Submitted:
21 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
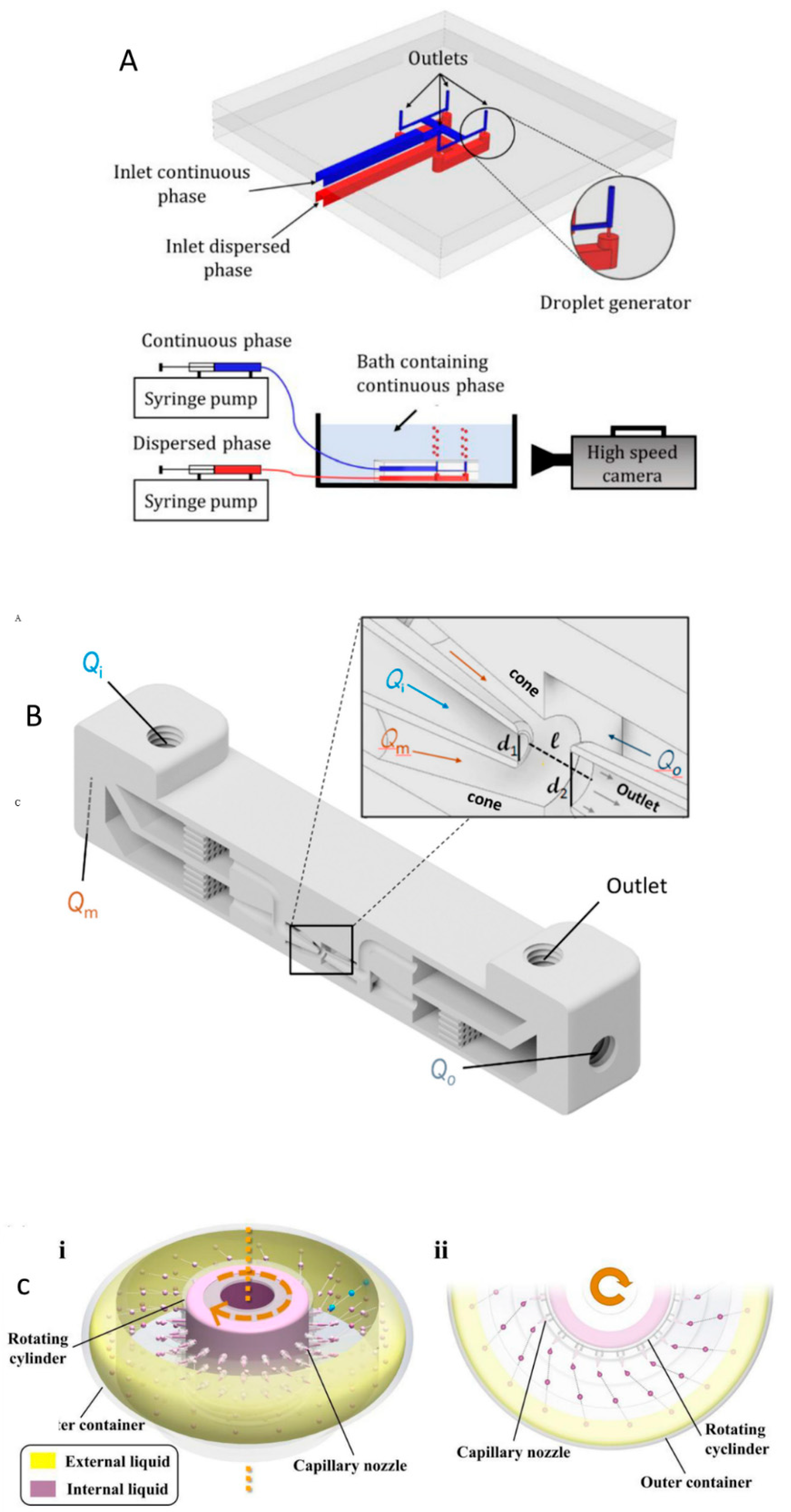
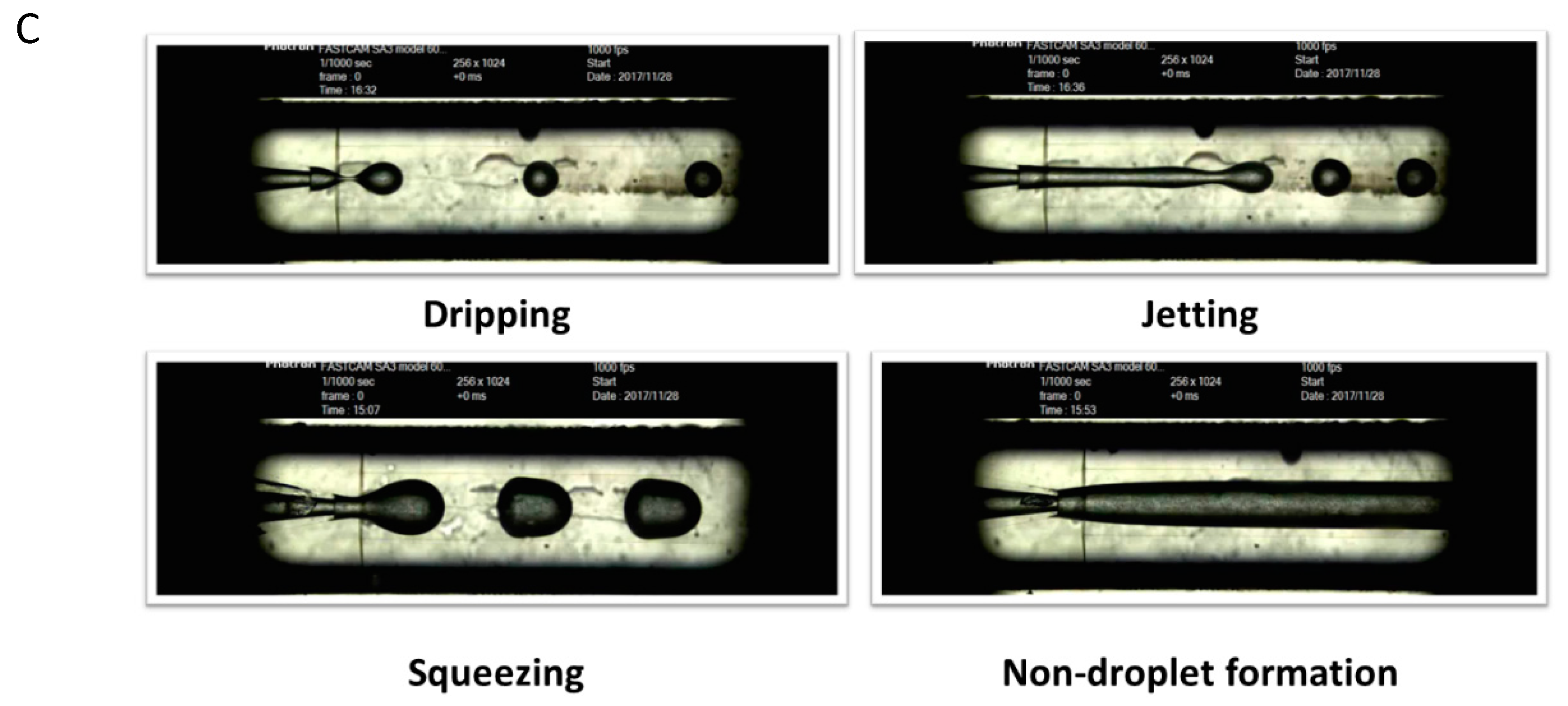
2. Principles of droplets-based microfluidics
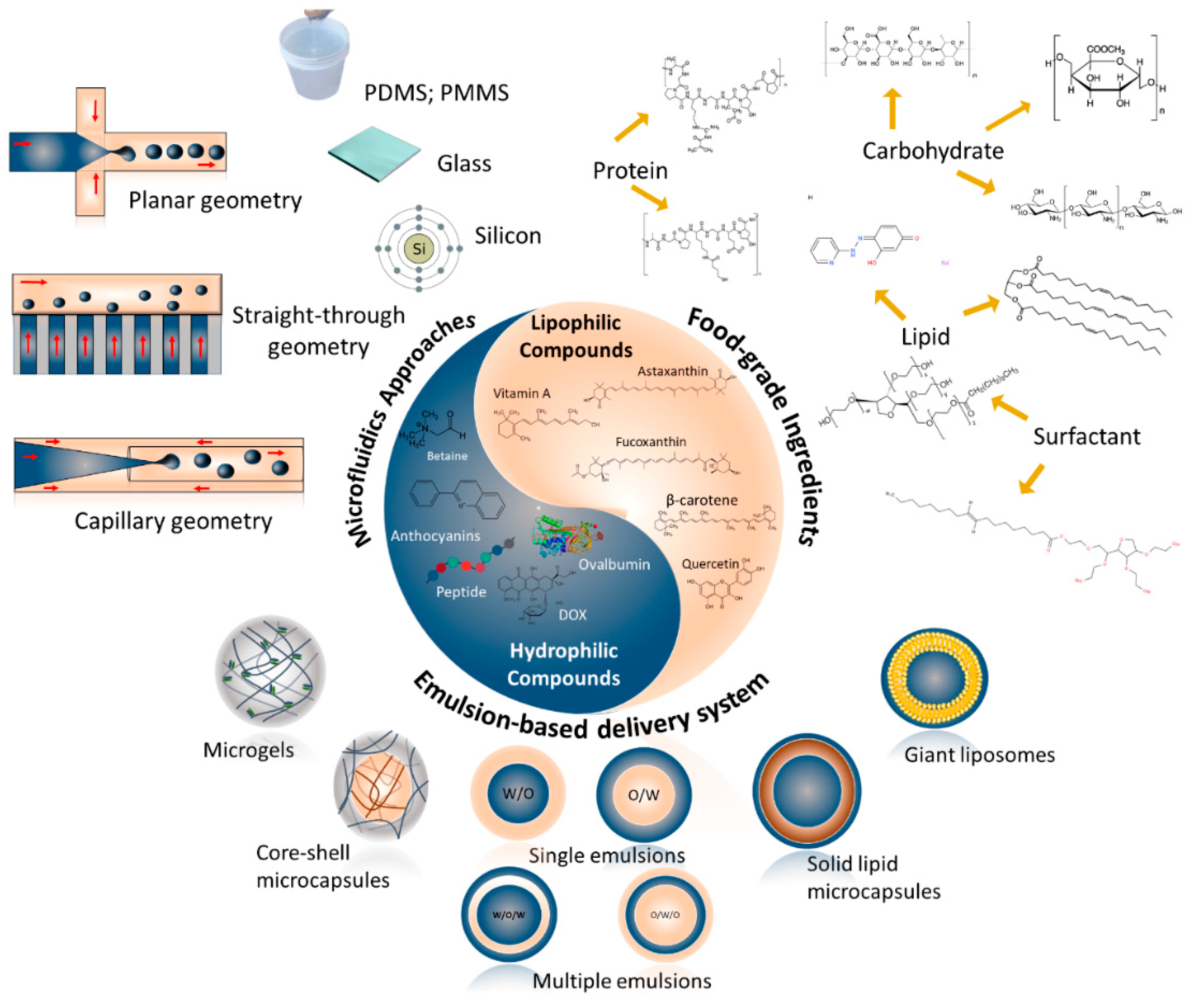
3. Microfluidic assembly of food-grade delivery systems based on entrapped compound type
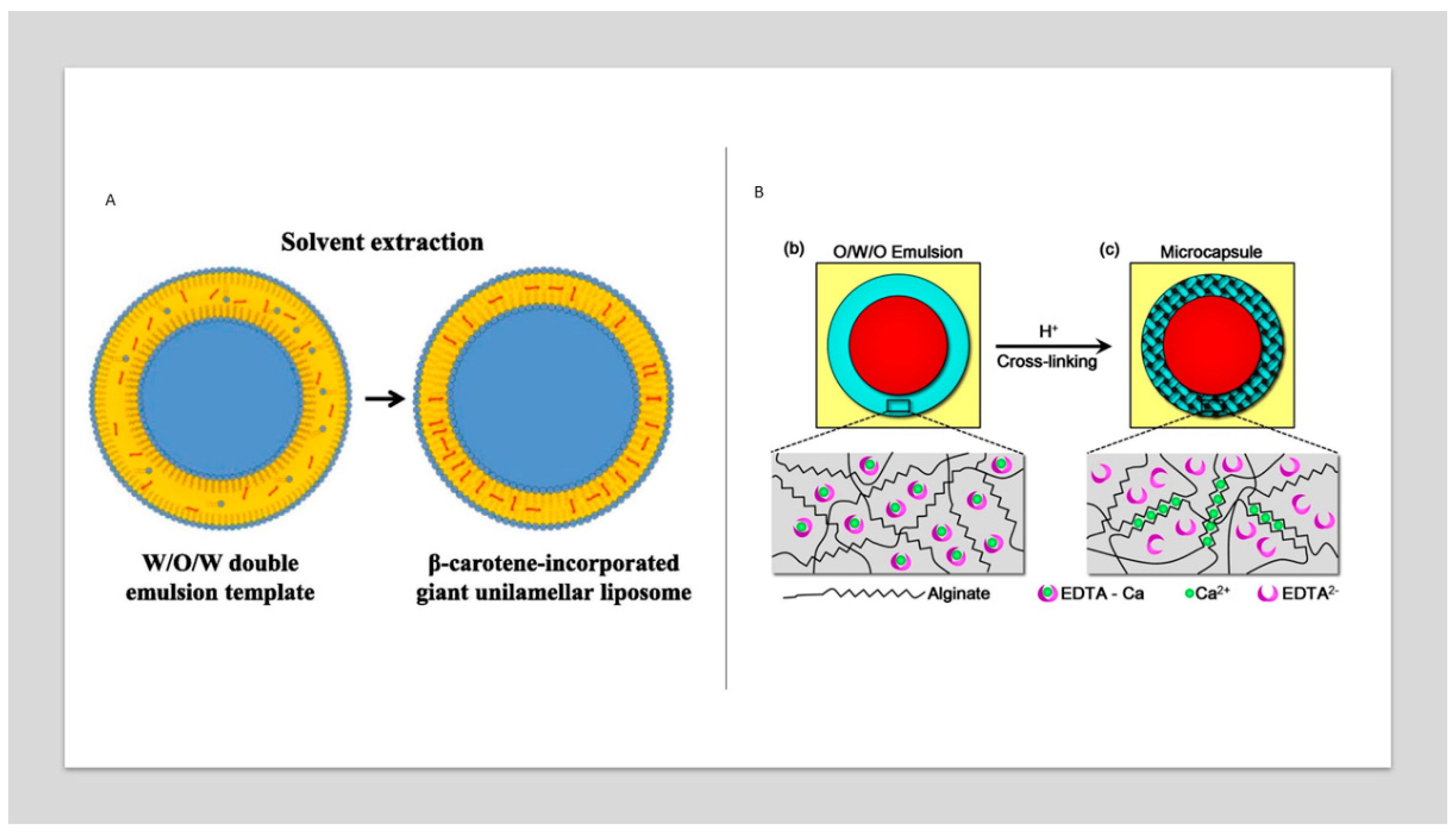
3.1. Lipophilic functional compounds
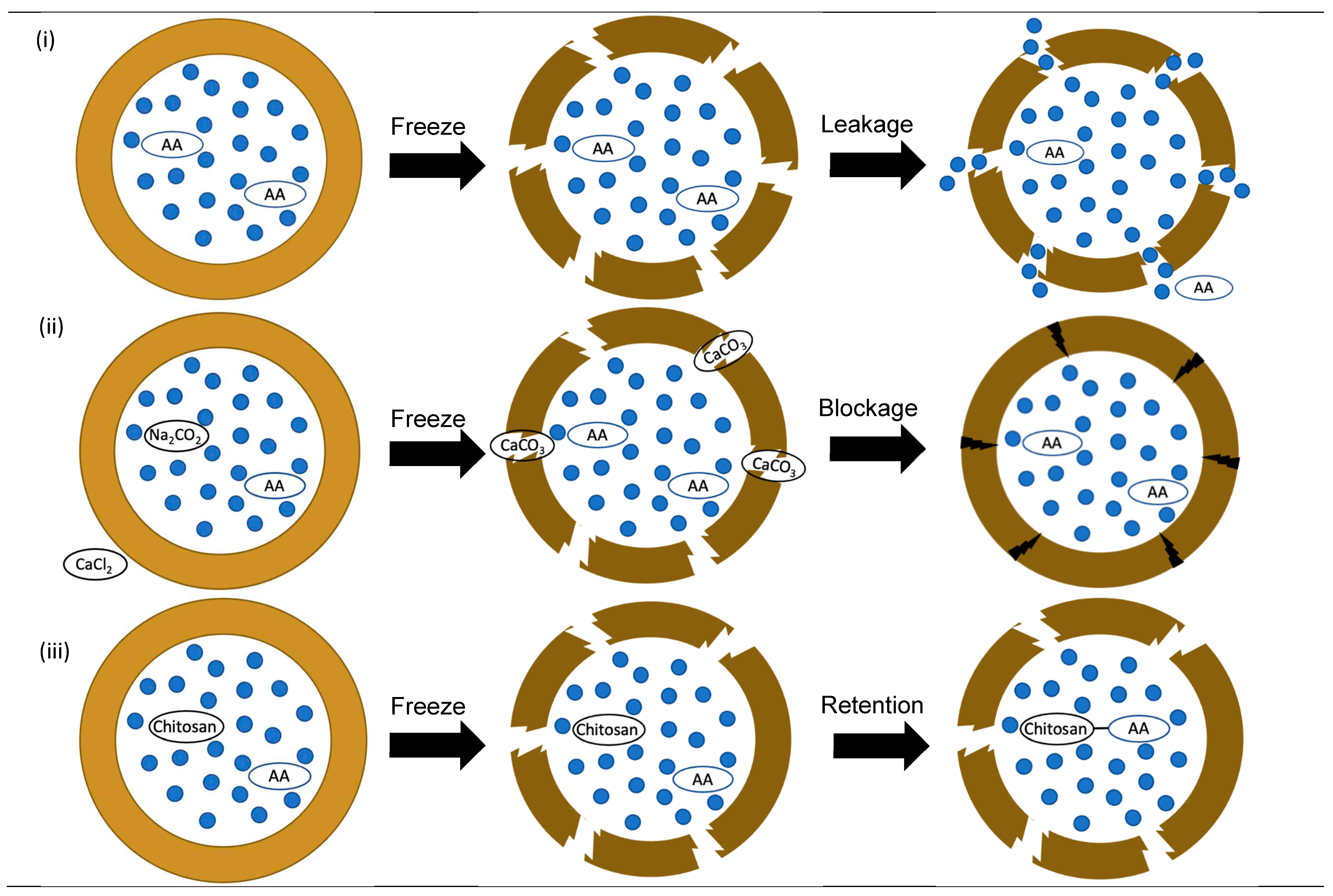
3.2. Hydrophilic functional compounds
4. Limitations and perspectives of scale-up in droplet-based microfluidic approaches
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nat. 2006 4427101 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet Microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Invent. 2018, Vol. 3, Page 60 2018, 3, 60. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic Diagnostic Technologies for Global Public Health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Tachikawa, K.; Manz, A. Micro Total Analysis Systems. Latest Advancements and Trends. Anal. Chem. 2006, 78, 3887–3907. [Google Scholar] [CrossRef] [PubMed]
- Vit, F.F.; Nunes, R.; Wu, Y.T.; Prado Soares, M.C.; Godoi, N.; Fujiwara, E.; Carvalho, H.F.; Gaziola de la Torre, L. A Modular, Reversible Sealing, and Reusable Microfluidic Device for Drug Screening. Anal. Chim. Acta 2021, 1185, 339068. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening-a-Chip; Organ-on-a-Chip; Human-on-a-Chip. 2018. [CrossRef]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet Based Microfluidics. Reports Prog. Phys. 2012, 75. [Google Scholar] [CrossRef]
- Yamamoto, F.; Cunha, R.L. Acid Gelation of Gellan: Effect of Final PH and Heat Treatment Conditions. Carbohydr. Polym. 2007, 68, 517–527. [Google Scholar] [CrossRef]
- Comunian, T.A.; Abbaspourrad, A.; Favaro-Trindade, C.S.; Weitz, D.A. Fabrication of Solid Lipid Microcapsules Containing Ascorbic Acid Using a Microfluidic Technique. Food Chem. 2014, 152, 271–275. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, Vol. 10, Page 812 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.R.; Gomes, A.; Ushikubo, F.Y.; Cunha, R.L. Gellan Microgels Produced in Planar Microfluidic Devices. J. Food Eng. 2017, 209, 18–25. [Google Scholar] [CrossRef]
