1. Introduction
Immunization is a cornerstone of public health, credited with dramatically reducing vaccine-preventable diseases related childhood morbidity and mortality worldwide [
1]. Despite concerted global and regional efforts to improve vaccine coverage, many children, especially in low- and middle-income countries, remain zero-dose, meaning they have not received any vaccinations, notably the first dose of diphtheria-tetanus-pertussis containing vaccine (DTP1).
In 2023, 14.5 million infants did not receive an initial dose of the DTP vaccine, and an additional 6.5 million are partially vaccinated, pointing to current gaps in access and continuity of immunization and other essential health services [
2].
The Global Immunization Agenda 2030 emphasizes coverage and equity as a key strategic priority, aiming to achieve high and equitable immunization coverage nationwide and across all subnational levels. Its goal is to ensure that everyone, regardless of location, age, socioeconomic status, or gender-related barriers, is fully protected through immunization [
3]. Reasons for non-vaccination and under-vaccination of children in low- and middle-income countries include issues within the health system and immunization service delivery, missed opportunities for vaccinations, limited access to vaccination services, family dynamics and beliefs, parental attitudes and knowledge, and challenges in immunization-related communication and information dissemination [
4,
5]. Mozambique, one of the ten countries with a notable proportion of zero-dose children [
6], exemplifies the challenges associated with reaching children in hard-to-access areas. Geographic, socioeconomic, and systemic barriers contribute significantly to this problem [
7,
8].
The presence of zero-dose and under-immunized children often reflects a web of interconnected factors. Research highlights the need to address immunization inequalities comprehensively, acknowledging that barriers like geographic isolation, gender inequality, and economic deprivation combine to create distinct challenges for caregivers. Mothers or female caregivers typically bear the responsibility of securing vaccination for their children, but they often face obstacles related to limited healthcare access, financial constraints, and adverse health service experiences [
9]. Additionally, perceptions of immunization, influenced by societal norms and personal experiences, can shape caregivers’ willingness to seek vaccination for their children [
10].
Mozambique's Expanded Programme on Immunization (EPI), launched in 1979, offers free immunization services. Public sector health workers administer vaccines at fixed health posts, through routine mobile outreach activities, and occasionally during specific vaccine campaigns [
11]. Since the early 2000s, Mozambique has adopted strategies such as the WHO/UNICEF Reaching Every District/Reaching Every Community (RED/REC) approach to enhance vaccination outreach in remote areas [
11]. According to the Mozambique EPI manual, mobile vaccination brigades are expected to serve communities located more than 8 km from a health facility on a quarterly basis [
12]. These outreach efforts are crucial for improving vaccine access, as approximately 67% of the population resides in rural areas [
13]. However, nationwide implementation of these strategies has been hindered by insufficient funding and a lack of material and human resources, leading to coverage gaps.
Routine vaccination coverage in Mozambique saw a significant rise from 47% in 1997 to 63% in 2003. However, progress has slowed, with the 2015 Survey of Indicators on Immunization, Malaria, and HIV/AIDS reporting that only 66% of children were fully vaccinated [
13], and in 2022-23, the Demographic and Health Survey showed that the proportion of fully vaccinated children dropped dramatically to reaching 38%, Its all-time lowest level [
14]. The same report indicated that about 24% of the target population had not received the first dose of the pentavalent vaccine, the so-called zero-dose children. Zero-dose refers to children who didn’t receive any dose of scheduled routine vaccine. For operational purposes, Gavi defines zero-dose children as those who lack the first dose of the pentavalent vaccine. An under-immunized children refer to those missing the third dose. When drilling down to the subnational level, Zambezia province has the lowest vaccination coverage in the country. The same 2022-23 DHS report estimated Pentavalent 1 vaccination at 46,8%, implying that about 53% of the target population were zero-dose. Low coverage of the initial set of recommended vaccines reflects limited access to services or low acceptance of vaccination, while high dropout rates point to systemic failures in delivering subsequent doses effectively. In addition, only 24% of children in the Zambezia province receive three doses of the pentavalent vaccine, leading to a huge dropout rate between pentavalent 1 and pentavalent 3. In Mozambique, there is limited knowledge about the specific factors driving routine immunization dropouts. Available research indicates that the determinants of immunization dropout are highly context-specific, often stemming from a combination of individual, interpersonal, and health system factors [
15].
