Introduction
Vascular endothelium lining on the inner surface of blood vessel wall plays significant role in controlling vasomotor tone, cells and nutrient trafficking, maintaining blood fluidity, and angiogenesis(1). When disrupted, caused by infection, toxin, trauma, irradiation, and ischemia, etc., endotheliopathy develops, resulting in the release of several adhesion molecular such as von Willebrand factor (VWF)(2), proteoglycan syndecan-1(3, 4), and soluble thrombomodulin(5), etc.
VWF, a multimeric glycoprotein, is stored in the Weibel-Palade bodies of endothelial cells and alpha granules of megakaryocytes. Upon stimulation by inflammatory cytokines or vascular injury, a large amount of VWF will be released that remains anchored on endothelial membrane(2, 6). The released VWF multimers may form unusually large (UL) VWF bundles or strings that attract platelets and neutrophils from circulation for hemostasis and inflammation. The endothelium bound ULVWF bundles or strings appear to exhibit an “open” conformation, which can be rapidly cleaved by plasma metalloprotease ADAMTS13(7, 8), which is primarily synthesized in hepatic stellate cells(9) and endothelial cells(10).
ADAMTS13 synthesis can be negatively regulated by tumor necrosis factor-α (TNF-α), interferon-r, and inteleukin-6 (IL-6)(11). Deficiency of plasma ADAMTS13 activity, resulting from mutations in ADAMTS13(12) or autoantibodies against ADAMTS13(13), may result in thrombotic thrombocytopenic purpura (TTP). Mild to moderate reduced levels of plasma ADAMTS13 activity are associated with an increased risk of other inflammatory thrombotic disorders, including ischemic stroke(14, 15), myocardial infarction(15, 16), preeclampsia(17), trauma(18, 19), and COVID-19 associated coagulopathy(20, 21), etc.
However, the data published to date on the relationship between VWF/ADAMTS13 and adverse events or complications of COVID-19 remains inconclusive, largely due to small sample sizes and single time blood sampling on admission. To better understand the role of endothelial activation or injury predicting long-term outcome, we performed a prospective and longitudinal assessment of plasma VWF and VWF/ADAMTS13 ratios in a large cohort of hospitalized patients with SARS-CoV2 infection with hospitalized but non-COVID19 patients as controls. We show that dynamic assessment of plasma VWF and VWF/ADAMTS13 ratios may predict mortality of patients with SARS-CoV2 infection. These results may have clinical implication when considering early intervention with anti-VWF nanobody such as caplacizumab(22) or supplementation of recombinant ADAMTS13(23) to improve the short and long-term outcome.
Methods
Patients and sampling: The Institutional Review Board (IRB) of University of Kansas Medical Center (KUMC) has approved the study (#00148313). A total of 124 consecutive hospitalized patients with a positive SARS-CoV-2 PCR test from January 2022 to March 2022 were prospectively recruited for the study. Citrated or EDTA-anticoagulated blood samples were obtained on admission (D1), 3-4 days following treatment (D2), and 1-2 days prior to discharge or death (D3). The exclusion criteria included those under 18 years old, those who had undergone a surgery before initial sample collection or whose initial samples were collected more than 48 hours after the hospital admission. Negative control samples were those admitted for a medical condition (such as orthopedic surgery, depression, and coronary artery disease, etc.) that was not primarily COVID-19 related but tested positive for SARS-CoV-2 as the asymptomatic group. Patient demographic, clinical, and laboratory data were collected throughout hospitalization and follow up from the electronic medical record. All patients were followed up for 60 days for obtaining survival information. Additionally, we included 23 local healthy controls who are not acutely ill but matched for their age, gender, and race.
Plasma VWF antigen: An in-house enzyme-linked immunosorbent assay (ELISA) determined the plasma VWF antigen as described previously(24).
Plasma ADAMTS13 antigen: Plasma ADAMTS13 antigen was determined by a commercially available ELISA according to the manufacturers’ instructions (R&D Systems, Minneapolis, MN). The intra-assay and inter-assay CVs of this assay were also <8% and <10%, respectively.
Plasma ADAMTS13 activity: An in-house fluorescence resonance energy transfer (FRETS)-VWF73 assay determined plasma ADAMTS13 activity as previously described(25, 26).
Statistical Analysis: Means and standard deviations (SD) were determined for continuous variables, but medians and interquartile ranges (IQR) were determined for non-continuous variables. Mann-Whitney and Kruskal-Wallis tests were performed for comparison of two and ≥ 3 groups, respectively. Kaplan Meier survival analysis with a log-rank test were used to determine the difference in survival rates of two groups. Cox proportional hazard ratio regression determined the predict value of these biomarkers at different sample collection timepoints for the mortality rate. All analyses were performed using either SPSS statistics version 26.0 (IBM) or Prism 8.0.
Results
Demographic, clinical, and laboratory characteristics of the COVID-19 patient cohort. 124 patients with SARS-CoV-2 infection were included in the study. These patients were divided into 4 groups: asymptomatic, moderate, severe, and critical groups based on an updated WHO guideline. The demographic, clinical, and laboratory characteristics of this cohort of patient were previously described(4).
