Submitted:
18 August 2023
Posted:
21 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Isolation and Culturing of Bacteria
2.2. Supplement of Plant Extracts
2.3. Antibacterial Susceptibility Test
2.4. Minimum Inhibitory Concentration (MIC) determination protocol
2.5. Biofilm Inhibition and Disruption Assay
2.6. Quorum Sensing Inhibition Assay
2.7. Statistical Analysis
3. Results
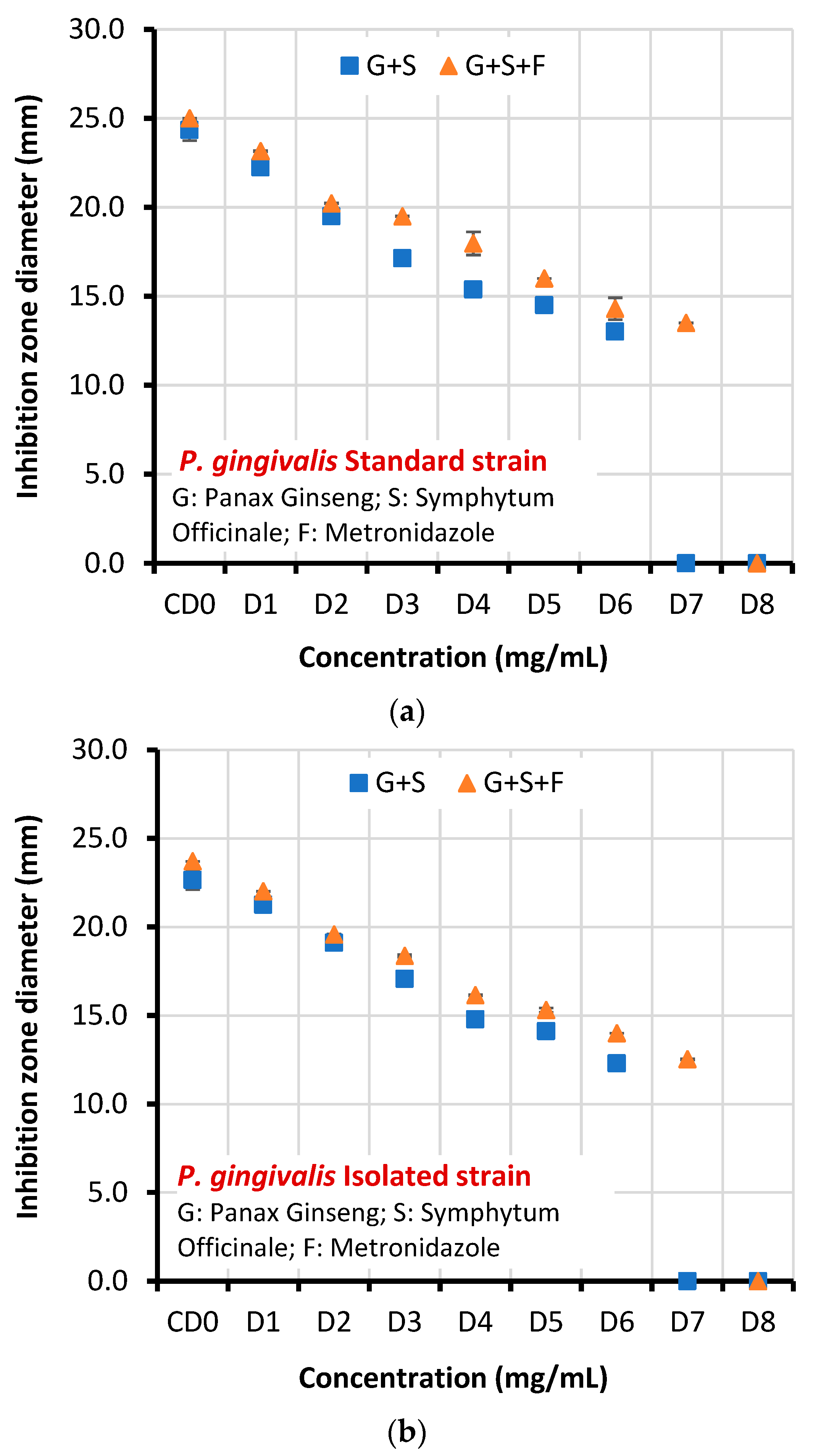
3.1. Antibacterial Activity of Plant Extracts and Metronidazole
3.2. MIC determination
3.3. Biofilm disruption of P. gingivalis
3.4. Quorum Sensing Inhibition
4. Discussion
5. Conclusions
Funding
Acknowledgments
References
- Adler et al. Helicobacter pylori and oral pathology: relationship with the gastric infection. World J. Gastroenterol. WJG 2014, 20, 9922. [CrossRef] [PubMed]
- Bazaz, M.R.; Rahman, Z.; Qadir, I.; Pasam, T.; Dandekar, M.P. Importance of Gut Microbiome-Based Therapeutics in Cancer Treatment. In Targeted Cancer Therapy in Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2023; pp. 831–885. [Google Scholar]
- Cha, J.-D.; Jeong, M.-R.; Choi, K.-M.; Park, J.-H.; Cha, S.-M.; Lee, K.-Y. Synergistic effect between cryptotanshinone and antibiotics in oral pathogenic bacteria. Adv. Biosci. Biotechnol. 2013, 4, 283–294. [Google Scholar] [CrossRef]
- Cha, S.M.; Han, S.B.; Lee, Y.S.; Cha, J.D. Synergistic Effect of the Ethanol Extract of Alismatis rhizoma against Oral Pathogens. J. Oral. Bio 2015, 2, 7. [Google Scholar]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Rahman, N.A.; Karsani, S.A.; et al. Boesenbergia rotunda: From Ethnomedicine to Drug Discovery. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ermolenko, E.; Koroleva, I.; Suvorov, A. Microbial Therapy with Indigenous Bacteria: From Idea to Clinical Evidence. In Microbiome in 3P Medicine Strategies: The First Exploitation Guide; Springer: Berlin/Heidelberg, Germany, 2023; pp. 251–274. [Google Scholar]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lai, T.; Huang, R.Y.; Su, K.W.; Lai, S.R.; Lan, A. Effect of an herbal preparation fermented by Lactobacillus reuteri LR107 in preventing periodontal inflammation in an experimental gingivitis model. Asian J Complement Altern. Med. 2014, 2, 12–18. [Google Scholar]