Caregivers in these contexts often face extended travel distances to health facilities, frequently experience stockouts of vaccines, and report concerns over service quality, including disrespectful treatment from healthcare providers [
16].
In order to address these barriers, in Mozambique the PNAPS, the National Programme for Polyvalent Health Agents helps to improve health services and health education at community level in remote areas. The PNAPS relies on digital health through the upSCALE mobile platform, which empowers community health workers (CHWs) to deliver effective, data-driven patient-centered care, particularly in remote and rural settings [
17,
18]. The platform equips community health workers with mobile tools to guide patient registration, diagnosis, and treatment of common illnesses such as malaria, pneumonia, and diarrhea. The system enhances adherence to clinical protocols, enables real-time data collection for DHIS2 integration, and supports supervisors in monitoring performance, planning visits, and managing stock levels. It also assists in delivering behaviour change messages to patients and was successfully adapted for the COVID-19 response. By addressing long-standing challenges, such as inconsistent guideline adherence, commodity shortages, limited supervision, and data gaps, upSCALE has become a model for digital health innovation in Mozambique, paving the way for broader digital transformation and more equitable access to quality healthcare in remote areas [
19]. The main aim of this study is to assess the effectiveness, feasibility, and implementation learnings linked with the use of the upscale platform by CHWs for Zero-dose children Identification and Reach (ZIDER) in Mozambique. The findings of this initiative will inform both programmatic scale-up and national policy.
Specifically, this implementation research aims to evaluate the effectiveness of the enhanced upSCALE digital immunization module and improved workflows between the community and health services in reducing the proportion of zero-dose and under-immunized children. Secondly, it will assess the impact of the enhanced upSCALE module on strengthening the capacity of Agentes Polivalentes de Saúde (CHWs) and the EPI program to identify and vaccinate zero-dose and under-immunized children. In addition, there will be an assessment of the feasibility and acceptability of the enhanced upSCALE immunization module, while identifying its strengths, weaknesses, workflows, and processes, to provide recommendations for scalability.
2. Materials and Methods
The Intervention
In Mozambique, the first port of call for many sick children is through the CHWs, known as APS, who form the core cadre of the country’s CHWs’ Program — known locally as PNAPS. More than 5,300 APSs are working in Mozambique. Following a six-month training period, each APS provides care for as many as 1,000 inhabitants in vulnerable communities. In addition, each APS offers an Essential Health Care package in its respective community. APS assesses the status of several conditions, including immunization for under-five children. Since 2016, APSs are equipped with