Submitted:
17 August 2023
Posted:
21 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. The ADME-Tox studies
2.2. The influence of astragaloside IV on the acquisition and consolidation of long-term memory impairment induced by the SCOP administration in mice
2.3. Influence of astragaloside IV on the acquisition and consolidation of long-term memory impairment induced by the LSP administration in mice
2.4. Locomotor activity
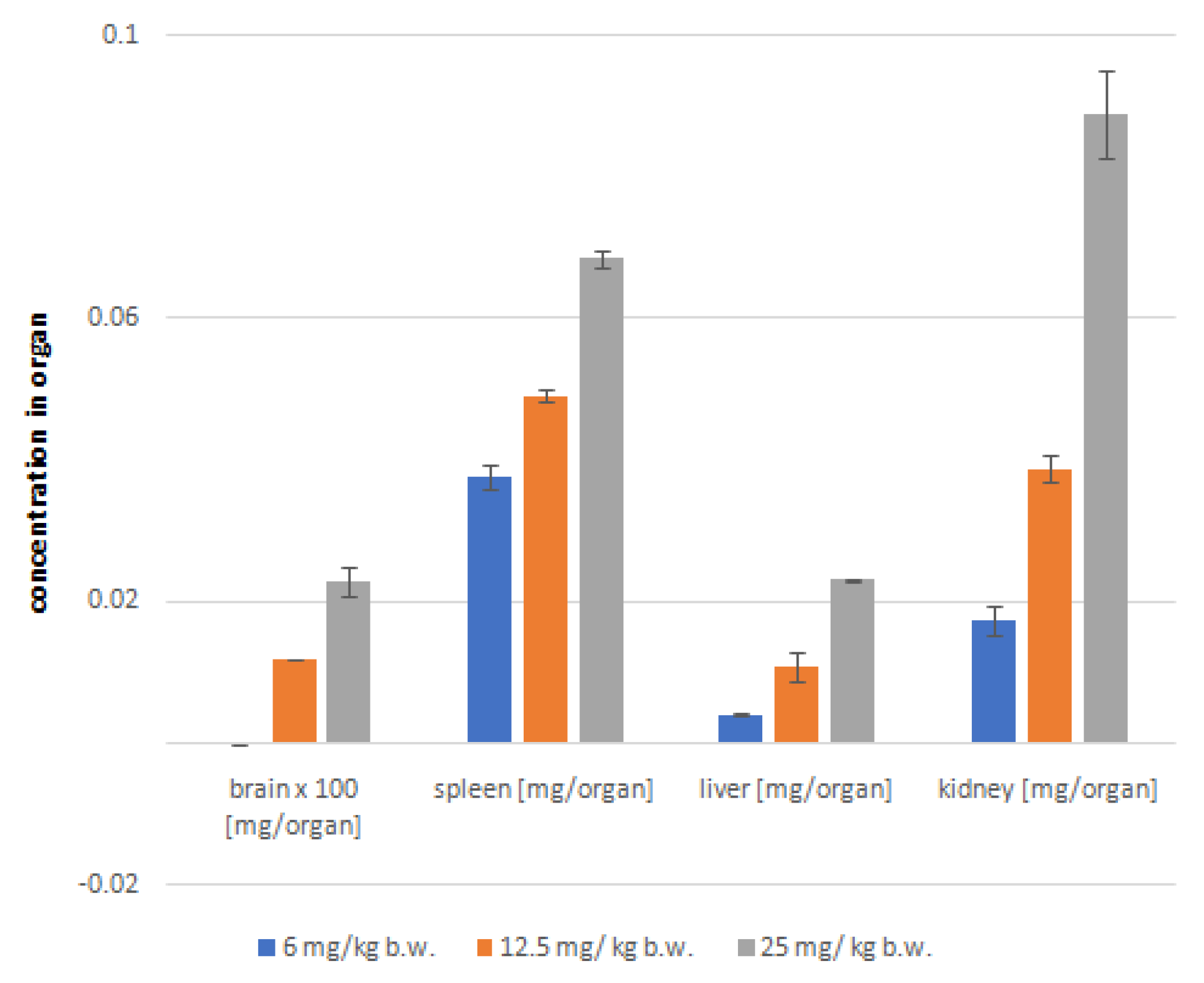
2.5. Post-mortem analysis of astragaloside IV distribution in soft organs
3. Discussion
4. Materials and Methods
4.1. The ADME-Toxicity profiling
4.2. Animal studies
4.2.1. Mice
4.2.2. Drugs
4.2.3. The passive avoidance test
- Acquisition of long-term memory
- 2.
