1. Introduction
Food allergy is defined as an abnormal, excessive reaction that occurs every time a subject is exposed to a specific food at a dose tolerated by healthy individuals [
1]. Unlike food intolerance, food allergy is an IgE-mediated or non-IgE-mediated adverse immune response. Although immediate hypersensitivity plays the most important role in the pathogenesis of food allergy, cytotoxic reactions and immune complex-type reactions may also be involved. The pathophysiology of food allergy results from complex interactions between the gastrointestinal mucosa, local and systemic immune responsivity, and the microbiome [
1,
2,
3]. The first case reports on food allergies date back to the turn of the 20th century. At present, the prevalence of food allergies in developed countries is estimated at 8% in children and ca. 5% in adults [
3,
4]. A worrying upward trend has been observed in the global prevalence of this pathology in recent years [
1].
The nasal provocation test belongs to the group of methods in which the course of the examination is evaluated based on subjective measures for reporting complaints (e.g. visual-analog scale), as well as on objective techniques for assessing nasal patency (e.g. optical rhinometry) [
5]. It is one of the few available allergological testing methods that reproduce the natural response of the mucous membrane to local application of an allergen in controlled conditions. The response may be either IgE-mediated or eosinophilic. Early and late phases are observed in allergic reactions, as resulting from a cascade of events regulated by the sympathetic and parasympathetic sensory systems as well as the receptors present within the nasal epithelium (histamine, leukotriene, prostaglandin receptors) [
5,
6,
7,
8,
9]. Following a series of complex reactions, the allergen is presented (by the allergen presenting cells, APCs) to Th2 lymphocytes; resulting in the release of IL-4 and IL-13, which in turn stimulate B lymphocytes to produce IgE and cytokines that mobilize eosinophilic cells for their specific migration within the submucosal layer as well as the mucosa. This leads to complex consequences, including the allergen being bound by sIgE and coating the mast cells within the effector tissue; contributing to the release of preformed mediators, including tryptase. Simultaneously, mast cells synthesize and release mediators; such as the platelet activation factor (PAF), LTC4 and LTD4 formed from arachidonic acid, PGD2, PGI2, as well as substance P. In the late phase, the stimulated cells release cytokines and chemokines into the blood, resulting in eosinophilic leukocytes and their precursor release from bone marrow. Inflammatory cell infiltrates are observed within nasal mucosa as the consequence of the inflow of eosinophils and proliferation of precursor cells [
6,
7,
8]. Due to the cognitive potential of nasal provocation tests, increased attention has focused on possible broadening of the indications for its implementation, including diagnostics for food allergies. Evidence is available for local response to food allergens within the nasal mucosa in nasal food allergen provocation tests [
6,
7,
8]. Furthermore, typical symptoms—such as those observed in the early allergic reaction phase—are observed in nasal food challenge (NFC) tests with inhalatory allergens. The aim of the study was to assess the risk of nasal mucosal hypersensitivity in the nasal challenge test using incremental doses of raw chicken egg white allergen, as a premise for developing and implementing NFC in the procedure of diagnosis of food allergies. The study is a research experiment and is one of the first of its kind to detail the NFC methodology, including the extraction of a substance (chicken egg) for intranasal application of an allergen. It provides, in a way, a foundation for further research in the area of standardization of NFC and expansion of its capabilities in the differential diagnosis of food allergy, as highlighted in EAACI’s consensus statement Position paper on the standardization of nasal allergen challenges. One of the indications in this document for performing nasal allergen challenge tests is further evidence diagnosing food allergy [
5].
2. Materials and Methods
2.1. Ethical Statements
The study was conducted in accordance with the guidelines in the Declaration of Helsinki. Approval of the Bioethics Committee of the Medical University of Warsaw (decision no. KB 63/2022) was obtained. All participants provided signed informed consent.
2.2. Study Participants
The study consisted of 25 healthy adults (18 women and 7 men, residents of a large urban agglomeration), not allergic to common environmental or food allergens, as described next (Table 1). The study group demonstrated significant variability in terms of height and weight, and the majority of study participants were noted to be residents of large urban agglomerations. The selection of the group was targeted and was mainly based on a negative clinical history of allergic diseases (including, but not limited to, chicken egg allergy) and screening tests in the area of skin prick test (a panel of 13 common Allergopharma environmental allergens was used: birch, alder, hazel, grasses / corn, rye, mugwort, plantain, Alternaria tenius, Cladosporium herbarum, Dermatophagoides pteronyssinus, Dermatophagoides farinae, dog, cat and food allergens: chicken egg, milk, soy, histamine, negative control).
