Introduction
Albumin (ALB), the most abundant protein in human plasma (4.0 gr/dl), is synthesized in the liver as a long peptide of 585-aminoacid, with a half-life of approximately 25 days [
1].
ALB has the highest number of citations in PubMed, being one of the most studied physiological and laboratory parameters in medicine. In this narrative review, we have deliberately focused on non-gastroenterological and non-hepatic diseases, to highlight its role in medicine, as a biomarker in many acute and chronic diseases, and to emphasize perspectives on how to obtain the best information in terms of pathophysiology and therapeutic hints in clinical practice.
Albumin synthesis and physiology
The synthesis of human albumin begins in the hepatocyte nucleus where its genes are transcribed into mRNA. The mRNA is secreted into the cytoplasm, where it binds to ribosomes that synthesize Pre-Pro-Albumin (Pre-Pro-A) [
2]. The Pre-Pro-A has a N-terminal peptide (24 aminoacids), which is processed in the lumen of the endoplasmic reticulum, resulting in an extension of 6 still bound aminoacid (Pro-Albumin), and releasing the Pre-Albumin. The Pre-Albumin molecule, due to its short half-life (1.9 days) is considered the best parameter for assessing nutritional status and due to its binding to triiodothyronine (T3) and thyroxine (T4), it is also called transthyretin and thyroxine-binding prealbumin (TBPA). Pro-Albumin is exported into the Golgi apparatus, where the 6 aminoacids extension is in turn removed to produce albumin, a single polypeptide containing 585 amino acids.
ALB has a molecular weight of 66.5 kD and is strongly negatively charged. Structurally, it consists of three domains (I, II, III), each of which has two additional subdomains (A, B), containing 4 and 6 α-helices, respectively [
1].
Only a very small amount of ALB is stored in the liver and most of it is readily excreted into the blood plasma. Physiologically, 10 to 15 g of ALB are daily released into the bloodstream, accounting for almost half of the total protein content (3.5 to 5 g/dL) of plasma in healthy humans. The synthesis of ALB is stimulated by hormonal factors, such as insulin, cortisol and growth hormone (GH), while pro-inflammatory mediators, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, exert an inhibitory effect. Once produced, only 30-40% of ALB circulates in the blood, while the remaining (70-60%) leaves the vascular compartment at a rate of 5% per hour (transcapillary passage rate) and returns through the lymphatic system; the amount of ALB returning to the vascular compartment is almost equivalent to the amount leaving it. For these reasons, the circulatory half-life of albumin is 16-18 hours, while its overall half-life is approximately 3 weeks in healthy young adults. The catabolism of albumin is ubiquitous, mainly taking place at the muscles, liver, and kidneys vascular endothelium level. Serum ALB serves as the primary modulator of fluid distribution throughout the body, being responsible for about 80% of colloidal osmotic pressure (oncotic pressure) [
1,
2]. This function is exerted both by a direct osmotic effect, related to its molecular weight and high plasma concentration, and by an indirect ability to attract positively charged molecules into the bloodstream, due to its negative charge (
Gibbs-Donnan effect) [
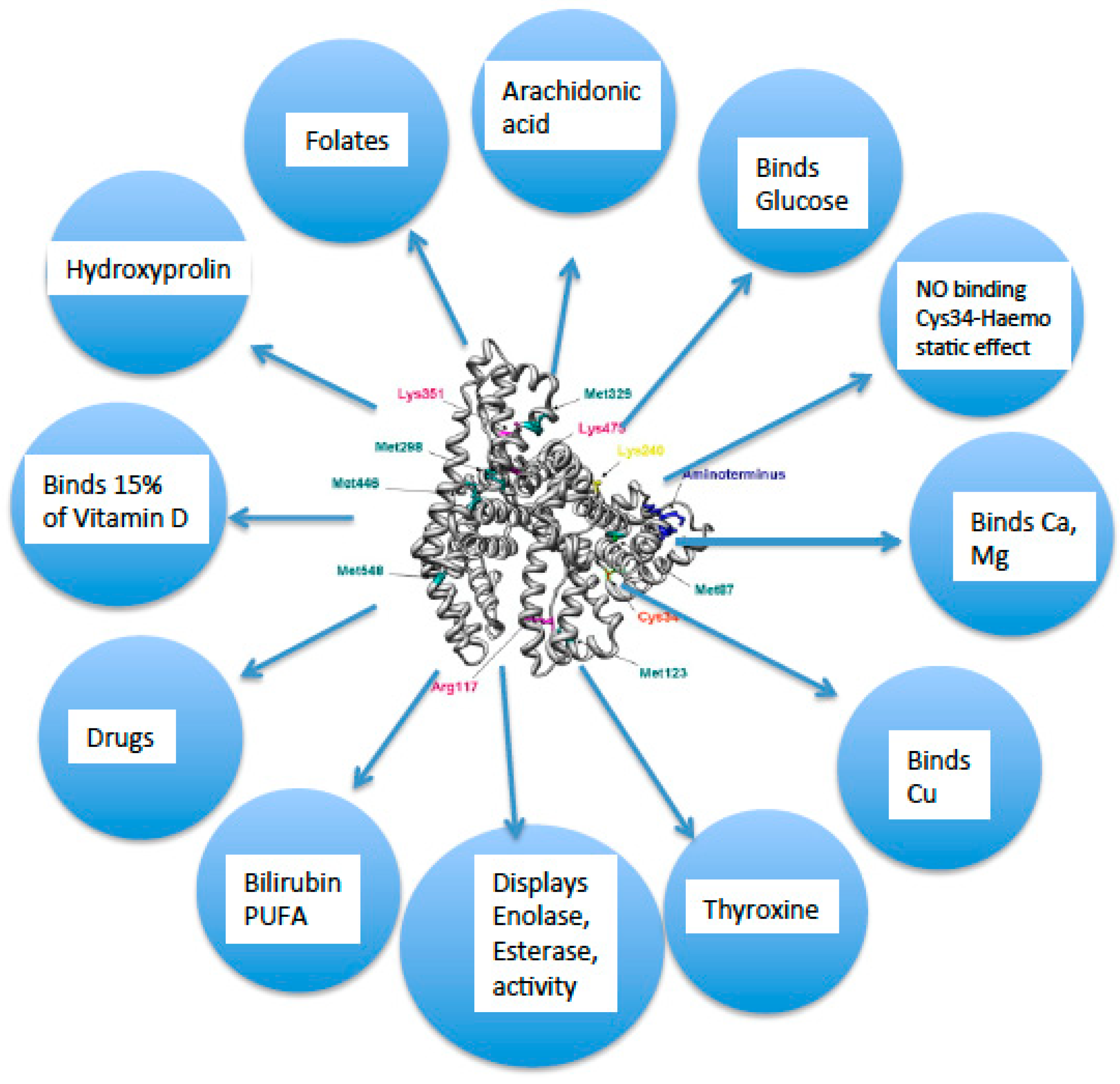
1]. Due to its chemical structure, serum ALB acts as the main carrier of a broad range of endogenous hydrophobic molecules, such as vitamin D, bilirubin, long-chain fatty acids and divalent cations (calcium, magnesium, copper, zinc, thyroxine) and plays a critical role in the delivery of drugs throughout the body [
3]. Albumin depletion for any reason [
4,
5] can lead to extravascular leakage of water and the occurrence of oedema, although a close relationship between albumin level and oedema was not observed in 50 consecutive hospitalized patients in a medical ward in Pretoria-South Africa. In fact, out of 24 patients with ALB levels below 3 gr/dl, only 6 had oedema and none of the patients with levels below 1.5 gr/dl, had any sign of oedema [
6]. This means that other causes should be considered when evaluating the origin of oedema (salt retention from renal disease, cor pulmonale, malignancy, chronic inflammatory diseases, malnutrition, etc.).
