Submitted:
25 August 2023
Posted:
28 August 2023
You are already at the latest version
Abstract
Keywords:

1. Background
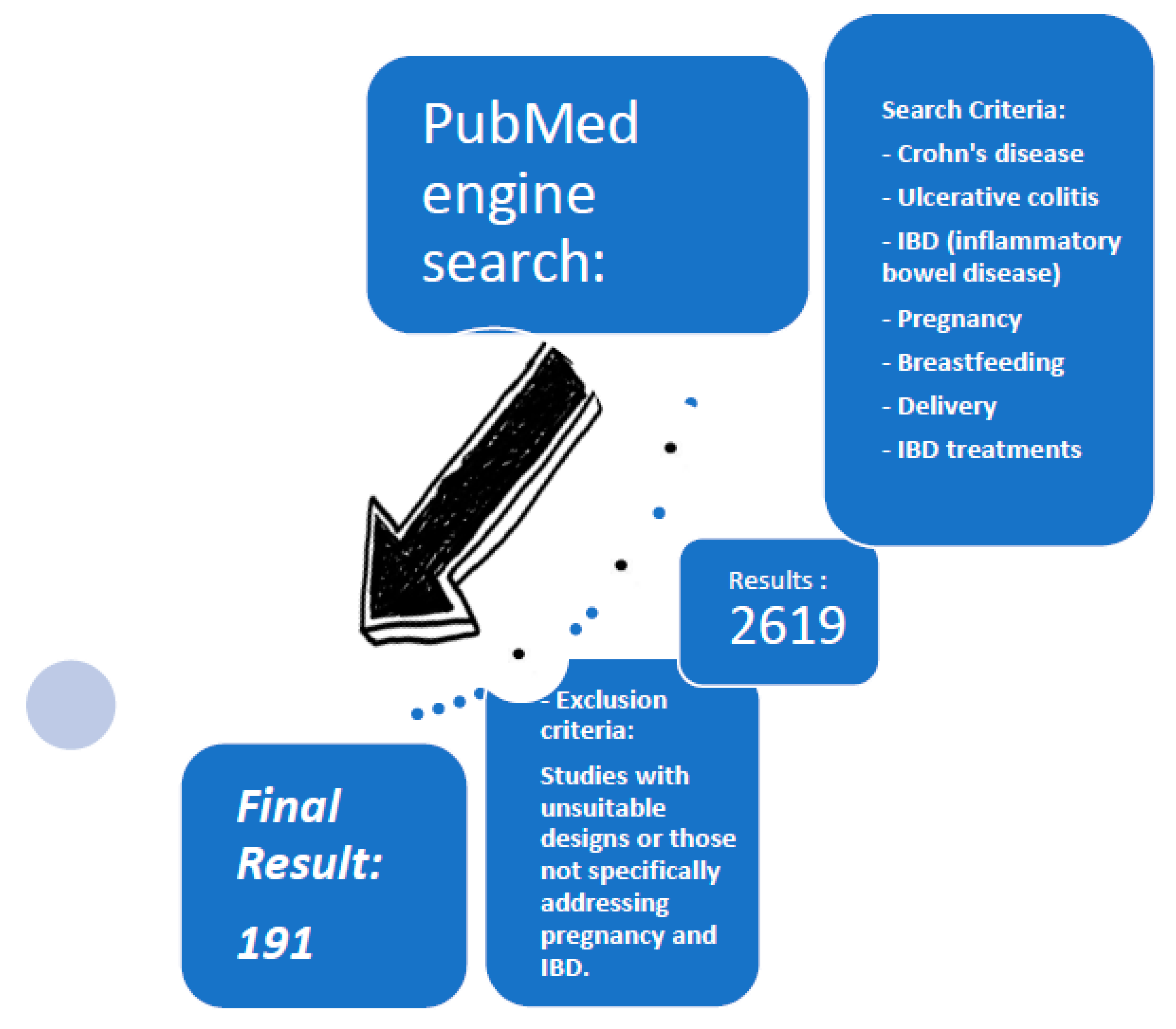
2. Methodology
3. Fertility in IBD Patients
4. Effect of IBD on Pregnancy
5. Effect of Pregnancy on IBD
6. Monitoring and Management of IBD During Pregnancy
7. Treatments and Pregnancy:
| Mechanism of Action | Medication | Recommendation for Initiation or Short-term Treatment | Recommendation for Maintenance Treatment |
|---|---|---|---|
| Aminosalicylates | Mesalazine and sulfasalazine | Low risk | Low risk |
| Corticosteroids | Budesonide and prednisone | Moderate risk, consider alternatives | Not recommended |
| Thiopurines | 6-Mercaptopurine and Azathioprine | Not recommended | Low risk |
| Antimetabolites | Methotrexate | Contraindicated | Contraindicated |
| Calcineurin Inhibitors | Cyclosporine | Low risk | Not recommended |
| Anti-TNF | Adalimumab, golimumab, and Infliximab | Low risk | Low risk |
| Anti-integrin | Vedolizumab | Low risk | Low risk |
| Anti-IL-23 | Risankizumab | Probably low risk, limited data | Probably low risk, limited data |
| Anti-IL-12/IL-23 | Ustekinumab | Low risk | Low risk |
| JAK Inhibitors | Tofacitinib, Upadacitinib, and Filgotinib | Not recommended | Not recommended |
| S1P Inhibitors | Ozanimod | Not recommended | Not recommended |
7.1. Steroids
7.2. Aminosalicylates (5- ASA)
7.3. Immunomodulators
7.4. Anti-TNFa
| Study | Pregnancies | Live Births (%) | Spontaneous Abortion (%) | Premature Birth | Congenital Anomalies (%) | Low Birth Weight | C-Section |
|---|---|---|---|---|---|---|---|
| Argüelles et al. (104) | 12 | 12 | 0 | 0 | 0 | 0 | 0 |
| Casanova et al. (88) | 29 | 0 (9.1%) | 0 (6.1%) | 0 (1.7%) | 0 (3%) | 0 | 0 |
| Chaparro et al. (96) | 388 | - | - | 10.6% | 5.4% | 10.6% | 43.8% |
| Correia LM (105) | 2 | 2 | 0 | 50% (1) | 0 | 100% (2) | 0 |
| Deepak et al. (106) | 783 | 237 (30%) | 3.3% (26) | 1.7% (13) | 1% (8) | 9.83% (77) | 0 |
| Kane et al. (107) | 3 | 3 | 0 | 33% (1) | 0 | 33% (1) | 0 |
| Kanis et al. | 131 | 131 | 6.8% (9) | 2.29% (3) | 0 | 0 | 43.51% (57) |
| Katz et al. (108) | 55 | 0 (58) | 20% (11) | 0 | 0 | 0 | 0 |
| Kiely et al. (109) | 21 | 0 (9.52%) | 0 | 0 (2) | 0 (9.52%) | 0 | 57.14% (12) |
| Lichtenstein et al. (110) | 162 | 81 (81.8%) | 16 (16.2%) | - | 1 (1.2%) | - | - |
| Mahadevan et al.(93) | 10 | 10 | 0 | 30% (3) | 0 | 10% (1) | 80% (8) |
| Moens et al. (111) | 186 | 162 | 24 | 14 | 44 | 2 | - |
| Schnitzler et al. (112) | 35 | 27 (77.1%) | 7 (20%) | 17.14% (6) | 0 | 14.28% (5) | 0 |
| Seirafi et al. (113) | 133 | 117 (87.96%) | 16 (12%) | 20% (23) | 1% (1) | 16% (19) | 0 |
| Zelinkova et al. (114) | 4 | 4 | 0 | 0 | 1 (25%) | 0 | 0 |
7.5. Anti-integrins: Vedolizumab
| Study | Pregnancies | Live Births | Spontaneous Abortions | Congenital Anomalies | Premature Births | Low Birth Weight | C-Section |
|---|---|---|---|---|---|---|---|
| Moens et al. (111) | 24 | 23 | 1 | 3 | 4 | 1 | 5 |
| Bar et al. (117) | 24 | 19 | 5 | 1 | 5 | 0 | 0 |
| Mitrova et al. (1320) | 24 | 22 | 2 | 0 | 0 | 1 | 9 |
| Julsgaard et al. (11) | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| Sheridan et al. (122) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chambers et al. (123) | 73 | 68 | 12.6% | 3 (4.2%) | 13.6% | - | - |
