Submitted:
22 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Microglia in homeostasis
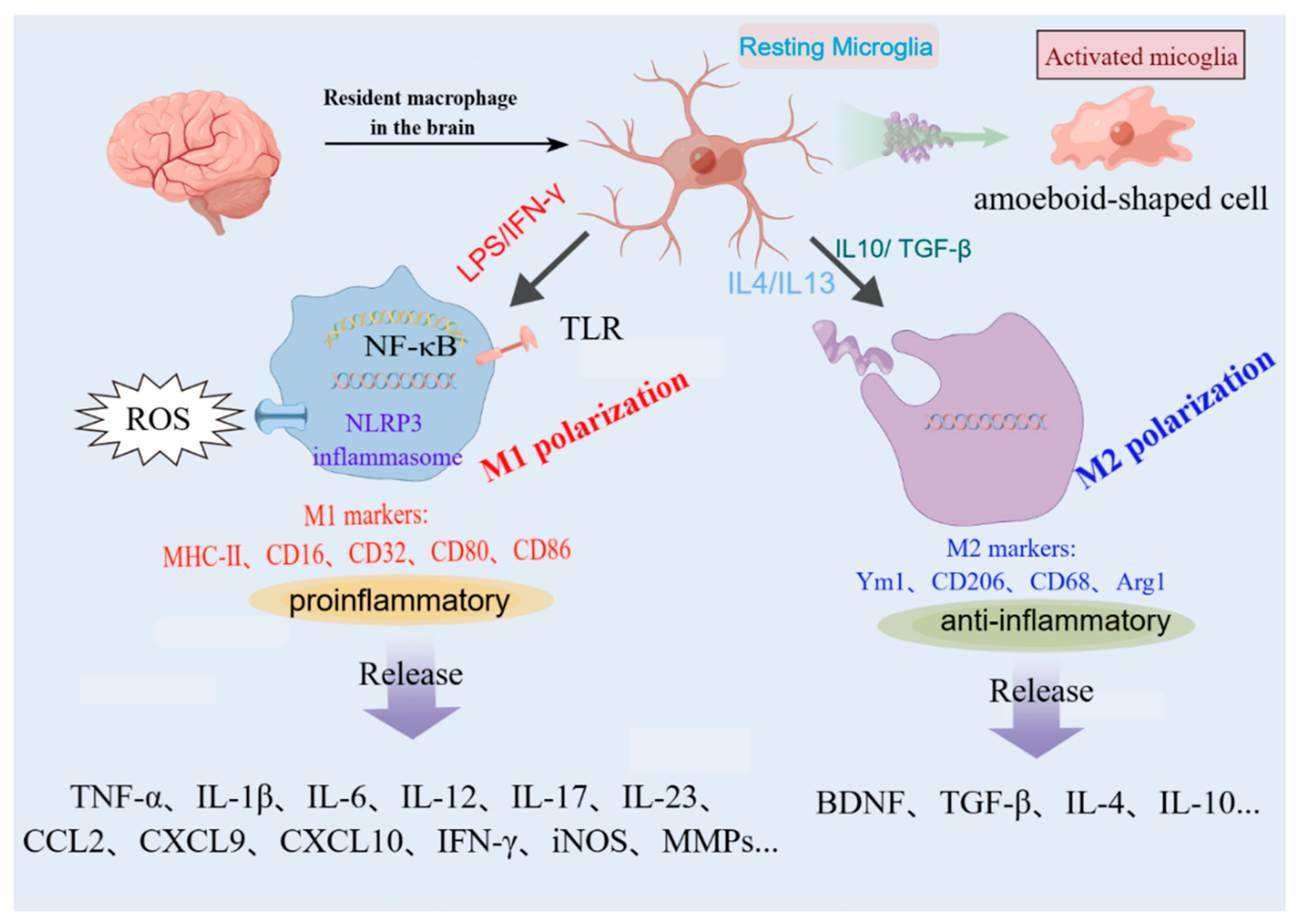
3. M1/M2 microglial polarization
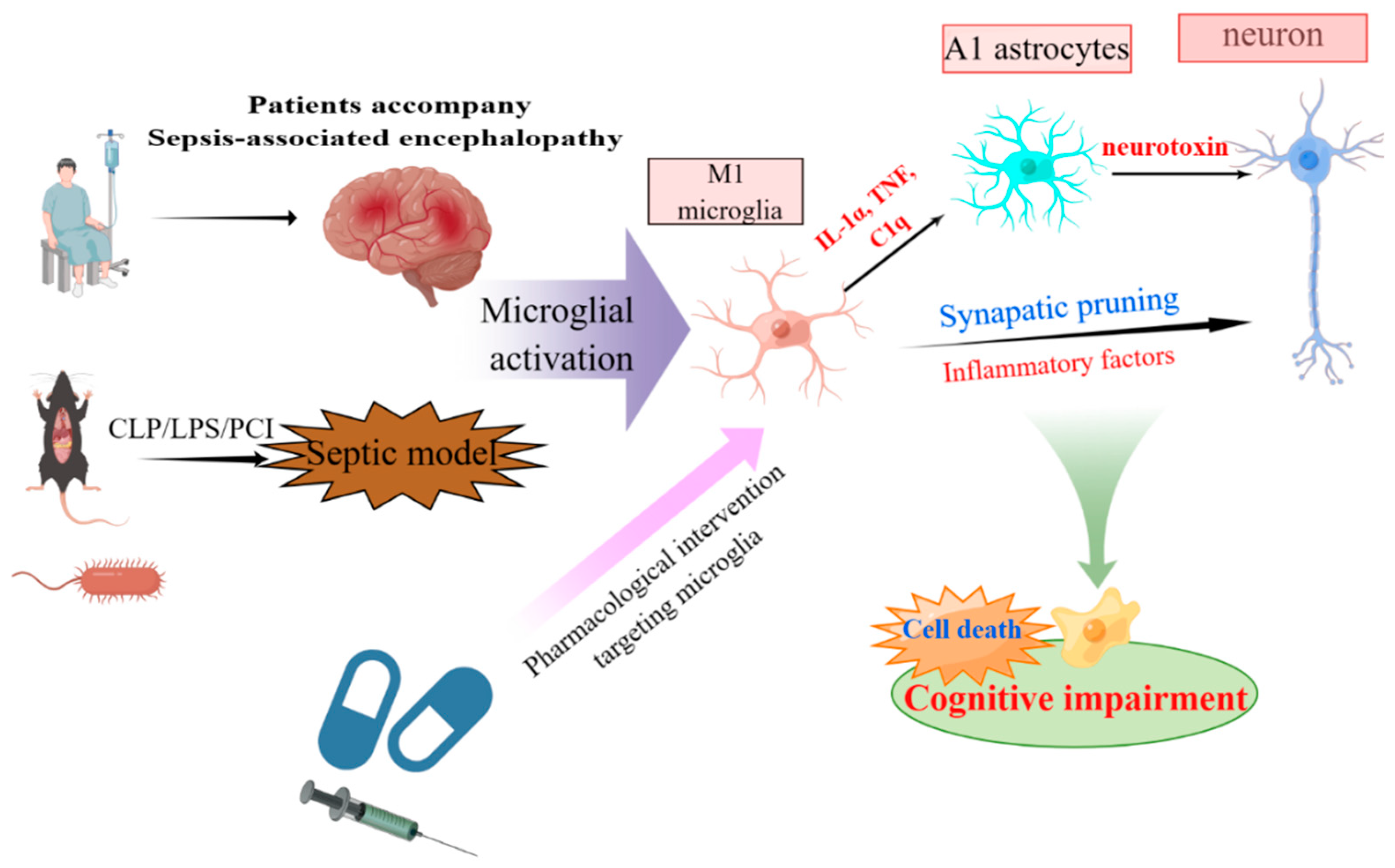
4. Microglia as Central Actors in SAE
4.1. Experimental techniques
4.2. Crosstalk between microglia, neurons, and astrocytes
4.3. Microglia activation in cognitive impairment
5. Pharmacological intervention targeting microglia
5.1. Blockers of inflammatory factors and pyroptosis
5.2. Signaling pathway inhibitors
5.3. Mitochondrial targeting drugs
5.4. Traditional Chinese medicine
6. Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet (London, England) 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital deaths in patients with sepsis from 2 independent cohorts. Jama 2014, 312, 90–92. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. The New England journal of medicine 2017, 377, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Golzari, S.E.; Mahmoodpoor, A. Sepsis-associated encephalopathy versus sepsis-induced encephalopathy. Lancet Neurol 2014, 13, 967–968. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, R.; de Montmollin, E.; Poujade, J.; Garrouste-Orgeas, M.; Souweine, B.; Darmon, M.; Mariotte, E.; Argaud, L.; Barbier, F.; Goldgran-Toledano, D.; et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med 2017, 43, 1075–1084. [Google Scholar] [CrossRef]
- Gofton, T.E.; Young, G.B. Sepsis-associated encephalopathy. Nature reviews. Neurology 2012, 8, 557–566. [Google Scholar] [CrossRef]
- Bozza, F.A.; D'Avila, J.C.; Ritter, C.; Sonneville, R.; Sharshar, T.; Dal-Pizzol, F. Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 2013, 39 Suppl 1, 10–16. [Google Scholar] [CrossRef]
- Bustamante, A.C.; Opron, K.; Ehlenbach, W.J.; Larson, E.B.; Crane, P.K.; Keene, C.D.; Standiford, T.J.; Singer, B.H. Transcriptomic Profiles of Sepsis in the Human Brain. Am J Respir Crit Care Med 2020, 201, 861–863. [Google Scholar] [CrossRef]
- Michels, M.; Abatti, M.R.; Ávila, P.; Vieira, A.; Borges, H.; Carvalho Junior, C.; Wendhausen, D.; Gasparotto, J.; Tiefensee Ribeiro, C.; Moreira, J.C.F.; et al. Characterization and modulation of microglial phenotypes in an animal model of severe sepsis. J Cell Mol Med 2020, 24, 88–97. [Google Scholar] [CrossRef]
- Lemstra, A.W.; Groen in't Woud, J.C.; Hoozemans, J.J.; van Haastert, E.S.; Rozemuller, A.J.; Eikelenboom, P.; van Gool, W.A. Microglia activation in sepsis: a case-control study. J Neuroinflammation 2007, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Zrzavy, T.; Höftberger, R.; Berger, T.; Rauschka, H.; Butovsky, O.; Weiner, H.; Lassmann, H. Pro-inflammatory activation of microglia in the brain of patients with sepsis. Neuropathology and applied neurobiology 2019, 45, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annual review of immunology 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat Med 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Kim, I.S.; Choi, D.K. Microglial Turnover in Ageing-Related Neurodegeneration: Therapeutic Avenue to Intervene in Disease Progression. Cells 2021, 10. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science (New York, N.Y.) 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 2014, 49, 1422–1434. [Google Scholar] [CrossRef]
- Barakat, R.; Redzic, Z. The Role of Activated Microglia and Resident Macrophages in the Neurovascular Unit during Cerebral Ischemia: Is the Jury Still Out? Medical principles and practice : international journal of the Kuwait University, Health Science Centre 2016, 25(Suppl 1), 3–14. [Google Scholar] [CrossRef]
- Galea, I.; Bechmann, I.; Perry, V.H. What is immune privilege (not)? Trends in immunology 2007, 28, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Nayak, D.; Atanasijevic, T.; Koretsky, A.P.; Latour, L.L.; McGavern, D.B. Transcranial amelioration of inflammation and cell death after brain injury. Nature 2014, 505, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature neuroscience 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.; Koizumi, S.; Moorhouse, A.J.; Yoshimura, Y.; Nabekura, J. Microglia contact induces synapse formation in developing somatosensory cortex. Nature communications 2016, 7, 12540. [Google Scholar] [CrossRef]
- Michels, M.; Sonai, B.; Dal-Pizzol, F. Polarization of microglia and its role in bacterial sepsis. Journal of neuroimmunology 2017, 303, 90–98. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: unique stimuli, specialized responses. Annual review of immunology 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Masuda, T.; Amann, L.; Monaco, G.; Sankowski, R.; Staszewski, O.; Krueger, M.; Del Gaudio, F.; He, L.; Paterson, N.; Nent, E.; et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 2022, 604, 740–748. [Google Scholar] [CrossRef]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e256. [Google Scholar] [CrossRef]
- Madry, C.; Kyrargyri, V.; Arancibia-Cárcamo, I.L.; Jolivet, R.; Kohsaka, S.; Bryan, R.M.; Attwell, D. Microglial Ramification, Surveillance, and Interleukin-1β Release Are Regulated by the Two-Pore Domain K(+) Channel THIK-1. Neuron 2018, 97, 299–312.e296. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science (New York, N.Y.) 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. A polarizing question: do M1 and M2 microglia exist? Nature neuroscience 2016, 19, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar; Scheiwe, C. ; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nature neuroscience 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Xu, Z.; Zhang, B.; Liu, A.; He, Q.; Zheng, F.; Zhan, J. Protective Effects of Cannabinoid Type 2 Receptor Activation Against Microglia Overactivation and Neuronal Pyroptosis in Sepsis-Associated Encephalopathy. Neuroscience 2022, 493, 99–108. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Guo, Y.; Huang, P.; Ha, Y.; Zhang, R.; Bai, Y.; Cui, X.; He, S.; Liu, Q. Qiang Xin 1 Formula Suppresses Excessive Pro-Inflammatory Cytokine Responses and Microglia Activation to Prevent Cognitive Impairment and Emotional Dysfunctions in Experimental Sepsis. Front Pharmacol 2020, 11, 579. [Google Scholar] [CrossRef]
- Gosselin, D.; Skola, D.; Coufal, N.G.; Holtman, I.R.; Schlachetzki, J.C.M.; Sajti, E.; Jaeger, B.N.; O'Connor, C.; Fitzpatrick, C.; Pasillas, M.P.; et al. An environment-dependent transcriptional network specifies human microglia identity. Science (New York, N.Y.) 2017, 356. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278–293.e279. [Google Scholar] [CrossRef]
- Mueller, K.L.; Hines, P.J.; Travis, J. Neuroimmunology. Science (New York, N.Y.) 2016, 353, 760–761. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annual review of immunology 2021, 39, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Shulyatnikova, T.; Tumanskyi, V.; Hayden, M.R. Reactive Microgliosis in Sepsis-Associated and Acute Hepatic Encephalopathies: An Ultrastructural Study. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Wickel, J.; Hahn, N.; Mein, N.; Schwarzbrunn, M.; Koch, P.; Ceanga, M.; Haselmann, H.; Baade-Büttner, C.; von Stackelberg, N.; et al. Microglia mediate neurocognitive deficits by eliminating C1q-tagged synapses in sepsis-associated encephalopathy. Science advances 2023, 9, eabq7806. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xu, H.; Zhang, H.; Tang, R.; Lan, Y.; Xing, R.; Li, S.; Christian, E.; Hou, Y.; Lorello, P.; et al. Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting microglial metabolic adaptation and phagocytic function. Immunity 2022, 55, 159–173.e159. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W., 2nd; Mochly-Rosen, D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nature neuroscience 2019, 22, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, Y.; Peng, Z.; Xie, L.; Zhong, X.; Liang, F.; Yuan, C.; Lu, B. Silencing IFNγ inhibits A1 astrocytes and attenuates neurogenesis decline and cognitive impairment in endotoxemia. Biochem Biophys Res Commun 2020, 533, 1519–1526. [Google Scholar] [CrossRef]
- Xiao, T.; Ji, H.; Shangguan, X.; Qu, S.; Cui, Y.; Xu, J. NLRP3 inflammasome of microglia promotes A1 astrocyte transformation, neo-neuron decline and cognition impairment in endotoxemia. Biochem Biophys Res Commun 2022, 602, 1–7. [Google Scholar] [CrossRef]