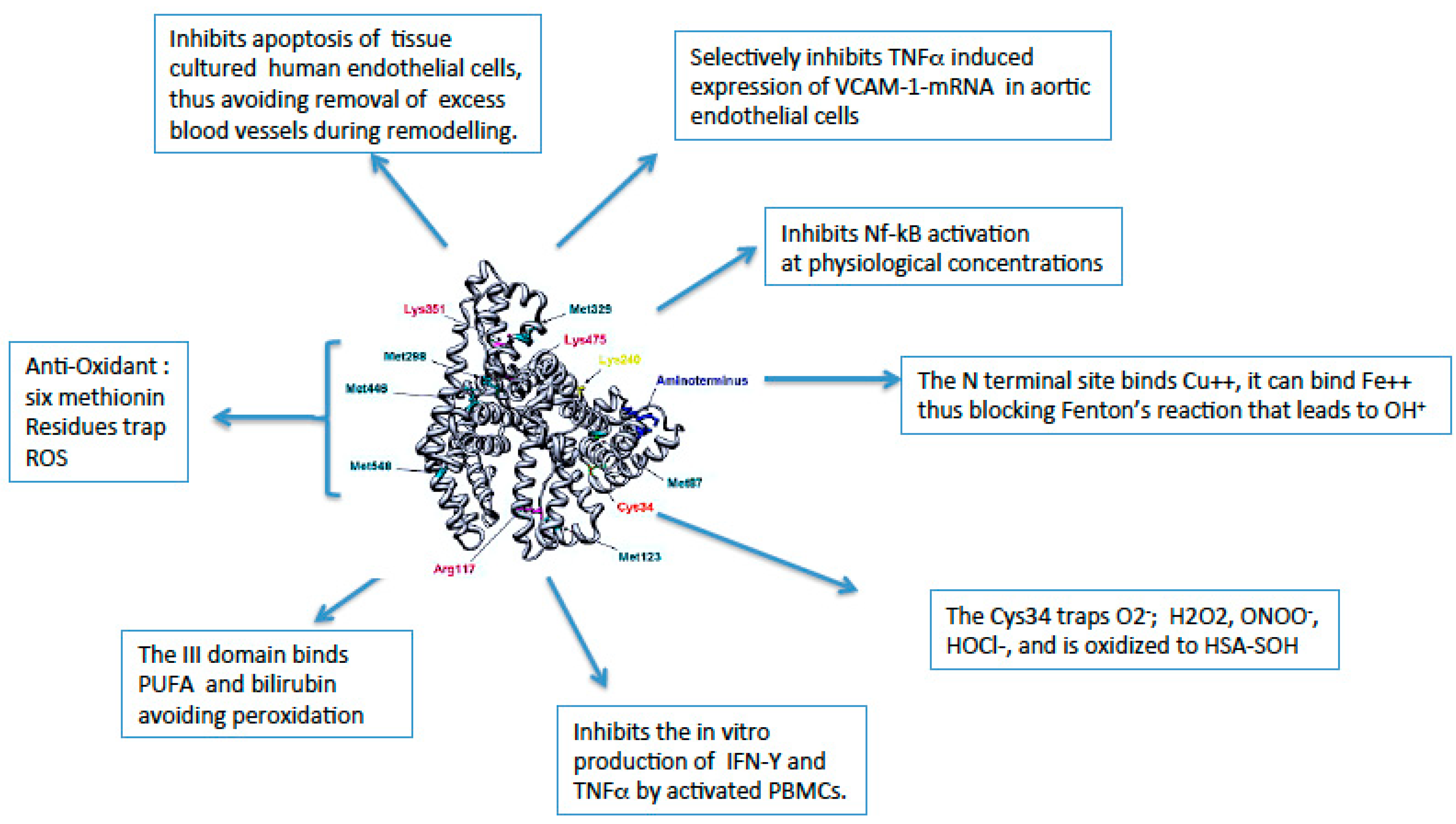
Human serum ALB (HSA) is an appealing anti-oxidant molecule [
1]. Its antioxidant properties rely on the capacity of binding free redox-active transition metal ions (mainly Cu(II) and Fe(II)) at the N-terminal site and serving as a free radical scavenger [
7]. Indeed, these free metal ions may have a high pro-oxidative role through the interaction with hydrogen peroxide (H2O2), leading to the formation of aggressive ROS (
Fenton reaction) [
8]. Thus, their binding by HSA limits availability for the Fenton reaction. Moreover, containing 6 methionines and 35 cysteine residues involved in the formation of 17 disulfide bonds [
9]. This is based on the reduced sulphydryl groups that can scavenge nitric oxide (NO), hypochlorous acid (HOCL), and other reactive oxigen species (ROS), and on the high affinity site for Cu(II) ions, which together with Fe(II) can be extremely pro-oxidant [
10].
The binding of free transition metals limits their availability for the Fenton reaction that catalyzes the production of aggressive ROS [
11]. The study of synthetic fractional albumin (and fibrinogen) rates using simultaneous infusions of intravenous [1-14C] leucine and intraduodenal [4,5-3H] leucine, after 22 hours of fasting and during glucose and amino acids absorption, showed an increase of albumin synthesis by 28% [
12]. Conversely, inducing a mild acidosis (pH=7.3) in a normal subject through a 7-day infusion of NH4Cl, resulted in a 20% decrease in ALB synthesis [
13]. These studies highlight the dynamics of liver synthetic capacity and suggest that nutrition is certainly important, however most studies support the idea that albumin is not a good biomarker of nutritional status given its longer half-life [
14], while data suggest that pre-albumin levels, due to its shorter half-life, more rapidly reflect the overall level of malnutrition in clinics [
15]. A useful algorithm has been proposed to define the pre-albumin status and the risk of poor outcomes (
Table 1) [
15,
16]. Among the hormones that exert a profound effect on the protein synthesis, insulin arose as an important one. Studying, in diabetic patients, whole body protein synthesis and fractional synthetic rate of albumin and fibrinogen (using simultaneous 5-hour infusions of [14C]leucine and [13C]bicarbonate) during continous insulin infusion (to maintain euglycemia), and after short-term insulin deprivation, insulin deficiency decreased albumin synthesis by 29%, and increased fibrinogen levels by 50%, increasing whole body proteolysis by 35%.