7.6. Anti Il 12/23 and Anti Il 23: Ustekinumab and Risankizumab
| Study | Pregnancies | Live Births | Spontaneous Abortions | Congenital Anomalies | Premature Births | Low Birth Weight | C-Section |
|---|---|---|---|---|---|---|---|
| Abraham (127) | 39 | 26 | 8 | 0 | - | 3 | - |
| Avni-Biron (128) | 27 | 25 | 2 | 1 | 1 | 1 | 10 |
| Chugh R (129) | 43 | 43 | 0 | 7 | 0 | 1 | 12 |
| Cortes et al. (130) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galli-Novak (131) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Klenske et al. (124) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lukesova et al. (121) | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mitrova et al. (120) | 32 | 27 | 5 | 3 | 0 | 1 | 8 |
| Rowan et al. (132) | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Scherl et al. (133) | 24 | 15 | 4 | 0 | 0 | 0 | - |
| Venturin et al. (134) | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
7.7. Small Molecules: JAK and S1P Inhibitors
8. Childbirth
9. Breastfeeding
10. Children of Mothers with IBD
11. Conclusions
Author Contributions
Funding
Conflicts of interest
References
- de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017 Dec;14(12):739–49.
- Ahmad T, Tamboli CP, Jewell D, Colombel JF. Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology. 2004 May;126(6):1533–49. [CrossRef]
- Kim DH, Cheon JH. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017;17(1):25. [CrossRef]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012 Nov 31;491(7422):119–24.
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46-54.e42; quiz e30.
- Loftus E, V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004 May;126(6):1504–17. [CrossRef]
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46-54.e42; quiz e30.
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011 May;140(6):1785–94. [CrossRef]
- Bernstein CN, Rawsthorne P, Cheang M, Blanchard JF. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006 May;101(5):993–1002.
- De Dombal FT, Watts JM, Watkinson G, Goligher JC. Ulcerative colitis and pregnancy. Lancet. 1965 Sep 25;2(7413):599–602.
- Korelitz, BI. Inflammatory bowel disease and pregnancy. Gastroenterol Clin North Am. 1998 Mar;27(1):213–24.
- Ørding Olsen K, Juul S, Berndtsson I, Oresland T, Laurberg S. Ulcerative colitis: female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology. 2002 Jan;122(1):15–9.
- Bokemeyer B, Hardt J, Hüppe D, Prenzler A, Conrad S, Düffelmeyer M, et al. Clinical status, psychosocial impairments, medical treatment and health care costs for patients with inflammatory bowel disease (IBD) in Germany: an online IBD registry. J Crohns Colitis. 2013 Jun;7(5):355–68. [CrossRef]
- Bel LGJ, Vollebregt AM, Van der Meulen-de Jong AE, Fidder HH, Ten Hove WR, Vliet-Vlieland CW, et al. Sexual Dysfunctions in Men and Women with Inflammatory Bowel Disease: The Influence of IBD-Related Clinical Factors and Depression on Sexual Function. J Sex Med. 2015 Jul;12(7):1557–67. [CrossRef]
- King JA, Rosal MC, Ma Y, Reed GW. Association of stress, hostility and plasma testosterone levels. Neuro Endocrinol Lett. 2005 Aug;26(4):355–60.
