Submitted:
22 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Objectives of the review
- To describe current knowledge on the risk of HBV MTCT in the WHO Africa region.
- To describe the status of HBV MTCT mitigation strategies including hepatitis B birth-dose vaccination programs.
- To explore health systems capacity to support hepatitis B birth-dose vaccination programs in the WHO Africa region.
2.2. Methods
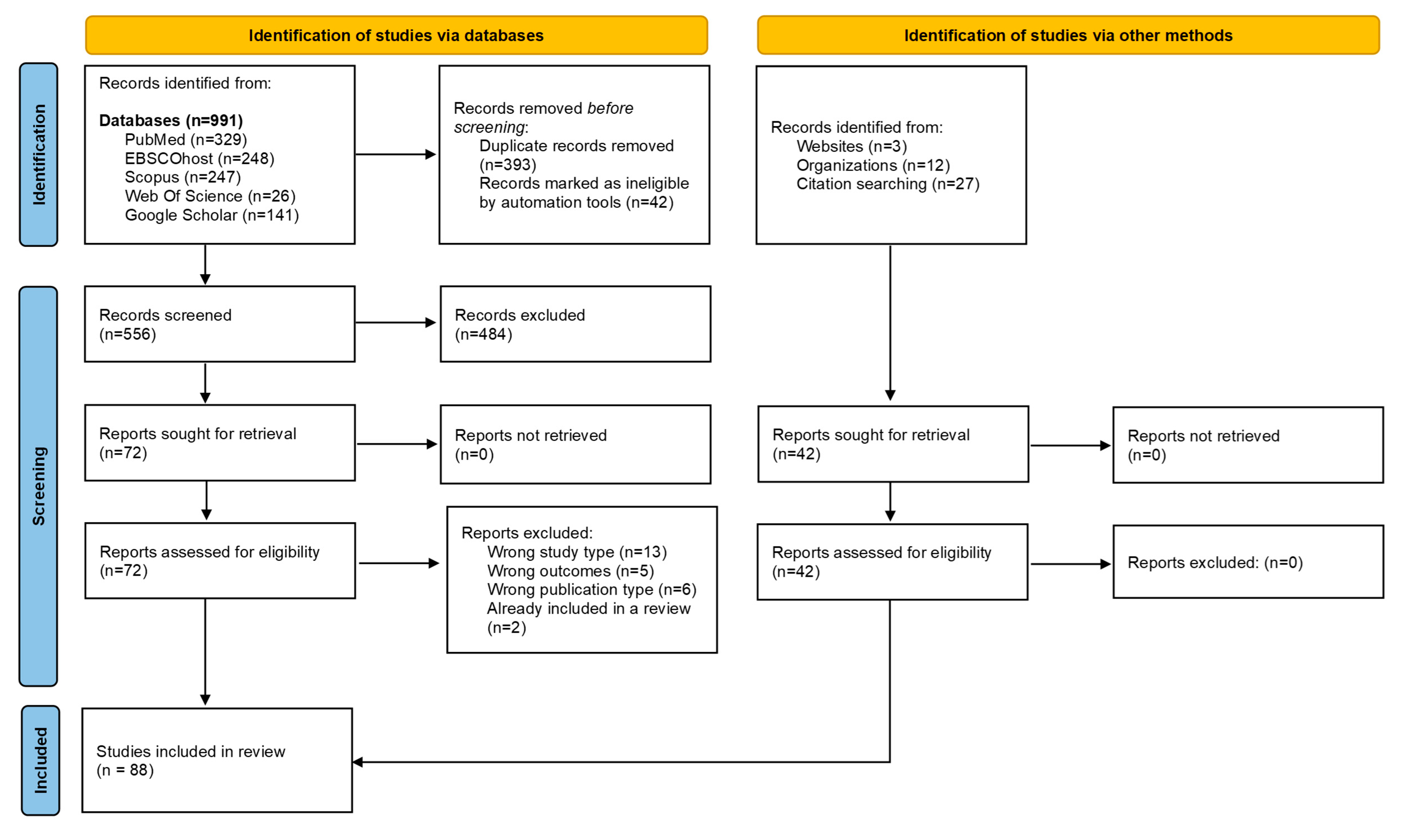
3. Results
3.1. Status of HBV MTCT in the WHO Africa region
3.1.2. Growing prevalence of HBV MTCT
3.1.3. HIV-HBV co-infection and the increased risk of HBV MTCT
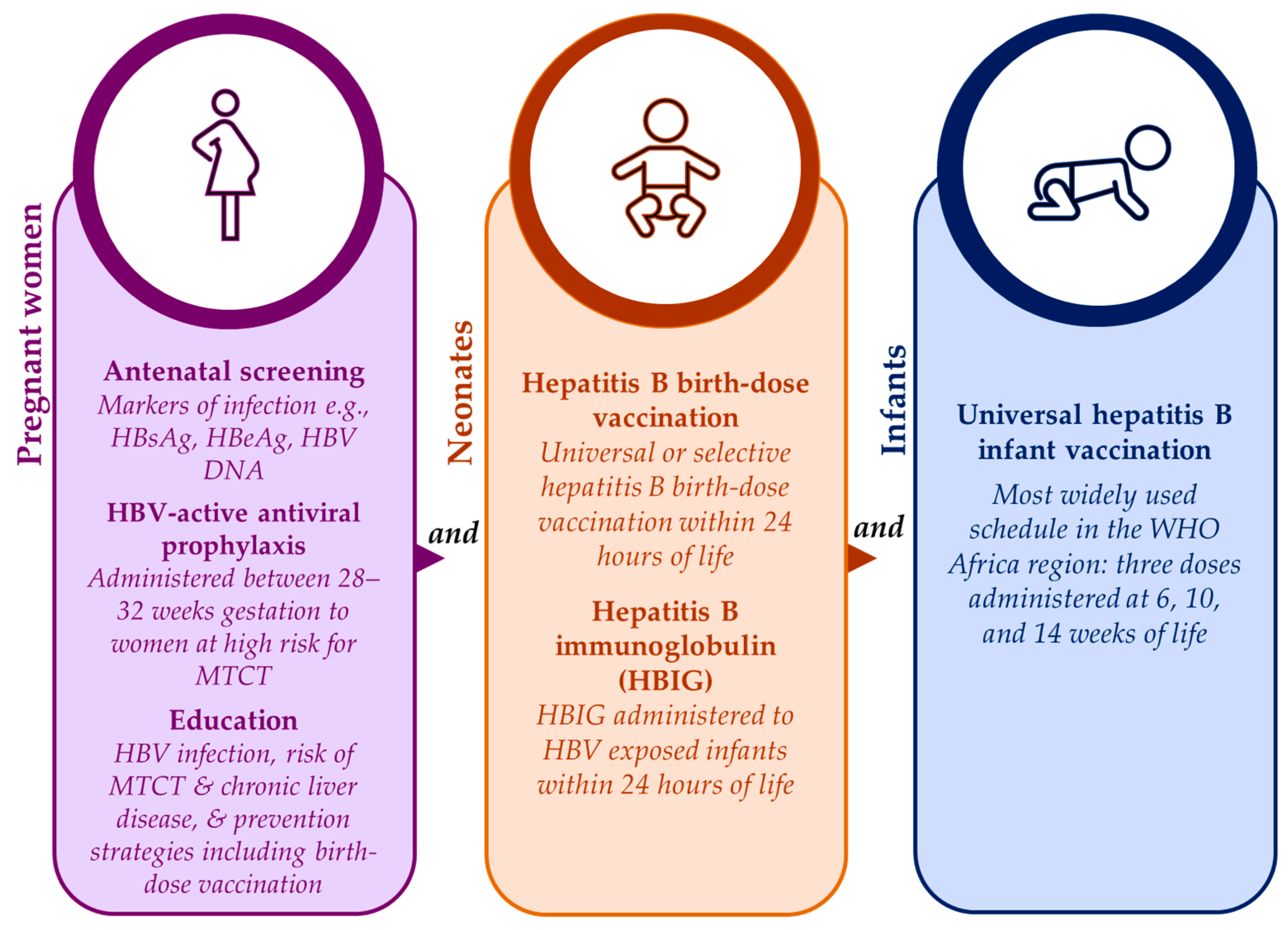
3.2. Mitigation strategies for prevention of HBV MTCT in the WHO Africa region
3.2.1. Barriers to adopting universal hepatitis B birth-dose vaccination programs
3.2.2. Challenges faced by established hepatitis B birth-dose vaccination programs
3.2.3. Poor adherence to timely hepatitis B birth-dose vaccination
3.3. State of health systems and hepatitis B birth-dose vaccination programs in the WHO Africa region
3.3.1. Conceptual models for the assessment of health systems capacity
3.3.2. Complexity as a characteristic of hepatitis B birth-dose vaccination programs
3.3.3. A systems-based logic model for assessing complexity within hepatitis B birth-dose vaccination programs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dionne-Odom, J.; Njei, B.; Tita, A. Elimination of Vertical Transmission of Hepatitis B in Africa: A Review of Available Tools and New Opportunities. Clin Ther 2018, 40, 1255–1267. [Google Scholar] [CrossRef]
- Breakwell, L.; Tevi-Benissan, C.; Childs, L.; Mihigo, R.; Tohme, R. The Status of Hepatitis B Control in the African Region. Pan Afr Med J 2017, 27, 17. [Google Scholar] [CrossRef]
- Tamandjou Tchuem, C.R.; Andersson, M.I.; Wiysonge, C.S.; Mufenda, J.; Preiser, W.; Cleary, S. Prevention of Hepatitis B Mother-to-Child Transmission in Namibia: A Cost-Effectiveness Analysis. Vaccine 2021, 39, 3141–3151. [Google Scholar] [CrossRef]
- Chotun, N.; Nel, E.; Cotton, M.F.; Preiser, W.; Andersson, M.I. Hepatitis B Virus Infection in HIV-Exposed Infants in the Western Cape, South Africa. Vaccine 2015, 33, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Lemoine, M.; Thursz, M. Prevention of Materno-Foetal Transmission of Hepatitis B in Sub-Saharan Africa: The Evidence, Current Practice and Future Challenges. J Viral Hepat 2014, 21, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Sadoh, A.; Sadoh, W. Does Nigeria Need the Birth Dose of the Hepatitis B Vaccine? Niger J Paediatr 2014, 41, 104–109. [Google Scholar] [CrossRef]