- Consolidation of long-term memory
4.2.4. The locomotor activity test
4.2.5. Statistical analysis
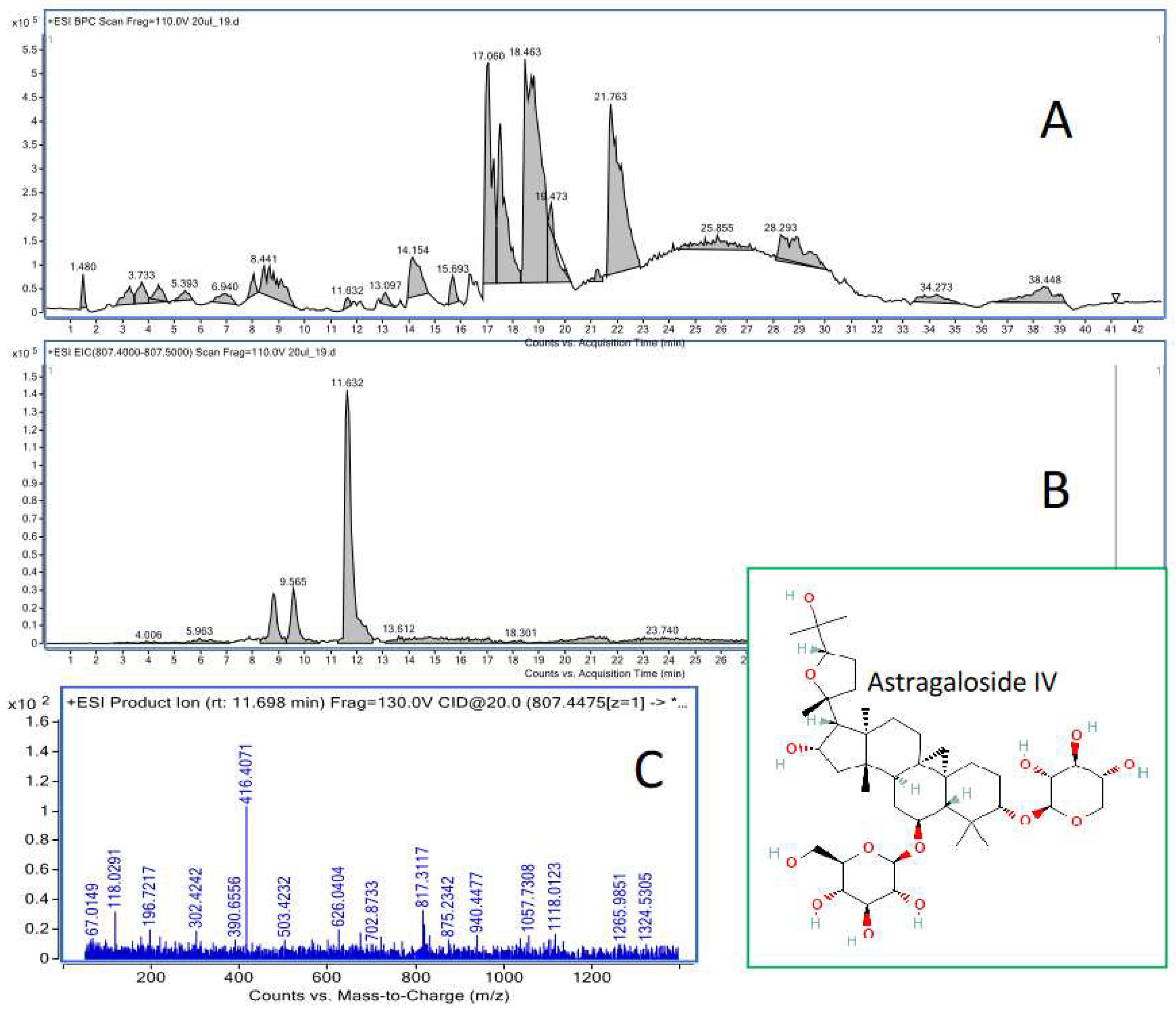
4.3. HPLC-ESI-QTOF-MS/MS determination of astragaloside IV in the animal tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization, Global action plan on the public health response to dementia 2017–2025. Available online: https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025 (accessed on 10 April 2023).