- O’Toole A, de Silva PS, Marc LG, Ulysse CA, Testa MA, Ting A, et al. Sexual Dysfunction in Men With Inflammatory Bowel Disease: A New IBD-Specific Scale. Inflamm Bowel Dis. 2018 Jan 18;24(2):310–6. [CrossRef]
- Eluri S, Cross RK, Martin C, Weinfurt KP, Flynn KE, Long MD, et al. Inflammatory Bowel Diseases Can Adversely Impact Domains of Sexual Function Such as Satisfaction with Sex Life. Dig Dis Sci. 2018 Jun 21;63(6):1572–82.
- Hashash JG, Kane S. Pregnancy and Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2015 Feb;11(2):96–102.
- van der Woude CJ, Ardizzone S, Bengtson MB, Fiorino G, Fraser G, Katsanos K, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015 Feb;9(2):107–24. [CrossRef]
- Ban L, Tata LJ, Humes DJ, Fiaschi L, Card T. Decreased fertility rates in 9639 women diagnosed with inflammatory bowel disease: a United Kingdom population-based cohort study. Aliment Pharmacol Ther. 2015 Oct;42(7):855–66. [CrossRef]
- Ørding Olsen K, Juul S, Berndtsson I, Oresland T, Laurberg S. Ulcerative colitis: female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology. 2002 Jan;122(1):15–9. [CrossRef]
- Oresland T, Palmblad S, Ellström M, Berndtsson I, Crona N, Hultén L. Gynaecological and sexual function related to anatomical changes in the female pelvis after restorative proctocolectomy. Int J Colorectal Dis. 1994 May;9(2):77–81. [CrossRef]
- Friedman S, Nielsen J, Nøhr EA, Jølving LR, Nørgård BM. Comparison of Time to Pregnancy in Women With and Without Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020 Jun;18(7):1537-1544.e1. [CrossRef]
- Lee S, Crowe M, Seow CH, Kotze PG, Kaplan GG, Metcalfe A, et al. The impact of surgical therapies for inflammatory bowel disease on female fertility. Cochrane Database Syst Rev. 2019 Jul 23;7(7):CD012711.
- Marri SR, Ahn C, Buchman AL. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm Bowel Dis. 2007 May;13(5):591–9.
- Roseira J, Magro F, Fernandes S, Simões C, Portela F, Vieira AI, et al. Sexual Quality of Life in Inflammatory Bowel Disease: A Multicenter, National-Level Study. Inflamm Bowel Dis. 2020 Apr 11;26(5):746–55. [CrossRef]
- Roseira J, Magro F, Fernandes S, Simões C, Portela F, Vieira AI, et al. Sexual Quality of Life in Inflammatory Bowel Disease: A Multicenter, National-Level Study. Inflamm Bowel Dis. 2020 Apr 11;26(5):746–55. [CrossRef]
- Mountifield R, Bampton P, Prosser R, Muller K, Andrews JM. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm Bowel Dis. 2009 May;15(5):720–5. [CrossRef]
- Gallinger ZR, Rumman A, Nguyen GC. Perceptions and Attitudes Towards Medication Adherence during Pregnancy in Inflammatory Bowel Disease. J Crohns Colitis. 2016 Aug;10(8):892–7. [CrossRef]
- Nguyen GC, Seow CH, Maxwell C, Huang V, Leung Y, Jones J, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology. 2016 Mar;150(3):734-757.e1. [CrossRef]
- Vieujean S, De Vos M, D’Amico F, Paridaens K, Daftary G, Dudkowiak R, et al. Inflammatory bowel disease meets fertility: A physician and patient survey. Dig Liver Dis. 2023 Jan 23.
- Walldorf J, Brunne S, Gittinger FS, Michl P. Family planning in inflammatory bowel disease: childlessness and disease-related concerns among female patients. Eur J Gastroenterol Hepatol. 2018 Mar;30(3):310–5.
- Hernandez-Nieto C, Sekhon L, Lee J, Gounko D, Copperman A, Sandler B. Infertile patients with inflammatory bowel disease have comparable in vitro fertilization clinical outcomes to the general infertile population. Gynecol Endocrinol. 2020 Jun;36(6):554–7. [CrossRef]
- Kim MA, Kim YH, Chun J, Lee HS, Park SJ, Cheon JH, et al. The Influence of Disease Activity on Pregnancy Outcomes in Women With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J Crohns Colitis. 2021 May 4;15(5):719–32. [CrossRef]
- Abdul Sultan A, West J, Ban L, Humes D, Tata LJ, Fleming KM, et al. Adverse Pregnancy Outcomes Among Women with Inflammatory Bowel Disease: A Population-Based Study from England. Inflamm Bowel Dis. 2016 Jul;22(7):1621–30.
- Mahadevan U, Long MD, Kane S V, Roy A, Dubinsky MC, Sands BE, et al. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology. 2021 Mar;160(4):1131–9. [CrossRef]
- Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014 Jun;20(6):1091–8.
- Bengtson MB, Martin CF, Aamodt G, Vatn MH, Mahadevan U. Inadequate Gestational Weight Gain Predicts Adverse Pregnancy Outcomes in Mothers with Inflammatory Bowel Disease: Results from a Prospective US Pregnancy Cohort. Dig Dis Sci. 2017 Aug;62(8):2063–9.
- Kammerlander H, Nielsen J, Kjeldsen J, Knudsen T, Friedman S, Nørgård B. The Effect of Disease Activity on Birth Outcomes in a Nationwide Cohort of Women with Moderate to Severe Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Jun;23(6):1011–8. [CrossRef]
- Riis L, Vind I, Politi P, Wolters F, Vermeire S, Tsianos E, et al. Does pregnancy change the disease course? A study in a European cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2006 Jul;101(7):1539–45. [CrossRef]
- Vestergaard T, Julsgaard M, Røsok JF, Vestergaard S V, Helmig RB, Friedman S, et al. Predictors of disease activity during pregnancy in women with inflammatory bowel disease-a Danish cohort study. Aliment Pharmacol Ther. 2023 Feb;57(3):335–44. [CrossRef]
- Hashash JG, Kane S. Pregnancy and Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2015 Feb;11(2):96–102.
- Abhyankar A, Ham M, Moss AC. Meta-analysis: the impact of disease activity at conception on disease activity during pregnancy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013 Sep;38(5):460–6.