- Kim, T.-H.; Kim, S.-C.; Jung, W.-K. Therapeutic effect of marine bioactive substances against periodontitis based on in vitro, in vivo, and clinical studies. Fish. Aquat. Sci. 2023, 26, 1–23. [Google Scholar] [CrossRef]
- Juiz, P.J.L.; Lucchese, A.M.; Gambari, R.; Piva, R.; Penolazzi, L.; DI Ciano, M.; Uetanabaro, A.P.T.; Silva, F.; Avila-Campos, M.J. Essential oils and isolated compounds from Lippia alba leaves and flowers: Antimicrobial activity and osteoclast apoptosis. Int. J. Mol. Med. 2014, 35, 211–217. [Google Scholar] [CrossRef]
- Menchicchi, B.; Hensel, A.; Goycoolea, F. Polysaccharides as Bacterial Antiadhesive Agents and “Smart” Constituents for Improved Drug Delivery Systems Against Helicobacter pylori Infection. Curr. Pharm. Des. 2015, 21, 4888–4906. [Google Scholar] [CrossRef]
- Meresta, A.; Folkert, J.; Gaber, T.; Miksch, K.; Buttgereit, F.; Detert, J.; Pischon, N.; Gurzawska, K. Plant-derived pectin nanocoatings to prevent inflammatory cellular response of osteoblasts following Porphyromonas gingivalis infection. Int. J. Nanomed. 2017, ume 12, 433–445. [Google Scholar] [CrossRef]
- Mihm, C. Beeinflussung der Adhärenz und er Internalisierung von Porphyromonas gingivalis durch. Proteinaseinhibitoren. Dissertation, Friedrich-Schiller-Universität Jena, Jena, 2011. [Google Scholar]
- Fauzi, F.M.; Mischon, A.; Zain, N.M.; Baharuddin, I. The Therapeutic Potential of Plant Extraction in Oral Health - A Systematic Review. Compend. Oral Sci. 2022, 9, 88–104. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.-A.; Mukavi, J.; Omosa, L.K.; Kuete, V. Phytochemistry and antibacterial potential of the genus Garcinia. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2023; pp. 105–175. [Google Scholar] [CrossRef]
- E. O. F. H. O. N. PERIODONTITIS–A and S. GUM. International Journal of Pharmacology Research.