These dynamic data proved that albumin synthesis (as well as fibrinogen) is an insulin-sensitive process, and that the increase in fibrinogen reflects an acute phase protein response [
17]. These data are of fundamental clinical importance.
Albumin exerts several anti-inflammatory effects (
Figure 1) and possesses several non-oncotic binding properties (
Figure 2) [
1,
18,
19].
Critical levels of albumin in hospitalized and intensive care unit (ICU) patients.
Hypoalbuminemia, defined as a serum albumin concentration below 3.5 gr/dl, is commonly detected in hospitalized adult patients [
20], being reported with a prevalence higher than 70% in the elderly [
21]. In patients hospitalized for acute illness, albumin emerged as a prognostic factor of death, length of stay, and readmission in 156,511 patients older than 40 years; 21% of patients had albumin < 3.5 gr/dl, in-hospital mortality was 14% compared to 4% of those with normal levels (3.5 times higher), and these patients were more likely to stay longer and to be readmitted sooner and more frequently [
22].
Hypoalbuminemia can result from a wide range of clinical conditions leading to impaired hepatic synthesis, increased catabolism, or leakage via the gastrointestinal (GI) tract, kidney, skin, or extravascular space, or a combination of these mechanisms [
1]. A wide range of clinical conditions may underlie each of these pathophysiological mechanisms. Addressing the first point, liver cirrhosis can result in impaired hepatic ALB synthesis [
23], although hypoalbuminemia is clinically evident only in the presence of chronic and severe hepatic insufficiency. Increased catabolism of serum ALB may occur in septic patients, in whom low serum ALB levels result from the synergy of all pathogenetic mechanisms [
1]. The third case is typically observed in malabsorption syndromes [
24] or in nephrotic syndromes [
25]. Moreover, the close connection between serum ALB and inflammation is well recognized. Indeed, inflammatory processes, regardless of the etiopathogenesis, increase capillary permeability, promoting the leakage of serum ALB and, consequently, the expansion of the interstitial space [
4,
5].
Aside from these typical cases, low serum albumin levels are important predictors of morbidity and mortality even after hospital discharge. Several studies have reported that serum albumin is associated with poor clinical outcomes in severely acute ill patients. Specifically, it arose as a prognostic factor of morbidity, mortality and prolonged hospitalization in acutely ill patients [
22].
In a meta-analysis, Vincent et al. reported that each 10 g/L decrease in serum ALB levels is related to an increase in the odds of mortality by 137%, morbidity by 89%, and prolonged ICU stay by 28%, respectively, regardless of nutritional status [
20].
Interestingly, analysis of dose dependence in controlled trials of albumin therapy indicated that the complication rate may be lower if serum ALB concentration exceeds 30 g/L during ALB supplementation [
26].
Moreover, Eckart et al. prospectively investigated the association between nutritional status, inflammation, and low serum ALB levels (<3.4 gr/dl) and 30-day mortality in a cohort of 2465 patients in the emergency department of a Swiss tertiary care center, finding that hypoalbuminemia correlated with systemic inflammation and high nutritional risk, and independently predicted 30-day mortality (OR:2.87, 95% CI:1.70-4.84, p< 0.001) [
27].
All these data suggest that albumin levels in the emergency department can already be considered a strong biomarker of an overall outcome in the hospitalized patients. At the same time, inflammation must be determined in various disease states [
28,
29,
30]. In fact, TNF has shown effects on acute phase proteins on its own [
31], IL-1 has been shown to stimulate hepatic synthesis of some proteins up to 1000-fold (serum amyloid A-SAA), and 2-10 fold of fibrinogen, complement components, factor B, metallothionins, but also to decrease the transcription of RNA encoding albumin (and transferrin, liprotein lipase, and cytochromes) [
32]. In primary cultures of human hepatocytes, it has been shown that IL-6 induces C-reactive protein (CRP) and SAA in a dose dependent manner, as well as fibrinogen, 1-acid glycoprotein, 2 macroglobulin, haptoglobin, and to decrease albumin and pre-albumin expression [
33]. The strongest downregulation of the albumin gene and the increase of CRP were observed with the combination of IL1 and IL6 [
34].
Based on the above, serum ALB concentration appears to be a reliable tool for mortality risk stratification in the context of medical emergency.
Albumin and cardiovascular disease
Conceptually, serum ALB levels can have a massive impact on cardiovascular (CV) pathophysiology, due to its oncotic, antioxidant, anti-inflammatory, and anticoagulant/antiplatelet aggregation actions.
Although the prevalence of hypoalbuminemia in CV diseases has not been precisely elucidated, its frequency has been reported to be consistent and extremely varied in several meta-analyses addressing deleterious conditions such as heart failure (HF), coronary artery disease (CAD), atrial fibrillation (AF), cerebrovascular accident (CVA) and peripheral artery disease (PAD), ranging from nearly 13% in stable CAD to 90% in elderly patients with severe HF [
35,
36].
Interestingly, in this setting of CV diseases, low serum ALB levels have arisen as a robust poor prognostic factor independent of the most common modifiable and non-modifiable risk factors and confounders including systemic inflammation and malnutrition, thus suggesting a causative role of other physiological functions of serum ALB [
35,
36,
37].
In particular, in the Copenhagen General population study (100,520 subjects with a follow-up of 8.5 years) it emerged that for each 1 gr/dl lower albumin level (hypoalbuminemia defined as < 3.5 gr/dl), the HRs were 1.17 for ischemic heart disease, 1.25 for myocardial infarction, 1.37 for any stroke and 1.46 for ischemic stroke [
35].
Specifically, hypoalbuminemia has been shown to be common in heart failure patients, emerging as a reliable tool to identify patients at high risk of in-hospital and long-term mortality, with a similar predictive value to that reported for serum BNP (brain natriuretic peptide) [
38,
39].
Analysis of 576 consecutive patients with HF and preserved ejection fraction (HFPEF), hospitalized and followed-up for 30 days, revealed hypoalbuminemia (< 3.4 gr/dl) in 160 (28%) patients. After 30 days, CV mortality was 21.8% in hypoalbuminemic with respect to 8.9% in normoalbuminemic patients (2.44-fold higher risk, p<0.001) [
40]. Renal dysfunction has been considered the pathophysiological mechanism leading to hypoalbuminemia.
Data from a prospective cohort study enrolling 734 patients with stable coronary artery disease stratified by baseline serum ALB concentration in the low serum albumin group (<3.5 g/dL, n=98) and in the normal albumin group (≥3.5 g/dL, n=636), demonstrated that lower baseline serum albumin was related to an increased risk of all-cause mortality (10.2 vs 0.5%, p<0.001) and serious CV events (7.1 vs 1.4%, P<0.001) regardless of comorbidities and demographic characteristics (all-cause mortality, HR 6.81, 95% CI 1.01-45.62; hard CV events, HR 3.68, 95% CI 1.03-13.19) [
41]. Moreover, when analysing 1303 patients with acute coronary syndrome (patients with ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina, undergoing coronary angiography, a baseline albumin level <3.65 mg/dl, elevated systolic blood pressure, and a high SYNTAX score emerged as independent predictors of in-hospital mortality. Albumin level
per se was strongly predictive of the SYNTAX score [
42].