- van der Woude CJ, Ardizzone S, Bengtson MB, Fiorino G, Fraser G, Katsanos K, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015 Feb;9(2):107–24. [CrossRef]
- P085. Pregnancy-onset IBD is not associated with adverse maternal or neonatal pregnancy outcomes. J Crohns Colitis. 2015 Feb 1;9(suppl 1):S120–1.
- Julsgaard M, Hvas CL, Gearry RB, Vestergaard T, Fallingborg J, Svenningsen L, et al. Fecal Calprotectin Is Not Affected by Pregnancy: Clinical Implications for the Management of Pregnant Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Jul;23(7):1240–6.
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002 Feb;122(2):512–30. [CrossRef]
- Tandon P, Lee EY, Maxwell C, Hitz L, Ambrosio L, Dieleman L, et al. Fecal Calprotectin May Predict Adverse Pregnancy-Related Outcomes in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2021 May;66(5):1639–49. [CrossRef]
- Kammerlander H, Nielsen J, Kjeldsen J, Knudsen T, Gradel KO, Friedman S, et al. Fecal Calprotectin During Pregnancy in Women With Moderate-Severe Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018 Mar 19;24(4):839–48. [CrossRef]
- Kim ES, Tarassishin L, Eisele C, Barre A, Nair N, Rendon A, et al. Longitudinal Changes in Fecal Calprotectin Levels Among Pregnant Women With and Without Inflammatory Bowel Disease and Their Babies. Gastroenterology. 2021 Mar;160(4):1118-1130.e3. [CrossRef]
- Flanagan E, Wright EK, Begun J, Bryant R V, An YK, Ross AL, et al. Monitoring Inflammatory Bowel Disease in Pregnancy Using Gastrointestinal Ultrasonography. J Crohns Colitis. 2020 Oct 5;14(10):1405–12. [CrossRef]
- Leung Y, Shim HH, Wilkens R, Tanyingoh D, Afshar EE, Sharifi N, et al. The Role of Bowel Ultrasound in Detecting Subclinical Inflammation in Pregnant Women with Crohn’s Disease. J Can Assoc Gastroenterol. 2019 Dec;2(4):153–60. [CrossRef]
- Nguyen GC, Seow CH, Maxwell C, Huang V, Leung Y, Jones J, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology. 2016 Mar;150(3):734-757.e1. [CrossRef]
- Ludvigsson JF, Lebwohl B, Ekbom A, Kiran RP, Green PHR, Höijer J, et al. Outcomes of Pregnancies for Women Undergoing Endoscopy While They Were Pregnant: A Nationwide Cohort Study. Gastroenterology. 2017 Feb;152(3):554-563.e9. [CrossRef]
- Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol. 2020 Mar 28;26(12):1231–41. [CrossRef]
- Kim YH, Pfaller B, Marson A, Yim HW, Huang V, Ito S. The risk of venous thromboembolism in women with inflammatory bowel disease during pregnancy and the postpartum period: A systematic review and meta-analysis. Medicine. 2019 Sep;98(38):e17309.
- Chaparro M, Kunovský L, Aguas M, Livne M, Rivière P, Bar-Gil Shitrit A, et al. Surgery due to Inflammatory Bowel Disease During Pregnancy: Mothers and Offspring Outcomes From an ECCO Confer Multicentre Case Series [Scar Study]. J Crohns Colitis. 2022 Sep 8;16(9):1428–35.
- Killeen S, Gunn J, Hartley J. Surgical management of complicated and medically refractory inflammatory bowel disease during pregnancy. Colorectal Dis. 2017 Feb;19(2):123–38. [CrossRef]
- Gaidos JKJ, Kane S V. Medication Adherence During Pregnancy in IBD: Compliance Avoids Complications. Dig Dis Sci. 2021 Feb;66(2):336–7.
- Watanabe C, Nagahori M, Fujii T, Yokoyama K, Yoshimura N, Kobayashi T, et al. Non-adherence to Medications in Pregnant Ulcerative Colitis Patients Contributes to Disease Flares and Adverse Pregnancy Outcomes. Dig Dis Sci. 2021 Feb;66(2):577–86. [CrossRef]
- Singh RR, Cuffe JSM, Moritz KM. Short- and long-term effects of exposure to natural and synthetic glucocorticoids during development. Clin Exp Pharmacol Physiol. 2012 Nov;39(11):979–89. [CrossRef]
- De Felice KM, Kane S V. Inflammatory bowel disease in women of reproductive age. Expert Rev Gastroenterol Hepatol. 2014 May;8(4):417–25.
- Mahadevan, U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut. 2006 Aug;55(8):1198–206.
- Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum Dis Clin North Am. 2017 Aug;43(3):489–502. [CrossRef]
- Chaparro M, Kunovský L, Aguas M, Livne M, Rivière P, Bar-Gil Shitrit A, et al. Surgery due to Inflammatory Bowel Disease During Pregnancy: Mothers and Offspring Outcomes From an ECCO Confer Multicentre Case Series [Scar Study]. J Crohns Colitis. 2022 Sep 8;16(9):1428–35.
- Carmichael SL, Shaw GM. Maternal corticosteroid use and risk of selected congenital anomalies. Am J Med Genet. 1999 Sep 17;86(3):242–4. [CrossRef]
- Rodríguez-Pinilla E, Martínez-Frías ML. Corticosteroids during pregnancy and oral clefts: a case-control study. Teratology. 1998 Jul;58(1):2–5. [CrossRef]
- Skuladottir H, Wilcox AJ, Ma C, Lammer EJ, Rasmussen SA, Werler MM, et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2014 Jun;100(6):499–506.
- Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol. 2004;18(1):93–101. [CrossRef]
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011 Apr 19;183(7):796–804. [CrossRef]
- Homar V, Grosek S, Battelino T. High-dose methylprednisolone in a pregnant woman with Crohn’s disease and adrenal suppression in her newborn. Neonatology. 2008;94(4):306–9. [CrossRef]
- Fedorak RN, Bistritz L. Targeted delivery, safety, and efficacy of oral enteric-coated formulations of budesonide. Adv Drug Deliv Rev. 2005 Jan 6;57(2):303–16. [CrossRef]
- Beaulieu DB, Ananthakrishnan AN, Issa M, Rosenbaum L, Skaros S, Newcomer JR, et al. Budesonide induction and maintenance therapy for Crohn’s disease during pregnancy. Inflamm Bowel Dis. 2009 Jan;15(1):25–8.
- Gluck PA, Gluck JC. A review of pregnancy outcomes after exposure to orally inhaled or intranasal budesonide. Curr Med Res Opin. 2005 Jul;21(7):1075–84. [CrossRef]
- De Felice KM, Kane S V. Inflammatory bowel disease in women of reproductive age. Expert Rev Gastroenterol Hepatol. 2014 May;8(4):417–25.
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008 Feb;25(2):271–5. [CrossRef]
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16(5):693–7. [CrossRef]
- Baggott JE, Morgan SL, Ha T, Vaughn WH, Hine RJ. Inhibition of folate-dependent enzymes by non-steroidal anti-inflammatory drugs. Biochem J. 1992 Feb 15;282 ( Pt 1)(Pt 1):197–202. [CrossRef]
- Levi AJ, Fisher AM, Hughes L, Hendry WF. Male infertility due to sulphasalazine. Lancet. 1979 Aug 11;2(8137):276–8. [CrossRef]
- O’Moráin C, Smethurst P, Doré CJ, Levi AJ. Reversible male infertility due to sulphasalazine: studies in man and rat. Gut. 1984 Oct;25(10):1078–84. [CrossRef]
- Nassan FL, Coull BA, Skakkebaek NE, Williams MA, Dadd R, Mínguez-Alarcón L, et al. A crossover-crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environ Int. 2016 Oct;95:120–30. [CrossRef]
- O’Morain C, Smethurst P, Dore CJ, Levi AJ. Reversible male infertility due to sulphasalazine: studies in man and rat. Gut. 1984 Oct 1;25(10):1078–84. [CrossRef]
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008 Feb;25(2):271–5. [CrossRef]
- Saarikoski S, Seppälä M. Immunosuppression during pregnancy: transmission of azathioprine and its metabolites from the mother to the fetus. Am J Obstet Gynecol. 1973 Apr 15;115(8):1100–6.
- Cleary BJ, Källén B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res A Clin Mol Teratol. 2009 Jul;85(7):647–54. [CrossRef]
- Nørgård B, Pedersen L, Fonager K, Rasmussen SN, Sørensen HT. Azathioprine, mercaptopurine and birth outcome: a population-based cohort study. Aliment Pharmacol Ther. 2003 Mar 15;17(6):827–34. [CrossRef]
- Akbari M, Shah S, Velayos FS, Mahadevan U, Cheifetz AS. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013 Jan;19(1):15–22. [CrossRef]
- Casanova MJ, Chaparro M, Domènech E, Barreiro-de Acosta M, Bermejo F, Iglesias E, et al. Safety of thiopurines and anti-TNF-α drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013 Mar;108(3):433–40. [CrossRef]
- Mahadevan U, Martin CF, Sandler RS, Kane S V., Dubinsky M, Lewis JD, et al. 865 PIANO: A 1000 Patient Prospective Registry of Pregnancy Outcomes in Women With IBD Exposed to Immunomodulators and Biologic Therapy. Gastroenterology. 2012 May;142(5):S-149. [CrossRef]
- Lloyd ME, Carr M, McElhatton P, Hall GM, Hughes RA. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999 Oct;92(10):551–63. [CrossRef]
- Martínez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding). Clin Exp Rheumatol. 2009;27(4):678–84.
- Weber-Schoendorfer C, Chambers C, Wacker E, Beghin D, Bernard N, Network of French Pharmacovigilance Centers, et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 2014 May;66(5):1101–10. [CrossRef]
- Dalrymple JM, Stamp LK, O’Donnell JL, Chapman PT, Zhang M, Barclay ML. Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2008 Nov;58(11):3299–308. [CrossRef]
- Reddy D, Murphy SJ, Kane S V, Present DH, Kornbluth AA. Relapses of inflammatory bowel disease during pregnancy: in-hospital management and birth outcomes. Am J Gastroenterol. 2008 May;103(5):1203–9. [CrossRef]
- Paziana K, Del Monaco M, Cardonick E, Moritz M, Keller M, Smith B, et al. Ciclosporin use during pregnancy. Drug Saf. 2013 May;36(5):279–94. [CrossRef]
- Branche J, Cortot A, Bourreille A, Coffin B, de Vos M, de Saussure P, et al. Cyclosporine treatment of steroid-refractory ulcerative colitis during pregnancy. Inflamm Bowel Dis. 2009 Jul;15(7):1044–8.
- Chaparro M, Verreth A, Lobaton T, Gravito-Soares E, Julsgaard M, Savarino E, et al. Long-Term Safety of In Utero Exposure to Anti-TNFα Drugs for the Treatment of Inflammatory Bowel Disease: Results from the Multicenter European TEDDY Study. Am J Gastroenterol. 2018 Mar;113(3):396–403. [CrossRef]
- Mahadevan U, Long MD, Kane S V, Roy A, Dubinsky MC, Sands BE, et al. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology. 2021 Mar;160(4):1131–9. [CrossRef]
- Martin PL, Oneda S, Treacy G. Effects of an anti-TNF-alpha monoclonal antibody, administered throughout pregnancy and lactation, on the development of the macaque immune system. Am J Reprod Immunol. 2007 Aug;58(2):138–49.