- Hullinger, R.; Puglielli, L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav. Brain Res. 2017, 322, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Kluever, V.; Fornasiero, E.F. Principles of brain aging: status and challenges of modeling human molecular changes in mice. Ageing Res. Rev. 2021, 72, 101465. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Phan, Ch.W.; Lim, S.J.; Babji, A.S. Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents. Food Sci. Hum. Wellness 2023, 12, 1008–1019. [Google Scholar] [CrossRef]
- Farooqui, A.A. Contribution of neuroinflammation, resolution, and neuroprotection in neurotraumatic diseases. Farooqui, A.A., Ed.; In Neuroinflammation, Resolution, and Neuroprotection in the Brain; Elsevier: Amsterdam, 2022; pp. 83–119. [Google Scholar]
- Rapposelli, S.; Digiacomo, M.; Balsamo, A. P-gp Transporter and its Role in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2009, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-Derived Neuroprotective Agents: Cutting the Cycle of Cell Death through Multiple Mechanisms. Evid. Based Complement. Alternat. Med. 2017, 3574012. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s Disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, P.V.; Marzloff, K.; Hyman, B.T. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 1992, 42, 1681–1688. [Google Scholar] [CrossRef]
- Mrak, R.E.; Griffin, W.S.T. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol. Aging. 2001, 22, 903–908. [Google Scholar] [CrossRef]
- Cole, G.; Teter, B.; Frautschy, S. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 595–197. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Dodwell, S.A.; Borrelli, L.A.; Raju, S.; Bacskai, B. In vivo reduction of plaque size in APPswe/PS1D9 mice treated with curcumin. Alzheimer’s & Dementia 2006, 2, S617. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; Khachaturian, Z.S. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Houser, C.R.; Crawford, G.D.; Barber, R.P.; Salvaterra, P.M.; Vaughn, J.E. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983, 266, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.J.; Butcher, L.L. Cholinergic systems mediate action from movement to higher consciousness. Behav. Brain Res. 2011, 221, 488–498. [Google Scholar] [CrossRef]