- Salas-Jara, M.J.; Sanhueza, E.A.; Retamal-Díaz, A.; González, C.; Urrutia, H.; García, A. Probiotic Lactobacillus fermentum UCO-979C biofilm formation on AGS and Caco-2 cells and Helicobacter pylori inhibition. Biofouling 2016, 32, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, U.; Elango, S.; Jayesh, R. Herbals and green synthesized nanoparticles in dentistry. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 617–646. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, S.; Ai, D.; Qin, H.; Sun, Y.; Xia, X.; Xu, X.; Ji, W.; Song, J. Rational Design of Bioactive Hydrogels toward Periodontal Delivery: From Pathophysiology to Therapeutic Applications. Adv. Funct. Mater. 2023, 33. [Google Scholar] [CrossRef]
- Mahdi Ibrahim, S.; sabri Al-mizragchi, A.; Haider, J. Metronidazole Potentiation by Panax Ginseng and Symphidium Officinale: A New Strategy for P. gingivalis Infection Control. 2023. [Google Scholar] [CrossRef]
- Ibraheem, D.R.; Hussein, N.N.; Sulaiman, G.M. Antibacterial Activity of Silver nanoparticles Against Pathogenic Bacterial Isolates from Diabetic Foot Patients. Iraqi Journal of Science 2023, 64, 2223–2239. [Google Scholar] [CrossRef]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Brazilian journal of infectious diseases 2011, 15, 305–311. [Google Scholar] [CrossRef]
- Christensen, G.D.; A Simpson, W.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Al-Hayanni, H.S. The Antibacterial and anti-biofilm effects of Sumac (Rhus coriaria L) fruits extracts against some multidrug-resistant pathogenic bacteria. Journal of the Faculty of Medicine Baghdad 2022, 64, 183–188. [Google Scholar]
- Bakir, S.H.; Fattma, A.A. Comparison of different methods for detection of biofilm production in multi-drug resistance bacteria causing pharyngotonsillitis. International journal of research in pharmacy and biosciences 2016, 3, 13–22. [Google Scholar]
- Baldiris, R.; TeherÃ, V; Vivas-Reyes, R.; Montes, A.; Arzuza, O. Anti-biofilm activity of ibuprofen and diclofenac against some biofilm producing Escherichia coli and Klebsiella pneumoniae uropathogens. African Journal of Microbiology Research 2016, 10, 1675–1684. [Google Scholar]
- Shallal, L.F.; A Ahmed, M. Experimental In vitro Study to Assess the Antibacte-rial Activity of Thymus vulgaris Oil on Streptococ-cus Sanguinis. J. Baghdad Coll. Dent. 2022, 34, 17–27. [Google Scholar] [CrossRef]
- M’ebid, A.K. Application of Combined Chlorhexidine and Hydrogen Peroxide In Periodontal Pockets. Bacteriological and Clinical Outcomes.