Concerning cardiac arrhythmias, serum albumin has been shown to impact the electrical function of the myocardium [
43], although there are few data assessing its correlation and cardiac rhythm abnormalities. A retrospective case-control study in China, aimed to evaluate the clinical features of 950 patients suffering from atrial fibrillation and 963 age-and sex-matched non-AF individuals with sinus rhythm, reported a higher incidence of low serum ALB concentration in adults men who experienced AF, regardless of potential confounding covariates [
44].
There are still limited long-term studies focusing on the association between hypoalbuminemia and cerebrovascular accident. According to the data from the Third China National Stroke Registry (CNSR- III), low baseline serum ALB levels (< 3.5 g/dl) arose as a risk factor associated with poorer outcomes and mortality in acute ischaemic stroke (AIS) or transient ischaemic attack (TIA) patients at 3-month follow-up [
45].
Interesting data on peripheral artery disease and CV outcomes have been published. In particular, Chahrour MA et al. retrospectively explored data from 35,383 patients undergoing lower limb amputation to investigate the relationship between preoperative hypoalbuminemia and postoperative mortality; the authors concluded that the mortality rate was higher in patients with very low serum albumin concentration (<2.5 g/dL) compared to low (2.5-3.39 g/dl) and normal levels (≥3.4 g/dl) (11%, 6.8% and 3.9%, respectively), even after adjusting for confounding variables [
46].
As far as the drugs most commonly used in the setting of CV diseases, severe hypoalbuminemia has been shown experimentally to be involved in the mechanisms of resistance to diuretics. Although it has not yet been satisfactorily interpreted in the clinical setting, the role of human serum ALB as a major drug carrier appears to be an additional weapon to influence the etiopathogenesis of CV disease [
47].
All mentioned data promote serum ALB levels as a potential modifiable risk factor as well as a powerful prognostic marker in the context of CV diseases. Although the clinical efficacy of ALB supplementation is still debated, it is conceivable to consider the treatment of all possible underlying conditions leading to hypoalbuminemia as a huge priority in the management of CV diseases.
Albumin and COVID-19
Research encompassing biomarkers for predicting COVID-19 patient outcomes is rapidly increasing, and higher albumin levels upon admission have emerged as predictors of better prognosis in hospitalized patients with confirmed COVID-19 infection. Biologically, ALB has been shown to exert a down-regulating effect on the production of angiotensin converting enzyme 2 (ACE2), which is the target receptor for COVID-19 [
48].
Moreover, glycated-ALB serves as a high affinity binding protein for SARS-CoV-2 spike proteins, and this may contribute to immune evasion and influence the severity and the pathology of SARS-CoV2 infection, especially in prediabetes and diabetes [
49].
Prior to the COVID-19 pandemic, hypoalbuminemia was already considered a poor prognostic factor in patients hospitalized for community-acquired pneumonia (CAP) [
50], significantly affecting the 30-day mortality rate [
51,
52].
Hypoalbuminemia appears to impact outcomes in COVID-19 infected patients regardless of age and morbidity, being related to a greater prevalence of cardiac injury and hypercoagulability, thereby increasing the burden of inflammatory disease [
53].
Of interest, Violi F et al. provided evidence that ALB supplementation reduces hypercoagulability in hospitalized patients with laboratory-confirmed COVID-19 and SARS-CoV-2-related pneumonia in a small Italian cohort [
54].
Furthermore, Arnau-Barres I et al. evaluated the impact of serum ALB levels on COVID-19-related in-hospital mortality adjusted for potential confounders, reporting that deceased patients were older, had more comorbidities, higher inflammatory status, and lower serum albumin levels at time of hospitalization [3.10 g/dL (0.51) vs. 3.45 g/dL (0.45); p <0.01]. Interestingly, severe hypoalbuminemia (<3 g/dl) at baseline strongly predicted in-hospital mortality in a multivariate logistic regression model controlled for age, inflammation, comorbidities, and severity at admission (OR 2.18 95% CI 1.03–4.62; p = 0.039) [
55].
By transferring these data to the emergency department, Turcato et al. observed that in patients infected with COVID-19, serum ALB levels <3.5 g/dL were independently associated with the presence of severe infection and 30-day mortality [
56].
However, here are still few studies addressing the correlation between serum ALB levels and COVID-19 disease severity, but it is conceivable that serum ALB could serve as a reliable tool to detect in-hospital complications in COVID-19 disease.
Albumin in Nephrology
Serum ALB serves as an established and risk-adjusted predictor of progression to end-stage renal disease (ESRD) and all-cause mortality in patients with chronic kidney disease (CKD), especially in more vulnerable populations, such as HIV-infected and elderly adults [
57].
In this context, hypoalbuminemia mainly occurs as a manifestation of protein-energy wasting, which is a state of metabolic and nutritional alterations characterized by loss of protein and energy stores leading to cachexia [
58].
Notably, hypoalbuminemia represents an established risk factor for infection-related in-hospital death in patients with end-stage renal disease undergoing hemodyalisis [
59].
Additionally, ESRD patients undergoing hemodialysis face an even greater risk of hypoalbuminemia due to ALB losses during dialysis [
60].
Admur RL et al. demonstrated that in the context of chronic kidney disease, baseline serum ALB level can act as a valid tool to predict the occurrence of atherosclerotic vascular disease beyond the conventional assessment of cardiovascular risk factors [
61].
Kawai et al. pictorially reported the risk-adjusted correlation between low baseline serum ALB concentration and worsening renal outcome in patients suffering from Immunoglobulin A (IgA) nephropathy [
62], which is the the most common type of idiopathic glomerulonephritis worldwide [
63].
Remarkably, oxidative stress on mesangial cells has been shown to be a key pathogenic factor in the onset and progression of IgA nephropathy [
62], thus suggesting that the protective action of serum ALB in this context mostly relies on its massive antioxidant capability [
64].
Additionally, in primary membranous nephropathy, patients who have achieved partial remission (proteinuria <3.5 g/day and relative reduction ≥50% with preserved glomerular filtration rate) with serum ALB levels >3.5 g/dl showed a lower risk of relapsing disease if compared than those with lower serum ALB concentration [
65].
In conclusion, data suggest that serum ALB could be a feasible tool for early identification of patients at higher risk regardless of the ehtiopatogenesis of renal damage. However, in such a range of plausible mechanisms leading to hypoalbuminemia in CKD patients (i.e., proteinuria, chronic inflammation, inappropriate nutrition), further studies are needed to detect in which type of patients the ALB supplementation may be more beneficial [
66].
Albumin in Oncology
Malnutrition and cachexia in cancer patients are major challenges, negatively impacting response to treatment and overall quality of life [
67]. Among the broad spectrum of methods to assess nutritional status in cancer, serum ALB levels have emerged as one of the most accurate [
68].