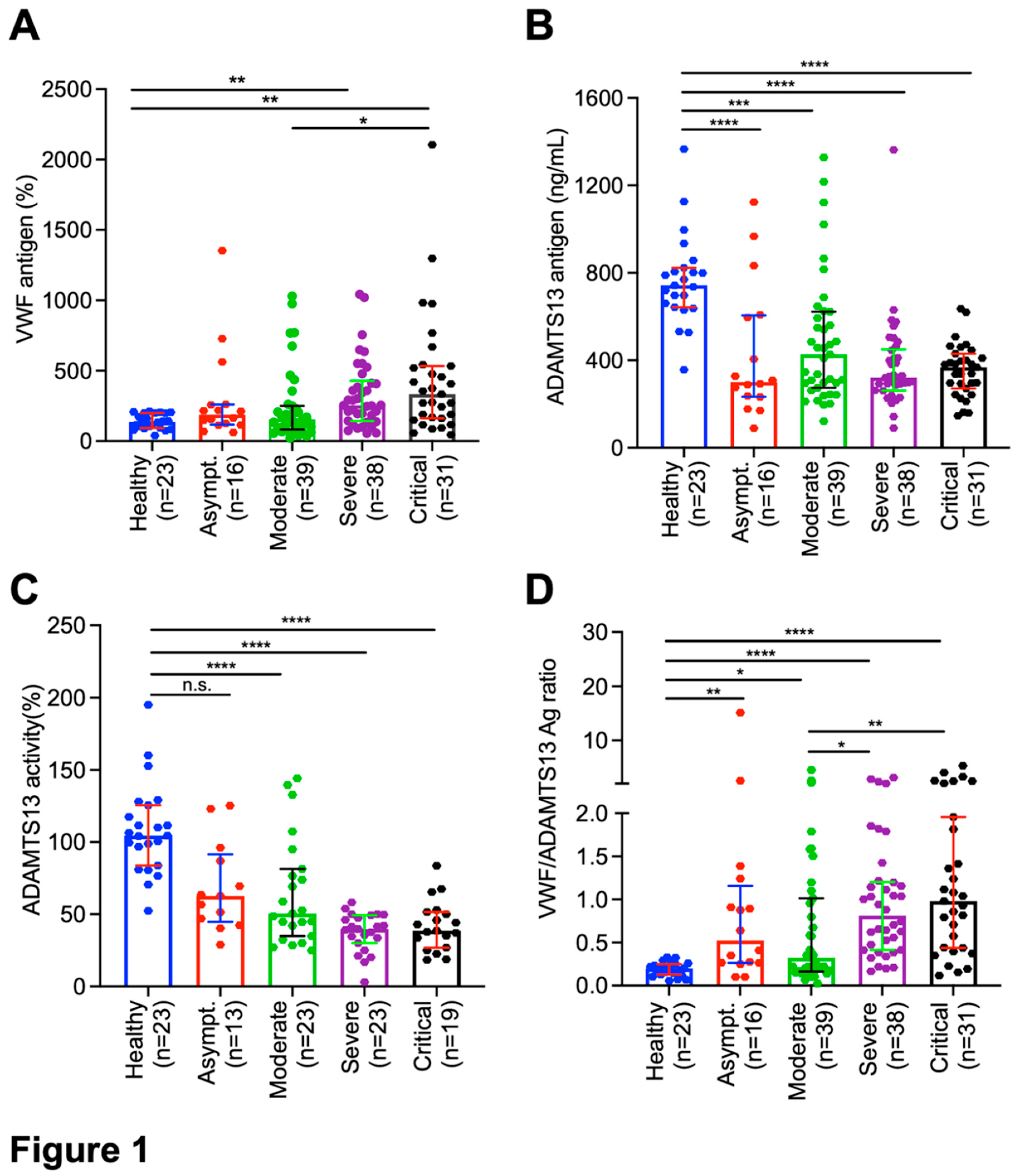
Plasma levels of VWF antigen, ADAMTS13 activity, and ADAMTS13 antigen in hospitalized patients with SARS-CoV2 infection. The median (IQR) admission levels of plasma VWF antigen in asymptomatic, moderate, severe, and critical groups were 136% (93-200%), 188% (120-259%), 153% (186-240%), 255% (143-411%), and 334% (165-526%), respectively. However, statistically significant difference in the plasma levels of VWF antigen was only detected between critical and moderate groups (p<0.05) and between severe/critical group and healthy controls (p<0.01) (
Figure 1A). Concomitantly, admission plasma levels of ADAMTS13 antigen (
Figure 1B) and activity (
Figure 1C) were reduced in all hospitalized patients regardless of their disease severity (p<0.005~0.001). More interestingly, the ratios (median, IQR) of plasma VWF/ADAMTS13 antigen in patients with asymptomatic (0.5, 0.3-1.2) (p<0.01), moderate (0.3, 0.2-1.0) (p<0.05), severe (0.8, 0.4-1.2) (p<0.001), and critical (1.0, 0.4-2.0) (p<0.001) COVID-19 were significantly higher than those in the healthy controls. Furthermore, the ratios of VWF/ADAMTS13 antigen in patients with severe/critical were significantly higher than those in patients with mild to moderate disease (p<0.05-0.01) (
Figure 1D).
Wilcoxon test between two groups. Here, n.s., * and ** indicate p>0.05, <0.05, and <0.01, respectively.