Skin testing was performed in accordance with EAACI guidelines: standardized allergen extracts were applied to the inner side of the forearm, preserving adequate spacing, and punctured with a standardized lancet. Positive reaction (reaction ≤3mm) was assessed based on the control solution and histamine test. [
10,
11]. Moreover, the assessment of inflammation risk in the nasal mucosa was performed by means of physical examination, including endoscopy of the nasal cavity, as well as by measuring the acoustic rhinometry (MCA-1, MCA-2;
Minimal Cross Sectional Area for nasal vestibule and nasal concha), spirometry (FEV
1/FVC, FEV
1) and nitric oxide concentration in the air exhaled from the upper respiratory tract (Table 2). The examination was carried out at the Department of Allergology and Immunology of the University Clinical Center of the Medical University of Warsaw by qualified medical personnel: an allergologist and a nurse who both possessed the necessary knowledge, skills and equipment for providing first aid if any life-threatening complications occur. The study group was recruited by special announcements (patient information sheet) which are enclosed in the documentation submitted to the Bioethics Committee of the Medical University of Warsaw.
The exclusion criteria included bacterial infection within the nasal and sinus cavities, severe comorbidities (circulatory or respiratory), severe systemic diseases (malignant tumors, autoimmune diseases), systemic immunotherapy, and pregnancy [
5,
9]. The study was performed with reference to the NFC protocol (Table 2) in accordance with the current standard for nasal challenge tests. After local acclimatization to room conditions (temperature 21
0C, relative humidity 43%), the test was performed in accordance with the recommendations provided by the consensus on the standardization of provocation tests [
5]. The added value was the allergen extracted under laboratory conditions from a solid form (chicken egg) to a lyophilized form.
2.3. Data Collection
The NFC extract was prepared from two raw chicken eggs purchased from an organic chicken farm so as to ensure the absence of admixtures that could ultimately affect the test results. Whites obtained from the two eggs (total weight 50 g) were separated from the yolks and mixed with phosphate-buffered saline in a 1:4 (m/v) ratio. Aliquots of mixtures were then homogenized for at least 30 s; the homogenates were sequentially filtered through a polyethersulfone (PES) bottle top filter with a diameter of 90 mm and pore size of 0.45 µm (qpore Plastic Vacuum Filter, Bionovo, Poland) as well as cellulose acetate (CA) filters with the diameter of 50 mm and pore size of 0.22 µm (qpore Plastic Vacuum Filter, Bionovo, Poland) under reduced pressure (Ca-MI New Askir20, Frazione Pilastro, Italy). Following filtration, the protein was lyophilized (Christ LCG Lyo Chamber Guard, Alpha 2-4 LSC plus, Martin Christ Gefriertrocknungsanlagen GmbH, Germany) to achieve 6.6 g of freeze-dried raw chicken egg white powder. The powder was then used for preparation of doses to be used in the challenge test. Since the weight of the stock raw egg white was 50 g, it was calculated that 1 g of raw egg white translated to 135 mg of the lyophilizate; this amount was subsequently dissolved in 1 mL of PBS. The presence of five protein fractions of potential allergens was confirmed by capillary electrophoresis (Beckman Coulter P/ACE MDQ) (
Figure 2). Separation of the lyophilizate was carried out using capillary electrophoresis in a phosphate buffer environment at a concentration of 100 mM and pH=2.5. The detection was carried out at a wavelength of 200 nm and a separation voltage of 10 kV.
The burette method was used to determine the total protein content in 135 mg/mL of RCEWP. Thus, 135 mg of lyophilizate contained 88.4 mg of pure protein. First, the stock solution of RCEWP at the concentration of 1.52 mg/mL was prepared in PBS. The following doses of pure freeze-dried powdered chicken egg proteins were predefined within the NFC protocol: 200 µg/305.4 µg dry weight (d.w.) of RCEWP, 150/229.0, 100/152.7, 50/76.3, 25/38.2, and 12.5/19.1 µg/µg. The samples were dissolved in 200 µL of saline for intranasal application.
2.4. Study Design and Setting
As part of the NFC, the allergen was administered intranasally using a calibrated atomizer (maximum variation in the intranasally applied volume of ±10%, standard deviation of ±4%) in the amount of 0.02 mL bilaterally into each nostril. The assessments were made as per the standard protocol 15 minutes after local application using the OR technique and a 10-cm VAS scale to evaluate nasal symptoms; including itching, watery discharge, and nasal obstruction (for the total nasal symptoms: ≥55 mm extremely positive FNC, ≥23 mm moderately positive) [
4].