- Woolf, N.J. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 1991, 37, 475–524. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 2017, 1670, 173–184. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Dunnett, S.B.; Everitt, B.J.; Robbins, T.W. The basal forebraincortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991, 14, 494–501. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Anderson, B.P.; Bower, J.M. Cholinergic modulation of cortical associative memory function. J. Neurophysiol. 1992, 67, 1230–1246. [Google Scholar] [CrossRef]
- Lucas-Meunier, E.; Fossier, P.; Baux, G.; Amar, M. Cholinergic modulation of the cortical neuronal network. Pflugers Arch. 2003, 446, 17–29. [Google Scholar] [CrossRef]
- Sarter, M.; Bruno, J.P. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res. Rev. 1997, 23, 28–46. [Google Scholar] [CrossRef]
- Bucci, D.J.; Holland, P.C.; Gallagher, M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J. Neurosci. 1998, 18, 8038–8046. [Google Scholar] [CrossRef]
- Voytko, M.L.; Olton, D.S.; Richardson, R.T.; Gorman, L.K.; Tobin, J.R.; Price, D.L. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J. Neurosci. 1994, 14, 167–186. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G.; Bracco, L. Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 2013, 203, 361–364. [Google Scholar] [CrossRef]
- Bracco, L.; Bessi, V.; Padiglioni, S.; Marini, S.; Pepeu, G. Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer’s disease patients. J. Alzheimers Dis. 2014, 40, 737–742. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeon, S.J.; Jung, J.W.; Lee, S.; Yoon, B.H.; Shin, B.Y.; Son, K.H.; Cheong, J.H.; Kim, Y.S.; Kang, S.S.; Ko, K.H.; Ryu, J.H. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur. J. Pharmacol. 2007, 574, 140–147. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Khurelbat, D.; Boguszewska-Czubara, A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor-From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. Int J Mol Sci. 2023, 24, 9152. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W. In Silico Studies on Triterpenoid Saponins Permeation through the Blood-Brain Barrier Combined with Postmortem Research on the Brain Tissues of Mice Affected by Astragaloside IV Administration. Int. J. Mol. Sci. 2020, 21, 2534. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials. 2017, 129, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.A.; Dietschy, J.M. The intestinal unstirred layer: Its surface area and effect on active transport kinetics. Biochim. Biophys. Acta. 1974, 363, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Wils, P.; Warnery, A.; Phung-Ba, V.; Legrain, S.; Scherman, D. High lipophilicity decreases drug transport across intestinal epithelial cells. J. Pharmacol. Exp. Ther. 1994, 269, 654–658. [Google Scholar] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 1996, 22, 67–84. [Google Scholar] [CrossRef]
- New, R. Oral Delivery of Biologics via the Intestine. Pharmaceutics. 2020, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Weickert, C.S.; Garner, B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J. Neurochem. 2008, 104, 1145–1166. [Google Scholar] [CrossRef]
- Tatsuta, T.; Naito, M.; Oh-hara, T.; Sugawara, I.; Tsuruo, T. Functional involvement of P-glycoprotein in blood-brain barrier. J. Biol. Chem. 1992, 267, 20383–20391. [Google Scholar] [CrossRef]
- Didziapetris, R.; Japertas, P.; Avdeef, A.; Petrauskas, A. Classification Analysis of P-Glycoprotein Substrate Specificity. J. Drug Target. 2003, 11, 391–406. [Google Scholar] [CrossRef]
- Lochner, M.; Thompson, A.J. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacol. 2016, 108, 220–228. [Google Scholar] [CrossRef]
- Misane, I.; Ögren, S. Selective 5-HT1A Antagonists WAY 100635 and NAD-299 Attenuate the Impairment of Passive Avoidance Caused by Scopolamine in the Rat. Neuropsychopharmacol. 2003, 28, 253–264. [Google Scholar] [CrossRef]