- Santana, R.C.; Perrechil, F.A.; Cunha, R.L. High- and Low-Energy Emulsifications for Food Applications: A Focus on Process Parameters. Food Eng. Rev. 2013 52 2013, 5, 107–122. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Encapsulation of β-Sitosterol plus γ-Oryzanol in O/W Emulsions: Formulation Characteristics and Stability Evaluation with Microchannel Emulsification. Food Bioprod. Process. 2017, 102, 222–232. [Google Scholar] [CrossRef]
- Santos, T.P.; Michelon, M.; Carvalho, M.S.; Cunha, R.L. Formation and Stability of Oil-in-Water Emulsions Based on Components of Bioprocesses: A Microfluidic Analysis. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 126994. [Google Scholar] [CrossRef]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-Specific Drug Delivery: New Approaches and in Vitro/in Vivo Evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Forigua, A.; Kirsch, R.L.; Willerth, S.M.; Elvira, K.S. Recent Advances in the Design of Microfluidic Technologies for the Manufacture of Drug Releasing Particles. J. Control. Release 2021, 333, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.Y.; Kim, J.W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer Emulsions Using Microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Shao, C.; Chi, J.; Shang, L.; Fan, Q.; Ye, F. Droplet Microfluidics-Based Biomedical Microcarriers. Acta Biomater. 2022, 138, 21–33. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Velikov, K.P. Choosing the Right Delivery Systems for Functional Ingredients in Foods: An Industrial Perspective. Curr. Opin. Food Sci. 2018, 21, 15–25. [Google Scholar] [CrossRef]
- Hinman, S.S.; Wang, Y.; Kim, R.; Allbritton, N.L. In Vitro Generation of Self-Renewing Human Intestinal Epithelia over Planar and Shaped Collagen Hydrogels. Nat. Protoc. 2020 161 2020, 16, 352–382. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Wu, H.; Neish, A.S.; Champion, J.A. Alginate/Chitosan Microparticles for Gastric Passage and Intestinal Release of Therapeutic Protein Nanoparticles. J. Control. Release 2019, 295, 174–186. [Google Scholar] [CrossRef]