- Shi, J.; Xu, H.; Cavagnaro, M.J.; Li, X.; Fang, J. Blocking HMGB1/RAGE Signaling by Berberine Alleviates A1 Astrocyte and Attenuates Sepsis-Associated Encephalopathy. Front Pharmacol 2021, 12, 760186. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Zhou, Q.; Li, Q.; Liu, N.; Li, Z.; Chen, C.; Deng, Y. Melatonin ameliorates axonal hypomyelination of periventricular white matter by transforming A1 to A2 astrocyte via JAK2/STAT3 pathway in septic neonatal rats. Journal of Inflammation Research 2021, 14, 5919–5937. [Google Scholar] [CrossRef]
- Calsavara, A.J.C.; Nobre, V.; Barichello, T.; Teixeira, A.L. Post-sepsis cognitive impairment and associated risk factors: A systematic review. Australian critical care : official journal of the Confederation of Australian Critical Care Nurses 2018, 31, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.A.; Santos, G.; de Sampaio e Spohr, T.C.; D'Avila, J.C.; Lima, F.R.; Benjamim, C.F.; Bozza, F.A.; Gomes, F.C. Activated Microglia-Induced Deficits in Excitatory Synapses Through IL-1β: Implications for Cognitive Impairment in Sepsis. Mol Neurobiol 2015, 52, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Munster, B.C.; Aronica, E.; Zwinderman, A.H.; Eikelenboom, P.; Cunningham, C.; Rooij, S.E. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation research 2011, 14, 615–622. [Google Scholar] [CrossRef]
- Andonegui, G.; Zelinski, E.L.; Schubert, C.L.; Knight, D.; Craig, L.A.; Winston, B.W.; Spanswick, S.C.; Petri, B.; Jenne, C.N.; Sutherland, J.C.; et al. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Jing, G.; Zuo, J.; Fang, Q.; Yuan, M.; Xia, Y.; Jin, Q.; Liu, Y.; Wang, Y.; Zhang, Z.; Liu, W.; et al. Erbin protects against sepsis-associated encephalopathy by attenuating microglia pyroptosis via IRE1α/Xbp1s-Ca(2+) axis. J Neuroinflammation 2022, 19, 237. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Fang, M.; Zhu, G.F.; Zhou, Y.; Zeng, H.K. Role of microglia in the pathogenesis of sepsis-associated encephalopathy. CNS Neurol Disord Drug Targets 2013, 12, 720–725. [Google Scholar] [CrossRef]
- Hoshino, K.; Hayakawa, M.; Morimoto, Y. Minocycline Prevents the Impairment of Hippocampal Long-Term Potentiation in the Septic Mouse. Shock 2017, 48, 209–214. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Chen, J.; Zhang, S.; Le, Y.; Xie, Z.; Ouyang, W.; Tong, J. Subcutaneous administration of β-hydroxybutyrate improves learning and memory of sepsis surviving mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2020, 17, 616–626. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Cooke, C.R.; Wunsch, H.; Kahn, J.M. Population burden of long-term survivorship after severe sepsis in older Americans. Journal of the American Geriatrics Society 2012, 60, 1070–1077. [Google Scholar] [CrossRef]
- Armstrong, B.A.; Betzold, R.D.; May, A.K. Sepsis and Septic Shock Strategies. The Surgical clinics of North America 2017, 97, 1339–1379. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, K.; Xiao, Q.; Hou, R.; Pan, X.; Zhu, X. Central role of microglia in sepsis-associated encephalopathy: From mechanism to therapy. Front Immunol 2022, 13, 929316. [Google Scholar] [CrossRef] [PubMed]