- Sone, L.; Voufo, R.; Dimodi, H.; Kengne, M.; Gueguim, C.; Nnanga, N.; Oben, J.; Ngondi, J. Prevalence and Identification of Serum Markers Associated with Vertical Transmission of Hepatitis B in Pregnant Women in Yaounde, Cameroon. Int. j MCH AIDS 2017, 6, 69–74. [Google Scholar] [CrossRef]
- Bittaye, M.; Idoko, P.; Ekele, B.A.; Obed, S.A.; Nyan, O. Hepatitis B Virus Sero-Prevalence amongst Pregnant Women in the Gambia. BMC Infect Dis 2019, 19, 259. [Google Scholar] [CrossRef]
- Umare, A.; Seyoum, B.; Gobena, T.; Haile Mariyam, T. Hepatitis B Virus Infections and Associated Factors among Pregnant Women Attending Antenatal Care Clinic at Deder Hospital, Eastern Ethiopia. PLoS One 2016, 11, e0166936. [Google Scholar] [CrossRef]
- Bayo, P.; Ochola, E.; Oleo, C.; Mwaka, A.D. High Prevalence of Hepatitis B Virus Infection among Pregnant Women Attending Antenatal Care: A Cross-Sectional Study in Two Hospitals in Northern Uganda. BMJ Open 2014, 4, e005889. [Google Scholar] [CrossRef]
- Kirbak, A.L.S.; Ng’ang’a, Z.; Omolo, J.; Idris, H.; Usman, A.; Mbabazi, W.B. Sero-Prevalence for Hepatitis B Virus among Pregnant Women Attending Antenatal Clinic in Juba Teaching Hospital, Republic of South Sudan. Pan Afr. Med. J 2017, 26. [Google Scholar] [CrossRef]
- Rashid, S.; Kilewo, C.; Aboud, S. Seroprevalence of Hepatitis B Virus Infection among Antenatal Clinic Attendees at a Tertiary Hospital in Dar Es Salaam, Tanzania. Tanzan J Health Res 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guingané, A.N.; Bougouma, A.; Sombié, R.; King, R.; Nagot, N.; Meda, N.; Van de Perre, P.; Tuaillon, E. Identifying Gaps across the Cascade of Care for the Prevention of HBV Mother-to-child Transmission in Burkina Faso: Findings from the Real World. Liver Int. 2020, 40, 2367–2376. [Google Scholar] [CrossRef]
- Thompson, P.; Morgan, C.E.; Ngimbi, P.; Mwandagalirwa, K.; Ravelomanana, N.L.R.; Tabala, M.; Fathy, M.; Kawende, B.; Muwonga, J.; Misingi, P.; et al. Arresting Vertical Transmission of Hepatitis B Virus (AVERT-HBV) in Pregnant Women and Their Neonates in the Democratic Republic of the Congo: A Feasibility Study. Lancet Glob Health 2021, 9, e1600–9. [Google Scholar] [CrossRef] [PubMed]
- Chotun, N.; Preiser, W.; van Rensburg, C.J.; Fernandez, P.; Theron, G.B.; Glebe, D.; Andersson, M.I. Point-of-Care Screening for Hepatitis B Virus Infection in Pregnant Women at an Antenatal Clinic: A South African Experience. PLoS One 2017, 12, e0181267. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016-2021: Actions for Impact.; World Health Organization: Geneva, 2021. [Google Scholar]