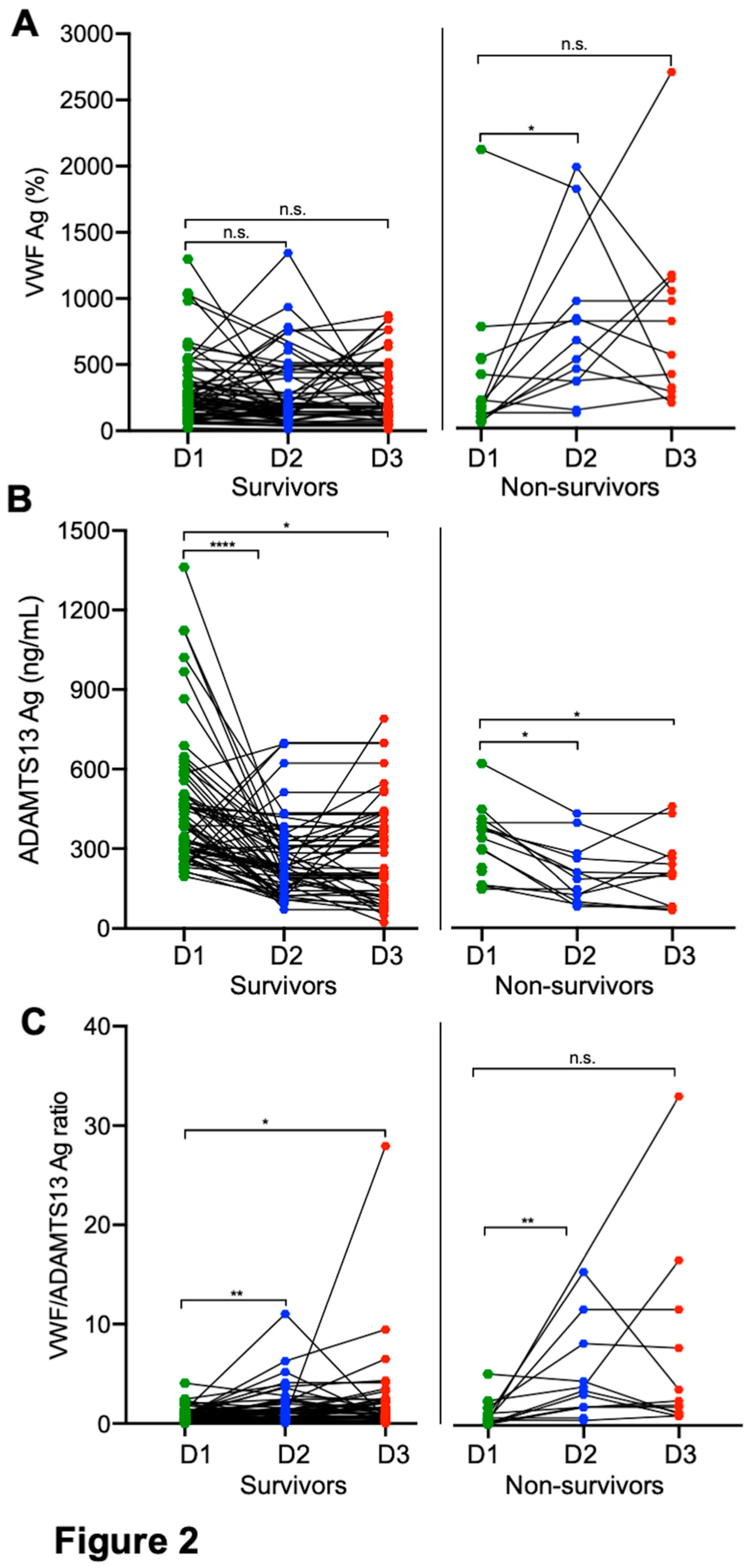
Longitudinal changes of plasma VWF and ADAMTS13 in patients with SARS-CoV2 infection. Serial blood samples were obtained and determined in patients who survived or died of severe/critical COVID-19. Our results showed that while there was a significant individual variability in plasma levels of VWF and ADAMTS13 antigen among all hospitalized patients infected with SARS-CoV-2, plasma levels of VWF antigen (
Figure 2A) tended to increase with a concomitant decrease of plasma ADAMTS13 antigen (
Figure 2B) 3-5 days following treatments. This might be better presented by the ratios of VWF/ADAMTS13 antigen in
Figure 2C. However, plasma VWF levels or the ratios of VWF/ADAMTS13 antigen in patients with severe/critical COVID-19 remained persistently higher than the normal over entire hospitalization, particularly in those who died (
Figure 1A and
Figure 1C, right panels). These results suggest that persistent endotheliopathy in patients with severe/critical COVID-19 may be associated with an increased mortality rate.
Figure 1.
Plasma levels of biomarkers in hospitalized patients with SARS-CoV-2 infection and healthy controls. Admission plasmas VWF antigen (A), ADAMTS13 activity (B), ADAMTS13 antigen (C), and the ratio of VWF/ADAMTS13 antigen in healthy controls, hospitalized patients with SARS-CoV-2 infection on admission (D1) with various disease severities (e.g., asymptomatic, moderate, severe, and critical) (D). The data shown are the median (bar) and interquartile range (IQR) (horizontal lines), as well as individual values (dots). Kruskal-Willis test was performed to determine the statistical significance of the difference among various groups. Here, n.s., *, **, *** and **** indicate a p-value >0.05, <0.05, <0.01, <0.005 and <0.001, respectively.
Figure 1.
Plasma levels of biomarkers in hospitalized patients with SARS-CoV-2 infection and healthy controls. Admission plasmas VWF antigen (A), ADAMTS13 activity (B), ADAMTS13 antigen (C), and the ratio of VWF/ADAMTS13 antigen in healthy controls, hospitalized patients with SARS-CoV-2 infection on admission (D1) with various disease severities (e.g., asymptomatic, moderate, severe, and critical) (D). The data shown are the median (bar) and interquartile range (IQR) (horizontal lines), as well as individual values (dots). Kruskal-Willis test was performed to determine the statistical significance of the difference among various groups. Here, n.s., *, **, *** and **** indicate a p-value >0.05, <0.05, <0.01, <0.005 and <0.001, respectively.
Figure 2.
Longitudinal changes of plasma biomarkers in patients who survived and died of COVID-19. Each solid line depicts the change of plasma levels of VWF antigen (A), ADAMTS13 antigen (B), and the ratio of VWF/ADAMTS13 antigen (C) in patients who survived (survivors on the left) or died (non-survivors on the right) in each panel on admission (D1), 3-4 days following therapy (D2), and before discharge or death (D3), respectively. Data were analyzed by.
Figure 2.
Longitudinal changes of plasma biomarkers in patients who survived and died of COVID-19. Each solid line depicts the change of plasma levels of VWF antigen (A), ADAMTS13 antigen (B), and the ratio of VWF/ADAMTS13 antigen (C) in patients who survived (survivors on the left) or died (non-survivors on the right) in each panel on admission (D1), 3-4 days following therapy (D2), and before discharge or death (D3), respectively. Data were analyzed by.