- Salah, R.; Abdulbaqi, H.R.; Mohammed, A.; Abdulkareem, A.A. Four-day randomized controlled crossover trial evaluating the antiplaque effect of a combination of green tea and Salvadora persica L. mouthwash. J. Herb. Med. 2020, 23, 100357. [Google Scholar] [CrossRef]
- Enad, H.H.; Nahidh, M. Salivary Cortisol as a Stress Biomarker and Total Viable Count of Salivary Bacterial Microbiome among COVID-19 Patients. J. Baghdad Coll. Dent. 2021, 33, 6–10. [Google Scholar] [CrossRef]
- Kadhum, W.N.; Al-Ogaidi, I.A.Z. Evaluation of Chitosan-Alginate Nanoparticle as A Stable Antibacterial Formula in Biological Fluids. Iraqi J. Sci. 2022, 2398–2418. [Google Scholar] [CrossRef]
- A Abdulkareem, A.; Abdulbaqi, H.R.; Milward, M.R. In Vitro Homeostasis of Rat Oral Epithelial Cell Cultures Following Withdrawal of Periodontal Pathogens. Braz. Dent. J. 2020, 31, 135–142. [Google Scholar] [CrossRef]
- Alaamery, S.K.; Al-Hayanni, H.S.A. Antibacterial and antibiofilm effect of sumac (Rhus coriaria L) fruits extracts against some multidrug_resistant pathogenic bacteria. J. Fac. Med. Baghdad 2022, 64, 183–188. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef]
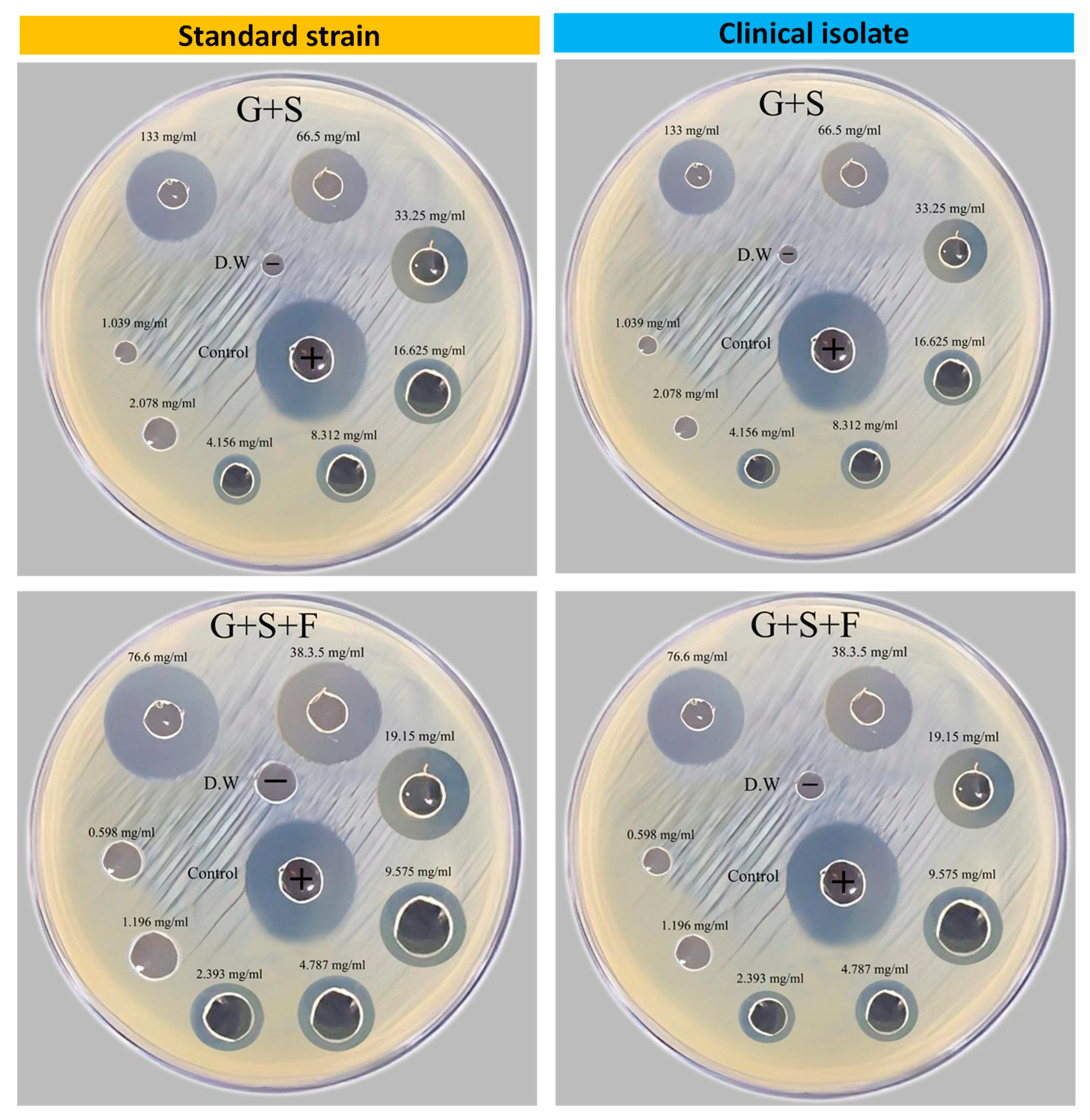
| Dilution | Total concentrations (mg/mL) | |
| G+S | G+S+F | |
| CD0: Control | 266 | 153.2 |
| D1: first dilution | 133 | 76.6 |
| D2: second dilution | 66.5 | 38.3 |
| D3: third dilution | 33.25 | 19.15 |
| D4: fourth dilution | 16.625 | 9.575 |
| D5: fifth dilution | 8.312 | 4.787 |
| D6: sixth dilution | 4.156 | 2.393 |
| D7: seventh dilution | 2.078 | 1.196 |
| D8: eighth dilution | 1.039 | 0.598 |
| Microorganisms | Concentration | Mean inhibition zone diameter ± SD (mm) | T-test | P-value | |
| G+S | G+S+F | ||||
| Standard strain | CD0 | 24.341±0.593 | 25.000±0.001 | 13.618 | 0.000 |
| D1 | 22.247±0.382 | 23.154±0.030 | |||
| D2 | 19.503±0.205 | 20.221±0.012 | |||
| D3 | 17.136±0.361 | 19.503±0.020 | |||