- World Health Organization Web Annex 1. Key Data at a Glance. In Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact; World Health Organization: Geneva, 2021. [Google Scholar]
- United Nations Children’s Fund Global and Regional Trends Available online:. Available online: https://data.unicef.org/topic/hivaids/global-regional-trends/ (accessed on 15 August 2023).
- Andersson, M.I.; Maponga, T.G.; Ijaz, S.; Barnes, J.; Theron, G.B.; Meredith, S.A.; Preiser, W.; Tedder, R.S. The Epidemiology of Hepatitis B Virus Infection in HIV-Infected and HIV-Uninfected Pregnant Women in the Western Cape, South Africa. Vaccine 2013, 31, 5579–5584. [Google Scholar] [CrossRef]
- Maponga, T.G.; Matteau Matsha, R.; Morin, S.; Scheibe, A.; Swan, T.; Andrieux-Meyer, I.; Spearman, C.W.; Klein, M.B.; Rockstroh, J.K. Highlights from the 3rd International HIV/Viral Hepatitis Co-Infection Meeting - HIV/Viral Hepatitis: Improving Diagnosis, Antiviral Therapy and Access. Hepatol Med Policy 2017, 2, 1–9. [Google Scholar] [CrossRef]
- United Nations Children’s Fund Immunization Coverage Estimates Data Visualization Available online:. Available online: https://data.unicef.org/resources/immunization-coverage-estimates-data-visualization/ (accessed on 15 August 2023).
- Anderson, S.; Harper, L.M.; Dionne-Odom, J.; Halle-Ekane, G.; Tita, A.T.N.; Dionne-Odom, J.; Halle-Ekane, G. A Decision Analytic Model for Prevention of Hepatitis B Virus Infection in Sub-Saharan Africa Using Birth-Dose Vaccination. Int J of Gynae Obstet 2018, 141, 126–132. [Google Scholar] [CrossRef]
- Diale, Q.; Pattinson, R.; Chokoe, R.; Masenyetse, L.; Mayaphi, S. Antenatal Screening for Hepatitis B Virus in HIV-Infected and Uninfected Pregnant Women in the Tshwane District of South Africa. South African Medical Journal 2016, 106, 97–100. [Google Scholar] [CrossRef]
- World Health Organization Hepatitis B Vaccines: WHO Position Paper, July 2017 – Recommendations. Vaccine 2019, 37, 223–225. [CrossRef]
- World Health Organization Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards Ending Viral Hepatitis.; World Health Organization: Geneva, 2016; ISBN 9789241503501. [Google Scholar]
- de Villiers, M.J.; Nayagam, S.; Hallett, T.B. The Impact of the Timely Birth Dose Vaccine on the Global Elimination of Hepatitis B. Nat Commun 2021, 12, 6223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Immunization Coverage Available online:. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 15 August 2022).
- World Health Organization Regional Office for Africa Prevention, Care and Treatment of Viral Hepatitis in the African Region: Framework for Action, 2016-2020; World Health Organization: Geneva, 2017.