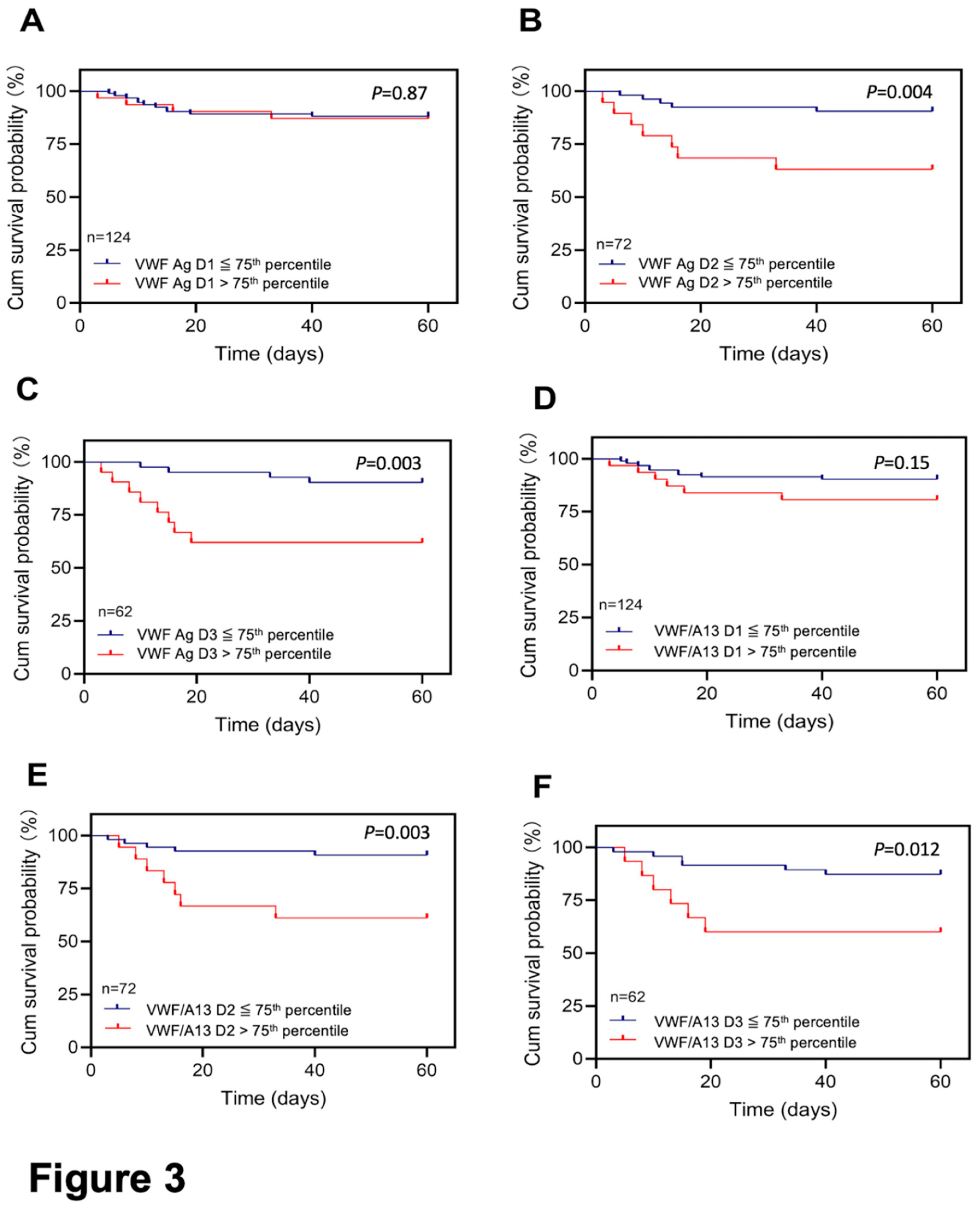
Elevated VWF levels or VWF/ADAMTS13 antigen ratios predict 60-day mortality in patients with severe/critical COVID-19. To determine if elevated plasma VWF levels or increased ratios of VWF/ADAMTS13 at various time points during hospitalization would predict long-term outcomes, we performed Kaplan-Meier survival and Cox proportional hazard risk analyses. Our results demonstrated that while the admission plasma levels of VWF had no or little predictive value (p>0.05) (Figure 3A), patients with high plasma levels of VWF antigen (>75th percentile) at D2 (>459%) (p<0.005) (Figure 3B) and D3 (>513%) (p<0.005) (Figure 3C) had a dramatically higher 60-day mortality rate than those with their plasma levels of VWF antigen below 75th percentile. Similarly, the elevated ratios of plasma VWF/ADAMTS13 antigen at D2 (1.98) (Figure 3E) and D3 (2.31) (Figure 3F), but not D1 (Figure 3D) also predicted the 60-day mortality rate.