Optical rhinometry (transmission spectroscopy: Rhinolux; Rhios GmbH, Grosserkmannsdorf, Germany) is an objective examination technique for assessing the response of nasal mucosa. The principle of the technique is overall (bilateral) measurement of blood passing through the blood vessels within the nasal cavity (swelling of the nasal cavity is characterized by an increase in blood volume. This change in volume indicates an increase in light absorbed by the mucous membrane in OR) within a predefined period, using a beam of infrared radiation at a specified wavelength (600–800 µm). The device provides a continuous measurement of blood flow changes, and thus provides an objective assessment. A beam of infrared radiation is emitted from the transmitter placed on the nasal bridge in a manner resembling the placement of eyeglasses. The optical curve precisely determines the onset of the response (T1) and the peak response to the locally applied allergen (T2). The optical density (OD) parameter is important for the definition of strongly positive, moderately positive, or negative results of the test. In the case of a positive response to the applied allergen, an increase in the absorption of infrared radiation—and thus an upward trend of the optic curve—is observed. On the other hand, negative values are observed in tests involving e.g. use of xylometazoline; alternating negative and positive values are observed within a particular time frame in the nasal cycle. Positive results of allergen challenge tests were defined on the basis of the OD, as measured by OR increasing by 0.52 [
12,
13] and on the basis of VAS scores of at least 55 mm [
5,
9]. The total duration of the study was 120 minutes. The study was performed by qualified personnel in hospital conditions. The study was conducted and funded under the National Science Center Miniature-5 grant (2021/05/X/NZ5/01/099).
2.5. Statistical Analyses
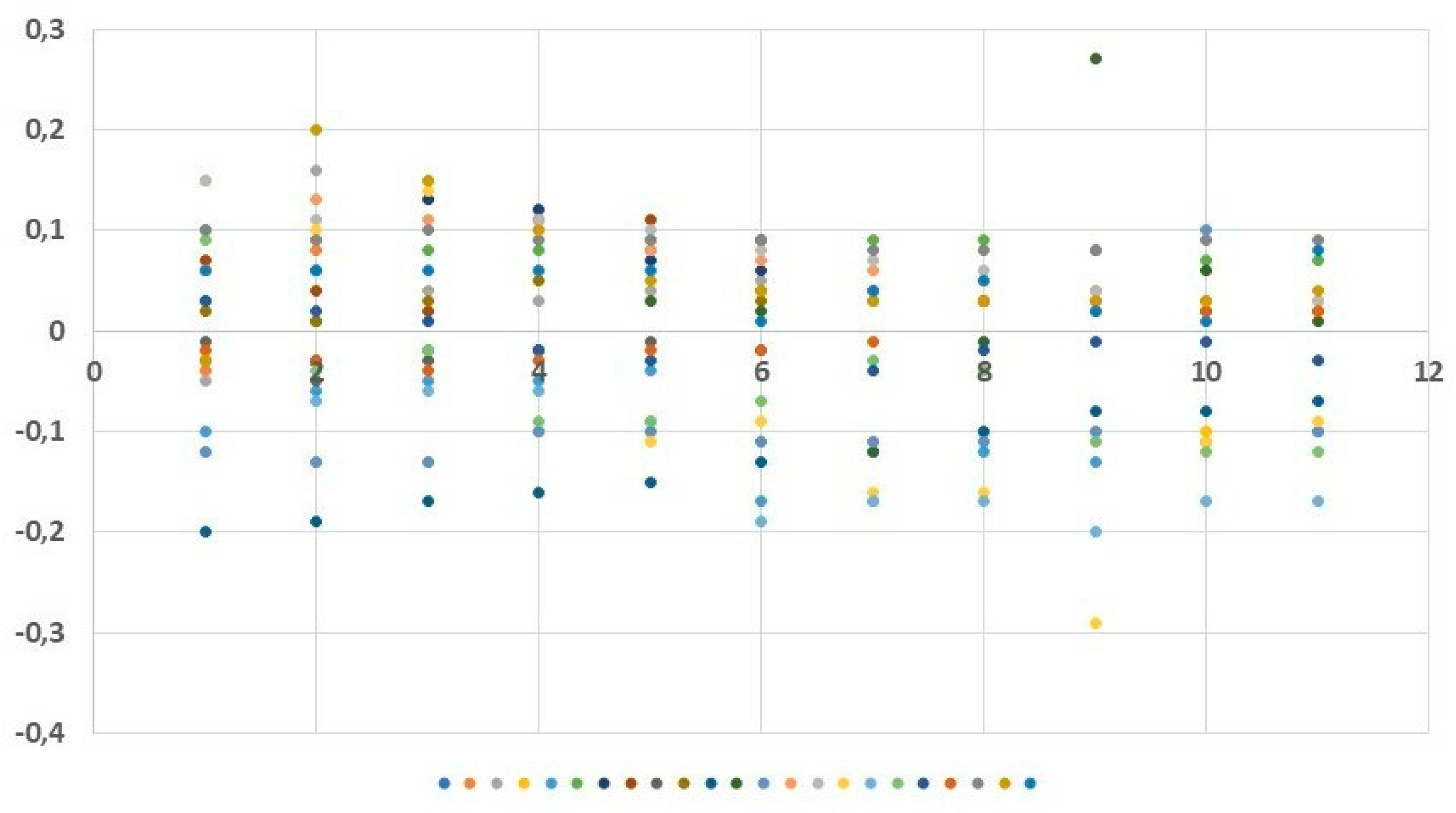
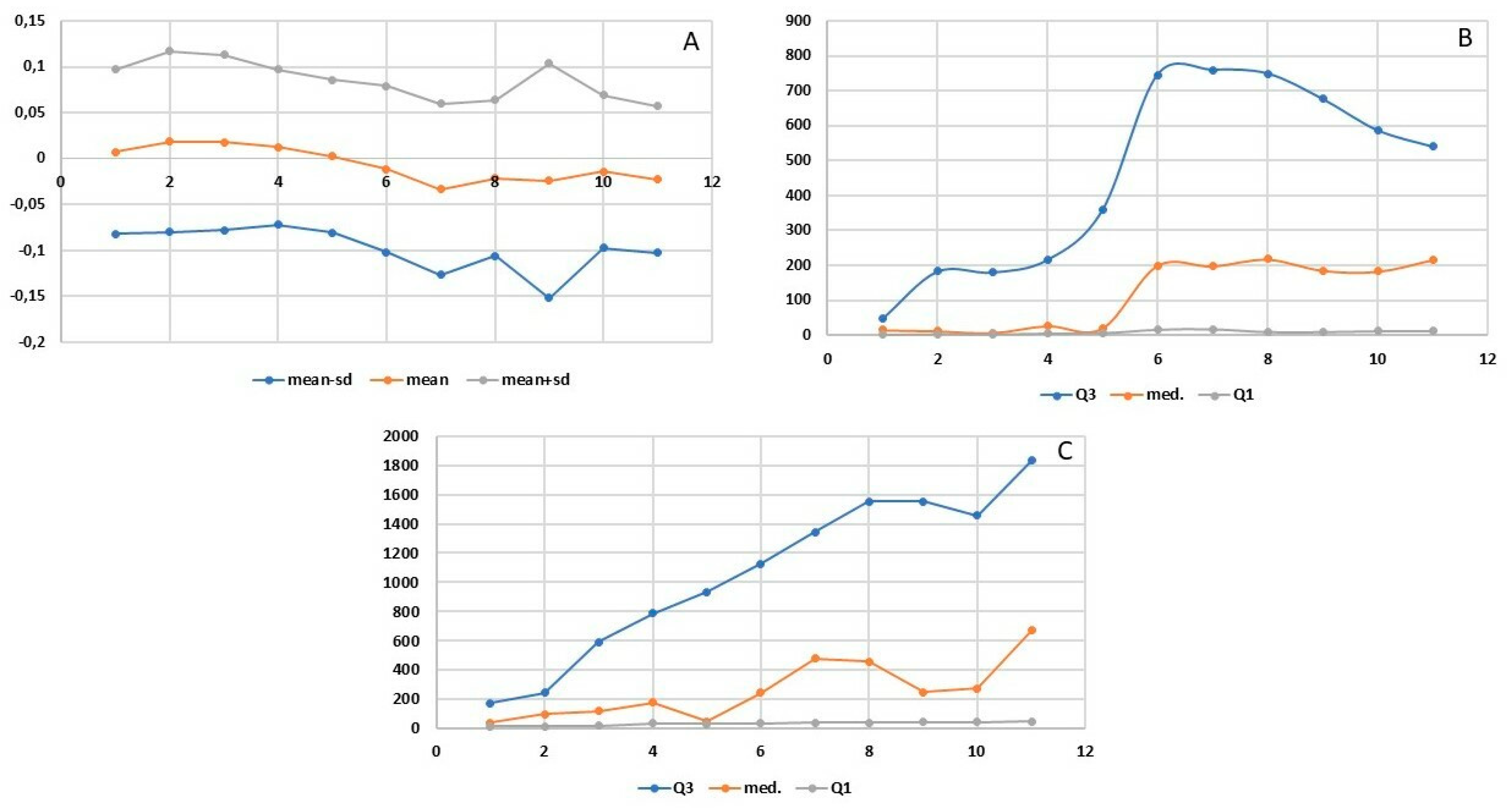
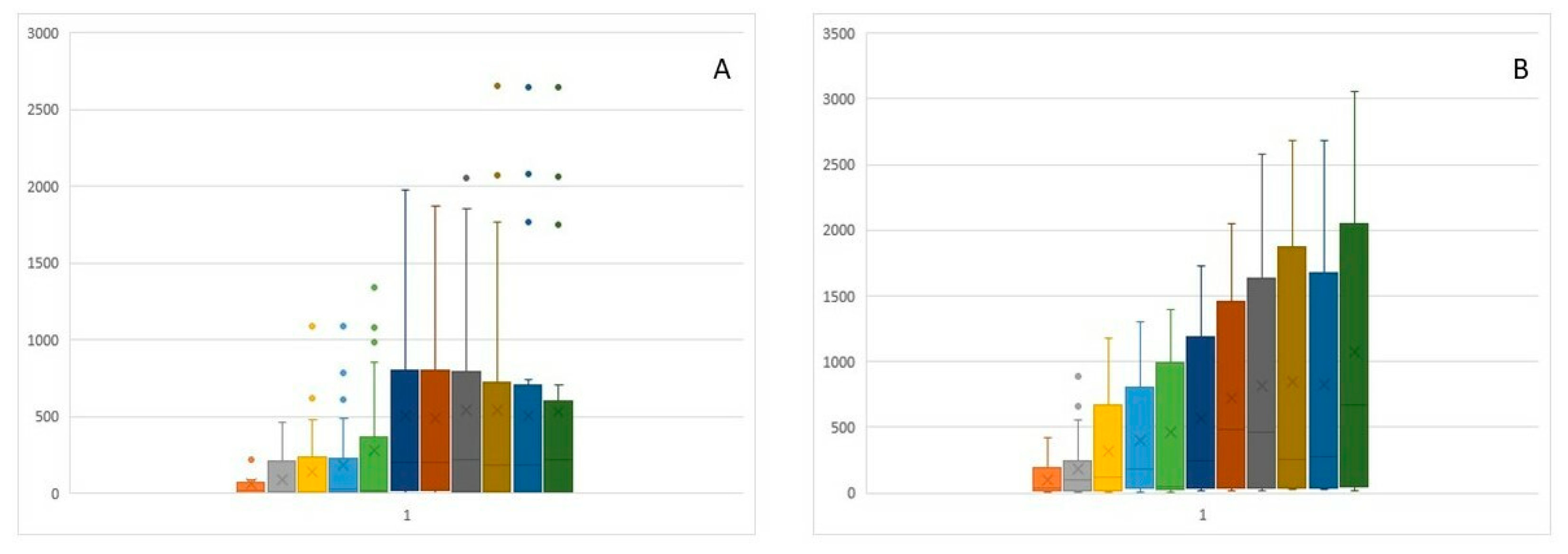
Statistical analyses included basic descriptive statistics (mean, standard deviation (SD)] as well as positional statistics (quartiles). An analysis of empirical distributions of the OD differences (ΔE) as well as the T1 and T2 parameters is also presented as variability charts and box plots (Figure 1,
Figure 3 and
Figure 4a,b). We used the Welch Two Sample t-test for comparisons. Statistical significance was determined with a p value < 0.05
3. Results
3.1. Variability of nasal flow at individual time points in relation to an increasing dose of allergen administered
The increasing doses of the allergen did not cause any statistically significant subjective ailments as measured by the VAS scale. Nasal itching was the only symptom observed at the level of 0.95 mm in two subjects following the administered dose of 50 µg. Slight variability of nasal flows as measured by means of OR was observed during the early allergic reaction phase: following local application of the saline solution and following the increasing doses of the allergen (12.5, 25, 50, and 100 µg). The mean values of the OD (measured regardless of the allergen dose