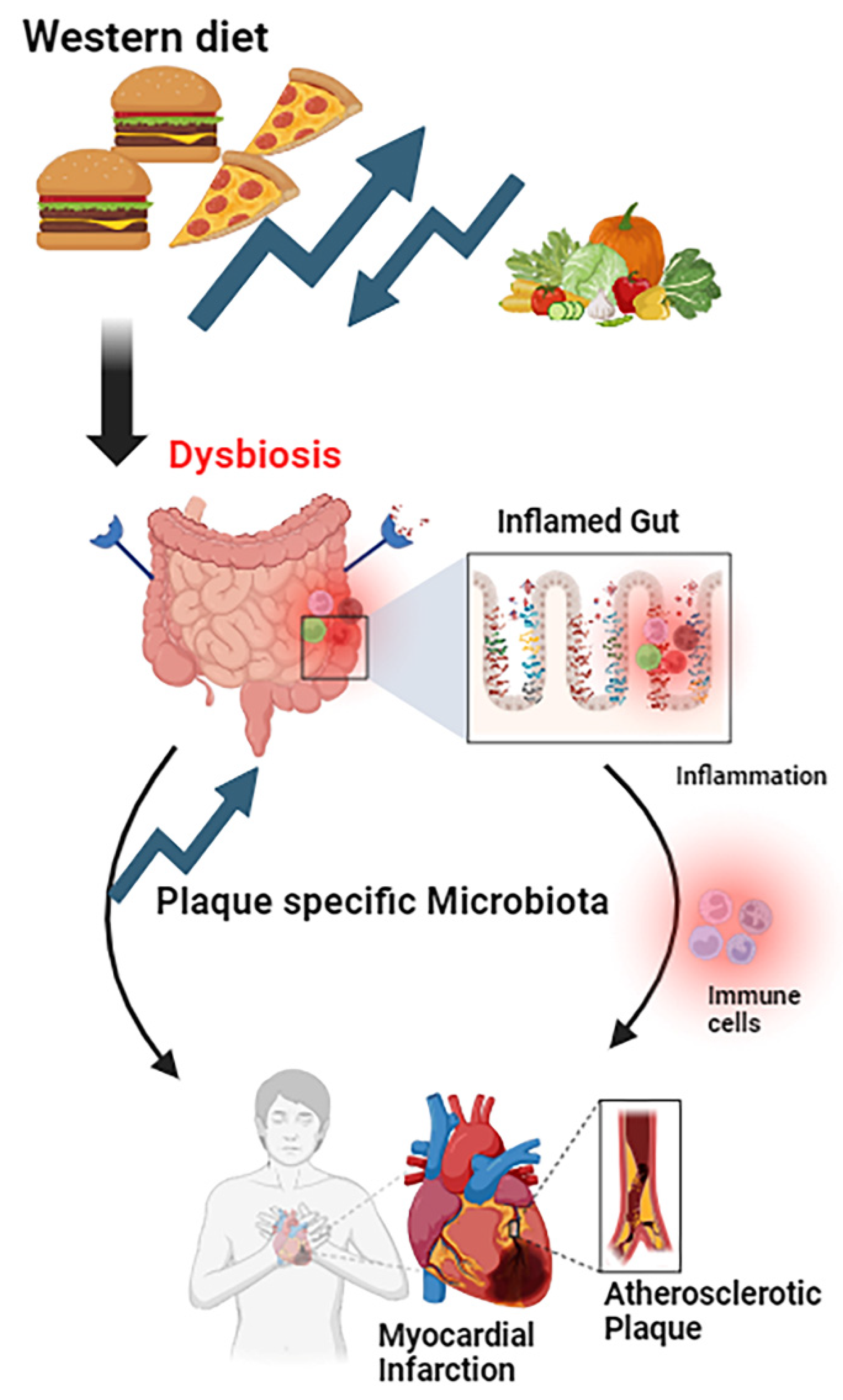
1. Introduction
2. Diversity and Composition the Gut Microbiota
3. Emerging Insights: Gut Microbiota and Cardiovascular Health:
3.1. The Inflammation: Unveiling the Role of Gut Health in Cardiovascular Diseases
3.2. Microbial Architects of Cholesterol: Gut Microbiota's Influence on Lipid Metabolism
3.3. Gut microbiota obesity, a major risk factor for heart disease
4. Impact of gut microbiota on Blood Pressure Regulation:
4.1. The connection between gut bacteria and blood pressure (BP) regulation
- a.