digital applications (upSCALE) to guide them to perform patient registration, checking for symptoms, nutrition, and immunization status of the child and advising on treatment, patient counseling, referral, and follow-up. The enhancement of the immunization module specifically targeting zero-dose children aligns with the broader Reaching Every District (RED) and Reaching Every Child (REC) strategy, which emphasize inclusive community-based healthcare delivery to strengthen the link between communities and health facilities [
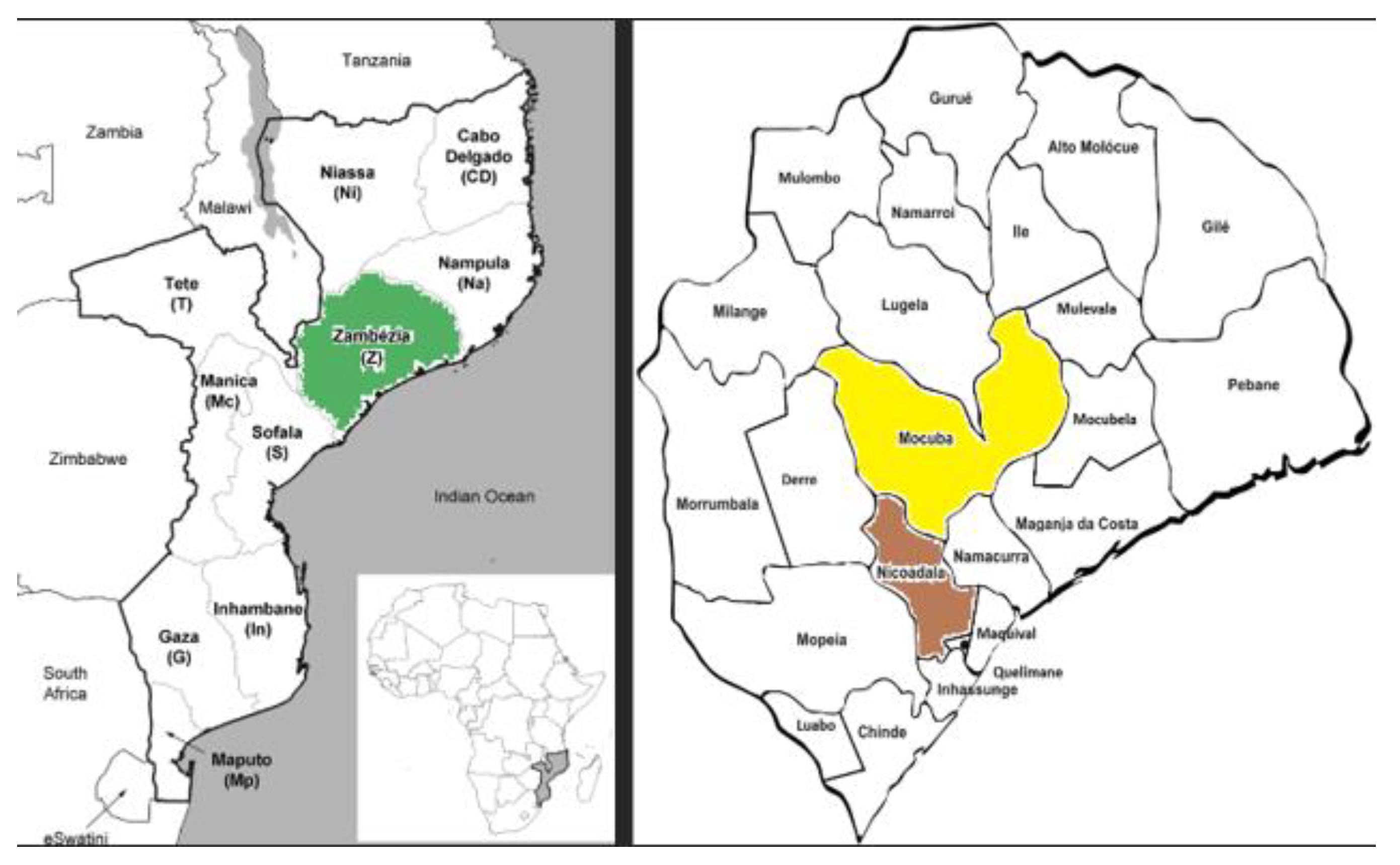
20]. This enhancement includes conducting an analysis of current gaps in the upSCALE digital platform and defining the optimal state of the platform to support the immunization program; developing and revising business processes, data exchanges, and workflows to link zero-dose and under-immunized children identified through the upSCALE application with immunization services, pilot testing the modified workflows and processes in one district. APSs and health facility supervisors in the select district will be trained on the enhanced EPI module and workflows, and not more than six months of implementation will be considered to measure success. Routinizing the timely detection and outreach to zero-dose and underimmunised children will help Mozambique’s EPI program maintain strong performance and prevent children from remaining unvaccinated into older ages, when catch-up becomes more difficult, thereby placing them at higher risk of morbidity and death from vaccine-preventable diseases. The implementation will be conducted in the Mocuba district, in the Zambezia province, which implements the RED/REC approach and the upSCALE platform. This study aims to evaluate the effectiveness, feasibility, and acceptability of the enhanced upSCALE module in reducing zero-dose prevalence in Mozambique before planning for scale-up.
Study Design
This is an embedded implementation research study using mixed-methods design, combining quantitative and qualitative components. There will be three different sub-studies to address the study objectives. The first sub-study is a non-randomized controlled trial (nRCT) to assess the effectiveness of the ZIDER initiative in identifying and linking zero-dose children to immunization services. The second sub-study utilises a before-and-after design to quantitatively assess Knowledge, Attitudes, and Practices (KAP) among community health workers (APSs) to explore frontline perceptions and practices related to immunization delivery. The third study component will involve qualitative implementation research through key informant interviews (KIIs) and focus group discussions (FGDs) to assess stakeholder experiences and system-level facilitators and barriers. The study follows a Type 2 hybrid effectiveness-implementation design, allowing simultaneous evaluation of both the outcomes of the intervention (identification and referral of zero-dose children) and the process through which it is implemented (
Table 1). The study design and methodological approaches are described in greater detail for each specific objective in a later section.