Nevertheless, serum ALB is not only a tool to investigate the nutritional status of cancer patients: in the hospital setting, it is well-known that serum ALB is contingent upon length of stay and all-cause mortality in cancer patients, regardless of type of malignancy, considered alone or in combination with other factors [
69].
Notably, while serum ALB concentration usually remains in the normal range in the early stages of cancer, it has been reported to dramatically decrease as the disease progresses, thereby serving as a valuable prognostic marker.
The modified Glasgow Prognostic Score (mGPS), a widely used composite score including CRP and serum ALB to estimate postoperative outcome in cancer patients, has been shown to be a predictor of mortality among patients undergoing chemotherapy for unresectable colorectal cancer [
70].
Choi et al. investigated the risk factors of early recurrence after curative resection of hepatocellular carcinoma, reporting that serum ALB level < or equal to 3.5 g/dL at time of recurrence is a poor prognostic factor for overall survival at 1, 3, and 5 years [
71].
An elegant study prospectively evaluating 3-year survival in localized non-small cell lung cancer reported serum ALB levels among factors associated with the worst outcome [
72].
For female cancers, in a cohort of 213 histologically confirmed cases of ovarian cancer, serum ALB levels ≥ 3.6 g/dL were associated with a median survival of 23.3 months (95% CI, 16.5-30.1 months), compared to a median survival of 7.3 months (95% CI, 4.8-9.8 months) in those with low serum ALB levels, regardless of disease stage, treatment, and serum cancer antigen-125 [
73].
Moreover, low preoperative serum ALB levels (<4.0 g/dl) were associated with shorter recurrence-free survival and overall survival in a cohort of 157 patients who underwent surgery for breast cancer, irrespective of canonical poor prognostic factors including histological and receptor expression [
74].
Recently, Yoo SK et al. reported elevated pretreatment serum ALB concentrations to favorably predict radiographic response to immune checkpoint blockade (ICB) among 16 cancer types, proposing baseline serum ALB as a valuable biomarker for determining ICB outcome and patients prognosis along with genomic factors [
75].
In conclusion, in the era of transcriptomic, proteomic and metabolomic analyses towards precision medicine in oncology, serum ALB might arise as costless and readily available predictor of cancer progression and survival.
Albumin in Rheumatologic Autoimmune Diseases.
The well-know antioxidant capacity of serum ALB is pivotal in such a range of immune-regulatory mechanisms. Since the majority of immune-mediated inflammatory diseases (IMIDs) are characterized by a down-regulation of serum ALB in response to the inflammatory milieu [
76], it is conceivable that its assessment could arise as a useful marker for monitoring the ongoing activity as well as response to treatment.
Decreased serum ALB levels have been extensively described in rheumatoid arthritis (RA), mainly due to suppression of hepatic production by inflammatory cytokines storm, hemodilution and malnutrion status [
77].
Ganeb S et al. observed that serum ALB levels are significantly lower in RA subjects (median 3.9; 3.5-4.35; p <0.001) than in healthy controls; moreover, when stratifying RA patients by disease activity, as assessed by Disease Activity Score-28 (DAS28), serum ALB was significantly decreased in patients with high disease activity compared with both those in moderate activity and those in remission [
77].
Additionally, a case-control study in Japan described low serum ALB levels (≤4.2 g/dl) to be independently associated with the prevalence of osteoporosis in a cohort of 197 postmenopausal women with RA [
78].
Hypoalbuminemia has been frequently reported in systemic lupus erythematosus (SLE) [
79,
80,
81]. In this context, apart from the canonical underlying pathogenic mechanisms, nephritis may account for nephrotic range proteinuria and, consequently, low serum ALB levels.
In a cohort of 1078 patients diagnosed with SLE, serum ALB concentration inversely correlated to overall SLE disease activity, especially in those suffering from lupus nephritis and proteinuria [
82].
Furthermore, data from the NYU SAMPLE (Specimen and Matched Phenotype Linked Evaluation) Lupus Registry demonstrated that in lupus nephritis serum ALB levels >3.7 g/dL at 12 months of follow-up after renal biopsy predicted good long-term renal outcome [
83].
Recently, Ahn SS et al. prospectively evaluated the relantionship between disease activity and serum ALB, prealbumin, and ischemia-modified albumin in patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), revealing that only serum ALB concentrations increased consensually when disease activity improved [
84].
All these evidences support the possibility of including serum ALB measurement in such a composite score range to better and easily stratify patients and improve their outcomes in rheumatic diseases.
Conclusions and Future Directions
Due to the aforementioned physiological functions, a robust correlation between serum ALB levels and general health results understandable. The levels of ALB depend on gene function: to date, 77 of its mutations are known, of which 65 resulting in bis-albuminemia, and 5 leading to analbuminemia (< 1 gr/L) [
85]. These are very rare, thus supporting ALB levels as a strong indicator of normal physiology in the clinical practice [
1].
Low serum ALB levels have emerged as a detrimental factor in a broad range of clinical settings, serving as an independent baseline predictor of poor outcomes (
Table 2). Although there is no clear evidence on the benefits of ALB supplementation nor stringent indications to assess serum levels during the follow-up of chronic diseases, it is evident that any treatment capable of preserving the intravascular ALB pool could improve the prognosis of patients, particularly when performed at an early stage.
Regarding future perspectives, the ischemia-modified albumin (IMA) is a variant of ALB characterized by an altered structural conformation of the N-terminus, which mainly occurs during prolonged tissue ischemia and inflammation [
86].
This variant has a lower binding capacity to cobalt, and studies suggest that it may sensitively reflect myocardial ischemia (it can be measured by an albumin-cobalt binding assay). Moreover, recent studies report IMA as a valid biomarker of collateral vessels formation in coronary myocardial disease [
87].
Of interest, IMA has also been observed to be upregulated in patients with autoimmune disorders including ankylosing spondylitis, Behcet’s disease, and inflammatory bowel diseases [
88,
89,
90].
Furthermore, oxidative forms of human ALB [human mercapto-albumin (HMA), human non-mercapto-albumin (HNA)] appear to be promising biomarkers of oxidative stress. Although previous studies have demonstrated the upregulation of HNA in a variety of chronic conditions such as CKD, diabetes mellitus, and liver cirrhosis [
91,
92,
93], there are no published data investigating it in the context of rheumatic diseases. Considering the pivotal role of oxidative stress in the pathogenesis of atherosclerosis and the well-known increased risk of CVD in patients suffering from rheumatic diseases, oxidative forms of human ALB could help clinicians earlier identify patients in whom a more stringent follow-up is needed.
Author contribution: (1) EG, DB, VV, SP, LP, GFF: conception and design of the study; (2) DB, VV, SP, LP: acquisition of data, (3) EG, GFF: analysis and interpretation of data, (4) EG, DB, VV, GFF: drafting the article and revising it critically for important intellectual content, (5) EG, DB, VV, SP, LP, GFF: final approval of the version to be submitted.
This research received no external funding
The authors declare no conflict of interest.