| D4 | 15.381±0.146 | 17.970±0.645 | |||
| D5 | 14.500±0.004 | 16.005±0.001 | |||
| D6 | 13.022±0.013 | 14.302±0.620 | |||
| D7 | 0.0 | 13.502±0.011 | |||
| D8 | 0.0 | 0.0 | |||
| Clinical isolate | CD0 | 22.654±0.534 | 23.712±0.004 | 12.796 | 0.000 |
| D1 | 21.254±0.125 | 22.0189±0.013 | |||
| D2 | 19.105±0.010 | 19.5890±0.013 | |||
| D3 | 17.071±0.004 | 18.3801±0.074 | |||
| D4 | 14..791±0.015 | 16.155±0.033 | |||
| D5 | 14.123±0.105 | 15.311±0.123 | |||
| D6 | 12.301±0.060 | 14.002±0.001 | |||
| D7 | 0.0 | 12.533±0.033 | |||
| D8 | 0.0 | 0.0 | |||
| Microorganisms | Minimum Inhibitory Concentration (MIC) mg/mL (Doses) | |
| G+S | G+S+F | |
| Standard strain | 8.312 (D5) | 2.393 (D6) |
| Clinical Isolate | 8.312 (D5) | 2.393 (D6) |
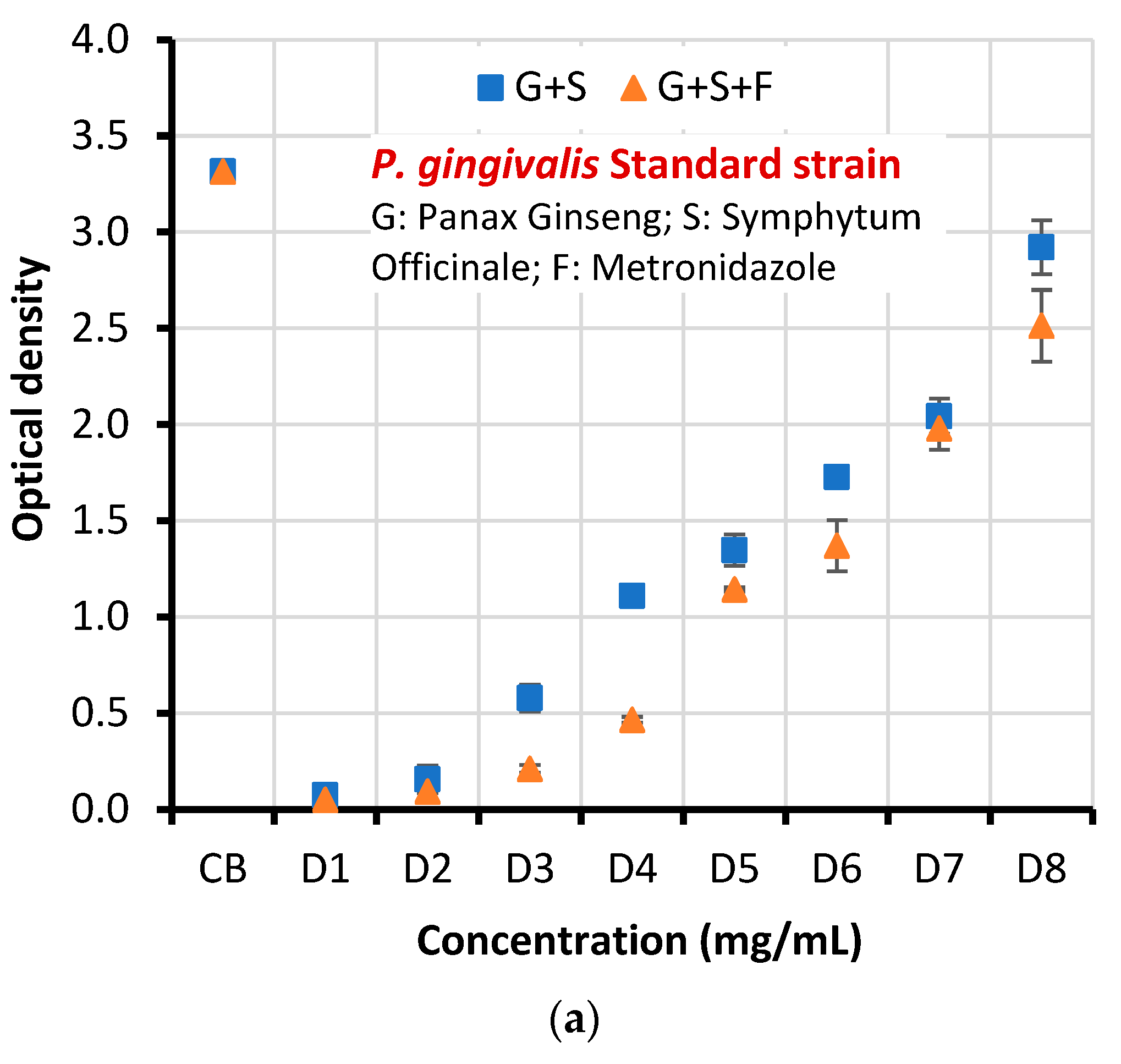
| Microorganism | Concentration | Mean optical density (OD) of biofilm ±SD | T-test | P-value | |
| G+S | G+S+F | ||||
| Standard strain | CB | 3.315±0.098 | 3.315±0.098 | 3.859 | 0.005 |