- Short-Chain Fatty Acids (SCFAs): Short-chain fatty acids (SCFAs) are a class of gut microbial metabolites known to activate multiple signaling pathways in the host. Short-chain fatty acids (SCFAs) are a result of the fermentation of dietary fiber by gut bacteria. Blood vessel relaxation and blood pressure reduction have both been linked to SCFAs. SCFAs enhance the production of nitric oxide that helps dilate blood arteries, boosting blood flow and lowering blood pressure [56].
- b.
- Inflammation and Immune Response: Chronic inflammation as a result of dysbiosis might contribute to the development of hypertension. The immune system's reaction is regulated by the gut microbiota, and inflammation is controlled, both of which have been linked to indirect effects on blood pressure. National Center for Cardiovascular Diseases of China has done a study in 2017 to observe, how Gut microbiota dysbiosis contributes to the development of hypertension [57]. They observed, dysbiosis an important pathogenic factor for the high BP of the host. To further investigate the precise processes underlying the impact of gut microbiome in BP regulation, bacteria including Prevotella, Klebsiella, Enterobacter, and Fusobacterium are potential candidates for further bacteria transfer research [58].
- c.
- Salt Sensitivity: According to several research, a person's sensitivity to dietary salt (sodium) may be influenced by the bacteria in their stomach. The development and treatment of hypertension are significantly influenced by salt sensitivity [59].
4.2. Production of nitric oxide by gut bacteria in maintaining healthy blood vessels
- a.
- Dietary Nitrate Conversion: Dietary nitrate, which is widely found in vegetables such as spinach, beetroot, and lettuce, can be converted into nitrite by particular gut flora. Nitrite can then be further converted into nitric oxide under certain conditions, such as tissue hypoxia. This conversion is mediated by gut enzyme [63,64,65].
- b.
- Influence on Nitric Oxide Synthase (NOS) Activity: Endothelial nitric oxide synthase (eNOS), an enzyme that generates nitric oxide in blood vessel walls, may be affected by certain gut flora [66]. Shandong Provincial Key Laboratory of Animal Cell and Developmental Biology conduct a study on how Gut Microbiota Homeostasis effects on Nitric Oxide Synthase (NOS) activity. They observed, the concentration NOS in the gastrointestinal tract were increased significantly in shrimp infected with Vibrio anguillarum. A gut microbiota dysbiosis situation [67].
- c.
- Anti-Inflammatory Effects: A healthy gut microbiota composition helps to minimize inflammation, which is essential for maintaining healthy blood vessels. Chronic inflammation has been linked to endothelial dysfunction. The inflammatory mediators enter into the bloodstream, through the blood–intestinal barrier which results effects on circulatory system including stiffness of the vessel due to inflammatory reponse. These results increase the blood pressure [68,69]. NO reduced inflammation which indirectly helps blood pressure regulation.
- d.
- Gut-Endothelium Axis: Gut-endothelium axis, or connection between gut microbes and the endothelium, involves a variety of signaling molecules, including those involved in NO generation [70].
5. Diet, Lifestyle, and Gut Health
- a.
- Consumption of Fiber rich diet: Dietary fiber can have a significant impact on the diversity, richness, and composition of the microbiome by offering a wide range of substrates for fermentation events carried out by particular microbe species that have the enzyme machinery required to break down these complex polysaccharides. [72]. A prebiotic is a compound that feeds the healthy bacteria in the gut, and fiber is one such material. In the process of fermenting dietary fiber, these bacteria create short-chain fatty acids (SCFAs), which have a number of health advantages, including enhancing gut health and lowering inflammation [73].
- b.
- Plant-Based vs. Animal-Based Diets: A plant-based diet rich in vegetables, fruits, whole grains, and nuts promotes a more diversified and healthier gut flora. Animal-based diets, especially those heavy in saturated fats and processed meats, might result in unfavorable alterations in gut microbial composition [74,75].
- c.
- Probiotic and Fermented Foods: Probiotics are advantageous, active bacteria that colonize the human intestines and alter the flora in specific regions of the host. Probiotics may improve or prevent gut inflammation and other intestinal or systemic disease phenotypes by restoring the composition of the gut microbiome and introducing beneficial functionalities to gut microbial communities [76].
- d.
- Sugar and Refined Carbohydrates: Excess sugar consumption has been associated to increased morbidity and mortality and has been connected to the development of numerous illnesses, including metabolic, cardiovascular, neurological, and even some malignancies [77,78]. sugars in the form of nutritious and non-nutritional sweeteners consumption have negative effects on the gut microbial ecosystem, including abnormal short-chain fatty acid synthesis, altered intestinal barrier integrity, and chronic inflammation, which frequently fuels a variety of metabolic conditions [79].