- Santos, T.P.; Costa, A.L.R.; Michelon, M.; Costa, L.P.; Cunha, R.L. Development of a Microfluidic Route for the Formation of Gellan-Based Microgels Incorporating Jabuticaba (Myrciaria Cauliflora) Extract. J. Food Eng. 2020, 276, 109884. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, Y. Microfluidic Assembly of Food-Grade Delivery Systems: Toward Functional Delivery Structure Design. Trends Food Sci. Technol. 2019, 86, 465–478. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, J.; Bak, D.H.; Lee, B.C.; Lee, E.; Choi, M.J.; Ryu, C.H.; Lee, S.; Mun, S.K.; Park, B.C.; et al. Development of Finasteride Polymer Microspheres for Systemic Application in Androgenic Alopecia. Int. J. Mol. Med. 2019, 43, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ryu, C.H.; Chon, C.H.; Kim, S.; Lee, S.; Maharjan, R.; Kim, N.A.; Jeong, S.H. Three Months Extended-Release Microspheres Prepared by Multi-Microchannel Microfluidics in Beagle Dog Models. Int. J. Pharm. 2021, 608, 121039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ren, Y.; Jiang, T.; Jiang, H. Thermal Field-Actuated Multifunctional Double-Emulsion Droplet Carriers: On-Demand Migration, Core Release and Released Particle Focusing. Chem. Eng. J. 2022, 431, 134200. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and Active Droplet Generation with Microfluidics: A Review. Lab Chip 2016, 17, 34–75. [Google Scholar] [CrossRef]
- Shi, M.; Shi, Y.L.; Li, X.M.; Yang, R.; Cai, Z.Y.; Li, Q.S.; Ma, S.C.; Ye, J.H.; Lu, J.L.; Liang, Y.R.; et al. Food-Grade Encapsulation Systems for (−)-Epigallocatechin Gallate. Mol. 2018, Vol. 23, Page 445 2018, 23, 445. [Google Scholar] [CrossRef]
- Samal, J.; Segura, T. Injectable Biomaterial Shuttles for Cell Therapy in Stroke. Brain Res. Bull. 2021, 176, 25–42. [Google Scholar] [CrossRef]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of Microfluidic Droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Controlled Microfluidic Interfaces. Nat. 2005 4377059 2004, 437, 648–655. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Cunha, R.L. Studies of Droplets Formation Regime and Actual Flow Rate of Liquid-Liquid Flows in Flow-Focusing Microfluidic Devices. Exp. Therm. Fluid Sci. 2017, 85, 167–175. [Google Scholar] [CrossRef]
- Kobayashi, I.; Nakajima, M.; Chun, K.; Kikuchi, Y.; Fujita, H. Silicon Array of Elongated Through-Holes for Monodisperse Emulsion Droplets. AIChE J. 2002, 48, 1639–1644. [Google Scholar] [CrossRef]