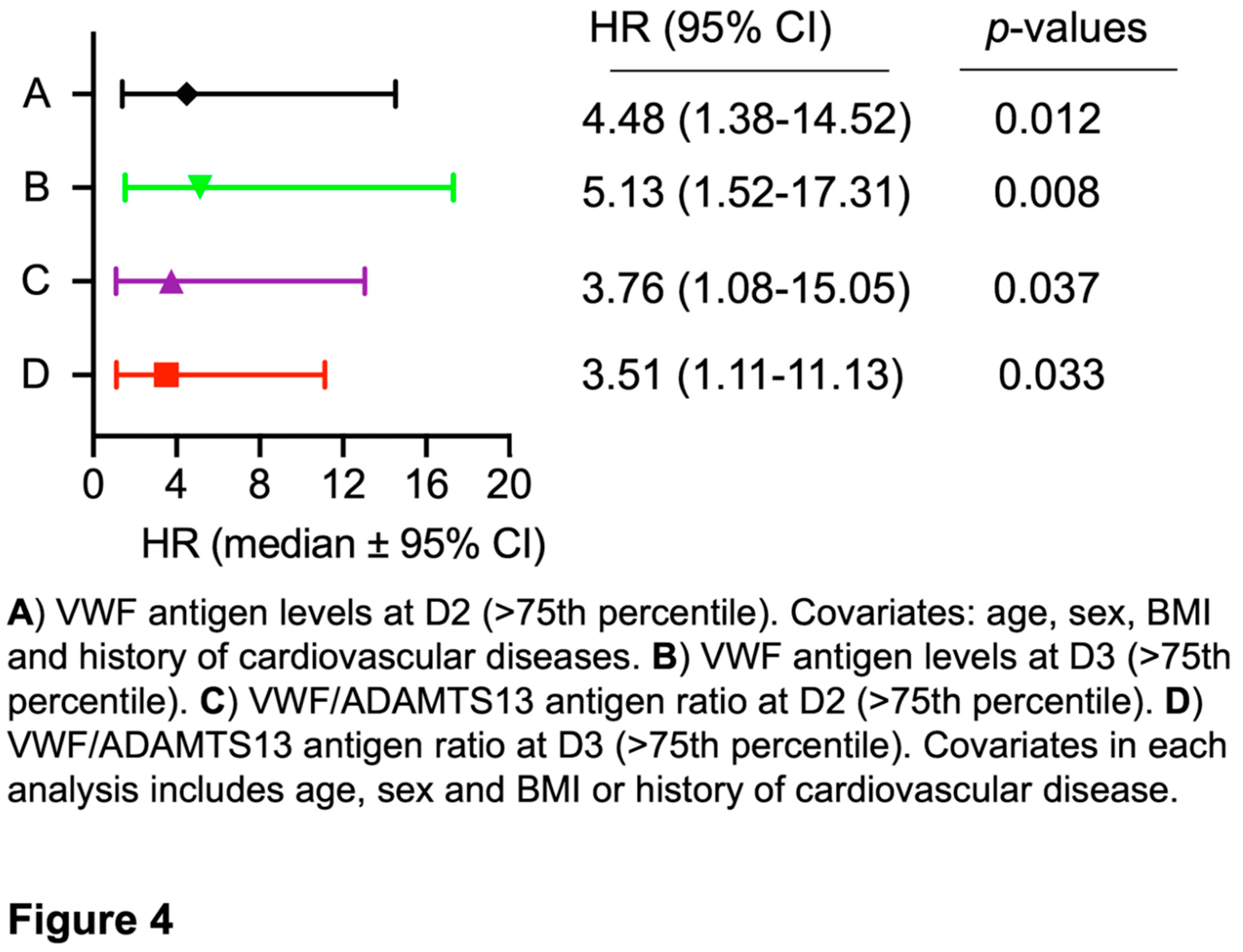
Cox proportional hazard (mortality) regression analysis revealed that the elevated plasma levels of VWF at D2 had a hazard ratio (HR) of 4.5 (95% CI, 0.38-14.52) (p<0.05) after age, gender, body mass index, and cardiovascular disease were adjusted (Figure 4A). At D3, the elevated plasma VWF levels had an HR of 5.13 (95% CI, 1.52-17.31) (p<0.01), also after age, gender, and body mass index were adjusted in the analysis (Figure 4B). Similarly, a predictive value was found with an increased ratio of plasma VWF/ADAMTS13 antigen at D2 (Figure 4C) and D3 (Figure 4D) for mortality, although low levels of plasma ADAMTS13 antigen (<25th percentile) alone had no such a predictive value, regardless of sampling time points (data not shown). These results indicate that the dynamic assessment of endothelial VWF or VWF/ADAMTS13 ratios may have a prognostic value for the long-term adverse outcome in patients with severe/critical COVID-19.
Figure 3.
Kaplan-Meier survival analysis in patients with COVID-19 based on plasma levels of VWF or the VWF/ADAMTS13 ratio at different time points. The 60-day survival probabilities in patients with high (>75th percentile) and low (≤75th percentile) levels of plasma VWF antigen on admission (D1) (A), 3 to 4 days following therapy (D2) (B), and prior to being discharged or death (D3) (C) are shown. Similarly, the 60-day survival probabilities in patients with high (>75th percentile) and low (≤75th percentile) ratios of plasma VWF/ADAMTS13 antigen at D1 (D), D2 (E), and D3 (F). Here, p<0.05 and <0.01 are statistically significant and highly significant, respectively.
Figure 3.
Kaplan-Meier survival analysis in patients with COVID-19 based on plasma levels of VWF or the VWF/ADAMTS13 ratio at different time points. The 60-day survival probabilities in patients with high (>75th percentile) and low (≤75th percentile) levels of plasma VWF antigen on admission (D1) (A), 3 to 4 days following therapy (D2) (B), and prior to being discharged or death (D3) (C) are shown. Similarly, the 60-day survival probabilities in patients with high (>75th percentile) and low (≤75th percentile) ratios of plasma VWF/ADAMTS13 antigen at D1 (D), D2 (E), and D3 (F). Here, p<0.05 and <0.01 are statistically significant and highly significant, respectively.
Figure 4.
Cox proportional hazard regression analysis. The hazard ratios (HRs) for death were determined in patients with high VWF antigen (>75th percentile) on 3-4 days following therapy (D2) (A) and prior to discharge or death (T3) (B). Additionally, the HRs were also determined in patients with high ratios of VWF/ADAMTS13 antigen (>75th percentile) on D2 (C) and D3 (D). The HRs were adjusted for covariates (age, sex, and body mass index, BMI) as indicated in each panel. Here, p<0.05 and 0.01 are statistically significant and highly significant, respectively.