used) were both positive and negative: 0.007 OD (SD 0.089) at 5 minutes, 0.019 OD (SD 0.098) at 10 minutes, 0.018 OD (SD 0.095) at 15 minutes, 0.012 OD (SD 0.084) at 20 minutes, 0.003 OD (SD 0.083) at 25 minutes, −0.011 OD (SD 0.090) at 30 minutes, −0.033 OD (SD 0.093) at 35 minutes, −0.021 OD (SD 0.085) at 40 minutes, −0.024 OD (SD 0.128) at 45 minutes, −0.014 OD (SD 0.083) at 50 minutes, and −0.023 OD (SD 0.080) at 55 minutes (
Figure 3 and
Figure 4a). Similarly, no statistically significant differences were observed at predefined time points corresponding to the assessment of mucosal response to the allergen used (at 15, 30, 45, and 50 minutes into the study). The slight variability as observed in the assessment was identical to that observed in the nasal cycle.
3.2. Mean optical density, onset and greatest change in flow measured by optical rhinometry
The onset of flow variability was observed at 8.48–8.97 minutes into the study, whereas the peak change was observed at 13.73–17.88 minutes (
Figure 4b,c). Due to the low variability of nasal flows during the early allergic reaction phase, no significant differences were recorded at individual study time points of 15, 30, 45, and 60 minutes after application. The T1 and T2 parameters (
Figure 4b,c) are characterized by high variability of the third quartile and median values at individual measurement points. The wide distribution of these values results translates to the absence of significant differences being observed in these parameters over time. This effect is evident in the box plot charts in
Figure 5a and
Figure 4b.