- Kabore, J. Hepatitis B Birth Dose (HepB BD) Vaccination in the WHO African Region. In Proceedings of the Building a community of practice to assist hepB birth dose introduction in African countries, 2021., March 17-18 2021.; Coalition for Global Hepatitis Elimination. [Google Scholar]
- Njuguna, H. HepB-BD and Infant HepB3 Coverage Status in Africa. In Proceedings of the Hepatitis B Birth Dose in the African region: Bridging science and advocacy to eliminate mother-to-child transmission of HBV, August 22 2022. [Google Scholar]
- Mounier-Jack, S.; Griffiths, U.K.; Closser, S.; Burchett, H.; Marchal, B. Measuring the Health Systems Impact of Disease Control Programmes: A Critical Reflection on the WHO Building Blocks Framework. BMC Public Health 2014, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Travis, P.; Bennet, S.; Haines, A.; Pang, T.; Bhutta, Z.; Hyder, A.; Pielemeier, N.; Mills, A.; Evans, T. Overcoming Health-Systems Constraints to Achieve the Millenium Development Goals. Lancet 2004, 364, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Shiell, A.; Hawe, P.; Gold, L. Complex Interventions or Complex Systems? Implications for Health Economic Evaluation. BMJ 2008, 336, 1281. [Google Scholar] [CrossRef] [PubMed]
- Systems Thinking for Health Systems Strengthening; de Savigny, D. , Adam, T., Eds.; World Health Organization: Geneva, 2009. [Google Scholar]
- Evidence Synthesis for Health Policy and System: A Method Guide; Langlois, E. V, Daniels, K., Akl, E.A., Eds.; World Health Organization: Geneva, 2018; ISBN 9789241514552. [Google Scholar]
- Petticrew, M. When Are Complex Interventions “Complex”? When Are Simple Interventions “Simple”? Eur J Public Health 2011, 21, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Dieppe, P.; Macintyre, S.; Mitchie, S.; Nazareth, I.; Petticrew, M. Developing and Evaluating Complex Interventions: The New Medical Research Council Guidance. Bmj 2008, 337, 979–983. [Google Scholar] [CrossRef]
- Petticrew, M.; Anderson, L.; Elder, R.; Grimshaw, J.; Hopkins, D.; Hahn, R.; Krause, L.; Kristjansson, E.; Mercer, S.; Sipe, T.; et al. Complex Interventions and Their Implications for Systematic Reviews: A Pragmatic Approach. J Clin Epidemiol 2013, 66, 1209–1214. [Google Scholar] [CrossRef]
- Hawe, P.; Shiell, A.; Riley, T. Theorising Interventions as Events in Systems. Am J Community Psychol 2009, 43, 267–276. [Google Scholar] [CrossRef]
- JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI, 2020; ISBN 978-0-6488488-0-6.
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Reiswig, J. Mendeley. Journal of the Medical Library Association 2010, 98, 193–194. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a Web and Mobile App for Systematic Reviews. Syst Rev 2016, 5, 210. [Google Scholar] [CrossRef]
- Shimakawa, Y.; Bottomley, C.; Njie, R.; Mendy, M. The Association between Maternal Hepatitis B e Antigen Status, as a Proxy for Perinatal Transmission, and the Risk of Hepatitis B e Antigenaemia in Gambian Children. BMC Public Health 2014, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Keane, E.; Funk, A.L.; Shimakawa, Y. Systematic Review with Meta-Analysis: The Risk of Mother-to-Child Transmission of Hepatitis B Virus Infection in Sub-Saharan Africa. Aliment Pharmacol Ther 2016, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Ekra, D.; Herbinger, K.-H.; Konate, S.; Leblond, A.; Fretz, C.; Cilote, V.; Douai, C.; Da Silva, A.; Gessner, B.D.; Chauvin, P. A Non-Randomized Vaccine Effectiveness Trial of Accelerated Infant Hepatitis B Immunization Schedules with a First Dose at Birth or Age 6 Weeks in Côte d’Ivoire. Vaccine 2008, 26, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Tamandjou Tchuem, C.R.; Maponga, T.G.; Chotun, N.; Preiser, W.; Andersson, M.I. Is Hepatitis B Birth Dose Vaccine Needed in Africa? Pan Afr Med J 2017, 27, 18. [Google Scholar] [CrossRef]
- World Health Organization Global Compliance with Hepatitis B Vaccine Birth Dose and Factors Related to Timely Schedule. A Review. 2016.
- Seremba, E.; Van Geertruyden, J.P.; Ssenyonga, R.; Opio, C.K.; Kaducu, J.M.; Sempa, J.B.; Colebunders, R.; Ocama, P. Early Childhood Transmission of Hepatitis B Prior to the First Hepatitis B Vaccine Dose Is Rare among Babies Born to HIV-Infected and Non-HIV Infected Mothers in Gulu, Uganda. Vaccine 2017, 35, 2937–2942. [Google Scholar] [CrossRef]
- Ott, J.J.; Stevens, G.A.; Groeger, J.; Wiersma, S.T. Global Epidemiology of Hepatitis B Virus Infection: New Estimates of Age-Specific HBsAg Seroprevalence and Endemicity. Vaccine 2012, 30, 2212–2219. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. International Journal of Surgery 2021, 88, 105906. [Google Scholar] [CrossRef]
- Platt, L.; French, C.E.; McGowan, C.R.; Sabin, K.; Gower, E.; Trickey, A.; McDonald, B.; Ong, J.; Stone, J.; Easterbrook, P.; et al. Prevalence and Burden of HBV Co-infection among People Living with HIV: A Global Systematic Review and Meta-analysis. J Viral Hepat 2020, 27, 294–315. [Google Scholar] [CrossRef]
- Sichone, V.; Vwalika, B. Prevalence of Hepatitis B Virus, HIV and HBV Coinfection and Associated Factors in Pregnant Women Attending Antenatal Care at the University Teaching Hospital, Lusaka, Zambia. Med J Zambia 2019, 46, 10–18. [Google Scholar] [CrossRef]
- Gueye, S.; Diop-Ndiaye, H.; Lo, G.; Guindo, I.; Dia, A.; Sow-Sall, A.; Gaye-Diallo, A.; Mboup, S.; Toure-Kane, C. HBV Carriage in Children Born From HIV-Seropositive Mothers in Senegal: The Need of Birth-Dose HBV Vaccination. J Med Virol 2016, 88, 815–819. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy; World Health Organization: Geneva, 2020; ISBN 9789240002708.