| D1 | 0.074±0.004 | 0.051±0.022 | |||
| D2 | 0.157±0.070 | 0.094±0.002 | |||
| D3 | 0.579±0.069 | 0.211±0.021 | |||
| D4 | 1.109±0.037 | 0.466±0.015 | |||
| D5 | 1.347±0.081 | 1.144±0.010 | |||
| D6 | 1.727±0.047 | 1.371±0.133 | |||
| D7 | 2.043±0.092 | 1.980±0.112 | |||
| D8 | 2.921±0.14 | 2.513±0.187 | |||
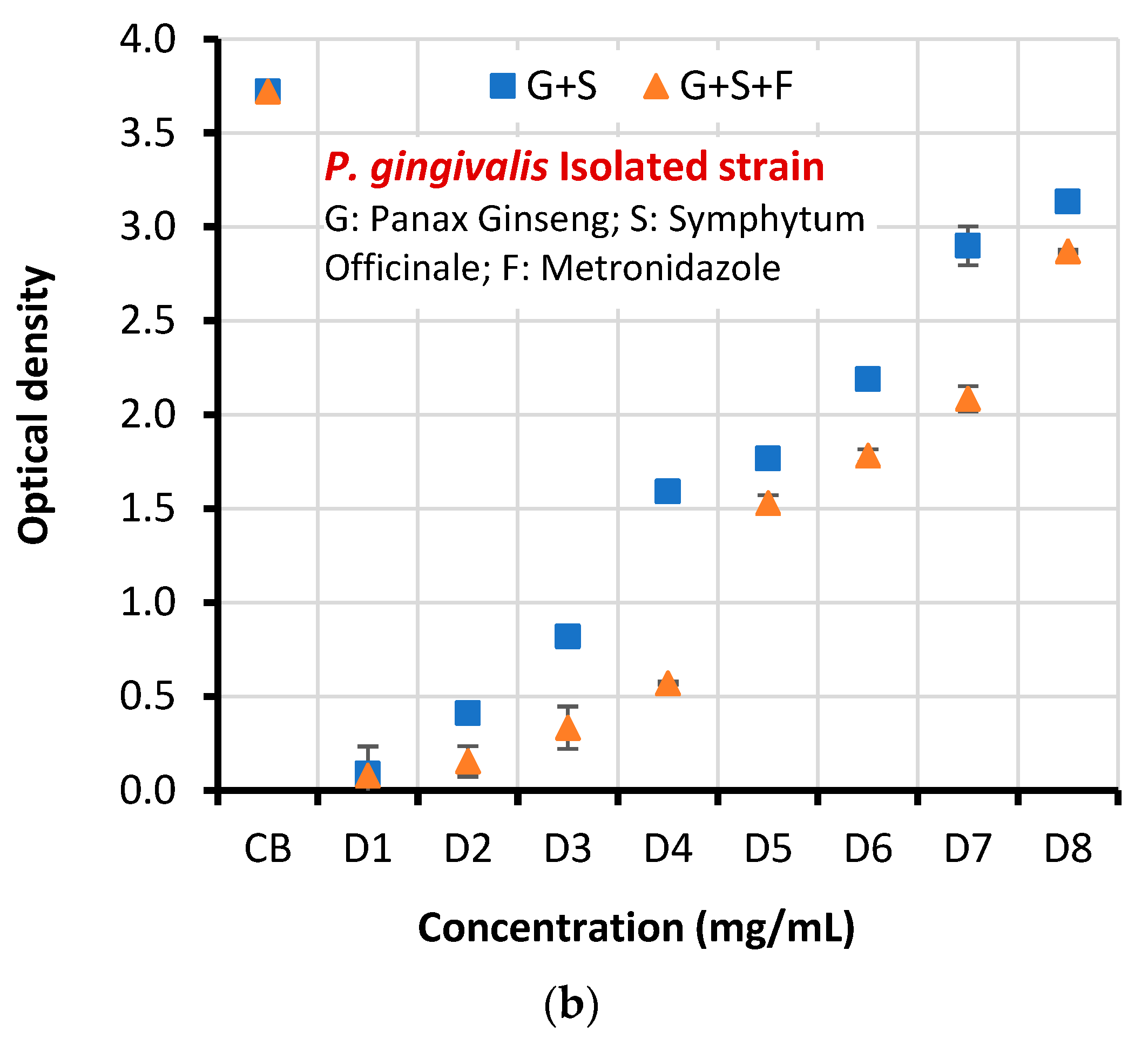
| Clinical isolate | CB | 3.722±0.069 | 3.722±0.069 | 4.402 | 0.002 |
| D1 | 0.090±0.145 | 0.080±0.001 | |||
| D2 | 0.411±0.001 | 0.155±0.081 | |||
| D3 | 0.820±0.040 | 0.334±0.113 | |||
| D4 | 1.591±0.005 | 0.571±0.008 | |||
| D5 | 1.766±0.208 | 1.530±0.041 | |||
| D6 | 2.189±0.050 | 1.782±0.034 | |||
| D7 | 2.900±0.103 | 2.085±0.068 | |||
| D8 | 3.135±0.022 | 2.870±0.011 | |||
| Microorganism | Concentration | Percentage inhibition of biofilm formation [(1-(OD with dilution/OD with control))×100] |
|
|---|---|---|---|
| G+S | G+S+F | ||
| Standard strain | D1 | 97.76 | 98.46 |