- Arsenescu R, Arsenescu V, de Villiers WJS. TNF-α and the development of the neonatal immune system: implications for inhibitor use in pregnancy. Am J Gastroenterol. 2011 Apr;106(4):559–62. [CrossRef]
- Fritzsche J, Pilch A, Mury D, Schaefer C, Weber-Schoendorfer C. Infliximab and adalimumab use during breastfeeding. J Clin Gastroenterol. 2012 Sep;46(8):718–9. [CrossRef]
- Luu M, Benzenine E, Doret M, Michiels C, Barkun A, Degand T, et al. Continuous Anti-TNFα Use Throughout Pregnancy: Possible Complications For the Mother But Not for the Fetus. A Retrospective Cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018 Nov;113(11):1669–77.
- Ng SW, Mahadevan U. Management of inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol. 2013 Feb;9(2):161–73; quiz 174. [CrossRef]
- Argüelles-Arias F, Castro-Laria L, Barreiro-de Acosta M, García-Sánchez MV, Guerrero-Jiménez P, Gómez-García MR, et al. Is safety infliximb during pregnancy in patients with inflammatory bowel disease? Revista espanola de enfermedades digestivas. 2012 Feb;104(2):59–64.
- Correia LM, Bonilha DQ, Ramos JD, Ambrogini O, Miszputen SJ. Inflammatory bowel disease and pregnancy: report of two cases treated with infliximab and a review of the literature. Eur J Gastroenterol Hepatol. 2010 Oct;22(10):1260–4. [CrossRef]
- Deepak P, Stobaugh DJ. Maternal and foetal adverse events with tumour necrosis factor-alpha inhibitors in inflammatory bowel disease. Aliment Pharmacol Ther. 2014 Nov;40(9):1035–43. [CrossRef]
- Kane S, Ford J, Cohen R, Wagner C. Absence of infliximab in infants and breast milk from nursing mothers receiving therapy for Crohn’s disease before and after delivery. J Clin Gastroenterol. 2009 Aug;43(7):613–6. [CrossRef]
- Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004 Dec;99(12):2385–92. [CrossRef]
- Kiely CJ, Subramaniam K, Platten J, Pavli P. Safe and effective: anti-tumour necrosis factor therapy use in pregnant patients with Crohn disease and ulcerative colitis. Intern Med J. 2016 May;46(5):616–9. [CrossRef]
- Lichtenstein GR, Feagan BG, Mahadevan U, Salzberg BA, Langholff W, Morgan JG, et al. Pregnancy Outcomes Reported During the 13-Year TREAT Registry: A Descriptive Report. Am J Gastroenterol. 2018 Nov;113(11):1678–88. [CrossRef]
- Moens A, van der Woude CJ, Julsgaard M, Humblet E, Sheridan J, Baumgart DC, et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: results of the European CONCEIVE study. Aliment Pharmacol Ther. 2020 Jan;51(1):129–38. [CrossRef]
- Schnitzler F, Fidder H, Ferrante M, Ballet V, Noman M, Van Assche G, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis. 2011 Sep;17(9):1846–54. [CrossRef]
- Seirafi M, de Vroey B, Amiot A, Seksik P, Roblin X, Allez M, et al. Factors associated with pregnancy outcome in anti-TNF treated women with inflammatory bowel disease. Aliment Pharmacol Ther. 2014 Aug;40(4):363–73. [CrossRef]
- Zelinkova Z, de Haar C, de Ridder L, Pierik MJ, Kuipers EJ, Peppelenbosch MP, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011 May;33(9):1053–8. [CrossRef]
- Flanagan E, Gibson PR, Wright EK, Moore GT, Sparrow MP, Connell W, et al. Infliximab, adalimumab and vedolizumab concentrations across pregnancy and vedolizumab concentrations in infants following intrauterine exposure. Aliment Pharmacol Ther. 2020 Sep 27. [CrossRef]
- Wils P, Seksik P, Stefanescu C, Nancey S, Allez M, Pineton de Chambrun G, et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study. Aliment Pharmacol Ther. 2021 Feb;53(4):460–70.
- Bar-Gil Shitrit A, Ben Yaʼacov A, Livovsky DM, Cuker T, Farkash R, Hoyda A, et al. Exposure to Vedolizumab in IBD Pregnant Women Appears of Low Risk for Mother and Neonate: A First Prospective Comparison Study. Am J Gastroenterol. 2019 Jul;114(7):1172–5. [CrossRef]
- Bell C, Tandon P, Lentz E, Marshall JK, Narula N. Systematic review and meta-analysis: Safety of vedolizumab during pregnancy in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2021 Oct;36(10):2640–8. [CrossRef]
- Gisbert JP, Chaparro M. Safety of New Biologics (Vedolizumab and Ustekinumab) and Small Molecules (Tofacitinib) During Pregnancy: A Review. Drugs. 2020 Jul 19;80(11):1085–100. [CrossRef]
- Mitrova K, Pipek B, Bortlik M, Bouchner L, Brezina J, Douda T, et al. Differences in the placental pharmacokinetics of vedolizumab and ustekinumab during pregnancy in women with inflammatory bowel disease: a prospective multicentre study. Therap Adv Gastroenterol. 2021 Jan 7;14:175628482110327. [CrossRef]
- Julsgaard M, Kjeldsen J, Baumgart DC. Vedolizumab safety in pregnancy and newborn outcomes. Gut. 2017 Oct;66(10):1866–7. [CrossRef]
- Sheridan J, Cullen G, Doherty G. Letter: Vedolizumab in Pregnancy. J Crohns Colitis. 2017 Aug 1;11(8):1025–6. [CrossRef]
- Chambers CD, Adams J, Xu R, Johnson DL, Luo Y, Jones KL. S0842 The Vedolizumab Pregnancy Exposure Registry: An OTIS Pregnancy Study Update. American Journal of Gastroenterology. 2020 Oct;115(1):S435–S435.
- Klenske E, Osaba L, Nagore D, Rath T, Neurath MF, Atreya R. Drug Levels in the Maternal Serum, Cord Blood and Breast Milk of a Ustekinumab-Treated Patient with Crohn’s Disease. J Crohns Colitis. 2019 Feb 1;13(2):267–9.