4. Discussion
4.1. Benefits of nasal provocation test in diagnosing food allergies
Among various indications for NFC, the following are worth noticing: diagnosis of persistent, chronic, occupational and local allergic rhinitis, identifying allergy to airborne allergens, determining the cause-and-effect relationship between the allergen and symptoms, especially in cases when interpretation of skin test results and sIgE concentration are difficult, determining the indications for immunotherapy, identifying allergens directly responsible for the symptoms and designing effective vaccine based on these findings, as well as monitoring the effectiveness of desensitization and pharmacotherapy, performing differential diagnosis of eye symptoms and finally, using the obtained information for scientific purposes (studying the pathophysiology of allergic reactions and examining the influence of various factors on the course of this reaction) [
5,
9]. An additional indication stated by the consensus of experts, involving the implementation of nasal provocation test in the diagnosis of food allergy, appears to be of particular importance for future clinical practice [
5]. As of present, little data is available in the literature on the use of nasal allergen provocation tests in the diagnostics of food allergies. In this study, the present authors presented a detailed protocol of the NFC test as a potentially useful and safe tool in diagnosing food allergy. The diagnosis of food allergy is complex due to the rich symptomatology and the multitude of symptom-triggering factors. The first documented report on the diagnosis of food allergy is from 1912, when scratch tests had been performed and probably first reported by an American pediatrician, Oscar Menderson Schloss [
14]. In the following years, the diagnostic methods used in this disease unit continued to develop and improve. Currently, the standards for the management of suspected food allergy include an interview, physical examination, skin tests, laboratory analyses, and component diagnostics. However, a double-blind, placebo-controlled food challenge remains the gold standard [
1,
2,
3,
4]. The main indication for the challenge test is to confirm the causal relationship between consumption of a particular food and development of hypersensitivity symptoms. As the result, the causative role of the suspected food product in the development of the disease can be confirmed. Another important indication for the test consists in documentation of the development of tolerance to food products that had previously triggered hypersensitivity reactions, including development of tolerance following specific immune therapy [
1,
15]. The main advantage of the challenge test consists in the fact that it reproduces the natural route of exposure to the allergen. Nonetheless, the oral challenge test is fraught with the possibility of burdensome, severe, and potentially life-threatening symptoms; and should therefore be carried out in hospital conditions, thus substantially increasing the procedure costs. Another important limitation consists in the necessity to precisely blind the examined foods [
16].
In the course of oral challenge tests, patients were found to frequently present with nasal symptoms—such as itching, sneezing, watery discharge, and nasal obstruction—in addition to symptoms from the digestive tract and skin. These observations have laid the foundation for research on the use of nasal challenge tests in the diagnosis of food allergies. Nasal allergen provocation tests are widely used in differential diagnostics of rhinitis. This is because such tests facilitate confirmation of the causal role and identification of factors responsible for IgE-dependent hypersensitivity reactions in allergic rhinitis, as well as confirmation of pharmacotherapy and specific immune therapy as used in treating allergic rhinitis. Nasal allergen provocation tests are relatively safe and can be performed in outpatient settings. The immediate reaction symptoms usually resolve within several minutes. Assessment of the challenge tests is based on clinical evaluation and objective measurement techniques; including rhinomanometry, acoustic rhinometry, peak nasal inspiratory flow (PNIF), and OR [
5,
17]. These considerations have instigated the search for safer, more accessible, and possibly outpatient-based diagnostic methods (compared with oral food challenge) that would facilitate reproduction of the natural route of allergen exposure. Kvenshagen and Jacobsen assessed the possibility of diagnosing food allergies by means of techniques other than the oral provocation test, which is a potentially dangerous, expensive, and time-consuming tool. According to the assumed premise, the new diagnostic tool should be cheaper, safer, and easier to perform than the oral provocation test while at the same time being painless and reliable. The authors had carried out a relevant query within full-text databases (Embase, PubMed and Cochrane) to obtain as few as seven publications on the use of mucosal allergen challenges (to nasal, conjunctival, labial, and gastrointestinal mucosa as assessed by endoscopic means) in the diagnostics of food allergies to be included in the analysis. Due to the availability of mucous membranes and the possibility of using small doses of allergen, they found these methods promising, but undoubtedly requiring validation and standardization in order to be used as alternatives to the oral provocative tests [
18]. A peculiar basis for the use of intranasal provocation tests in food allergies was laid by Amlot et al. in 1985. The authors presented the results of a study involving the use of nasal, labial, and gastric provocation in 39 patients with oral allergy to milk and hen’s eggs as diagnosed from the clinical history positive skin prick test results. The results of the nasal provocation tests were assessed on the basis of PNIF measurements and the number of sneezes. No oral food challenge test was performed. On the basis of the obtained results, the nasal allergen provocation test was considered to be the most sensitive modality [
19]. In the following years, only three other papers were published on the subject. Studies on nasal challenge tests with chicken egg and peanuts allergens had been published in 1993 by Seppey et al. and later on in 2007 and 2012 by Clark et al. Facial thermography was used by the authors to assess the test results; the examination was considered to be rapid, safe, and objective. However, no further studies were conducted [
20,
21,
22]. A breakthrough was made in 2021, when Gelis et al. presented the results of an innovative study assessing the usefulness of the nasal allergen provocation test in the diagnostics of shellfish allergies and in the differentiation of patients with allergy and non-allergic hypersensitivity as an alternative to the oral food challenge test. Included in the study were a total of 45 people with shrimp allergy confirmed by means of skin prick test, nasal allergen provocation test, history of anaphylaxis or intolerance of shrimp in medical history. The control group consisted of 10 healthy individuals. Boiled shrimp lyophilizate was used for the nasal test and the results were assessed on the basis of acoustic rhinometry and visual analog scale. The results confirmed the usefulness of the nasal allergen provocation test in the diagnostics of shrimp allergies [
23].