References
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Peters T, Jr. All About Albumin. Biochemistry, Genetics, and Medical Applications. Book, 1995. [Google Scholar]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Ann Clin Biochem. 2016, 53 (Pt 1), 106–111. [Google Scholar] [CrossRef]
- Ballmer, P.E. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001, 20, 271–273. [Google Scholar] [CrossRef]
- Steyl, C.; Van Zyl-Smit, R. Mechanisms of oedema formation: The minor role of hypoalbuminaemia. S Afr Med J. 2009, 99, 57–59. [Google Scholar] [PubMed]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013, 3, 4. [Google Scholar] [CrossRef]
- Khosravifarsani, M.; Monfared, A.S.; Pouramir, M.; Zabihi, E. Effects of Fenton Reaction on Human Serum Albumin: An In Vitro Study. Electron Physician. 2016, 8, 2970–2976. [Google Scholar] [CrossRef]
- Ginter, E.; Simko, V.; Panakova, V. Antioxidants in health and disease. Bratisl Lek Listy 2014, 115, 603–606. [Google Scholar] [CrossRef]
- Ambanelli, U.; Spisni, A.; Ferraccioli, G.F. Serum antioxidant activity and related variables in rheumatoid arthritis. Behaviour during sulphydrylant treatment. Scand J Rheumatol. 1982, 11, 203–207. [Google Scholar] [CrossRef]
- Halliwell, B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988, 37, 569–571. [Google Scholar] [CrossRef]
- De Feo, P.; Horber, F.F.; Haymond, M.W. Meal stimulation of albumin synthesis: A significant contributor to whole body protein synthesis in humans. Am J Physiol. 1992, 263 (4 Pt 1), E794–9. [Google Scholar] [CrossRef]
- Ballmer, P.E.; McNurlan, M.A.; Hulter, H.N.; Anderson, S.E.; Garlick, P.J.; Krapf, R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J Clin Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.K.; Rosenthal, T.C. Prealbumin: A marker for nutritional evaluation. Am Fam Physician. 2002, 65, 1575–1578. [Google Scholar] [PubMed]
- Measurement of visceral protein status in assessing protein and energy malnutrition: Standard of care. Prealbumin in Nutritional Care Consensus Group. Nutrition. 1995, 11, 169–171. [Google Scholar]
- De Feo, P.; Gaisano, M.G.; Haymond, M.W. Differential effects of insulin deficiency on albumin and fibrinogen synthesis in humans. J Clin Invest. 1991, 88, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Mateu, X.; Maseda, E.; Yébenes, J.C.; Aldecoa, C.; De Haro, C.; Ruiz-Rodriguez, J.C.; Garnacho-Montero, J. Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev Clin Pharmacol. 2018, 11, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Tufoni, M.; Baldassarre, M.; Zaccherini, G.; Antognoli, A.; Caraceni, P. Hemodynamic and Systemic Effects of Albumin in Patients with Advanced Liver Disease. Curr Hepatol Rep. 2020, 19, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Dubois, M.J.; Navickis, R.J.; Wilkes, M.M. Hypoalbuminemia in acute illness: Is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003, 237, 319–334. [Google Scholar] [CrossRef]
- FBrock, L.A. Bettinelli, T. Dobner, J. C. Stobbe, G. Pomatti, and C. T. Telles, ‘Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders’. Rev Lat Am Enfermagem 2016, 24, e2736. [Google Scholar] [CrossRef]
- Brock, F.; Bettinelli, L.A.; Dobner, T.; Stobbe, J.C.; Pomatti, G.; Telles, C.T. Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders. Rev Lat Am Enfermagem. 2016, 24, e2736. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology. 1988, 8, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Johnson, R. Malabsorption Syndromes. Nurs Clin North Am. 2018, 53, 361–374. [Google Scholar] [CrossRef]
- Kodner, C. Diagnosis and Management of Nephrotic Syndrome in Adults. Am Fam Physician. 2016, 93, 479–485. [Google Scholar]
- Sakr Y, Bauer M, Nierhaus A, Kluge S, Schumacher U, Putensen C, Fichtner F, Petros S, Scheer C, Jaschinski U, Tanev I, Jacob D, Weiler N, Schulze PC, Fiedler F, Kapfer B, Brunkhorst F, Lautenschlaeger I, Wartenberg K, Utzolino S, Briegel J, Moerer O, Bischoff P, Zarbock A, Quintel M, Gattinoni L; SepNet - Critical Care Trials Group. Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): Protocol for a randomized controlled trial. Trials. 2020, 21, 1002. [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; Schuetz, P. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am J Med. 2020, 133, 713–722. [Google Scholar] [CrossRef]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A.; Gelman, S. Raised interleukin-6 levels in obese patients. Obes Res. 2000, 8, 673–675. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991, 77, 1627–1652. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Van Damme, J.; Rieder, H.; Meyer zum Büschenfelde, K.H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988, 18, 1259–1264. [Google Scholar] [CrossRef]
- Ronit, A.; Kirkegaard-Klitbo, D.M.; Dohlmann, T.L.; Lundgren, J.; Sabin, C.A.; Phillips, A.N.; Nordestgaard, B.G.; Afzal, S. Plasma Albumin and Incident Cardiovascular Disease: Results From the CGPS and an Updated Meta-Analysis. Arterioscler Thromb Vasc Biol. 2020, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Arques, S. Serum albumin and cardiovascular disease: State-of-the-art review. Ann Cardiol Angeiol (Paris). 2020, 69, 192–200. [Google Scholar] [CrossRef]
- Schalk, B.W.; Visser, M.; Bremmer, M.A.; Penninx, B.W.; Bouter, L.M.; Deeg, D.J. Change of serum albumin and risk of cardiovascular disease and all-cause mortality: Longitudinal Aging Study Amsterdam. Am J Epidemiol. 2006, 164, 969–977. [Google Scholar] [CrossRef]
- El Iskandarani, M.; El Kurdi, B.; Murtaza, G.; Paul, T.K.; Refaat, M.M. Prognostic role of albumin level in heart failure: A systematic review and meta-analysis. Medicine (Baltimore). 2021, 100, e24785. [Google Scholar] [CrossRef]
- Horwich, T.B.; Kalantar-Zadeh, K.; MacLellan, R.W.; Fonarow, G.C. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008, 155, 883–889. [Google Scholar] [CrossRef]
- Liu, M.; Chan, C.P.; Yan, B.P.; Zhang, Q.; Lam, Y.Y.; Li, R.J.; Sanderson, J.E.; Coats, A.J.; Sun, J.P.; Yip, G.W.; Yu, C.M. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2012, 14, 39–44. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, C.Y.; Leu, H.B.; Su, C.H.; Yin, W.H.; Tseng, W.K.; Wu, Y.W.; Lin, T.H.; Chang, K.C.; Wang, J.H.; Wu, C.C.; Yeh, H.I.; Chen, J.W. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int J Cardiol. 2017, 241, 1–5. [Google Scholar] [CrossRef]