- Gorodensky JH, Bernatsky S, Afif W, Filion KB, Vinet É. Ustekinumab Safety in Pregnancy: A Comprehensive Review. Arthritis Care Res (Hoboken). 2023 Apr 16;75(4):930–5.
- Ferrante M, Feagan BG, Panés J, Baert F, Louis E, Dewit O, et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J Crohns Colitis. 2021 Dec 18;15(12):2001–10.
- Abraham BP, Ott E, Busse C, Murphy C, Miller L, Baumgart DC, et al. Ustekinumab Exposure in Pregnant Women From Inflammatory Bowel Disease Clinical Trials: Pregnancy Outcomes Through Up To 5 Years in Crohn’s Disease and 2 Years in Ulcerative Colitis. Crohns Colitis 360. 2022 Jul 1;4(3). [CrossRef]
- Avni-Biron I, Mishael T, Zittan E, Livne-Margolin M, Zinger A, Tzadok R, et al. Ustekinumab during pregnancy in patients with inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2022 Nov 27;56(9):1361–9. [CrossRef]
- Chugh, R. Maternal and Neonatal Outcomes in Vedolizumab and Ustekinumab Exposed Pregnancies: Results From the PIANO Registry. Gastroenterol Hepatol (N Y). 2022 Nov;18(11 Suppl 3):9–10. [CrossRef]
- Cortes X, Borrás-Blasco J, Antequera B, Fernandez-Martinez S, Casterá E, Martin S, et al. Ustekinumab therapy for Crohn’s disease during pregnancy: a case report and review of the literature. J Clin Pharm Ther. 2017 Apr;42(2):234–6. [CrossRef]
- Galli-Novak E, Mook SC, Büning J, Schmidt E, Zillikens D, Thaci D, et al. Successful pregnancy outcome under prolonged ustekinumab treatment in a patient with Crohn’s disease and paradoxical psoriasis. Journal of the European Academy of Dermatology and Venereology. 2016 Dec;30(12):e191–2. [CrossRef]
- Rowan CR, Cullen G, Mulcahy HE, Keegan D, Byrne K, Murphy DJ, et al. Ustekinumab Drug Levels in Maternal and Cord Blood in a Woman With Crohn’s Disease Treated Until 33 Weeks of Gestation. J Crohns Colitis. 2018 Feb 28;12(3):376–8. [CrossRef]
- Scherl E, Jacobstein D, Murphy C, Ott E, Gasink C, Baumgart DC, et al. A109 PREGNANCY OUTCOMES IN WOMEN EXPOSED TO USTEKINUMAB IN THE CROHN’S DISEASE CLINICAL DEVELOPMENT PROGRAM. J Can Assoc Gastroenterol. 2018 Mar 1;1(suppl_2):166–166. [CrossRef]
- Venturin C, Nancey S, Danion P, Uzzan M, Chauvenet M, Bergoin C, et al. Fetal death in utero and miscarriage in a patient with Crohn’s disease under therapy with ustekinumab: case-report and review of the literature. BMC Gastroenterol. 2017 Dec 19;17(1):80. [CrossRef]
- Pfizer Inc. Xeljanz prescribing information; 2016. https ://label ing. pfize r.com/ShowL abeli ng.aspx?id=959. Accessed Apr 02, 2020.
- Mahadevan U, Dubinsky MC, Su C, Lawendy N, Jones T V, Marren A, et al. Outcomes of Pregnancies With Maternal/Paternal Exposure in the Tofacitinib Safety Databases for Ulcerative Colitis. Inflamm Bowel Dis. 2018 Nov 29;24(12):2494–500. [CrossRef]
- Clowse MEB, Feldman SR, Isaacs JD, Kimball AB, Strand V, Warren RB, et al. Pregnancy Outcomes in the Tofacitinib Safety Databases for Rheumatoid Arthritis and Psoriasis. Drug Saf. 2016 Aug 9;39(8):755–62.
- Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. The Lancet. 2022 Jun;399(10341):2113–28. [CrossRef]
- Torres J, Chaparro M, Julsgaard M, Katsanos K, Zelinkova Z, Agrawal M, et al. European Crohn’s and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J Crohns Colitis. 2023 Jan 27;17(1):1–27.
- Dubinsky MC, Mahadevan U, Charles L, Afsari S, Henry A, Comi G, et al. DOP53 Pregnancy outcomes in the ozanimod clinical development program in relapsing multiple sclerosis, Ulcerative Colitis, and Crohn’s Disease. J Crohns Colitis. 2021 May 27;15(Supplement_1):S088–9. [CrossRef]
- Celgene. Highlights of prescribing information: Zeposia (ozanimod). FDA https://www. accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf (2020).
- Mahadevan U, Robinson C, Bernasko N, Boland B, Chambers C, Dubinsky M, et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report From the American Gastroenterological Association IBD Parenthood Project Working Group. Inflamm Bowel Dis. 2019 Mar 14;25(4):627–41.
- Burke KE, Haviland MJ, Hacker MR, Shainker SA, Cheifetz AS. Indications for Mode of Delivery in Pregnant Women with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 May;23(5):721–6. [CrossRef]
- Hashash JG, Kane S. Pregnancy and Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2015 Feb;11(2):96–102.