Figure 4.
Cox proportional hazard regression analysis. The hazard ratios (HRs) for death were determined in patients with high VWF antigen (>75th percentile) on 3-4 days following therapy (D2) (A) and prior to discharge or death (T3) (B). Additionally, the HRs were also determined in patients with high ratios of VWF/ADAMTS13 antigen (>75th percentile) on D2 (C) and D3 (D). The HRs were adjusted for covariates (age, sex, and body mass index, BMI) as indicated in each panel. Here, p<0.05 and 0.01 are statistically significant and highly significant, respectively.
Discussion
The present study demonstrates that plasma levels of VWF antigen are dramatically elevated in hospitalized patients with SARS-CoV-2 infection, regardless of their disease severity, and their median levels of plasma VWF antigen increase by ~2 fold compared with those in the healthy controls. Additionally, plasma ADAMTS13 activity and antigen levels in these patients are also significantly below the normal range, although only plasma ADAMTS13 activity is significantly reduced in patients with severe and critical COVID-19 compared with those with moderate disease. Kaplan-Meier survival and Cox proportional hazard ratio analyses demonstrate the predictive value of the elevated plasma VWF and the ratio of VWF/ADAMTS13 for 60-day mortality rate.
In the early COVID-19 outbreak, coagulopathy and fulminant thrombotic disorders emerge as life-threatening complications in patients with severe/critical disease and are associated with mortality(27). A meta-analysis shows that venous thromboembolism (VTE) occurred in 2.9%-5.7% of hospitalized COVID-19 patients despite venous thromboprophylaxis(28). Additionally, 0.3%-9.4% patients were found to develop thrombosis following hospital discharge, including arterial and venous events(29-31). As the Omicron subtypes became the dominant strain, which exhibits a relatively lower toxicity, 11.3% of our cohort experienced thrombotic events despite anticoagulation therapy. The vast variability of plasma VWF levels in patients with COVID-19 may be affected by the balance between increased synthesis and release of VWF from activated endothelium and consumption in thrombus formation. This may particularly be the case in patients with identifiable thromboembolic events when plasma levels of VWF may be reduced instead of increased. Nonetheless, the overall levels of plasma VWF at D1 in those with thrombotic events are still higher than the age-, gender-, and comorbidity-matched controls without thrombosis (data not shown). Thus, it appears that anticoagulation with unfractionated or low molecular weight heparin alone does not eliminate thrombosis in patients with COVID-19. This prophylactic strategy only addresses the secondary hemostasis (i.e., coagulation), but does not tackle the primary hemostasis (i.e., the VWF-platelet dominant thrombosis).
Endotheliopathy, marked by the increased levels of plasma VWF(32, 33) and syndecan-1(4), has been previously shown to be associated with the disease severity. It may predispose to a long-term cardiovascular complication. Endotheliopathy may be caused by direct infection with SARS-CoV-2 through binding to the outer membrane angiotensin-converting enzyme 2 (ACE2) receptor(34, 35). It may also be caused by a viral nucleocapsid protein(36), prolonged and overactive immune responses involving in cytotoxic T cells and monocytes(37, 38), and inflammatory cytokines including interleukin (IL)-4, IL-6, and tissue necrosis factor-α (TNF-α)(20, 39). It is known that inflammatory cytokines trigger release of VWF from endothelial cells while inhibiting hepatic stellate cells and endothelial cells to synthesize ADAMTS13(11, 40). Markedly increase in plasma VWF and modestly reduction of plasma ADAMTS13 activity create a prothrombotic status, which may lead to microvascular thrombosis and tissue damage in major organs such as brain, heart, kidney, pancreas, and adrenal glands, etc., leading to long-term complications and mortality. Our results are consistent with those reported previously from our laboratory in all hospitalized patients with a suspected heparin-induced thrombocytopenia (HIT) in whom high plasma levels of VWF and low ADAMTS13 are associated with mortality regardless of the HIT test results(41).
There are some limitations in this study. First, no data on VWF and ADAMTS13 before COVID-19 infection and values in the convalescent stage are available, which complicate the data analysis in the longitudinal study. Second, we did not recruit asymptomatic outpatients as controls, although this comparison has been previously performed by others(42). Thirdly, this is a single-center study, and the sample size remains relatively small although it is larger than any of those previously published for investigating the similar biomarkers(43-48). To overcome the individual variability and dynamic changes in VWF as a biomarker for endotheliopathy, a large multi-center study with a prolonged follow-up may be necessary to confirm our findings.
Nevertheless, we conclude that significantly elevated levels of plasma VWF antigen coupled with modestly reduced levels of plasma ADAMTS13 activity on admission and other time points during hospitalization in patients with severe/critical COVID-19 compared with those with mild/moderate disease. The dynamic assessment of plasma levels of VWF or the VWF/ADAMTS13 ratios predicts 60-day mortality. Our findings suggest the potential need for early therapeutic intervention with targeting VWF-platelet or supplementing recombinant ADAMTS13 to improve endotheliopathy and reduce long-term outcomes in patients with severe/critical COVID-19.
Author contributions
Q.Z. and X.L.Z. designed, executed, and interpreted the results and drafted manuscript. Other authors (A.B., N.Y., S.L., Z.Y.) helped patient recruitment, specimen collection, and processing. All authors revised and approved the final version of the manuscript for submission.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author at request.
Conflict of interests
X.L.Z. is a consultant for Alexion, Sanofi, BioMedica Diagnostics, Argenx, GC Biopharma, Kyowa Kirin, and Takeda, as well as a founder of Clotsolution. Other authors have nothing to declare.