Study Setting
The study is being conducted in two selected districts of Zambezia province, Mozambique (
Figure 1), prioritized based on a high burden of zero-dose children, poor immunization coverage, and operational feasibility. These areas were jointly identified in collaboration with the Ministry of Health and UNICEF Mozambique.
Study Period
This study is planned for a period of 6 months, from September 2025 to February 2026.
Objective-specific study design and methods
Non-Randomized Controlled Trial (nRCT)
This component will involve a non-randomized controlled trial (nRCT) based on a cluster sampling approach with repeated household surveys (at baseline and endline) of primary caregivers of children aged 3-59 months sampled in both the intervention and control arms.
Trial Arms and Sampling
The nRCT is conducted in Zambezia province across two arms:
Outcome Measures
Primary outcome: The proportion of zero-dose children, aged 3-59 months, defined as the proportion of children who have not received the first dose of the pentavalent vaccine (Penta1) in the study areas.
Secondary outcomes: The proportion of under-immunised children 6-59 who have not received the third dose of pentavalent vaccine (Penta3)
Study participants and sample size
Participants will be children aged 3-59 months, with their caregivers as respondents, whose (1) parent/guardian/caregiver has a child aged 3-59 months in the selected districts, (2) child has been resident in the selected district for at least one month prior to data collection; and (3) parent/guardian/caregiver is willing and able to give informed consent for their child’s participation in the trial in the selected districts. Children aged 3–59 months will be included because this group represents the standard target population for assessing zero-dose and under-immunization in immunization research. This age range will allow the study to capture both infants expected to have received birth and early-life vaccines, as well as older preschool children who should have completed their primary vaccination schedule. Including this entire window will ensure comparability with national immunization indicators and provide a comprehensive picture of missed opportunities for vaccination.
A minimum of 440 children per study arm per survey wave (baseline and endline) is required to detect a reduction in zero-dose prevalence from 30% to 15% among children aged 6–59 months, with 80% power and a 5% significance level. This accounts for a design effect of 2.8 (intra-cluster correlation of 0.2; cluster size of 10 households) and a 20% non-response rate. Effects size assumption is based on the Big Catch Up (BCU) intervention in Mozambique [
21]. Accordingly, 44 clusters of 10 households will be sampled per arm (district) at both baseline and endline. A multistage probability sampling design will be employed. In the first stage, districts will be purposively selected to represent intervention and control areas. Within each district, enumeration areas will be randomly selected using probability proportional to size. In places where it is not feasible to list households, an alternative approach, the ‘spin-the-pen' method, will be used. This design will ensure representativeness while maintaining feasibility in terms of time and resources.
Data Collection and analysis
A structured questionnaire will be administered to caregivers of children aged 3–59 months in both the intervention and control districts. Data will be collected using SurveyCTO programmed on tablets, with built-in skip patterns, range checks, and validation rules to minimize entry errors and ensure high data quality. The questionnaire will include the household background characteristics, access to healthcare, child immunization, healthcare seeking, and behavioural and social drivers of vaccination. Data will be collected at baseline and endline using household surveys and facility records. Children aged 3–59 months will constitute the unit of analysis. Primary outcomes will be the proportion of zero-dose and under-immunized children, while secondary outcomes will include timeliness of vaccination, caregiver knowledge, and health worker performance.
Descriptive statistics will be used to summarize baseline characteristics, with covariate balance between intervention and control districts assessed using ANOVA or chi-square tests, and non-parametric alternatives if needed. The effect of the intervention will be estimated using difference-in-differences analyses, fitted as extended mixed-effects logistic regression models with random intercepts for cluster units. This approach allows estimation of intervention effects while controlling for secular trends between districts over time. Both adjusted and unadjusted models will be presented, and results will be reported as odds ratios with 95% confidence intervals.
Knowledge, Attitudes, and Practices (KAP) Survey Among APSs
This component will include a KAP survey designed to assess the knowledge, attitudes, and practices of APSs involved in or exposed to the ZIDER strategy, with the aim of understanding how frontline health workers perceive their role in identifying and supporting zero-dose children using the new vaccine module in the upSCALE.