- Sugiura, S.; Nakajima, M.; Iwamoto, S.; Seki, M. Interfacial Tension Driven Monodispersed Droplet Formation from Microfabricated Channel Array. Langmuir 2001, 17, 5562–5566. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Birribilli, F.S.; Oliveira, D.R.B.; Cunha, R.L. Y- and T-Junction Microfluidic Devices: Effect of Fluids and Interface Properties and Operating Conditions. Microfluid. Nanofluidics 2014, 17, 711–720. [Google Scholar] [CrossRef]
- Shui, L.; Van Den Berg, A.; Eijkel, J.C.T. Interfacial Tension Controlled W/O and O/W 2-Phase Flows in Microchannel. Lab Chip 2009, 9, 795–801. [Google Scholar] [CrossRef]
- Khalid, N.; Shu, G.; Kobayashi, I.; Nakajima, M.; Barrow, C.J. Formulation and Characterization of Monodisperse O/W Emulsions Encapsulating Astaxanthin Extracts Using Microchannel Emulsification: Insights of Formulation and Stability Evaluation. Colloids Surfaces B Biointerfaces 2017, 157, 355–365. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, Y.; Khalid, N.; Shu, G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Comparative Study of Oil-in-Water Emulsions Encapsulating Fucoxanthin Formulated by Microchannel Emulsification and High-Pressure Homogenization. Food Hydrocoll. 2020, 108, 105977. [Google Scholar] [CrossRef]
- Pagano, A.P.E.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A.; Bastos, E.L. Microencapsulation of Betanin in Monodisperse W/O/W Emulsions. Food Res. Int. 2018, 109, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Microchannel Emulsification Study on Formulation and Stability Characterization of Monodisperse Oil-in-Water Emulsions Encapsulating Quercetin. Food Chem. 2016, 212, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dias Meirelles, A.A.; Rodrigues Costa, A.L.; Michelon, M.; Viganó, J.; Carvalho, M.S.; Cunha, R.L. Microfluidic Approach to Produce Emulsion-Filled Alginate Microgels. J. Food Eng. 2022, 315, 110812. [Google Scholar] [CrossRef]
- Duncanson, W.J.; Lin, T.; Abate, A.R.; Seiffert, S.; Shah, R.K.; Weitz, D.A. Microfluidic Synthesis of Advanced Microparticles for Encapsulation and Controlled Release. Lab Chip 2012, 12, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of Microfluidic Systems in Poly(Dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(Dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Abate, A.R.; Poitzsch, A.; Hwang, Y.; Lee, J.; Czerwinska, J.; Weitz, D.A. Impact of Inlet Channel Geometry on Microfluidic Drop Formation. Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys. 2009, 80, 026310. [Google Scholar] [CrossRef]