References
- Aird, WC. Endothelium as an organ system. Crit Care Med. 2004, 32, S271–279. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H, Townsend, L, Morrin, H, Ahmad, A, Comerford, C, Karampini, E, Englert, H, Byrne, M, Bergin, C, O'Sullivan, JM, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Rodriguez, E, Ostrowski, SR, Cardenas, JC, Baer, LA, Tomasek, JS, Henriksen, HH, Stensballe, J, Cotton, BA, Holcomb, JB, Johansson, PI, et al. Syndecan-1: A Quantitative Marker for the Endotheliopathy of Trauma. J Am Coll Surg. 2017, 225, 419–427. [Google Scholar] [CrossRef]
- Zhang, Q, Ye, Z, Bignotti, A, and Zheng, XL. Longitudinal Assessment of Plasma Syndecan-1 Predicts 60-
Day Mortality in Patients with COVID-19. J Clin Med. 2023, 12.
- Mori, Y, Wada, H, Okugawa, Y, Tamaki, S, Nakasaki, T, Watanabe, R, Gabazza, EC, Nishikawa, M, Minami, N, and Shiku, H. Increased plasma thrombomodulin as a vascular endothelial cell marker in patients with thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Clin Appl Thromb Hemost. 2001, 7, 5–9. [Google Scholar] [CrossRef]
- Wagner, DD, and Bonfanti, R. von Willebrand factor and the endothelium. Mayo Clin Proc. 1991, 66, 621–627. [Google Scholar] [CrossRef]
- Dong, JF, Moake, JL, Nolasco, L, Bernardo, A, Arceneaux, W, Shrimpton, CN, Schade, AJ, McIntire, LV, Fujikawa, K, and Lopez, JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002, 100, 4033–4039. [Google Scholar] [CrossRef]
- Chauhan, AK, Kisucka, J, Brill, A, Walsh, MT, Scheiflinger, F, and Wagner, DD. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008, 205, 2065–2074. [Google Scholar] [CrossRef]
- Uemura, M, Tatsumi, K, Matsumoto, M, Fujimoto, M, Matsuyama, T, Ishikawa, M, Iwamoto, TA, Mori, T, Wanaka, A, Fukui, H, et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005, 106, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Turner, N, Nolasco, L, Tao, Z, Dong, JF, and Moake, J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006, 4, 1396–1404. [Google Scholar] [CrossRef]
- Cao, WJ, Niiya, M, Zheng, XW, Shang, DZ, and Zheng, XL. Inflammatory cytokines inhibit ADAMTS13 synthesis in hepatic stellate cells and endothelial cells. J Thromb Haemost. 2008, 6, 1233–1235. [Google Scholar] [CrossRef]
- Levy, GG, Nichols, WC, Lian, EC, Foroud, T, McClintick, JN, McGee, BM, Yang, AY, Siemieniak, DR, Stark, KR, Gruppo, R, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001, 413, 488–494. [Google Scholar] [CrossRef]
- Tsai, HM, and Lian, EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Eng J Med. 1998, 339, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Bongers, TN, de Maat, MP, van Goor, ML, Bhagwanbali, V, van Vliet, HH, Gomez Garcia, EB, Dippel, DW, and Leebeek, FW. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006, 37, 2672–2677. [Google Scholar] [CrossRef] [PubMed]
- Andersson, HM, Siegerink, B, Luken, BM, Crawley, JT, Algra, A, Lane, DA, and Rosendaal, FR. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012, 119, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Horii, M, Uemura, S, Uemura, M, Matsumoto, M, Ishizashi, H, Imagawa, K, Iwama, H, Takeda, Y, Kawata, H, Nakajima, T, et al. Acute myocardial infarction as a systemic prothrombotic condition evidenced by increased von Willebrand factor protein over ADAMTS13 activity in coronary and systemic circulation. Heart Vessels. 2008, 23, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Aref, S, and Goda, H. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematology. 2013, 18, 237–241. [Google Scholar] [CrossRef]
- Genet, GF, Johansson, PI, Meyer, MA, Solbeck, S, Sorensen, AM, Larsen, CF, Welling, KL, Windelov, NA, Rasmussen, LS, and Ostrowski, SR. Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J Neurotrauma. 2013, 30, 301–306. [Google Scholar] [CrossRef]
- Russell, RT, McDaniel, JK, Cao, W, Shroyer, M, Wagener, BM, Zheng, XL, and Pittet, JF. Low Plasma ADAMTS13 Activity Is Associated with Coagulopathy, Endothelial Cell Damage and Mortality after Severe Paediatric Trauma. Thromb Haemost. 2018, 118, 676–687. [Google Scholar]
- Zhang, J, Tecson, KM, and McCullough, PA. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Dolgushina, N, Gorodnova, E, Beznoshenco, O, Romanov, A, Menzhinskaya, I, Krechetova, L, and Sukhikh, G. Von Willebrand Factor and ADAMTS-13 Are Associated with the Severity of COVID-19 Disease. J Clin
Med. 2022, 11.
- Peyvandi, F, Scully, M, Kremer Hovinga, JA, Cataland, S, Knobl, P, Wu, H, Artoni, A, Westwood, JP, Mansouri Taleghani, M, Jilma, B, et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med. 2016, 374, 511–522. [Google Scholar] [CrossRef]
- Scully, M, Knobl, P, Kentouche, K, Rice, L, Windyga, J, Schneppenheim, R, Kremer Hovinga, JA, Kajiwara, M, Fujimura, Y, Maggiore, C, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 2017, 130, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M, Cao, W, McDaniel, JK, Pham, HP, Raju, D, Nawalinski, K, Frangos, S, Kung, D, Zager, E, Kasner, SE, et al. Plasma ADAMTS13 activity and von Willebrand factor antigen and activity in patients with subarachnoid haemorrhage. Thromb Haemost. 2017, 117, 691–699. [Google Scholar] [CrossRef]
- Raife, TJ, Cao, W, Atkinson, BS, Bedell, B, Montgomery, RR, Lentz, SR, Johnson, GF, and Zheng, XL. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009, 114, 1666–1674. [Google Scholar] [CrossRef]
- Zhang, L, Lawson, HL, Harish, VC, Huff, JD, Knovich, MA, and Owen, J. Creation of a recombinant peptide substrate for fluorescence resonance energy transfer-based protease assays. Anal Biochem. 2006, 358, 298–300. [Google Scholar] [CrossRef]
- Rimmer, E, Houston, BL, Kumar, A, Abou-Setta, AM, Friesen, C, Marshall, JC, Rock, G, Turgeon, AF, Cook, DJ, Houston, DS, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014, 18, 699. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, E, Porfidia, A, Ageno, W, Spoto, S, Pola, R, and Di Nisio, M. High-dose versus low-dose venous thromboprophylaxis in hospitalized patients with COVID-19: a systematic review and meta-analysis. Intern
Emerg Med. 2022.