- Terrando, N.; Rei Fidalgo, A.; Vizcaychipi, M.; Cibelli, M.; Ma, D.; Monaco, C.; Feldmann, M.; Maze, M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care 2010, 14, R88. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Luo, Y. Ginsenoside Rg1 protects against sepsis-associated encephalopathy through beclin 1-independent autophagy in mice. J Surg Res 2017, 207, 181–189. [Google Scholar] [CrossRef]
- Tian, M.; Qingzhen, L.; Zhiyang, Y.; Chunlong, C.; Jiao, D.; Zhang, L.; Li, W. Attractylone attenuates sepsis-associated encephalopathy and cognitive dysfunction by inhibiting microglial activation and neuroinflammation. J Cell Biochem 2019, 120, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.E.; Liu, L.; Wang, Y.C.; Wang, C.T.; Zheng, Q.; Liu, Q.X.; Li, Z.F.; Bai, X.J.; Liu, X.H. Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav Immun 2019, 80, 859–870. [Google Scholar] [CrossRef]
- Heimfarth, L.; Carvalho, A.M.S.; Quintans, J.S.S.; Pereira, E.W.M.; Lima, N.T.; Bezerra Carvalho, M.T.; Barreto, R.S.S.; Moreira, J.C.F.; da Silva-Júnior, E.F.; Schmitt, M.; et al. Indole-3-guanylhydrazone hydrochloride mitigates long-term cognitive impairment in a neonatal sepsis model with involvement of MAPK and NFκB pathways. Neurochem Int 2020, 134, 104647. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, Y.; Wang, Y.; Meng, X.; Wang, Y.; Yu, Y.; Chen, H. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm Res 2020, 69, 697–710. [Google Scholar] [CrossRef]
- Rocha, M.; Vieira, A.; Michels, M.; Borges, H.; Goulart, A.; Fernandes, F.; Dominguini, D.; Ritter, C.; Dal-Pizzol, F. Effects of S100B neutralization on the long-term cognitive impairment and neuroinflammatory response in an animal model of sepsis. Neurochemistry International 2021, 142. [Google Scholar] [CrossRef]
- Bonfante, S.; Joaquim, L.; Fileti, M.E.; Giustina, A.D.; de Souza Goldim, M.P.; Danielski, L.G.; Cittadin, E.; De Carli, R.J.; de Farias, B.X.; Engel, N.A.; et al. Stanniocalcin 1 Inhibits the Inflammatory Response in Microglia and Protects Against Sepsis-Associated Encephalopathy. Neurotox Res 2021, 39, 119–132. [Google Scholar] [CrossRef]
- Wen, Q.; Ding, Q.; Wang, J.; Yin, Y.; Xu, S.; Ju, Y.; Ji, H.; Liu, B. Cortistatin-14 Exerts Neuroprotective Effect Against Microglial Activation, Blood-brain Barrier Disruption, and Cognitive Impairment in Sepsis-associated Encephalopathy. Journal of immunology research 2022, 2022, 3334145. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, Z.; Wang, Y.; Mao, M.; Deng, Y.; Shi, M.; Xu, Y.; Chen, L.; Cao, W. JQ1 attenuates neuroinflammation by inhibiting the inflammasome-dependent canonical pyroptosis pathway in SAE. Brain Res Bull 2022, 189, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liang, H.; Song, H.; Ding, X.; Wang, D.; Zhang, X.; Sun, T. Metformin Improves the Prognosis of Adult Mice with Sepsis-Associated Encephalopathy Better than That of Aged Mice. Journal of immunology research 2022, 2022, 3218452. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Ren, X.; Ai, Y.; Liu, Z. SS-31 Improves Cognitive Function in Sepsis-Associated Encephalopathy by Inhibiting the Drp1-NLRP3 Inflammasome Activation. Neuromolecular medicine 2022, 10.1007/s12017-022-08730-1. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Chen, S.; Wen, Y.; Zhang, S.; Luo, E.; Li, X.; Zhong, W.; Zeng, H. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis-associated encephalopathy. CNS Neurosci Ther 2022, 28, 247–258. [Google Scholar] [CrossRef]
- Scott, M.C.; Haase, C.M.; Olson, S.D.; Cox, C.S., Jr. Dexmedetomidine Alters the Inflammatory Profile of Rat Microglia In Vitro. Neurocrit Care 2023, 38, 688–697. [Google Scholar] [CrossRef]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nature reviews. Neuroscience 2014, 15, 84–97. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO molecular medicine 2019, 11. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res Rev 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Tang, X.; Bai, W.; Wang, G.; Tian, X. Repetitive transcranial magnetic stimulation regulates L-type Ca(2+) channel activity inhibited by early sevoflurane exposure. Brain Res 2016, 1646, 207–218. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Lou, Y.; Xu, J.; Feng, Z.; Chen, Y.; Tang, Q.; Zheng, G.; Zhang, Z.; Wu, Y.; et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med 2018, 22, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Yu, Y.; Jiang, Y.; Zhao, S.; Wang, Y.; Su, L.; Xie, K.; Yu, Y.; Lu, Y.; Lv, G. Molecular hydrogen attenuates sepsis-induced neuroinflammation through regulation of microglia polarization through an mTOR-autophagy-dependent pathway. Int Immunopharmacol 2020, 81, 106287. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Scheyltjens, I.; Frumer, G.R.; Kim, J.S.; Grozovski, J.; Ayanaw, S.; Dassa, B.; Van Hove, H.; Chappell-Maor, L.; Boura-Halfon, S.; et al. Interleukin-10 Prevents Pathological Microglia Hyperactivation following Peripheral Endotoxin Challenge. Immunity 2020, 53, 1033–1049.e1037. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Du, J.; Jiang, B.; Shan, T.; Li, H.; Bao, H.; Si, Y. CXCR5 down-regulation alleviates cognitive dysfunction in a mouse model of sepsis-associated encephalopathy: potential role of microglial autophagy and the p38MAPK/NF-κB/STAT3 signaling pathway. J Neuroinflammation 2021, 18, 246. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Deng, S.; Zhang, L.; Huang, Y.; Li, W.; Peng, Q.; Liu, Z.; Ai, Y. SS-31 reduces inflammation and oxidative stress through the inhibition of Fis1 expression in lipopolysaccharide-stimulated microglia. Biochem Biophys Res Commun 2019, 520, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Haileselassie, B.; Joshi, A.U.; Minhas, P.S.; Mukherjee, R.; Andreasson, K.I.; Mochly-Rosen, D. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J Neuroinflammation 2020, 17, 36. [Google Scholar] [CrossRef]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 2011, 107, 57–64. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, L.; Mo, Y.; Huang, Y.; Li, W.; Peng, Q.; Huang, L.; Ai, Y. Mdivi-1 attenuates lipopolysaccharide-induced acute lung injury by inhibiting MAPKs, oxidative stress and apoptosis. Pulm Pharmacol Ther 2020, 62, 101918. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Yang, Q.; Lu, Y.; Wang, G.; Yang, H.; Dong, J.; Zhang, H. Malvidin alleviates mitochondrial dysfunction and ROS accumulation through activating AMPK-α/UCP2 axis, thereby resisting inflammation and apoptosis in SAE mice. Front Pharmacol 2022, 13, 1038802. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; An, Z.; Ni, J. Active Components and Pharmacological Effects of Cornus officinalis: Literature Review. Front Pharmacol 2021, 12, 633447. [Google Scholar] [CrossRef] [PubMed]
| References | Species, strain, sex |
Model | Treatment and drug dose | Mode of administration and duration | Simplified treatment outcomes |
|---|---|---|---|---|---|
| Terrando 2010[62] | Mouse, WT C57BL/6 and IL-1R-/-, ♂ | LPS | IL-1 receptor antagonist (IL-1Ra), 100 mg/kg | Subcutaneous, immediately before LPS administration | Reduced plasma cytokines and hippocampal microgliosis and ameliorated cognitive dysfunction |
| Li 2017[63] | Mouse, C57 BL/6, ♂ | CLP | Ginsenoside Rg1, 40 and 200 mg/kg | I.p., 1 h before the CLP operation | Improved the survival rate; suppressed IBA1 activation and learning and memory impairments |
| Hoshino 2017[57] | Mouse, NA | CLP | Minocycline, 60 mg/kg | I.p., 3 consecutive days | Prevented impaired long-term potentiation in the hippocampus |
| Tian 2019 [64] | Mouse, C57 BL/6, ♂ | LPS | Attractylon, 25 mg/kg | I.p., with LPS injection | Attenuated LPS-induced cognitive impairment, neural apoptosis, inflammatory factors, and microglial activation |
| Xu 2019[65] | Mouse, BALB/c, ♂ | CLP | Caspase-1 inhibitor VX765, 0.2 mg per mouse | Intragastric administration, twice daily (10 a.m. and 4 p.m.) until mice were sacrificed | Reversed cognitive dysfunction and depressive-like behaviors; reduced microglia activation and BBB disruption and ultrastructure damages in the brain |
| Michels 2019[9] | Rat, Wistar, ♂ | CLP | Minocycline, 100 ug/kg | I.c.v, immediately after CLP operation | Induced a down-regulation of M1 markers |