- Roberts, C.C.; Rao, R.R.; Loewenberg, M.; Brooks, C.F.; Galambos, P.; Grillet, A.M.; Nemer, M.B. Comparison of Monodisperse Droplet Generation in Flow-Focusing Devices with Hydrophilic and Hydrophobic Surfaces. Lab Chip 2012, 12, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Vladisavljević, G.T.; Kobayashi, I.; Nakajima, M. Production of Uniform Droplets Using Membrane, Microchannel and Microfluidic Emulsification Devices. Microfluid. Nanofluidics 2012 131 2012, 13, 151–178. [Google Scholar] [CrossRef]
- Zhao, C.X. Multiphase Flow Microfluidics for the Production of Single or Multiple Emulsions for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1420–1446. [Google Scholar] [CrossRef]
- Tarchichi, N.; Chollet, F.; Manceau, J.F. New Regime of Droplet Generation in a T-Shape Microfluidic Junction. Microfluid. Nanofluidics 2013, 14, 45–51. [Google Scholar] [CrossRef]
- Wu, P.; Luo, Z.; Liu, Z.; Li, Z.; Chen, C.; Feng, L.; He, L. Drag-Induced Breakup Mechanism for Droplet Generation in Dripping within Flow Focusing Microfluidics. Chinese J. Chem. Eng. 2015, 23, 7–14. [Google Scholar] [CrossRef]
- Collins, D.J.; Neild, A.; deMello, A.; Liu, A.Q.; Ai, Y. The Poisson Distribution and beyond: Methods for Microfluidic Droplet Production and Single Cell Encapsulation. Lab Chip 2015, 15, 3439–3459. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.R.; Gomes, A.; Andrade, C.C.P. de; Cunha, R.L. Emulsifier Functionality and Process Engineering: Progress and Challenges. Food Hydrocoll. 2017, 68, 69–80. [Google Scholar] [CrossRef]
- Fu, T.; Wu, Y.; Ma, Y.; Li, H.Z. Droplet Formation and Breakup Dynamics in Microfluidic Flow-Focusing Devices: From Dripping to Jetting. Chem. Eng. Sci. 2012, 84, 207–217. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic Methods for Generating Continuous Droplet Streams. J. Phys. D. Appl. Phys. 2007, 40, R319. [Google Scholar] [CrossRef]
- Garti, N.; McClements, D.J. Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals. 2012, 612.