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD Guidelines for Treatment of Chronic Hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J Hepatol 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Jooste, P.; van Zyl, A.; Adland, E.; Daniels, S.; Hattingh, L.; Brits, A.; Wareing, S.; Goedhals, D.; Jeffery, K.; Andersson, M.; et al. Screening, Characterisation and Prevention of Hepatitis B Virus (HBV) Co-Infection in HIV-Positive Children in South Africa. Journal of Clinical Virology 2016, 85, 71–74. [Google Scholar] [CrossRef]
- Hipgrave, D.B.; Maynard, J.E.; Biggs, B.-A. Improving Birth Dose Coverage of Hepatitis B Vaccine. Bull World Health Organ 2006, 84, 65–71. [Google Scholar] [CrossRef]
- Dadari, I.K.; Zgibor, J.C. How the Use of Vaccines Outside the Cold Chain or in Controlled Temperature Chain Contributes to Improving Immunization Coverage in Low- and Middle-Income Countries (LMICs): A Scoping Review of the Literature. J Glob Health 2021, 11, 4004. [Google Scholar] [CrossRef]
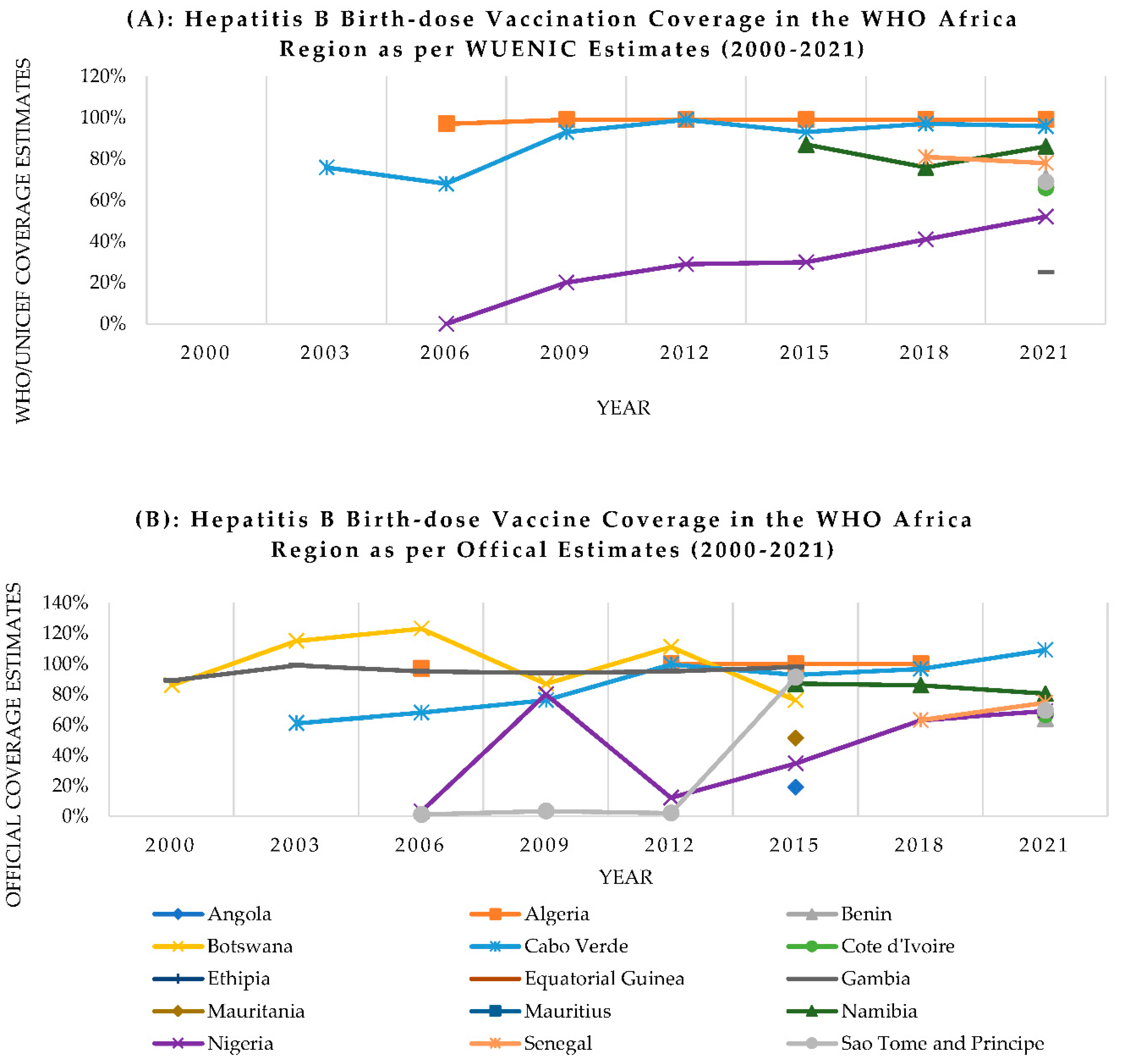
- Bassoum, O.; Kimura, M.; Dia, A.T.; Lemoine, M.; Shimakawa, Y. Coverage and Timeliness of Birth Dose Vaccination in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Vaccines (Basel) 2020, 8, 301. [Google Scholar] [CrossRef]
- World Health Organization Hepatitis B Vaccination Coverage Available online:. Available online: https://immunizationdata.who.int/pages/coverage/HEPB.html (accessed on 15 August 2023).