- Patell, R, Bogue, T, Koshy, A, Bindal, P, Merrill, M, Aird, WC, Bauer, KA, and Zwicker, JI. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020, 136, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D, Allen, SL, Tsang, J, Flint, S, Pinhasov, T, Williams, S, Tan, G, Thakur, R, Leung, C, Snyder, M, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021, 137, 2838–2847. [Google Scholar] [CrossRef]
- Ramacciotti, E, Barile Agati, L, Calderaro, D, Aguiar, VCR, Spyropoulos, AC, de Oliveira, CCC, Lins Dos Santos, J, Volpiani, GG, Sobreira, ML, Joviliano, EE, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022, 399, 50–59. [Google Scholar] [CrossRef]
- Seth, R, McKinnon, TAJ, and Zhang, XF. Contribution of the von Willebrand factor/ADAMTS13 imbalance to COVID-19 coagulopathy. Am J Physiol Heart Circ Physiol. 2022, 322, H87–H93. [Google Scholar] [CrossRef]
- Sinkovits, G, Reti, M, Muller, V, Ivanyi, Z, Gal, J, Gopcsa, L, Remenyi, P, Szathmary, B, Lakatos, B, Szlavik, J, et al. Associations between the von Willebrand Factor-ADAMTS13 Axis, Complement Activation, and COVID-19 Severity and Mortality. Thromb Haemost. 2022, 122, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z, Liu, F, Blair, R, Wang, C, Yang, H, Mudd, J, Currey, JM, Iwanaga, N, He, J, Mi, R, et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. 2021, 11, 8076–8091. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z, Flammer, AJ, Steiger, P, Haberecker, M, Andermatt, R, Zinkernagel, AS, Mehra, MR, Schuepbach, RA, Ruschitzka, F, and Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Qian, Y, Lei, T, Patel, PS, Lee, CH, Monaghan-Nichols, P, Xin, HB, Qiu, J, and Fu, M. Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J Virol. 2021, 95, e0139621. [Google Scholar] [CrossRef]
- Chioh, FW, Fong, SW, Young, BE, Wu, KX, Siau, A, Krishnan, S, Chan, YH, Carissimo, G, Teo, LL, Gao, F, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife. 2021, 10.
- Fogarty, H, Ward, SE, Townsend, L, Karampini, E, Elliott, S, Conlon, N, Dunne, J, Kiersey, R, Naughton, A, Gardiner, M, et al. Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction. J Thromb Haemost. 2022.
- Bernardo, A, Ball, C, Nolasco, L, Moake, JF, and Dong, JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004, 104, 100–106. [Google Scholar] [CrossRef]
- Bockmeyer, CL, Claus, RA, Budde, U, Kentouche, K, Schneppenheim, R, Losche, W, Reinhart, K, and Brunkhorst, FM. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica. 2008, 93, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Chan, M, Zhao, X, and Zheng, XL. Low ADAMTS-13 predicts adverse outcomes in hospitalized patients with suspected heparin-induced thrombocytopenia. Res Pract Thromb Haemost. 2021, 5, e12581. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M, Mansouritorghabeh, H, and Parsa-Kondelaji, M. High levels of Von Willebrand factor markers in COVID-19: a systematic review and meta-analysis. Clin Exp Med. 2022, 22, 347–357. [Google Scholar] [CrossRef]
- Zhang, D, Li, L, Chen, Y, Ma, J, Yang, Y, Aodeng, S, Cui, Q, Wen, K, Xiao, M, Xie, J, et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol Med. 2021, 27, 151. [Google Scholar] [CrossRef]
- Karampoor, S, Zahednasab, H, Farahmand, M, Mirzaei, R, Zamani, F, Tabibzadeh, A, Bouzari, B, Ajdarkosh, H, Nikkhah, M, Hashemi, MR, et al. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19). Int Immunopharmacol. 2021, 97, 107684. [Google Scholar] [CrossRef]
- Suzuki, K, Okada, H, Tomita, H, Sumi, K, Kakino, Y, Yasuda, R, Kitagawa, Y, Fukuta, T, Miyake, T, Yoshida, S, et al. Possible involvement of Syndecan-1 in the state of COVID-19 related to endothelial injury. Thromb J. 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Delrue, M, Siguret, V, Neuwirth, M, Joly, B, Beranger, N, Sene, D, Chousterman, BG, Voicu, S, Bonnin, P, Megarbane, B, et al. von Willebrand factor/ADAMTS13 axis and venous thromboembolism in moderate-to-severe COVID-19 patients. Br J Haematol. 2020.
- Mancini, I, Baronciani, L, Artoni, A, Colpani, P, Biganzoli, M, Cozzi, G, Novembrino, C, Boscolo Anzoletti, M, De Zan, V, Pagliari, MT, et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J
Thromb Haemost. 2020.
- Escher, R, Breakey, N, and Lammle, B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res. 2020, 192, 174–175. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).