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Inhibition of β-Carotene Degradation in Oil-in-Water Nanoemulsions: Influence of Oil-Soluble and Water-Soluble Antioxidants. Food Chem. 2012, 135, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; de Carvalho, R.A.; Trindade, C.S.F.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of Active Gelatin Films Incorporated with Rutin-Loaded Nanoemulsions. Int. J. Biol. Macromol. 2017, 98, 39–49. [Google Scholar] [CrossRef]
- Murillo, A.G.; Aguilar, D.; Norris, G.H.; DiMarco, D.M.; Missimer, A.; Hu, S.; Smyth, J.A.; Gannon, S.; Blesso, C.N.; Luo, Y.; et al. Compared with Powdered Lutein, a Lutein Nanoemulsion Increases Plasma and Liver Lutein, Protects against Hepatic Steatosis, and Affects Lipoprotein Metabolism in Guinea Pigs. J. Nutr. 2016, 146, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and Stabilization of Nanoemulsion-Based Vitamin E Delivery Systems Using Natural Biopolymers: Whey Protein Isolate and Gum Arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Furtado, G. de F.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Modulating in Vitro Digestibility of Pickering Emulsions Stabilized by Food-Grade Polysaccharides Particles. Carbohydr. Polym. 2020, 227, 115344. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Cardoso, D.D.; Náthia-Neves, G.; Meireles, M.A.A.; Cunha, R.L. Interactions of β-Carotene with WPI/Tween 80 Mixture and Oil Phase: Effect on the Behavior of O/W Emulsions during in Vitro Digestion. Food Chem. 2021, 341, 128155. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-Coalescence of Emulsion Droplets during High-Energy Emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Leser, M.E.; Sagalowicz, L.; Michel, M.; Watzke, H.J. Self-Assembly of Polar Food Lipids. Adv. Colloid Interface Sci. 2006, 123–126, 125–136. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Cunha, R.L. Impact of Oil Type and WPI/Tween 80 Ratio at the Oil-Water Interface: Adsorption, Interfacial Rheology and Emulsion Features. Colloids Surfaces B Biointerfaces 2018, 164, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Furtado, G.D.F.; Cunha, R.L. Bioaccessibility of Lipophilic Compounds Vehiculated in Emulsions: Choice of Lipids and Emulsifiers. J. Agric. Food Chem. 2019, 67, 13–18. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Ma, J.; Ling, S. Da; Wang, Y.D.; Kong, T.T.; Xu, J.H. Fabrication of Magnetic Core/Shell Hydrogels via Microfluidics for Controlled Drug Delivery. Chem. Eng. Sci. 2022, 248, 117216. [Google Scholar] [CrossRef]
- Mou, C.L.; Deng, Q.Z.; Hu, J.X.; Wang, L.Y.; Deng, H.B.; Xiao, G.; Zhan, Y. Controllable Preparation of Monodisperse Alginate Microcapsules with Oil Cores. J. Colloid Interface Sci. 2020, 569, 307–319. [Google Scholar] [CrossRef]
- Sek, L.; Porter, C.J.H.; Kaukonen, A.M.; Charman, W.N. Evaluation of the In-Vitro Digestion Profiles of Long and Medium Chain Glycerides and the Phase Behaviour of Their Lipolytic Products. J. Pharm. Pharmacol. 2010, 54, 29–41. [Google Scholar] [CrossRef]
- Santos, T.P.; Cunha, R.L. In Vitro Digestibility of Gellan Gels Loaded with Jabuticaba Extract: Effect of Matrix-Bioactive Interaction. Food Res. Int. 2019, 125, 108638. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Berling, C.L.; Garcia, A.O.; Queiroz, M.B.; Alvim, I.D.; Hubinger, M.D. Release of Anthocyanins from the Hibiscus Extract Encapsulated by Ionic Gelation and Application of Microparticles in Jelly Candy. Food Res. Int. 2019, 121, 542–552. [Google Scholar] [CrossRef]
- Soukoulis, C.; Tsevdou, M.; Andre, C.M.; Cambier, S.; Yonekura, L.; Taoukis, P.S.; Hoffmann, L. Modulation of Chemical Stability and in Vitro Bioaccessibility of Beta-Carotene Loaded in Kappa-Carrageenan Oil-in-Gel Emulsions. Food Chem. 2017, 220, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, W.; Xu, F.; Lu, W.; Hu, L.; Zhou, J.; Zhang, C.; Jiang, Z. Microfluidic Droplet Formation in Co-Flow Devices Fabricated by Micro 3D Printing. J. Food Eng. 2021, 290, 110212. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Mercadé-Prieto, R.; York, D.; Preece, J.A.; Zhang, Z. Structure and Mechanical Properties of Consumer-Friendly PMMA Microcapsules. Ind. Eng. Chem. Res. 2013, 52, 11253–11265. [Google Scholar] [CrossRef]
- Vu, H.T.H.; Streck, S.; Hook, S.M.; McDowell, A. Utilization of Microfluidics for the Preparation of Polymeric Nanoparticles for the Antioxidant Rutin: A Comparison with Bulk Production. Pharm. Nanotechnol. 2019, 7, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Keshavarz Moraveji, M. Microfluidic Assisted Synthesis of PLGA Drug Delivery Systems. RSC Adv. 2019, 9, 2055–2072. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wang, H.; Yan, L.; Zhang, X.; Cheng, Y. Continuous Preparation of Itraconazole Nanoparticles Using Droplet-Based Microreactor. Chem. Eng. J. 2020, 393, 124721. [Google Scholar] [CrossRef]