- World Health Organization Global Hepatitis Report 2017; World Health Organization: Geneva, 2017.
- Sanou, A.M.; Ilboudo, A.K.; Meda, Z.C.; Togozia, A.; Coulibaly, A.; Sagna Tani, A.C.; Dramane, K.; Tarnagda, Z. Hepatitis B Vaccination in Burkina Faso: Prevalence of HBsAg Carriage and Immune Response in Children in the Western Region. J Infect Dev Ctries 2018, 12, 1002–1008. [Google Scholar] [CrossRef]
- Apiung, T.; Ndanu, T.A.; Mingle, J.A.A.; Sagoe, K.W.C. Hepatitis B Virus Surface Antigen and Antibody Markers in Children at a Major Paediatric Hospital after the Pentavalent DTP-HBV-Hib Vaccination. Ghana Med J 2017, 51, 13–19. [Google Scholar] [CrossRef]
- Metodi, J.; Aboud, S.; Mpembeni, R.; Munubhi, E. Immunity to Hepatitis B Vaccine in Tanzanian Under-5 Children. Ann Trop Paediatr 2010, 30, 129–136. [Google Scholar] [CrossRef]
- Breakwell, L.; Marke, D.; Kaiser, R.; Tejada-Strop, A.; Pauly, M.D.; Jabbi, S.; Yambasu, S.; Kabore, H.J.; Stewart, B.; Sesay, T.; et al. Assessing the Impact of the Routine Childhood Hepatitis B Immunization Program and the Need for Hepatitis B Vaccine Birth Dose in Sierra Leone, 2018. Vaccine 2022, 40, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Gosset, A. Introducing Birth-Dose Hepatitis B Vaccination Would Be Cost Effective in Burkina Faso. PharmacoEcon Outcomes News 2021, 883, 21–24. [Google Scholar] [CrossRef]
- Gosset, A.; Nishimwe, M.L.; Diallo, M.Y.; Deroo, L.; Diallo, A.; Ba, E.H.; Carrieri, P.M.; Sokhna, C.; Vray, M.; Shimakawa, Y.; et al. The Costs of Introducing the Hepatitis B Birth Dose Vaccine into the National Immunization Programme in Senegal (NéoVac Study). Vaccines (Basel) 2021, 9, 521. [Google Scholar] [CrossRef]
- Memirie, S.T.; Desalegn, H.; Naizgi, M.; Nigus, M.; Taddesse, L.; Tadesse, Y.; Tessema, F.; Zelalem, M.; Girma, T. Introduction of Birth Dose of Hepatitis B Virus Vaccine to the Immunization Program in Ethiopia: An Economic Evaluation. Cost Eff Resour Alloc 2020, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Klingler, C.; Thoumi, A.I.; Mrithinjayam, V.S. Cost-Effectiveness Analysis of an Additional Birth Dose of Hepatitis B Vaccine to Prevent Perinatal Transmission in a Medical Setting in Mozambique. Vaccine 2012, 31, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.M.; O’Connor, S.M.; Njau, J.D.; Lam, E.K.; Staton, C.A.; Cookson, S.T. Cost-Effectiveness of Birth-Dose Hepatitis B Vaccination among Refugee Populations in the African Region: A Series of Case Studies. Confl Health 2019, 13, 5. [Google Scholar] [CrossRef]
- Hagan, J.E.; Carvalho, E.; Souza, V.; Queresma Dos Anjos, M.; Abimbola, T.O.; Pallas, S.W.; Tevi Benissan, M.C.; Shendale, S.; Hennessey, K.; Patel, M.K. Selective Hepatitis B Birth-Dose Vaccination in São Tomé and Príncipe: A Program Assessment and Cost-Effectiveness Study. Am J Trop Med Hyg 2019, 101, 891–898. [Google Scholar] [CrossRef]