| Wang 2020[58] | Mouse, C57 BL/6, ♂ | CLP | β-hydroxybutyrate, 250 mg/kg | Subcutaneous administration/i.c.v., every 6 hours from the fourth day to the seventh day after CLP/twice daily for 7 days | Increased survival and body weight recovery of sepsis mice and improved learning and memory; limited neuroinflammation and neuroplasticity damage |
| Heimfarth 2020[66] | Mouse, albino Swiss, ♂/♀ | LPS | Indole-3-guanylhydrazone hydrochloride, 50 mg/kg | I.p., after LPS administration and for 5 consecutive days | Attenuated inflammatory reactions through the MAPK and NFκB signaling pathways, and microglia activation suppression reduces anxiety-like behavior and cognitive impairment |
| Xie 2020[67] | Mouse, WT and Nrf2 KO, ♂ | CLP | MCC950/ Hydrogen-rich saline solution, 50 mg/kg/ 5 mL/kg | I.p., before operation/1 h and 6 h after CLP | Alleviated inflammation, neuronal apoptosis, and mitochondrial dysfunction via inhibiting Nrf2-mediated NLRP3 pathway. |
| Rocha 2021[68] | Rat, Wistar, ♂ | CLP | Anti-S100B monoclonal antibody, 10 μg/kg | I.c.v, 15 days after CLP | Increased the time of grooming; alleviated microglia activation |
| Bonfante 2021[69] | Rat, Wistar, ♂ | CLP | Stanniocalcin-1; 20/50/100 ng/kg | I.c.v, immediately after the CLP procedure | Improved hippocampal mitochondrial function and creatine kinase activity, reduced oxidative stress, neuroinflammation, and long-term memory impairment. |
| Wang 2022[36] | Mouse, C57 BL/6, ♂ | CLP | Qiang Xin 1, 0.5 /1/2 g/kg | Oral, 2 h after CLP | Attenuated cognitive deficits, emotional dysfunction, and reduces neuroinflammatory responses to improve survival. |
| Wen 2022[70] | Mouse, C57 BL/6 J, ♂ | CLP | Cortistatin-14, 200 ug/kg | I.p., 30 min after CLP | Relieved anxiety-related behaviors and the levels of various inflammatory cytokines; reduced BBB disruption and microglial activation |
| Zhong 2022[71] | Mouse, C57 BL/6, ♂ | LPS | JQ-1, 50 mg/kg | I.p., 1 h before LPS | Protected the hippocampal BBB and neuronal damage and microglia activation through the attenuation of neuroinflammation |
| Song 2022[72] | Mouse, C57 BL/6, ♂ | LPS | Metformin, 25 mg/kg | I.p., 1 h after LPS | Blocked microglial proliferation and production of inflammatory factors |
| Zhong 2022[73] | Mouse, C57 BL/J, ♂ | CLP | SS-31, 5 mg/kg | I.p., once daily for 1 week | Improved the survival rate and cognitive and memory dysfunctions in CLP mice |
| Yang 2022[35] | Mouse, C57 BL/6, ♂ | CLP | CB2R agonist HU308, 2.5 mg/kg | I.p., three consecutive days after CLP | Inhibited microglia activity and neuronal pyroptosis |
| Ding 2022[74] | Rat, NA, ♂ | CLP | Fisetin, 20 mg/kg | Intragastrical administration, once a day for three consecutive days before CLP | Blocked NLRP3 inflammasome activation by promoting mitophagy and ameliorating cognitive impairment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