- Kurtul, A.; Murat, S.N.; Yarlioglues, M.; Duran, M.; Ocek, A.H.; Koseoglu, C.; Celık, I.E.; Kilic, A.; Aksoy, O. Usefulness of Serum Albumin Concentration to Predict High Coronary SYNTAX Score and In-Hospital Mortality in Patients With Acute Coronary Syndrome. Angiology. 2016, 67, 34–40. [Google Scholar] [CrossRef]
- Kates, R.E.; Yee, Y.G.; Hill, I. Effect of albumin on the electrophysiologic stability of isolated perfused rabbit hearts. J Cardiovasc Pharmacol. 1989, 13, 168–172. [Google Scholar] [PubMed]
- Zhong X, Jiao H, Zhao D, Teng J, Yang M. A Retrospective Study to Determine the Association Between Serum Albumin Levels and Atrial Fibrillation by Sex in 950 Patients from a Single Center in China. Med Sci Monit. 2022, 28, e935347. [CrossRef]
- Zhou, H.; Wang, A.; Meng, X.; Lin, J.; Jiang, Y.; Jing, J.; Zuo, Y.; Wang, Y.; Zhao, X.; Li, H.; Wang, Y. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. 2021, 6, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.A.; Kharroubi, H.; Al Tannir, A.H.; Assi, S.; Habib, J.R.; Hoballah, J.J. Hypoalbuminemia is Associated with Mortality in Patients Undergoing Lower Extremity Amputation. Ann Vasc Surg. 2021, 77, 138–145. [Google Scholar] [CrossRef]
- Charokopos, A.; Griffin, M.; Rao, V.S.; Inker, L.; Sury, K.; Asher, J.; Turner, J.; Mahoney, D.; Cox, Z.L.; Wilson, F.P.; Testani, J.M. Serum and Urine Albumin and Response to Loop Diuretics in Heart Failure. Clin J Am Soc Nephrol. 2019, 14, 712–718. [Google Scholar] [CrossRef]
- Fagyas M, Úri K, Siket IM, Fülöp GÁ, Csató V, Daragó A, Boczán J, Bányai E, Szentkirályi IE, Maros TM, Szerafin T, Édes I, Papp Z, Tóth A. New perspectives in the renin-angiotensin-aldosterone system (RAAS) II: Albumin suppresses angiotensin converting enzyme (ACE) activity in human. PLoS ONE. 2014, 9, e87844. [Google Scholar] [CrossRef]
- IIles J, Zmuidinaite R, Sadee C, Gardiner A, Lacey J, Harding S, Ule J, Roblett D, Heeney J, Baxendale H, Iles RK. SARS-CoV-2 Spike Protein Binding of Glycated Serum Albumin-Its Potential Role in the Pathogenesis of the COVID-19 Clinical Syndromes and Bias towards Individuals with Pre-Diabetes/Type 2 Diabetes and Metabolic Diseases. Int J Mol Sci. 2022, 23, 4126. [Google Scholar] [CrossRef]
- Hedlund, J.U.; Hansson, L.O.; Ortqvist, A.B. Hypoalbuminemia in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 1995, 155, 1438–1442. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.; Kim, K.; Jo, Y.H.; Rhee, J.; Kim, T.Y.; Na, S.H.; Hwang, S.S. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care. 2011, 26, 287–294. [Google Scholar] [CrossRef]
- Viasus, D.; Garcia-Vidal, C.; Simonetti, A.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J Infect. 2013, 66, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cangemi, R.; Romiti, G.F.; Ceccarelli, G.; Oliva, A.; Alessandri, F.; Pirro, M.; Pignatelli, P.; Lichtner, M.; Carraro, A.; Cipollone, F.; D’ardes, D.; Pugliese, F.; Mastroianni, C.M. Is Albumin Predictor of Mortality in COVID-19? Antioxid Redox Signal. 2021, 35, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Ceccarelli, G.; Loffredo, L.; Alessandri, F.; Cipollone, F.; D’ardes, D.; D’Ettorre, G.; Pignatelli, P.; Venditti, M.; Mastroianni, C.M.; Pugliese, F. Albumin Supplementation Dampens Hypercoagulability in COVID-19: A Preliminary Report. Thromb Haemost. 2021, 121, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Arnau-Barrés, I.; Pascual-Dapena, A.; López-Montesinos, I.; Gómez-Zorrilla, S.; Sorlí, L.; Herrero, M.; Nogués, X.; Navarro-Valls, C.; Ibarra, B.; Canchucaja, L.; da Costa Venancio, E.; Blasco-Hernando, F.; Cruz, J.; Vázquez, O.; Miralles, R.; García-Giralt, N.; Güerri-Fernández, R. Severe Hypoalbuminemia at Admission Is Strongly Associated with Worse Prognosis in Older Adults with SARS-CoV-2 Infection. J Clin Med. 2021, 10, 5134. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Kostic, I.; Melchioretto, B.; Ciccariello, L.; Zaccaria, E.; Olivato, A.; Maccagnani, A.; Pfeifer, N.; Bonora, A. Severity of SARS-CoV-2 infection and albumin levels recorded at the first emergency department evaluation: A multicentre retrospective observational study. Emerg Med J. 2022, 39, 63–69. [Google Scholar] [CrossRef]
- Lang, J.; Scherzer, R.; Weekley, C.C.; Tien, P.C.; Grunfeld, C.; Shlipak, M.G. Serum albumin and short-term risk for mortality and cardiovascular disease among HIV-infected veterans. AIDS. 2013, 27, 1339–1343. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Minatoguchi S, Nomoura A, Imaizumi T; et al. Low serum albumin as a risk factor for infection-related in-hospital death among hemodialysis patients hospitalized on suspicion of infectious disease: A Japanese multicenter retrospective cohort study. Ren Replace Ther. 2018, 4, 30. [CrossRef]
- Kalantar-Zadeh, K.; Ficociello, L.H.; Bazzanella, J.; Mullon, C.; Anger, M.S. Slipping Through the Pores: Hypoalbuminemia and Albumin Loss During Hemodialysis. Int J Nephrol Renovasc Dis. 2021, 14, 11–21. [Google Scholar] [CrossRef]
- Amdur RL, Feldman HI, Dominic EA, Anderson AH, Beddhu S, Rahman M, Wolf M, Reilly M, Ojo A, Townsend RR, Go AS, He J, Xie D, Thompson S, Budoff M, Kasner S, Kimmel PL, Kusek JW, Raj DS; CRIC Study Investigators. Use of Measures of Inflammation and Kidney Function for Prediction of Atherosclerotic Vascular Disease Events and Death in Patients With CKD: Findings From the CRIC Study. Am J Kidney Dis. 2019, 73, 344–353. [Google Scholar] [CrossRef]
- Kawai, Y.; Masutani, K.; Torisu, K.; Katafuchi, R.; Tanaka, S.; Tsuchimoto, A.; Mitsuiki, K.; Tsuruya, K.; Kitazono, T. Association between serum albumin level and incidence of end-stage renal disease in patients with Immunoglobulin A nephropathy: A possible role of albumin as an antioxidant agent. PLoS ONE. 2018, 13, e0196655. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987, 64, 709–727. [Google Scholar] [PubMed]