Sampling
The KAP survey is conducted in Zambezia province, in both Mocuba and Nicoadala districts.
Outcome measures
The Knowledge, Attitudes, and Practices (KAP) survey will measure changes among APS, regarding immunization. Primary outcomes will include knowledge of the national vaccination schedule, ability to identify zero-dose and under-immunized children, and awareness of contraindications. Attitudinal outcomes will assess motivation, confidence, and perceived barriers to immunization service delivery. Practice outcomes will focus on use of immunization tools, recording and reporting accuracy, communication with caregivers, and follow-up of defaulters.
Study participants and sample size
All APSs working in both intervention and control arms are eligible. The survey is administered at baseline before the training and implementation of the use of the new module, and after six months at the endline of the study. Trained interviewers, as members of the research team, will administer the survey to eligible participants of this component.
Data Collection
The KAP survey will follow a pre-post cross-sectional design targeting all APS working in the intervention and control districts. Structured questionnaires will be administered at baseline and endline (six months apart) to assess changes in knowledge, attitudes, and practices related to immunization. All eligible APS working in the two districts will be invited to participate. The KAP survey will also be administered through SurveyCTO on tablets, using standardized digital forms for APS. This will ensure consistency across baseline and endline rounds and allow real-time monitoring of data completeness and quality.
The structured questionnaire includes sections on knowledge of zero-dose child identification and referral pathways; attitudes towards household-based strategies for immunization uptake; practices related to immunization outreach, follow-up, and caregiver engagement; exposure to training, perceived workload, and use of digital tools.
Data will be analyzed using descriptive statistics to summarize baseline characteristics and outcome distributions. Proportions of APS achieving satisfactory knowledge, attitude, and practice scores (≥75%) will be estimated at baseline and endline. Differences in proportions between intervention and control districts will be assessed using chi-square or Fisher’s exact tests, as appropriate. To estimate the effect of the intervention while accounting for potential confounders (e.g., age, gender, years of service, role, baseline score), multivariable logistic regression models will be fitted.
Qualitative study on the feasibility and acceptability of the enhanced module
This component will involve a qualitative study design to the feasibility and acceptability of this intervention, including an analysis of the key characteristics of the module that could influence its scalability, both positively and negatively. This involves identifying its strengths and weaknesses, evaluating how workflows are structured and interpreted, and determining whether they are smooth, efficient, and aligned with the intended goals.
For this component of the study, feasibility will be conceptualized according to the UK Medical Research Council framework for developing and evaluating complex interventions [
22]. The acceptability framework will be designed to address seven facets of acceptability: effective attitude, burden, ethicality, intervention coherence, opportunity costs, perceived effectiveness and self-efficacy will be used [
23].
Sampling
This component will recruit participants in Mocuba district among community members and health staff participating in the intervention.
Outcome
The qualitative component will explore the feasibility and acceptability of the enhanced upSCALE digital immunization module. It will identify common perceptions and ideas about the module’s feasibility and acceptability, while also documenting its strengths, weaknesses, workflows, and processes to generate recommendations for scalability.
Study participants and sample size
Purposive sampling will be used to ensure representation of the main actors involved in the intervention. Participants will be selected to capture a range of perspectives across levels of the health system, including APS and their supervisors, EPI focal points, IMCH nurses, facility and district directors, central-level policymakers, and caregivers of eligible children. Within each group, individuals will be identified based on their direct involvement with immunization activities, supervision of APS, or their experience as primary caregivers. This approach will allow the study to gather diverse experiences and viewpoints relevant to the feasibility and acceptability of the digital module.
Data collection and analysis
Data for exploring the feasibility, acceptability, and scalability of the initiative will be collected through Focus Group Discussions and Key Informant Interviews. Six to eight FGDs will be conducted in the intervention arm to explore perceptions about the feasibility and acceptability of the introduction of the new digital module used to identify zero-dose children among caregivers of zero-dose children and community leaders. Areas of questioning around acceptability will be developed based on the Theoretical Framework of Acceptability, guiding the assessment of the intervention across three temporal perspectives, that is, before, during, and after the intervention, and from the perspectives of intervention deliverers and recipients [
24,
25]. Key Informant Interviews (KIIs) will be performed with APS and APS Supervisors, IMCH nurses, and health facility directors. Each FGD will with 6 to 8 participants, with deliberate attention to age balance among participants to avoid any potential leadership bias during the discussion. Overall, 18 KIIs will be conducted to collect more insights about acceptability and feasibility from a range of people who have specific knowledge about the topic.