Allergy to chicken egg proteins is one of the most common food allergies encountered in an allergologist’s practice. The incidence of chicken egg protein allergy in the population of infants and young children ranges from 0.5% to 2% [
24]. As revealed by the results of a multicenter cohort Europrevall study carried out from 2005–2009, the incidence of chicken egg allergies in children aged up to 2 years was 1% [
23,
25]. In adults, food allergy to chicken eggs is much less common and accounts for 0.1%. It is believed that persistent allergy is the main cause of presentation in adults, whereas primary hypersensitivity to egg proteins is rare. The allergy to chicken eggs is particularly challenging to patients and their families, due to the necessity of abstaining from numerous food products and fear of accidental use; it is also an unfavorable prognostic factor of future inhalant allergens as well as the development of asthma and allergic rhinitis [
26,
27]. For this reason, an accurate diagnostic process is important, as is the safety of diagnostic procedures. Herein, the present authors describe the possibility of using freeze-dried chicken egg white and yolk powder in NFC, and reveal the lack of irritating effects of this form (raw chicken egg) on the nasal mucosa.
4.2. Limitation of study
One limitation of the study is the relatively small number of scientific reports conducted on a representative group of subjects, which would allow for including the FNC method in diagnostic guidelines for food allergies. Another issue is the lack of ready-made tools enabling scientists to prepare lyophilisate in a form that would meet the criteria for standardized nasal preparations. Those used in the form of skin tests often contain glycerin, which is not indifferent to nasal mucosa and may potentially increase the risk of false-positive results. Therefore, the use of this form of allergens in FNC is impossible for methodological reasons. On the other hand, the use of ready-made food preparations, e.g. milk, may contribute to increasing the risk of non-specific immune responses resulting from industrial technologies used in food processing [
28]. The method of obtaining allergen by means of filtration in laboratory conditions implemented by our research team creates new opportunities for further research in this field.
5. Conclusions
The freeze-dried chicken egg white powder as used in NFC did not increase the risk of nasal mucosal reactivity in the control group. The next stage of research will be based on the NFC model developed herein and will involve a group of subjects with allergies to chicken egg protein allergens.
Author Contributions
Conceptualization, E.K-F; methodology, E.K-F., S.B., M.E.C. and B.G.; software, K.F.; validation, E.K-F. and O.W.; formal analysis, E.K-F. and K.F.; investigation, E.K-F.; resources, E.K-F. and O.W.; data curation, E.K-F. and K.F.; writing—original draft preparation, E.K-F., S.B. and O.W.; writing—review and editing, E.K-F. and G.N.; visualization, E.KF.; supervision, E.K-F.; project administration, B.S., E.K-F. and A.S.; funding acquisition, E.K-F. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by the National Science Center Miniature-5 grant number 2021/05/X/NZ5/009.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Medical University of Warsaw (decision no. KB 63/2022) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to Ms. Agnieszka Górska (Department of Biochemistry and Pharmacogenomics of the Faculty of Pharmacy, Medical University of Warsaw) for valuable help in preparing freeze-dried chicken egg white powder and doses of pure protein for use in the nasal challenge tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Passanisi, S.; Lombardo, F.; Crisafulli, G.; Salzano, G.; Aversa, T.; Pajno, G.B. Novel diagnostic techniques and therapeutic strategies for IgE-mediated food allergy. Allergy Asthma Proc. 2021, 42, 124–130. [Google Scholar] [CrossRef]
- Eiwegger, T.; Hung, L.; San Diego, K.E.; O’Mahony, L.; Upton, J. Recent developments and highlights in food allergy. Allergy 2019, 74, 2355–2367. [Google Scholar] [CrossRef]
- Bartuzi, Z.; Kaczmarski, M.; Czerwionka-Szaflarska, M.; Małaczynska, T.; Krogulska, A. The diagnosis and management of food allergies. Position paper of the Food Allergy Section the Polish Society of Allergology. Adv Dermatol Allergol 2017, 34, 391–404. [Google Scholar] [CrossRef]