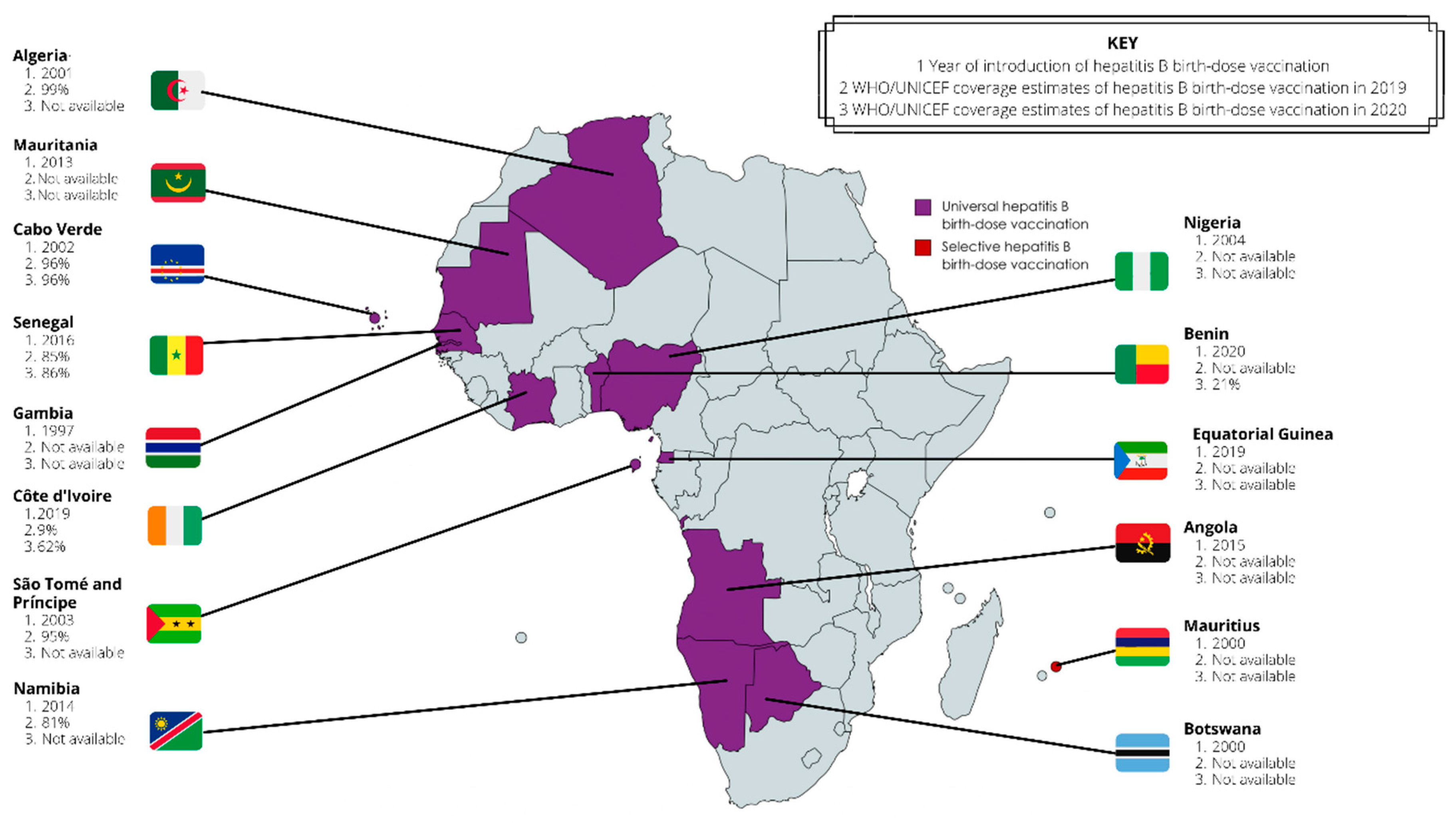
- Moturi, E.; Tevi-Benissan, C.; Hagan, J.E.; Shendale, S.; Mayenga, D.; Murokora, D.; Patel, M.; Hennessey, K.; Mihigo, R. Implementing a Birth Dose of Hepatitis B Vaccine in Africa: Findings from Assessments in 5 Countries. J Immunol Sci 2018, Suppl, 31–40. [Google Scholar] [CrossRef]
- Okenwa, U.J.; Dairo, M.D.; Uba, B.; Ajumobi, O. Maternal Reasons for Non-Receipt of Valid Hepatitis B Birth Dose among Mother-Infant Pairs Attending Routine Immunization Clinics, South-East, Nigeria. Vaccine 2019, 37, 6894–6899. [Google Scholar] [CrossRef]
- Nankya-Mutyoba, J.N.; Surkan, P.J.; Makumbi, F.; Aizire, J.; Kirk, G.D.; Ocama, P.; Atuyambe, L.M. Hepatitis B Birth Dose Vaccination for Newborns in Uganda: A Qualitative Inquiry on Pregnant Women’s Perceptions, Barriers and Preferences. J Virus Erad 2021, 7, 100039. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, R.; Jasseh, M.; Gomez, P.; Shimakawa, Y.; Greenwood, B.; Keita, K.; Ceesay, S.; D’Alessandro, U.; Roca, A. Barriers to Timely Administration of Birth Dose Vaccines in The Gambia, West Africa. Vaccine 2016, 34, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Boisson, A.; Goel, V.; Yotebieng, M.; Parr, J.B.; Fried, B.; Thompson, P. Implementation Approaches for Introducing and Overcoming Barriers to Hepatitis B Birth-Dose Vaccine in Sub-Saharan Africa. Glob Health Sci Pract 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Périères, L.; Marcellin, F.; Lo, G.; Protopopescu, C.; Ba, E.H.; Coste, M.; Kane, C.T.; Maradan, G.; Diallo, A.; Sokhna, C.; et al. Hepatitis B Vaccination in Senegalese Children: Coverage, Timeliness, and Sociodemographic Determinants of Non-Adherence to Immunisation Schedules (ANRS 12356 AmBASS Survey). Vaccines (Basel) 2021, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Olakunde, B.O.; Adeyinka, D.A.; Olakunde, O.A.; Ogundipe, T.; Oladunni, F.; Ezeanolue, E.E. The Coverage of Hepatitis B Birth Dose Vaccination in Nigeria: Does the Place of Delivery Matter? Trans R Soc Trop Med Hyg 2021, 116, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.D.; Patel, M.K.; Tohme, R.A. Hepatitis B Vaccine Birth Dose Coverage Correlates Worldwide with Rates of Institutional Deliveries and Skilled Attendance at Birth. Vaccine 2017, 35, 4094–4098. [Google Scholar] [CrossRef]
- Schweitzer, A.; Akmatov, M.K.; Krause, G. Hepatitis B Vaccination Timing: Results from Demographic Health Surveys in 47 Countries. Bull World Health Organ 2017, 95, 199–209G. [Google Scholar] [CrossRef]
- Maponga, T.G.; Nwankwo, C.; Matthews, P.C. Sustainable Development Goals for HBV Elimination in South Africa: Challenges, Progress, and the Road Ahead. South African Gastroenterol Rev 2019, 17, 15–25. [Google Scholar]
- Immunization Vaccines and Biologicals Practices to Improve Coverage of the Hepatitis B Birth Dose Vaccine; World Health Organization: Geneva, 2017.