Participants will be purposively selected to ensure representation across different implementation roles and geographic areas. Trained interviewers, as members of the research team, will administer interviews during the FGDs and KIIs with the support of research field coordinators.
Qualitative data will be coded thematically using a framework analysis approach based on RE-AIM dimensions (Reach, Effectiveness, Adoption, Implementation, Maintenance). Coding reliability will be ensured by dual coding of transcripts.
Implementation procedures
Procedures for baseline and endline surveys
Two surveyor teams of 20 interviewers each per study arm will visit selected households in each cluster. It is expected that each interviewer will complete 5 household visits per day. The data collection will last for five days, that is supposed to be sufficient to reach the sample size. The expected time for each household visit is estimated to be from thirty to sixty minutes. Interviews will be accompanied by community leaders in each community. The leaders of the selected communities in each district will be contacted in advance and will be informed about the survey by the Health Communication officer of the MoH at the district level, together with the research field coordinator. This will allow to inform all the communities about the visit of the interviewer and the main objective of the survey session. Questionnaires will be used to collect information on specific vaccine doses as recommended in the Mozambique national childhood routine immunization schedule. The immunization status of children will be validated during surveys using home-based child immunization records or the mother’s recall. Sociodemographic variables will include child-level characteristics such as sex, birth order, and birth size; maternal characteristics such as age, education, marital status, occupation, media exposure, and antenatal care attendance, place of child delivery (health facility or home).
Each member of the surveyor team will receive a three-day training including participant sampling, informed consent and data collections processes. Trainees will also have one day of pilot to test their understanding of field procedures. The study field coordinator and data management team will upload data to the protected online server and check for completeness and monitoring of corrections and backups. These procedures will be the same for the baseline and endline surveys.
The two surveyor teams of 20 interviewers per arm will interview also the APSs involved in the use of the new upSCALE module using a before-and-after survey. The baseline survey will take place shortly prior to the implementation of the enhanced digital immunization module, and an endline survey up to six months following the inception of the intervention in both study arms. It is expected to interview all the APSs in each study arms, during three days of data collection. The questionnaire will cover sections on respondents’ sociodemographic characteristics, vaccine knowledge, attitudes, practices, and motivation related to identifying and reaching zero-dose and under-immunised children. Knowledge questions will consist of Yes/No/Unsure response options. Attitude, behaviour and motivation sections will be based on questions or statements with responses on a 5-point Likert scale. The average time per interview will be around 45 minutes. The research team will contact in advance the Supervisors of APS and Health Facility’s heads to gather the APS in selected health facilities to facilitate the questionnaire administration. The administration will involve only the inquirer and the APS, no supervisors or FHF heads will participate during the interview.
Procedures for qualitative interviews
Participants will be provided with the information sheet to read and the informed consent form to sign. They will be given enough time to decide if they would like to participate.
The venue for the interview, both FGD and KII, will be agreed with the participants securing that it will allow for confidentiality and making participants feel comfortable for the duration such as a school, a church, and a private house’s courtyard for the FGD. The KIIs will be conducted in a quiet, private space that offers confidentiality, possibly at their office. An interviewer member of the research team, trained in qualitative procedures will facilitate the session by asking questions from the topic guide, whilst another research assistant audio records the interview and takes detailed field notes.
Ethics and Informed Consent
The study protocol was evaluated and approved by the Mozambique National Bioethics Committee for Health (40/CNBS/2025), authorized by Ministry of health of Mozambique. The trial's registration has been initiated with the Pan African Clinical Trials Registry (PACTR) and is currently pending issuance of a registration number, which will be provided once available.
All local health, administrative, and traditional authorities with jurisdiction over the targeted study area will be informed about the study and granted permission for its implementation. Written informed consent will be obtained from all participants, including for audio recording. Data confidentiality will be maintained by de-identifying data and restricting access to authorized personnel only.