- Lorenz, T.; Bojko, S.; Bunjes, H.; Dietzel, A. An Inert 3D Emulsification Device for Individual Precipitation and Concentration of Amorphous Drug Nanoparticles. Lab Chip 2018, 18, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Liu, F. Paclitaxel Nanocrystals for Overcoming Multidrug Resistance in Cancer. Mol. Pharm. 2010, 7, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; Desimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010 98 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.S.; Longmuir, K.J.; Yager, P. A Practical Guide to the Staggered Herringbone Mixer. Lab Chip 2008, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Michelon, M.; Huang, Y.; de la Torre, L.G.; Weitz, D.A.; Cunha, R.L. Single-Step Microfluidic Production of W/O/W Double Emulsions as Templates for β-Carotene-Loaded Giant Liposomes Formation. Chem. Eng. J. 2019, 366, 27–32. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Santana, A.S.; Monterrey-Quintero, E.S.; Trindade, M.A.; Netto, F.M. The Use of Spray Drying Technology to Reduce Bitter Taste of Casein Hydrolysate. Food Hydrocoll. 2010, 24, 336–340. [Google Scholar] [CrossRef]
- Kuang, S.S.; Oliveira, J.C.; Crean, A.M. Microencapsulation as a Tool for Incorporating Bioactive Ingredients into Food. https://doi.org/10.1080/10408390903044222 2010, 50, 951–968. [CrossRef]
- Yu, W.; Liu, X.; Zhao, Y.; Chen, Y. Droplet Generation Hydrodynamics in the Microfluidic Cross-Junction with Different Junction Angles. Chem. Eng. Sci. 2019, 203, 259–284. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, P.; Han, X.; Shi, R.; Tian, Y.; Wang, L. Microfluidic Generation of ATPS Droplets by Transient Double Emulsion Technique. Lab Chip 2021, 21, 2684–2690. [Google Scholar] [CrossRef]
- Tumarkin, E.; Kumacheva, E. Microfluidic Generation of Microgels from Synthetic and Natural Polymers. Chem. Soc. Rev. 2009, 38, 2161–2168. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B.; Nayak, A.K.; Hasnain, M.S. Gellan Gum in Drug Delivery Applications. Nat. Polysaccharides Drug Deliv. Biomed. Appl. 2019, 145–186. [Google Scholar] [CrossRef]
- Ogończyk, D.; Siek, M.; Garstecki, P. Microfluidic Formulation of Pectin Microbeads for Encapsulation and Controlled Release of Nanoparticles. Biomicrofluidics 2011, 5, 013405. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of Bioinspired Collagen/Alginate/Fibrin Based Hydrogels for Soft Tissue Engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.X. Microfluidic Formation of Core-Shell Alginate Microparticles for Protein Encapsulation and Controlled Release. J. Colloid Interface Sci. 2019, 539, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.H.; Chu, S.; Champion, J.A. Protein Nanoparticles for Intracellular Delivery of Therapeutic Enzymes. J. Pharm. Sci. 2014, 103, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Herrera Estrada, L.; Wu, H.; Ling, K.; Zhang, G.; Sumagin, R.; Parkos, C.A.; Jones, R.M.; Champion, J.A.; Neish, A.S. Bioengineering Bacterially Derived Immunomodulants: A Therapeutic Approach to Inflammatory Bowel Disease. ACS Nano 2017, 11, 9650–9662. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kim, J.; Kim, J.S.; Chung, Y.S.; Chang, S.T.; Park, J. Microencapsulation by Pectin for Multi-Components Carriers Bearing Both Hydrophobic and Hydrophilic Active Agents. Carbohydr. Polym. 2018, 182, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, F.; Zhu, C.; Xu, J.; Hua, X.; Xi, Z.; Shen, L.; Zhao, S.; Cen, L. Controllable Preparation of SB-3CT Loaded PLGA Microcapsules for Traumatic-Brain-Injury Pharmaco-Therapy. Chem. Eng. J. 2018, 339, 346–358. [Google Scholar] [CrossRef]
- Dingler, A.; Gohla, S. Production of Solid Lipid Nanoparticles (SLN): Scaling up Feasibilities. https://doi.org/10.1080/02652040010018056 2008, 19, 11–16. [CrossRef]
- Li, Y.; Yan, D.; Fu, F.; Liu, Y.; Zhang, B.; Wang, J.; Shang, L.; Gu, Z.; Zhao, Y. Composite Core-Shell Microparticles from Microfluidics for Synergistic Drug Delivery. Sci. China Mater. 2017 606 2017, 60, 543–553. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Mulligan, M.K.; Rothstein, J.P. Scale-up and Control of Droplet Production in Coupled Microfluidic Flow-Focusing Geometries. Microfluid. Nanofluidics 2012 131 2012, 13, 65–73. [Google Scholar] [CrossRef]
- Dolomite.
- Microfluidic ChipShop.