- Sharaf AA, Nguyen GC. Predictors of Cesarean Delivery in Pregnant Women with Inflammatory Bowel Disease. J Can Assoc Gastroenterol. 2018 Jun 13;1(2):76–81. [CrossRef]
- Foulon A, Dupas JL, Sabbagh C, Chevreau J, Rebibo L, Brazier F, et al. Defining the Most Appropriate Delivery Mode in Women with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 May;23(5):712–20. [CrossRef]
- Friedman S, Zegers FD, Riis Jølving L, Nielsen J, Nørgård BM. Postpartum Surgical Complications in Women with Inflammatory Bowel Disease After Caesarian Section: A Danish Nationwide Cohort Study. J Crohns Colitis. 2022 May 10;16(4):625–32. [CrossRef]
- Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, et al. Breastfeeding and the Use of Human Milk. Pediatrics. 2012 Mar 1;129(3):e827–41. [CrossRef]
- Moffatt DC, Ilnyckyj A, Bernstein CN. A Population-Based Study of Breastfeeding in Inflammatory Bowel Disease: Initiation, Duration, and Effect on Disease in the Postpartum Period. Am J Gastroenterol. 2009 Oct 23;104(10):2517–23. [CrossRef]
- Kane S, Lemieux N. The Role of Breastfeeding in Postpartum Disease Activity in Women with Inflammatory Bowel Disease. Am J Gastroenterol. 2005 Jan;100(1):102–5. [CrossRef]
- Tandon P, Lee E, Jogendran R, Kroeker KI, Dieleman LA, Halloran B, et al. Breastfeeding Patterns in Mothers with Inflammatory Bowel Disease: A Pilot Prospective Longitudinal Study. Inflamm Bowel Dis. 2022 Nov 2;28(11):1717–24. [CrossRef]
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016 May;75(5):795–810. [CrossRef]
- Klotz U, Harings-Kaim A. Negligible excretion of 5-aminosalicylic acid in breast milk. The Lancet. 1993 Sep;342(8871):618–9. [CrossRef]
- Öst L, Wettrell G, Björkhem I, Rane A. Prednisolone excretion in human milk. J Pediatr. 1985 Jun;106(6):1008–11. [CrossRef]
- Hutson JR, Matlow JN, Moretti ME, Koren G. The fetal safety of thiopurines for the treatment of inflammatory bowel disease in pregnancy. J Obstet Gynaecol (Lahore). 2013 Jan 21;33(1):1–8.
- Drugs and Lactation Database (LactMed). Cyclosporine (National Institute of Child Health and Human Development, 2021).
- Duricova D, Dvorakova E, Hradsky O, Mitrova K, Durilova M, Kozeluhova J, et al. Safety of Anti-TNF-Alpha Therapy During Pregnancy on Long-term Outcome of Exposed Children: A Controlled, Multicenter Observation. Inflamm Bowel Dis. 2019 Mar 14;25(4):789–96. [CrossRef]
- Meyer A, Taine M, Drouin J, Weill A, Carbonnel F, Dray-Spira R. Serious Infections in Children Born to Mothers With Inflammatory Bowel Disease With In Utero Exposure to Thiopurines and Anti-Tumor Necrosis Factor. Clinical Gastroenterology and Hepatology. 2022 Jun;20(6):1269-1281.e9. [CrossRef]
- Moens A, van der Woude CJ, Julsgaard M, Humblet E, Sheridan J, Baumgart DC, et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: results of the European CONCEIVE study. Aliment Pharmacol Ther. 2020 Jan;51(1):129–38. [CrossRef]
- Laharie D, Debeugny S, Peeters M, van Gossum A, Gower–Rousseau C, Bélaïche J, et al. Inflammatory bowel disease in spouses and their offspring. Gastroenterology. 2001 Mar;120(4):816–9. [CrossRef]
- Peeters M, Nevens H, Baert F, Hiele M, de Meyer A, Vlietinck R, et al. Familial aggregation in Crohn’s disease: Increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology. 1996 Sep;111(3):597–603. [CrossRef]
- Brondfield MN, Mahadevan U. Inflammatory bowel disease in pregnancy and breastfeeding. Nat Rev Gastroenterol Hepatol. 2023 Mar 31. [CrossRef]
- Cornish J, Tan E, Teare J, Teoh TG, Rai R, Clark SK, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007 Jun 1;56(6):830–7. [CrossRef]
- Grigorescu RR, Husar-Sburlan IA, Rosulescu G, Bobirca A, Cerban R, Bobirca F, et al. Pregnancy in Patients with Inflammatory Bowel Diseases—A Literature Review. Life. 2023 Feb 9;13(2):475. [CrossRef]
- Odufalu FD, Long M, Lin K, Mahadevan U. Exposure to corticosteroids in pregnancy is associated with adverse perinatal outcomes among infants of mothers with inflammatory bowel disease: results from the PIANO registry. Gut. 2022 Sep;71(9):1766–72.
- Dominitz, J. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002 Mar;97(3):641–8.
- Ban L, Tata LJ, Fiaschi L, Card T. Limited Risks of Major Congenital Anomalies in Children of Mothers With IBD and Effects of Medications. Gastroenterology. 2014 Jan;146(1):76–84. [CrossRef]
- Meyer A, Drouin J, Weill A, Carbonnel F, Dray-Spira R. Pregnancy in women with inflammatory bowel disease: a French nationwide study 2010-2018. Aliment Pharmacol Ther. 2020 Nov;52(9):1480–90. [CrossRef]
- Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth Outcomes in Women with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2014 May;1. [CrossRef]
- Boyd HA, Basit S, Harpsøe MC, Wohlfahrt J, Jess T. Inflammatory Bowel Disease and Risk of Adverse Pregnancy Outcomes. PLoS One. 2015 Jun 17;10(6):e0129567. [CrossRef]
- Friedman S, Nielsen J, Jølving LR, Nøhr EA, Nørgård BM. Long-term Motor and Cognitive Function in the Children of Women With Inflammatory Bowel Disease. J Crohns Colitis. 2020 Dec 2;14(12):1709–16. [CrossRef]
| Mechanism of Action | Medication | Breastfeeding Recommendation |
|---|---|---|
| Aminosalicylates | Mesalamine and Sulfasalazine | Low Risk |
| Corticosteroids | Budesonide and Prednisone | Low Risk |
| Thiopurines | 6-Mercaptopurine and Azathioprine | Low Risk |
| Antimetabolites | Methotrexate | Not Recommended |
| Calcineurin Inhibitors | Cyclosporine | Low Risk |
| Anti-TNF | Adalimumab and Infliximab | Low Risk |
| Anti-integrin | Vedolizumab | Low Risk |
| Anti-IL-23 | Risankizumab | Low Risk |
| Anti-IL-12/IL-23 | Ustekinumab | Low Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