- e.
- Mediterranean Diet: A gut microbiome composition linked to superior health outcomes, including decreased inflammation and improved cardiovascular health, has been found to be connected with the Mediterranean diet, which is high in vegetables, fruits, whole grains, lean proteins, and healthy fats like olive oil [80]. The Mediterranean diet produces gut microbiome regulation in rodents, nonhuman primates, and human subjects, and the potential function of gut microbiota and microbial metabolites as one of the key catalysts mediating the host's many favorable health benefits is discussed [81,82].
- f.
- Food Additives: In today's world, the use of food additives in food manufacturing is unavoidable. Some food additives and emulsifiers included in processed meals have been demonstrated to have a deleterious impact on gut flora and gut barrier integrity [83]. Artificial sweeteners are most likely to damage glucose tolerance and promote weight gain by negatively altering microbiota. The majority of sugar alcohols are fermentable by bacteria and may have qualities comparable to prebiotics[84] .
6. Future Implications and Research Directions
- a.
- Diet Modification: Plant-based diets that were processed or ultraprocessed were not connected with healthy clusters of gut bacteria. When selecting foods, think about whether they are processed or unprocessed, as well as if they are plant or animal food. Emphasizing minimally processed plant foods allows the gut microbiome to thrive, providing protection against, or decreasing the risk of, chronic diseases such as heart disease, diabetes, metabolic disease, and obesity. Certain diets, such as those high in fiber and plant-based foods, can help good gut flora proliferate. These bacteria may create short-chain fatty acids (SCFAs), which have anti-inflammatory properties and may benefit cardiovascular health [85].
- b.
- Probiotics and Prebiotics: Several human and animal research have explored the molecular mechanisms underlying the therapeutic benefit of various probiotic and prebiotic substances for gut health. Fibers like inulin and galactomannan have both independent and synergistic health benefits [86].
- c.
- Fecal Microbiota Transplantation (FMT): FMT includes transferring healthy donor feces to a recipient in order to restore a balanced gut flora. The treatment of recurrent Clostridium difficile infection with FMT has been proven effective [87]. There are early signs that it may potentially have therapeutic potential for other illnesses such obesity, metabolic syndrome, inflammatory bowel disease, and functional gastrointestinal disorders [88].
- d.
7. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Structure, function and diversity of the healthy human microbiome. Nature. 2012, 486:207-214. [CrossRef]
- Elizabeth Thursby NJ: Introduction to the human gut microbiota. Biochem J. 2017. [CrossRef]
- Analyses of the Stability and Core Taxonomic Memberships of the Human Microbiome | PLoS ONE. 2023. [CrossRef]
- Mohammed L, Jha G, Malasevskaia I, Goud HK, Hassan A: The Interplay Between Sugar and Yeast Infections: Do Diabetics Have a Greater Predisposition to Develop Oral and Vulvovaginal Candidiasis? Cureus. 2021, 13. [CrossRef]
- Akter S, Choubey M, Arbee S; et al.: Safeguarding Intimate Health: Decoding the Interplay of Diabetes and Erectile Dysfunction. 2023. [CrossRef]
- M C, A R, PS B, F B, A K: Direct actions of adiponectin on changes in reproductive, metabolic, and anti-oxidative enzymes status in the testis of adult mice. General and comparative endocrinology. 2019, 279. [CrossRef]
- M C, A R, PS B, A K: Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie. 2020, 168. [CrossRef]
- M C, A R, A K: Adiponectin/AdipoRs signaling as a key player in testicular aging and associated metabolic disorders. Vitamins and hormones. 2021, 115. [CrossRef]
- Growth Hormone and Insulin-like Growth Factor-I: Novel Insights into the Male Reproductive Health. (2023). Accessed: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=-oBBDNsAAAAJ&citation_for_view=-oBBDNsAAAAJ:ye4kPcJQO24C.