- Liu, Z.; Duan, C.; Jiang, S.; Zhu, C.; Ma, Y.; Fu, T. Microfluidic Step Emulsification Techniques Based on Spontaneous Transformation Mechanism: A Review. J. Ind. Eng. Chem. 2020, 92, 18–40. [Google Scholar] [CrossRef]
- Jans, A.; Lölsberg, J.; Omidinia-Anarkoli, A.; Viermann, R.; Möller, M.; De Laporte, L.; Wessling, M.; Kuehne, A.J.C. High-Throughput Production of Micrometer Sized Double Emulsions and Microgel Capsules in Parallelized 3D Printed Microfluidic Devices. Polym. 2019, Vol. 11, Page 1887 2019, 11, 1887. [Google Scholar] [CrossRef]
- de Rutte, J.M.; Koh, J.; Di Carlo, D. Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv. Funct. Mater. 2019, 29, 1900071. [Google Scholar] [CrossRef]
- Lee, S.; De Rutte, J.; Dimatteo, R.; Koo, D.; Di Carlo, D. Scalable Fabrication and Use of 3D Structured Microparticles Spatially Functionalized with Biomolecules. ACS Nano 2022, 16, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Hajji, I.; Serra, M.; Geremie, L.; Ferrante, I.; Renault, R.; Viovy, J.L.; Descroix, S.; Ferraro, D. Droplet Microfluidic Platform for Fast and Continuous-Flow RT-QPCR Analysis Devoted to Cancer Diagnosis Application. Sensors Actuators B Chem. 2020, 303, 127171. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Cai, L.; Shang, L.; Zhao, Y. High-Throughput Generation of Microgels in Centrifugal Multi-Channel Rotating System. Chem. Eng. J. 2022, 427, 130750. [Google Scholar] [CrossRef]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of Bioactive Salmon Protein Hydrolysates with Chitosan-Coated Liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Gelin, P.; Bihi, I.; Ziemecka, I.; Thienpont, B.; Christiaens, J.; Hellemans, K.; Maes, D.; De Malsche, W. Microfluidic Device for High-Throughput Production of Monodisperse Droplets. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
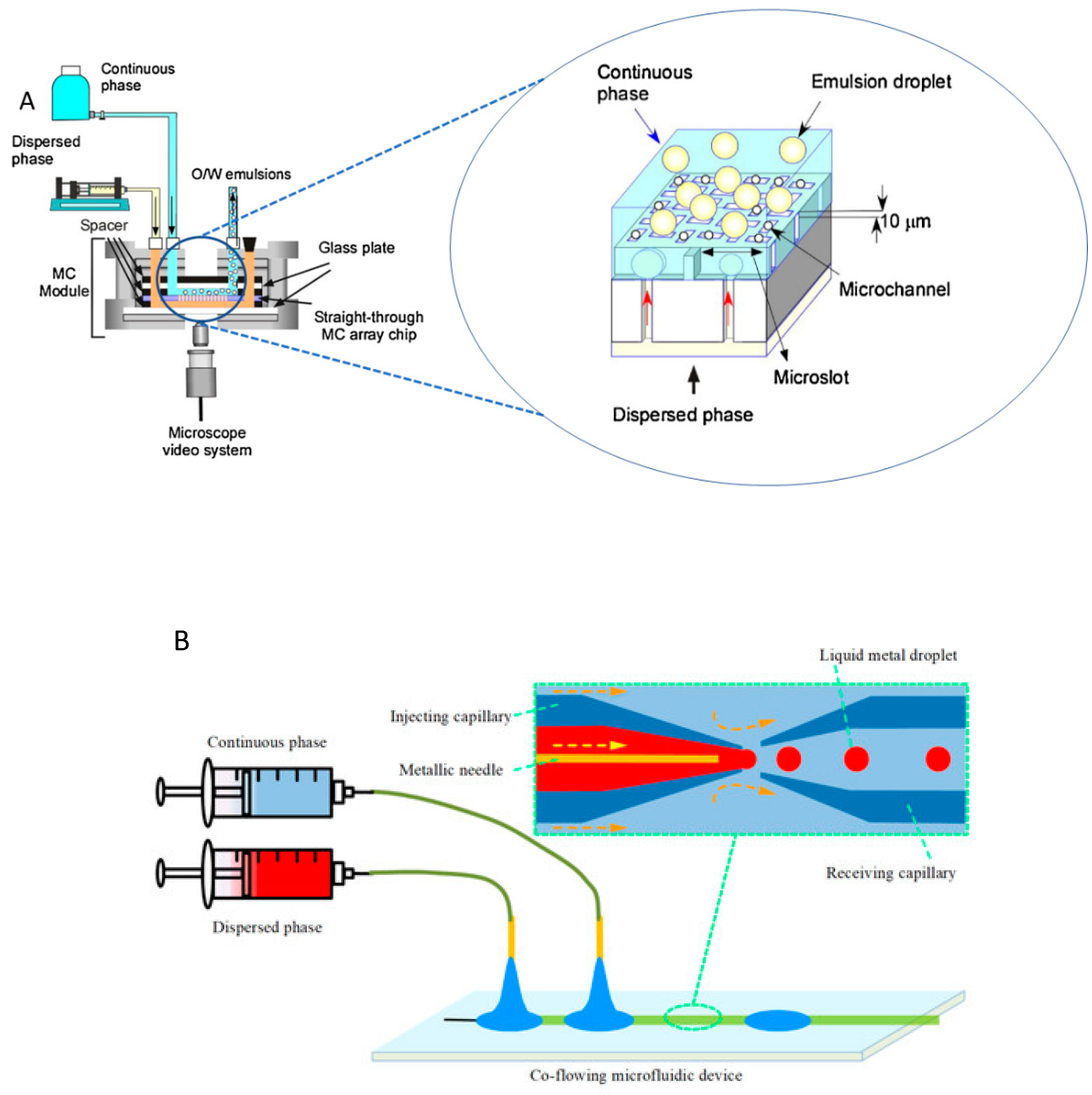
| geometry | Advantages | Limitations |
| Planar | - Can be manufactured in different geometries (T- and Y- junction) - Easy to modulate and can be made with different materials (e.g., PDMS, PMMA, and other polymers) - Allow manufacturing more complex configurations chips |
- Complex structures for industrial production - For multiple emulsions is necessary complex structure - Some based materials are not compatible with organic solvent - Rather sample microchannels structure |
| Capillary | - Good for multiple emulsions in one step. - Commonly manufactured of glass which has high transparency, high chemical resistance, inertness to most substances, biocompatibility |
- Complex structures for industrial production - Difficult reproducibility and manufacture - Low reproducibility - High cost - High time-consuming for fabricated |
| Straight-through | - droplet generation independent of flow rate. - good monodispersity - low energy consumption - easy amplification of the device - non-dead volume - reduced cost of chip design, processing, and operation. |
- Specific configuration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