- Camilla, R.; Suzuki, H.; Daprà, V.; Loiacono, E.; Peruzzi, L.; Amore, A.; Ghiggeri, G.M.; Mazzucco, G.; Scolari, F.; Gharavi, A.G.; Appel, G.B.; Troyanov, S.; Novak, J.; Julian, B.A.; Coppo, R. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 2011, 6, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Chung, Y.; Poulton, C.J.; Derebail, V.K.; Hogan, S.L.; Reich, H.N.; Falk, R.J.; Nachman, P.H. Serum Albumin at Partial Remission Predicts Outcomes in Membranous Nephropathy. Kidney Int Rep. 2020, 5, 706–717. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, X.; Han, Y.; Hao, J.; Hu, H.; Hao, L. The level of serum albumin is associated with renal prognosis and renal function decline in patients with chronic kidney disease. BMC Nephrol. 2023, 24, 57. [Google Scholar] [CrossRef]
- von Meyenfeldt, M. Cancer-associated malnutrition: An introduction. Eur J Oncol Nurs. 2005, 9 Suppl. 2, S35–S38. [Google Scholar] [CrossRef]
- Blackburn, G.L.; Thornton, P.A. Nutritional assessment of the hospitalized patient. Med Clin North Am. 1979, 63, 11103–11115. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Ishizuka, M.; Nagata, H.; Takagi, K.; Kubota, K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann Surg. 2009, 250, 268–272. [Google Scholar] [CrossRef]
- Choi, G.H.; Kim, D.H.; Kang, C.M.; Kim, K.S.; Choi, J.S.; Lee, W.J.; Kim, B.R. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol. 2008, 15, 618–629. [Google Scholar] [CrossRef]
- Win, T.; Sharples, L.; Groves, A.M.; Ritchie, A.J.; Wells, F.C.; Laroche, C.M. Predicting survival in potentially curable lung cancer patients. Lung. 2008, 186, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Asher, V.; Lee, J.; Bali, A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012, 29, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Tokuda, S.; Nakazawa, Y.; Kurozumi, S.; Obayashi, S.; Yajima, R.; Shirabe, K. Implications of Low Serum Albumin as a Prognostic Factor of Long-term Outcomes in Patients With Breast Cancer. In Vivo. 2020, 34, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.K.; Chowell, D.; Valero, C.; Morris, L.G.T.; Chan, T.A. Pre-treatment serum albumin and mutational burden as biomarkers of response to immune checkpoint blockade. NPJ Precis Oncol. 2022, 6, 23. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Ganeb, S.; Egaila, S.; Hamed, A.; Hassan, W. Significance of serum albumin and derived neutrophil-to-lymphocyte ratio score in assessment of disease activity in rheumatoid arthritis patients. Egypt Rheumatol Rehabil. 2020, 47, 5. [Google Scholar] [CrossRef]
- Nagayama, Y.; Ebina, K.; Tsuboi, H.; Hirao, M.; Hashimoto, J.; Yoshikawa, H.; Okada, S.; Nakata, K. Low serum albumin concentration is associated with increased risk of osteoporosis in postmenopausal patients with rheumatoid arthritis. J Orthop Sci. 2022, 27, 1283–1290. [Google Scholar] [CrossRef]
- Madda, R.; Lin, S.C.; Sun, W.H.; Huang, S.L. Differential expressions of plasma proteins in systemic lupus erythematosus patients identified by proteomic analysis. J Microbiol Immunol Infect. 2019, 52, 816–826. [Google Scholar] [CrossRef]
- Sule, S.D.; Fadrowski, J.J.; Fivush, B.A.; Gorman, G.; Furth, S.L. Reduced albumin levels and utilization of arteriovenous access in pediatric patients with systemic lupus erythematosus (SLE). Pediatr Nephrol. 2007, 22, 2041–2046. [Google Scholar] [CrossRef]
- Huang, S.T.; Chen, G.Y.; Wu, C.H.; Kuo, C.D. Effect of disease activity and position on autonomic nervous modulation in patients with systemic lupus erythematosus. Clin Rheumatol. 2008, 27, 295–300. [Google Scholar] [CrossRef]
- Yip, J.; Aghdassi, E.; Su, J.; Lou, W.; Reich, H.; Bargman, J.; Scholey, J.; Gladman, D.D.; Urowitz, M.B.; Fortin, P.R. Serum albumin as a marker for disease activity in patients with systemic lupus erythematosus. J Rheumatol. 2010, 37, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Domingues, V.; Levinson, B.A.; Bornkamp, N.; Goldberg, J.D.; Buyon, J.; Belmont, H.M. Serum albumin at 1 year predicts long-term renal outcome in lupus nephritis. Lupus Sci Med. 2018, 5, e000271. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Yoon, T.; Song, J.J.; Park, Y.B.; Lee, S.W. Serum albumin, prealbumin, and ischemia-modified albumin levels in patients with ANCA-associated vasculitis: A prospective cohort study. PLoS ONE. 2022, 17, e0271055. [Google Scholar] [CrossRef] [PubMed]
- Minchiotti L, Galliano M, Kragh-Hansen U, Peters T Jr. Mutations and polymorphisms of the gene of the major human blood protein, serum albumin. Hum Mutat. 2008, 29, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis Markers. 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Y.; Tian, L.; Wang, Z. Correlation between ischemia-modified albumin level and coronary collateral circulation. BMC Cardiovasc Disord. 2020, 20, 326. [Google Scholar] [CrossRef]
- Kaplan M, Yuksel M, Ates I, Kilic ZM, Kilic H, Kuzu UB, Kayacetin E. Is ischemia modified albumin a disease activity marker for inflammatory bowel diseases? J Gastroenterol Hepatol. 2016, 31, 1120–1125. [CrossRef]
- Capkin E, Karkucak M, Kola M, Karaca A, Aydin Capkin A, Caner Karahan S. Ischemia-modified albumin (IMA): A novel marker of vascular involvement in Behçet’s disease? Joint Bone Spine. 2015, 82, 68–69. [CrossRef]
- Sertpoyraz, F.M.; Colak, A.; Dikici, A.; Gunduz, N.E.; Aksit, M.Z. The relationship of ischemia-modified albumin levels to disease activity scores and HLA-B27 in patients with ankylosing spondylitis. North Clin Istanb. 2020, 8, 42–48. [Google Scholar] [CrossRef]
- Suzuki, E.; Yasuda, K.; Takeda, N.; Sakata, S.; Era, S.; Kuwata, K.; Sogami, M.; Miura, K. Increased oxidized form of human serum albumin in patients with diabetes mellitus. Diabetes Res Clin Pract. 1992, 18, 153–158. [Google Scholar] [CrossRef]
- Cingolani, F.; Czaja, M.J. Oxidized Albumin-A Trojan Horse for p38 MAPK-Mediated Inflammation in Decompensated Cirrhosis. Hepatology. 2018, 68, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Suda, K.; Matsuyama, Y.; Era, S.; Soejima, A. Close relationship between redox state of human serum albumin and serum cysteine levels in non-diabetic CKD patients with various degrees of renal function. Clin Nephrol. 2014, 82, 320–325. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).