- MS M, T H, Y Y; et al.: Glucagon Prevents Cytotoxicity Induced by Methylglyoxal in a Rat Neuronal Cell Line Model. Biomolecules. 2021, 11. [CrossRef]
- B C, O K, JK G; et al.: Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015, 519. [CrossRef]
- MB A: Could the menagerie of the gut microbiome really cure cancer? Hope or hype. Journal for immunotherapy of cancer. 2019, 7. [CrossRef]
- Ranjan A, Choubey M, Yada T, Krishna A: Immunohistochemical localization and possible functions of nesfatin-1 in the testis of mice during pubertal development and sexual maturation. Journal of Molecular Histology. 2019, 50:533-549. [CrossRef]
- A S, M C, P B, A K: Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reproductive sciences (Thousand Oaks, Calif). 2018, 25. [CrossRef]
- Mohib MM, Rabby SMF, Paran TZ; et al.: Protective role of green tea on diabetic nephropathy—A review. http://wwweditorialmanagercom/cogentbio. 2016. 1248166. [CrossRef]
- Mohiuddin MS, Himeno T, Inoue R; et al.: Glucagon-Like Peptide-1 Receptor Agonist Protects Dorsal Root Ganglion Neurons against Oxidative Insult. J Diabetes Res. 2019, 2019:9426014. [CrossRef]
- EJ B, SS V, CW C; et al.: Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018, 137. [CrossRef]
- SB D, OA K, T B; et al.: Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement From the American Heart Association. Circulation. 2018, 137. [CrossRef]
- Tasnim Sd, Auny FM, Hassan Y; et al.: Antenatal depression among women with gestational diabetes mellitus: A pilot study. Reproductive Health. 2022, 19:1-8. [CrossRef]
- Motegi M, Himeno T, Nakai-Shimoda H; et al.: Deficiency of glucagon gene-derived peptides induces peripheral polyneuropathy in mice. Biochem Biophys Res Commun. 2020, 532:47-53. [CrossRef]
- Munichandrababu T, Bhaskar BV, Ravi S, Bhuvaneswar C, Rajendra W: Structure based virtual screening of non-steroidal anti-inflammatory drugs (NSAIDs) against RNA-binding motif 6 (RBM6) involved in human lung cancer. Medicinal Chemistry Research. 2012, 22:2828-2839. [CrossRef]
- RR R, AC M, R K; et al.: Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives. European journal of medicinal chemistry. 2016, 123. [CrossRef]
- Rajendra W: Anti-Helicobacter pylori and anti-gastric cancer activity of Syzygium alternifolium fruits. https://wwwnutrafoodseu/indexphp/nutra. 2021. https://www.nutrafoods.eu/index.php/nutra/article/view/77.
- OC T-C, J M, A G: Colonization and impact of disease and other factors on intestinal microbiota. Digestive diseases and sciences. 2007, 52. [CrossRef]
- MJ C, Department of Microbiology UCC, Ireland., IB J; et al.: Gut microbiota composition correlates with diet and health in the elderly. Nature. 2023, 488:178-184. [CrossRef]
- Akter S, Tasnim Sd, Barua R; et al.: The Effect of COVID-19 on Gut Microbiota: Exploring the Complex Interplay and Implications for Human Health. Gastrointestinal Disorders. 2023, 5:340-355. [CrossRef]
- A F, G I, C M, E C, D S: NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. International journal of molecular sciences. 2019, 20. [CrossRef]
- Y F, Y H, Y W, P W, H S, F W: Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE. 2019, 14. [CrossRef]
- MK P, AL H, PG M: Metabolic endotoxaemia: Is it more than just a gut feeling? Current opinion in lipidology. 2013, 24. [CrossRef]
- Gionchetti P, Lammers KM, Rizzello F, Campieri M: Probiotics and barrier function in colitis. 2005. [CrossRef]
- CJ W, S K, S D; et al.: Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the Bruneck Study. Journal of the American College of Cardiology. 1999, 34. [CrossRef]
- Shah PK, Lecis D: Inflammation in atherosclerotic cardiovascular disease. F1000Research 2019 8:1402. 2019, 8. [CrossRef]
- SM C, PR V: Peripheral Arterial Disease. Heart, lung & circulation. 2018, 27. [CrossRef]
- TM B, A R, VB B, S D, D G, W R: Development of novel HER2 inhibitors against gastric cancer derived from flavonoid source of Syzygium alternifolium through molecular dynamics and pharmacophore-based screening. Drug design, development and therapy. 2016, 10. [CrossRef]
- G N, G L, RA M, F C: Advances in mechanisms, imaging and management of the unstable plaque. Atherosclerosis. 2014, 233. [CrossRef]
- Galkina E, Ley K: Immune and Inflammatory Mechanisms of Atherosclerosis*. 2009. [CrossRef]
- S Y, S H, S O; et al.: Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet (London, England). 2004, 364. [CrossRef]
- J S, ML D, DB G, AR D, P G, JD N: Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000, 284. [CrossRef]
- PW W, RB DA, D L, AM B, H S, WB K: Prediction of coronary heart disease using risk factor categories. Circulation. 1998, 97. [CrossRef]
- Steinberg D, dsteinberg@ucsd.edu: The LDL modification hypothesis of atherogenesis: An update. Journal of Lipid Research. 2009, 50. [CrossRef]
- Bhat BG, b.ganesh.bhat@pfizer.com, Rapp SR; et al.: Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. Journal of Lipid Research. 2003, 44:1614-1621. [CrossRef]
- The Critical Effect of Bile Acids in Atherosclerosis : Journal of Cardiovascular Pharmacology. 2023. [CrossRef]
- Ridlon JM, Kang, D.J., Hylemon, P.B., Bajaj, J.S.: Bile acids and the gut microbiome. Current Opinion in Gastroenterology. 2014. 10.1097/MOG.0000000000000057.