- Araujo, D.M.; Lapchak, P.A.; Robitaille, Y.; Gauthier, S.; Quirion, R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer’s disease. J. Neurochem. 1988, 50, 1914–1923. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Scheff, S.W.; Styren, S.D. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegener. 1996, 5, 417–421. [Google Scholar] [CrossRef]
- Kuhl, D.E.; Koeppe, R.A.; Minoshima, S.; Snyder, S.E.; Ficaro, E.P.; Foster, N.L. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology 1999, 52, 691–699. [Google Scholar] [CrossRef]
- Shinotoh, H.; Namba, H.; Fukushi, K.; Nagatsuka, S.; Tanaka, N.; Aotsuka, A. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer’s disease: a positron emission tomography study. Ann. Neurol. 2000, 48, 194–200. [Google Scholar] [CrossRef]
- Bartus, R.T. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, A.C.; Morris, H.; Buccafusco, J.J.; Kille, N.; Rosenzweig-Lipson, S.; Husbands, M.G.; Sabb, A.L.; Abou-Gharbia, M.; Moyer, J.A.; Boast, C.A. The preclinical pharmacological profile of WAY-132983, a potent M1 preferring agonist. J. Pharmacol. Exp. Ther. 2000, 292, 584–596. [Google Scholar] [PubMed]
- Elrod, K.; Buccafusco, J.J. An evaluation of the mechanism of scopolamine-induced impairment in two passive avoidance protocols. Pharmacol. Biochem. Behav. 1988, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Blin, O.; Audebert, C.; Pitel, S.; Kaladjian, A.; Casse-Perrot, C.; Zaim, M.; Micallef, J.; Tisne-Versailles, J.; Sokoloff, P.; Chopin, P.; Marien, M. Effects of dimethylaminoethanol pyroglutamate (DMAE p-Glu) against memory deficits induced by scopolamine: evidence from preclinical and clinical studies. Psychopharmacol. Berl. 2009, 207, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Blotnick-Rubin, E.; Anglister, L. Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction. Front. Mol. Neurosci. 2018, 11, 123. [Google Scholar] [CrossRef]