- Cheung, C.K. Proportion of Children Born to Infected Mothers at Risk of Contracting Hepatitis B, and Associated Risk Factors for Inadequate Hepatitis B Timely Birth Dose Vaccination: Analysis of the São Tomé and Príncipe Demographic Health Survey Program Data, 2008–2009, Uppsala Universitet, 2017.
- Kruk, M.; Freedman, L.P. Assessing Health System Performance in Developing Countries: A Review of the Literature. Health Policy (New York) 2008, 85, 263–276. [Google Scholar] [CrossRef]
- Samb, B.; Evans, T.; Dybul, M.; Atun, R.; Moatti, J.P.; Nishtar, S.; Wright, A.; Celletti, F.; Hsu, J.; Kim, J.Y.; et al. An Assessment of Interactions between Global Health Initiatives and Country Health Systems. The Lancet 2009, 373, 2137–2169. [Google Scholar] [CrossRef]
- World Health Organization Everybody’s Business: Strengthening Health Systems to Improve Health Outcomes: WHO’s Framework for Action; World Health Organization: Geneva. 2007.
- Arah, O.A.; Klazinga, N.S.; Delnoij, D.M.J.; Ten Asbroek, A.H.A.; Custers, T. Conceptual Frameworks for Health Systems Performance: A Quest for Effectiveness, Quality, and Improvement. Int J Qual Health Care 2003, 15, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Frenk, A.J. A Framework for Assessing the Performance of Health Systems. Bull World Health Organ 2000, 78, 717–731. [Google Scholar] [PubMed]
- Van Olmen, J.; Criel, B.; Bhojani, U.; Marchal, B.; Van Belle, S.; Chenge, F.; Hoeree, T.; Pirard, M.; Van Damme, W.; Kegels, G. The Health System Dynamics Framework: The Introduction of an Analytical Model for Health System Analysis and Its Application to Two Case-Studies. Health Culture and Society 2012, 2, 1–21. [Google Scholar] [CrossRef]
- Amponsah-Dacosta, E.; Amponsah-Dacosta, E.; Kagina, B.M.; Olivier, J. Health Systems Constraints and Facilitators of Human Papillomavirus Immunization Programmes in Sub-Saharan Africa: A Systematic Review. Health Policy Plan 2020, 35, 701–717. [Google Scholar] [CrossRef]
- Shen, A.K.; Fields, R.; McQuestion, M. The Future of Routine Immunization in the Developing World: Challenges and Opportunities. Glob Health Sci Pract 2014, 2, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, A.; Pfadenhauer, L.; Burns, J.; Brereton, L.; Gerhardus, A.; Booth, A.; Oortwijn, W.; Rehfuess, E. Series: Clinical Epidemiology in South Africa. Paper 3: Logic Models Help Make Sense of Complexity in Systematic Reviews and Health Technology Assessments. J Clin Epidemiol 2017, 83, 37–47. [Google Scholar] [CrossRef]

| Study No. | Author, year | Setting | Study design | Population | Summary of key findings |
|---|---|---|---|---|---|
| 1 | Rashid et al., 2007 [12] | Tanzania | Cross-sectional | Pregnant women |
|
| 2 | Bayo et al., 2014 [10] | Uganda | Cross-sectional | Pregnant women |
|
| 3 | Howell et al., 2014 [5] | Sub-Saharan Africa | Literature Review | Pregnant women and women of childbearing age |
|
| Mother-child pairs |
|
||||
| 4 | Sadoh et al., 2014 [6] | Nigeria | Literature Review | Pregnant women and women of childbearing age |
|
| Mother-child pairs |
|
||||
| 5 | Umare et al., 2016 [9] | Ethiopia | Cross-sectional | Pregnant women |
|
| 6 | Breakwell et al., 2017 [2] | WHO AFRO | Literature review | Pregnant women |
|
| Mother-child pairs |
|
||||
| 7 | Chotun et al., 2017 [15] | South Africa | Prospective cohort | Pregnant women |
|
| Infants |
|
||||
| 8 | Kirbak et al., 2017 [11] | South Sudan | Cross-sectional | Pregnant women |
|
| 9 | Seremba et al., 2017 [49] | Uganda | Cross-sectional | Mothers |
|
| Infants |
|
||||
| 10 | Sone et al., 2017 [7] | Cameroon | Prospective cross-sectional | Pregnant women |
|
| Infants |
|
||||
| 11 | Bittaye et al., 2019 [8] | The Gambia | Cross-sectional | Pregnant women |
|
| 12 | Guingané et al., 2020 [13] | Burkina Faso | Prospective cohort | Pregnant women |
|
| Infants |
|
||||
| 13 | Thompson et al., 2021 [14] | Democratic Republic of Congo | Cohort | Pregnant women |
|
| Infants |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