- David LA, Maurice CF, Carmody RN; et al.: Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013, 505:559-563. [CrossRef]
- DJ K, DR P, D S; et al.: Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell host & microbe. 2020, 28. [CrossRef]
- Wang Y, Wang M, Chen J; et al.: The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9. 2023. [CrossRef]
- Haghikia A, Department of Cardiology CUB, Campus Benjamin Franklin, Hindenburgdamm 30, 12203 Berlin, Germany, German Center for Cardiovascular Research (DZHK) PSB, Berlin, Germany; et al.: Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. European Heart Journal. 2023, 43:518-533. [CrossRef]
- Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ: Obesity paradox in cardiovascular disease: Where do we stand? Vascular Health and Risk Management. 2019, 15:89-100. [CrossRef]
- TM P-W, P P, LE B; et al.: Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021, 143. [CrossRef]
- P P, TD G, GA B; et al.: Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006, 113. [CrossRef]
- A K, G S, V M; et al.: Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC microbiology. 2017, 17. [CrossRef]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2023, 444:1027-1031. [CrossRef]
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E: Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Medicine. 2016, 8:1-12. [CrossRef]
- F B, H D, T W; et al.: The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004, 101. [CrossRef]
- A V, E VN, F H; et al.: Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012, 143. [CrossRef]
- Xu J, Moore BN, Pluznick JL: Short-Chain Fatty Acid Receptors and Blood Pressure Regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. 2022. [CrossRef]
- S A, JW N, NJ A; et al.: Alterations in the gut microbiota can elicit hypertension in rats. Physiological genomics. 2017, 49. [CrossRef]
- Li J, Zhao F, Wang Y; et al.: Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017, 5:1-19. [CrossRef]
- Bier A, Braun T, Khasbab R; et al.: A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 2018, 10:1154. [CrossRef]
- Bonetti G, Kiani AK, Medori MC; et al.: Dietary supplements for improving nitric-oxide synthesis. https://wwwjpmhorg/indexphp/jpmh. 2022. https://www.jpmh.org/index.php/jpmh/article/view/2766. [CrossRef]
- Mark H LH: Acute Effects of an Oral Nitric Oxide Supplement on Blood Pressure, Endothelial Function, and Vascular Compliance in Hypertensive Patients. J Clin Hypertens. 2014. [CrossRef]
- Najnin RA, Shafrin F, Polash AH; et al.: A diverse community of jute (Corchorus spp.) endophytes reveals mutualistic host–microbe interactions. Annals of Microbiology. 2014, 65:1615-1626. [CrossRef]
- Leclerc M, Bedu-Ferrari C, Etienne-Mesmin L; et al.: Nitric Oxide Impacts Human Gut Microbiota Diversity and Functionalities. 2021. msystems.00558-21. [CrossRef]
- Barua R, Sultana S, Talukder… M: Antioxidant and Cytotoxic Activity of Crude Flavonoid Fraction from the Fruits of Hybrid Variety of Momordica charantia (Bitter Gourd). 2014. [CrossRef]
- Babu TMC, Rajesh SS, Bhaskar BV; et al.: Molecular docking, molecular dynamics simulation, biological evaluation and 2D QSAR analysis of flavonoids from Syzygium alternifolium as potent anti-Helicobacter pylori agents. 2017. [CrossRef]
- M L, C B-F, L E-M; et al.: Nitric Oxide Impacts Human Gut Microbiota Diversity and Functionalities. mSystems. 2021, 6. [CrossRef]
- PP H, XX Z, WJ Y, GJ N, JX W: Nitric Oxide Synthase Regulates Gut Microbiota Homeostasis by ERK-NF-κB Pathway in Shrimp. Frontiers in immunology. 2021, 12. [CrossRef]
- Battson ML, Lee DM, Jarrell DK; et al.: Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. 2018. [CrossRef]
- W D, M C, S P, HA S, L O: Adipocyte CAMK2 deficiency improves obesity-associated glucose intolerance. Molecular metabolism. 2021, 53. [CrossRef]