- Gawel, K.; Labuz, K.; Gibula-Bruzda, E.; Jenda, M.; Marszalek-Grabska, M.; Silberring, J.; Kotlinska, J.H. Acquisition and reinstatement of ethanol-induced conditioned place preference in rats: Effects of the cholinesterase inhibitors donepezil and rivastigmine. J Psychopharmacol. 2016, 30, 676–687. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula-Bruzda, E.; Dziedzic, M.; Jenda-Wojtanowska, M.; Marszalek-Grabska, M.; Silberring, J.; Kotlinska, J.H. Cholinergic activation affects the acute and chronic antinociceptive effects of morphine. Physiol. Behav. 2017, 169, 22–32. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Yao, J.; Liu, J.; He, Y.; Liu, L.; Xu, Z.; Lin, X.; Liu, N.; Kai, G. Systems pharmacology reveals the mechanism of Astragaloside IV in improving immune activity on cyclophosphamide-induced immunosuppressed mice. J. Ethnopharmacol. 2023, 313, 116533. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jiang, H.; Tian, Y.; Zhao, W.; Wu, X. Astragaloside IV protects against polymicrobial sepsis through inhibiting inflammatory response and apoptosis of lymphocytes. J. Surg. Res. 2016, 200, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W-D; Zhang, Ch.; Liu, R-H.; Li, H-L.; Zhang, J-T.; Mao, Ch.; Moran, S.; Chen, Ch-L. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006, 79, 808–815. [CrossRef] [PubMed]
- Feher, M.; Sourial, E.; Schmidt, J.M. A simple model for the prediction of blood-brain partitioning. Int. J. Pharm. 2000, 201, 239–247. [Google Scholar] [CrossRef]
- Liu, R.; Sun, H.; So, S–S. Development of quantitative structure − property relationship models for early ADME evaluation in drug discovery. 2. Blood-brain barrier penetration. J Chem. Inf. Comput. Sci. 2001, 41, 1623–1632. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H. In Silico Models to Predict Oral Absorption. Taylor, J.B., Triggle, D.J. Eds. In Comprehensive Medicinal Chemistry II.; Elsevier: Amsterdam, 2007; pp. 669–697. [Google Scholar]
- Fischer, H.; Gottschlich, R.; Seelig, A. Blood-brain barrier permeation: molecular parameters governing passive diffusion. J. Membr. Biol. 1998, 165, 201–211. [Google Scholar] [CrossRef]