- Augé, J.; Vent, J.; Agache, I.; et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy 2018, 73, 1597–1608. [Google Scholar] [CrossRef]
- Samoliński, B.; Rapiejko, P.; Krzych-Fałta, E.; et al. Standardy wykonywania donosowych prób prowokacyjnych. Adv Dermatol Allergol 2010, XXVII, 141–160. [Google Scholar]
- Samoliński, B.; Rapiejko, P.; Krzych-Fałta, E.; et al. Definicja donosowej próby prowokacyjnej i donosowej próby prowokacyjnej z alergenem, klasyfikacja. Adv Dermatol Allergol 2010, XXVII, 166–169. [Google Scholar]
- Samoliński, B.; Rapiejko, P. The early reaction after nasal airway challenge with allergen. Adv Dermatol Allergol 2010, XXVII, 170–172. [Google Scholar]
- Pepper, A.N.; Ledford, D.K. Nasal and ocular challenges. J Allergy Clin Immunol 2018, 141, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Heinzerling, L.; Bachert, C.; et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012, 67, 18–24. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy. 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Wüstenberg, E.G.; Zahnert, T.; Huttenbrink, K.B.; et al. Comparison of Optical Rhinometry and Active Anterior Rhinomanometry Using Nasal Provocation Testing. Arch Otolaryngol Head Neck Surg 2007, 133, 344–349. [Google Scholar] [CrossRef]
- Mittenzwey, H.; Wustenberg, E.G.; Leupold, W. Optical rhinometry: Application on children and adolescents for nasal provocation tests. Pediatr Allergy Immunol 2007, 18, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, B. History of food allergy. Chem Immunol Allergy 2014, 100, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Cafarotti, A.; Giovannini, M.; Begìn, P.; Brough, H.A.; Arasi, S. Management of IgE-mediated food allergy in the 21st century. Clin Exp Allergy 2023, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Tang, M.L.K.; Wood, R.A. Food Allergy and Eosinophilic Gastrointestinal Diseases-The Next 10 Years. J Allergy Clin Immunol Pract. 2023, 11, 72–78. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Ansotegui, I.; Aberer, W.; et al. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: World Allergy Organization Statement. World Allergy Organ. J. 2016, 9, 33. [Google Scholar] [CrossRef]
- Kvenshagen, B.K.; Jacobsen, M. The value of mucosal allergen challenge for the diagnosis of food allergy. Curr Opin Allergy Clin Immunol. 2013, 13, 268–272. [Google Scholar] [CrossRef]
- Amlot, P.L.; Urbanek, R.; Youlten, L.J.F. Type I allergy to egg and milk proteins: Comparison of skin prick tests with nasal, buccal and gastric provocation tests. Int Archs Allergy Appl Immun 1985, 77, 171–173. [Google Scholar] [CrossRef]
- Clark, A.; Mangat, J.; Tay, S.; et al. Facial thermography is a sensitive and specific method for assessing food challenge outcome. Allergy 2007, 62, 744–749. [Google Scholar] [CrossRef]
- Clark, A.; Mangat, J.; King, Y.; et al. Thermographic imaging during nasal peanut challenge may be useful in the diagnosis of peanut allergy. Allergy 2012, 67, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Hessler, C.; Bruchez, M.; Savary, M.; Pécoud, A. Facial thermography during nasal provocation tests with histamine and allergen. Allergy 1993, 48, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Gelis, S.; Rueda, M.; Pascal, M.; et al. Usefulness of the Allergen Specific Nasal Provocation Test in the Diagnosis of Shellfish. J Investig Allergol Clin Immunol. 2022, 32, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Dona, D.W.; Suphioglu, C. Egg Allergy: Diagnosis and Immunotherapy. Int J Mol Sci. 2020, 21, 5010. [Google Scholar] [CrossRef]
- Keil, T.; McBride, D.; Grimshaw, K.; et al. The multinational birth cohort of EuroPrevall: Background, aims and methods. Allergy 2010, 65, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Leech, S.C.; Ewan, P.W.; Skypala, I.J.; et al. BSACI 2021 guideline for the management of egg allergy. Clin Exp Allergy 2021, 51, 1262–1278. [Google Scholar] [CrossRef]
- Trogen, B.; Jacobs, S.; Nowak-Wegrzyn, A. Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients 2022, 14, 2565. [Google Scholar] [CrossRef]
- Krzych-Fałta, E.; Wojas, O.; Namysłowski, A.; et al. Reactivity of nasal cavity mucosa in the nasal cow’s milk allergen provocation test. Adv Dermatol Allergol 2023, XL, 87–92. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).