- van Haver ER, Oste M, Thymann T; et al.: Enteral Feeding Reduces Endothelial Nitric Oxide Synthase in the Caudal Intestinal Microvasculature of Preterm Piglets. Pediatric Research. 2023, 63:137-142. [CrossRef]
- Nova E, Gómez-Martinez S, González-Soltero R: The Influence of Dietary Factors on the Gut Microbiota. Microorganisms. 2022, 10:1368. [CrossRef]
- Cronin P, Joyce SA, O’Toole PW, O’Connor EM: Dietary Fibre Modulates the Gut Microbiota. Nutrients. 2021, 13:1655. [CrossRef]
- Fu J, Zheng Y, Gao Y, Xu W: Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms. 2022, 10:2507. [CrossRef]
- Sidhu SRK, Kok CW, Kunasegaran T, Ramadas A: Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients. 2023, 15:1510. [CrossRef]
- Barua R, Mizuno K, Tashima Y; et al.: Bioinformatics and Functional Analyses Implicate Potential Roles for EOGT and L-fringe in Pancreatic Cancers. Molecules. 2021, 26:882. [CrossRef]
- Hemarajata P, Versalovic J: Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. 2012. [CrossRef]
- Garcia K, Ferreira G, Reis F, Viana S: Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology. 2022, 3:549-560. [CrossRef]
- Rashu Barua MEUT, Mohammad Sayedul Islam, Farhana Yesmin, Kanchan Chakma, Md Golam Kabir, Robiul Hasan Bhuiyan: Nutritional Analysis and Phytochemical Evaluation of Bitter Gourd (Momordica Charantia) from Bangladesh. 2020. [CrossRef]
- J S, T K, D Z; et al.: Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014, 514. [CrossRef]
- J D-L, P P-M, A G-R; et al.: CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: A clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on cardiovascular disease in coronary patients. American heart journal. 2016, 177. [CrossRef]
- Merra G, Noce A, Marrone G; et al.: Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020, 13:7. [CrossRef]
- Md. Ehsan Uddin Talukder FM, Rashu Barua, Samsad Sultana, Farhana Yesmin, Mohammad Sayedul Islam, Robiul Hasan Bhuiyan: In vitro Assessment of Cytotoxic Activity of Hybrid Variety of Momordica charantia (Bitter Gourd). 2023. [CrossRef]
- MB A-D, EM E-M, AA A-R, RE M, SS S: Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. Journal of toxicology and environmental health Part A. 2008, 71. [CrossRef]
- Campus G, and DI, WHO Collaborating Centre of Milan for Epidemiology and Community Dentistry M, Italy; et al.: Six Months of Daily High-Dose Xylitol in High-Risk Schoolchildren: A Randomized Clinical Trial on Plaque pH and Salivary Mutans Streptococci. Caries Research. 2023, 43:455-461. [CrossRef]
- Singh RK, Chang H-W, Yan D; et al.: Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine. 2017, 15:1-17. [CrossRef]
- R F, ER C, A P, F C: Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. European review for medical and pharmacological sciences. 2018, 22. [CrossRef]
- Benech N, Sokol H: Fecal microbiota transplantation in gastrointestinal disorders: Time for precision medicine. Genome Medicine. 2020, 12:1-4. [CrossRef]
- Gupta S, Allen-Vercoe E, Petrof EO: Fecal microbiota transplantation: In perspective. 2015. [CrossRef]
- Soontararak S, Center for Immune and Regenerative Medicine DoCS, College of Veterinary Medicine and Biomedical Sciences Colorado State University, Fort Collins, Colorado, USA, Chow L; et al.: Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Translational Medicine. 2023, 7:456-467. [CrossRef]
- X D, X F, J L; et al.: Characteristics of Intestinal Microecology during Mesenchymal Stem Cell-Based Therapy for Mouse Acute Liver Injury. Stem cells international. 2019, 2019. [CrossRef]
- Akter S, Choubey M, Mohib MM, Arbee S, Sagor MAT, Mohiuddin MS: Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sciences. 2023, 13:255. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