- Muehlbacher, M.; Spitzer, G.M.; Liedl, K.R.; Kornhuber, J. Qualitative prediction of blood-brain barrier permeability on a large and refined dataset. J. Comput. Aided Mol. Des. 2011, 25, 1095–1106. [Google Scholar] [CrossRef]
- Hutter, M.C. Prediction of blood-brain barrier permeation using quantum chemically derived information. J. Comput. Aided Mol. Des 2003, 17, 415–433. [Google Scholar] [CrossRef]
- Kotlinska, J.H.; Lopatynska-Mazurek, M.; Gawel, K.; Gabka, P.; Jenda-Wojtanowska, M.; Kruk-Slomka, M.; Marszalek-Grabska, M.; Danilczuk, Z.; Kedzierska, E.; Talarek, S.; Listos, J.; Gibula-Tarlowska, E. Impact of the metabotropic glutamate receptor7 (mGlu7) allosteric agonist, AMN082, on fear learning and memory and anxiety-like behavior. Eur. J. Pharmacol. 2019, 858, 172512. [Google Scholar] [CrossRef] [PubMed]
- Marszalek-Grabska, M.; Zakrocka, I.; Budzynska, B.; Marciniak, S.; Kaszubska, K.; Lemieszek, M.K.; Winiarczyk, S.; Kotlinska, J.H.; Rzeski, W.; Turski, W.A. Binge-like mephedrone treatment induces memory impairment concomitant with brain kynurenic acid reduction in mice. Toxicol. Appl. Pharmacol. 2022, 454, 116216. [Google Scholar] [CrossRef] [PubMed]
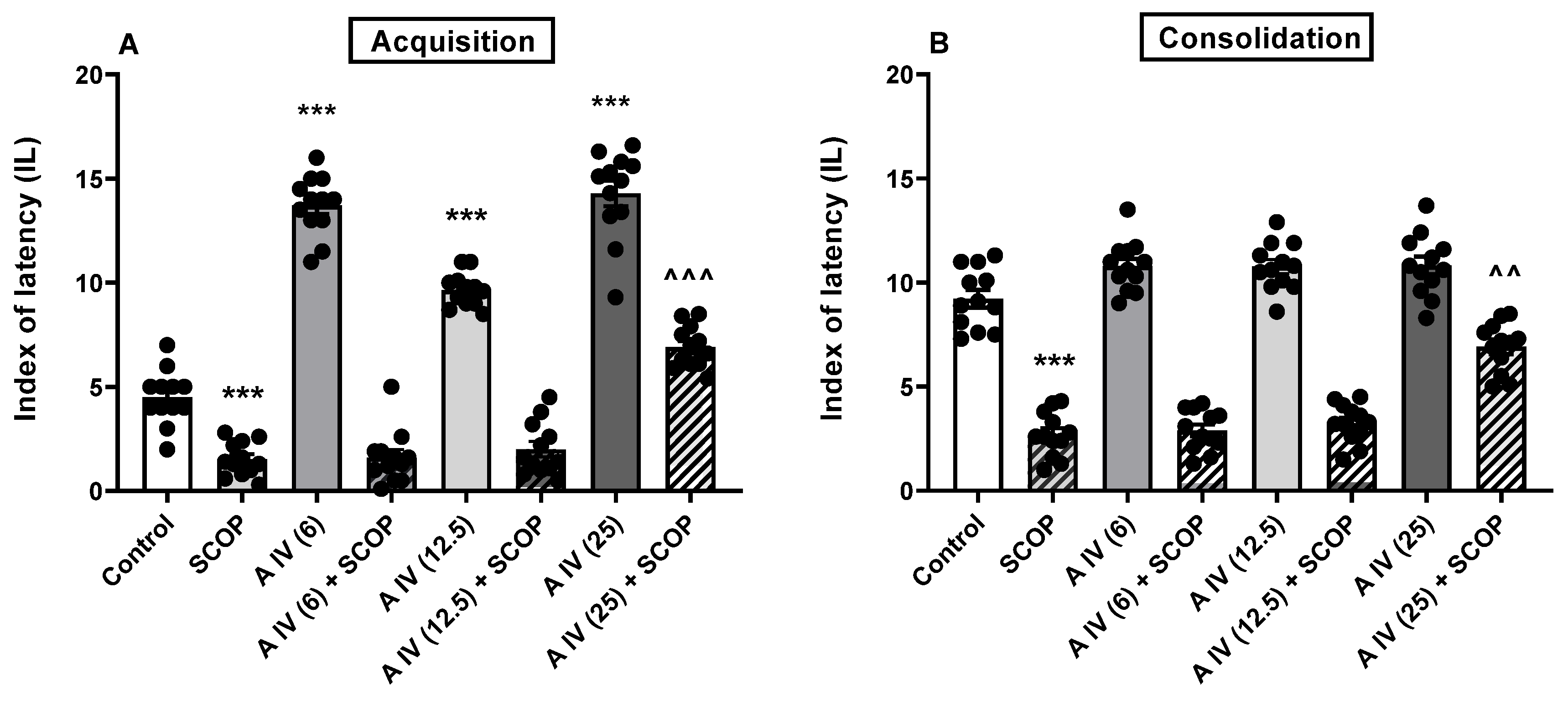
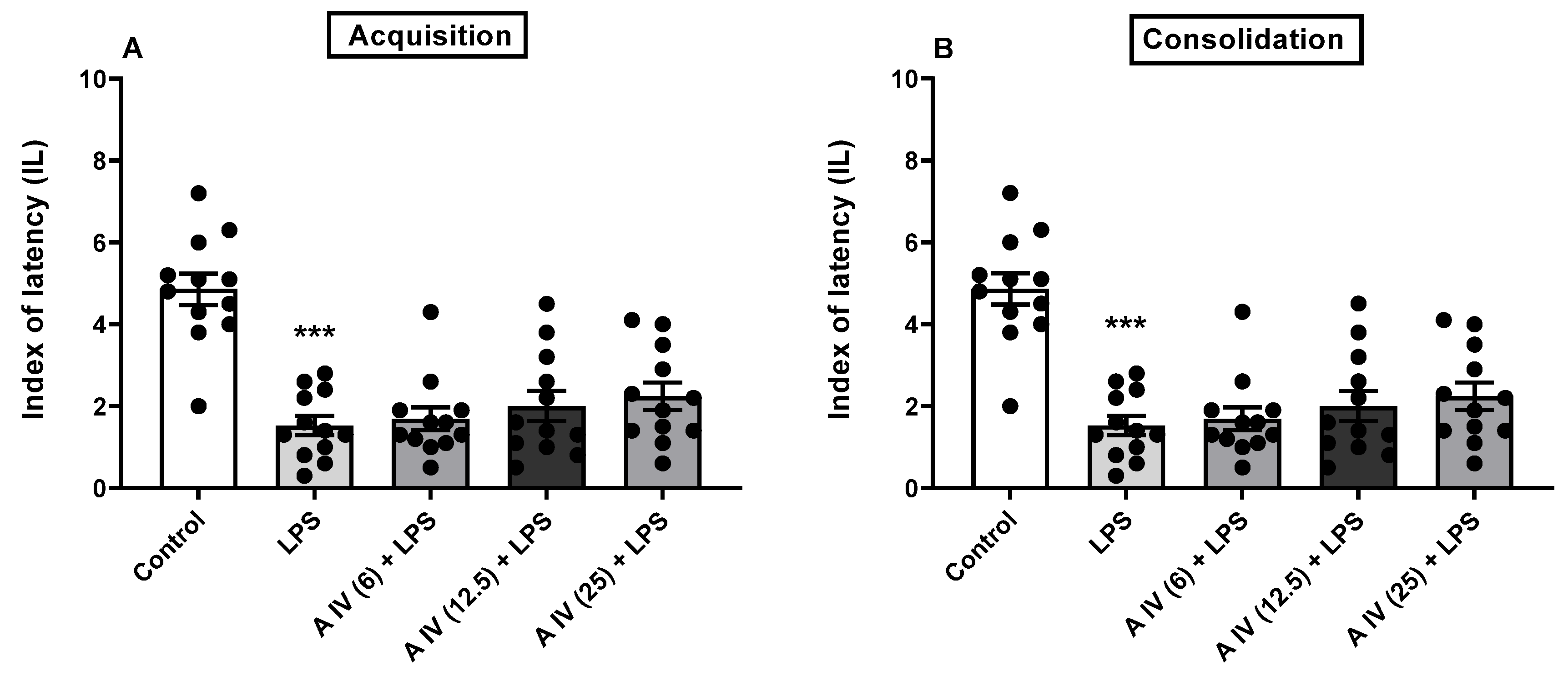
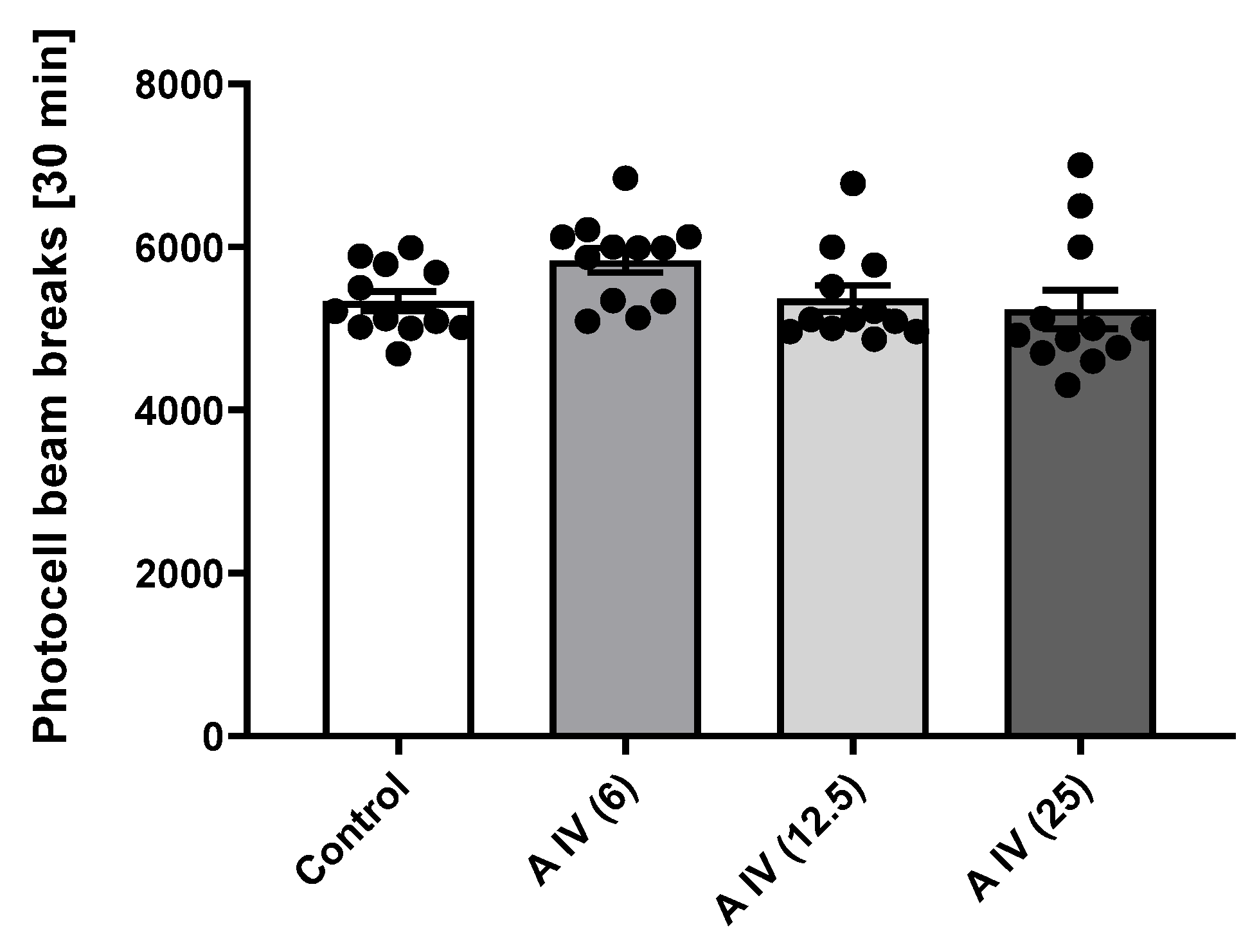
| Name | logPow | logBB | Pe,jejunum [10−4cm/s] | Pe,Caco-2 [10−6 cm/s] | Number of H-donors | Number of H-acceptors | P-gp substrate |
|---|---|---|---|---|---|---|---|
| AI | 5.020 | 0.46 | 1.05 | 2.0 | 7 | 16 | + |
| AII | 4.459 | 0.11 | 0.25 | 0.5 | 8 | 15 | + |
| AIII | 3.767 | 0.15 | 0.04 | 0.1 | 9 | 14 | - |
| AIV | 3.757 | 0.49* | 0.06 | 0.1 | 9 | 14 | - |
| Brain [mg/organ] |
Spleen [mg/organ] |
Liver [mg/organ] |
Kidney [mg/organ] |
|||||
|---|---|---|---|---|---|---|---|---|
| Animal group | Av | SD | Av | SD | Av | SD | Av | SD |
| 6 mg/kg | ND | ND | 0.03766 | 0.00186 | 0.00411 | 0.00011 | 0.01740 | 0.00209 |
| 12.5 mg/kg | 0.00012 | 0.00001 | 0.04919 | 0.00093 | 0.01084 | 0.00023 | 0.03882 | 0.00092 |
| 25 mg/kg | 0.00023 | 0.00002 | 0.06844 | 0.00133 | 0.02316 | 0.00016 | 0.08892 | 0.00613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