| D2 | 95.26 | 97.16 | |
| D3 | 82.53 | 93.63 | |
| D4 | 66.54 | 85.94 | |
| D5 | 59.36 | 65.49 | |
| D6 | 47.90 | 58.64 | |
| D7 | 38.37 | 40.27 | |
| D8 | 11.88 | 24.19 | |
| Clinical isolate | D1 | 97.58 | 97.85 |
| D2 | 88.95 | 95.83 | |
| D3 | 77.96 | 91.02 | |
| D4 | 57.25 | 84.65 | |
| D5 | 52.55 | 58.89 | |
| D6 | 41.18 | 52.12 | |
| D7 | 22.08 | 43.98 | |
| D8 | 15.77 | 22.89 | |
| X2 | 3.814 | ||
| p value | 0.00021 | ||
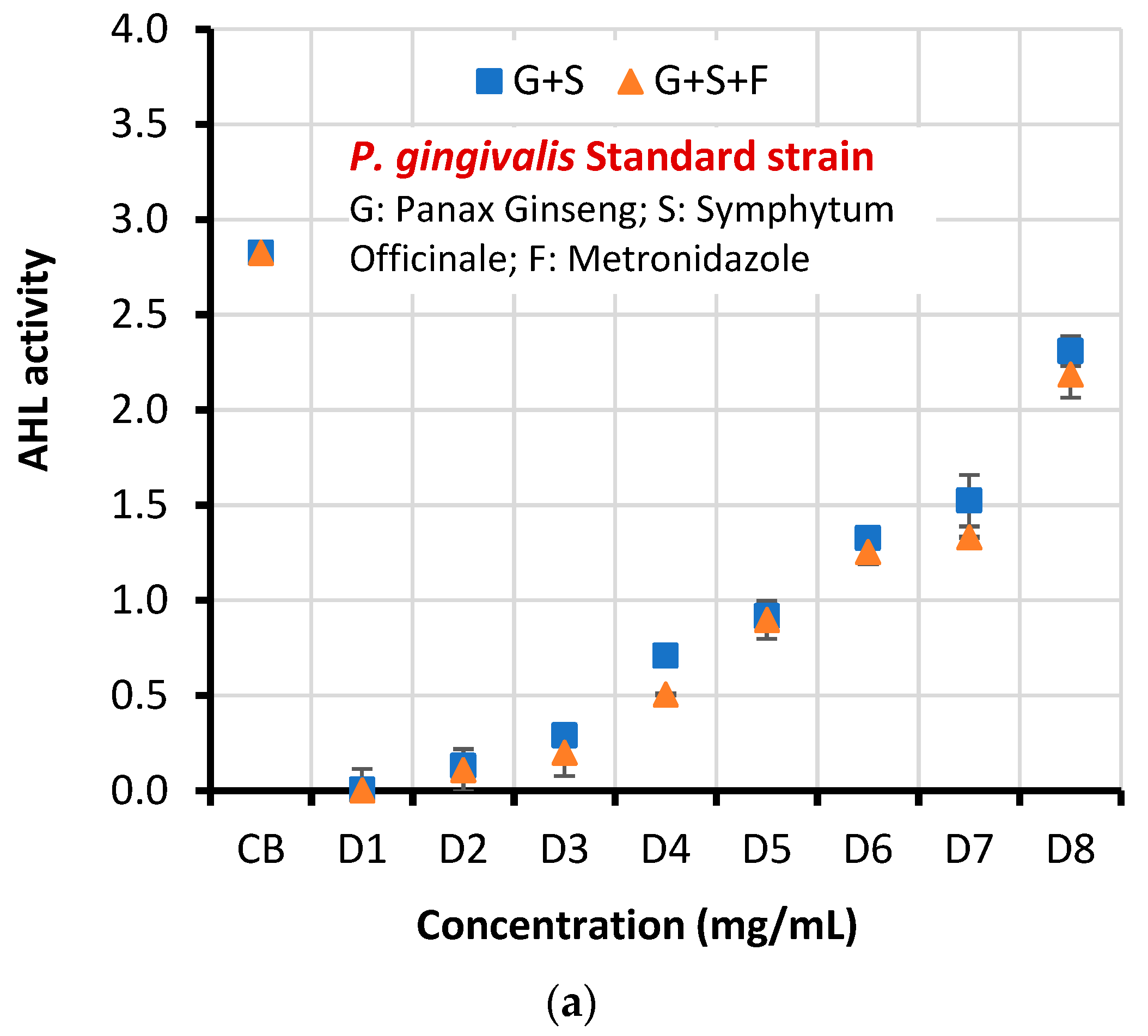
| Microorganism | Concentration | AHL activity ± SD | T-test | P-value | |
| G+S | G+S+F | ||||
| Standard strain | CB | 2.826±0.037 | 3.264 | 0.011 | |
| D1 | 0.006±0.110 | 0.003±0.001 | |||
| D2 | 0.130±0.033 | 0.109±0.110 | |||
| D3 | 0.290±0.031 | 0.201±0.122 | |||
| D4 | 0.709±0.001 | 0.506±0.004 | |||
| D5 | 0.917±0.025 | 0.898±0.100 | |||
| D6 | 1.327±0.039 | 1.254±0.062 | |||
| D7 | 1.524±0.135 | 1.332±0.004 | |||
| D8 | 2.310±0.078 | 2.187±0.122 | |||
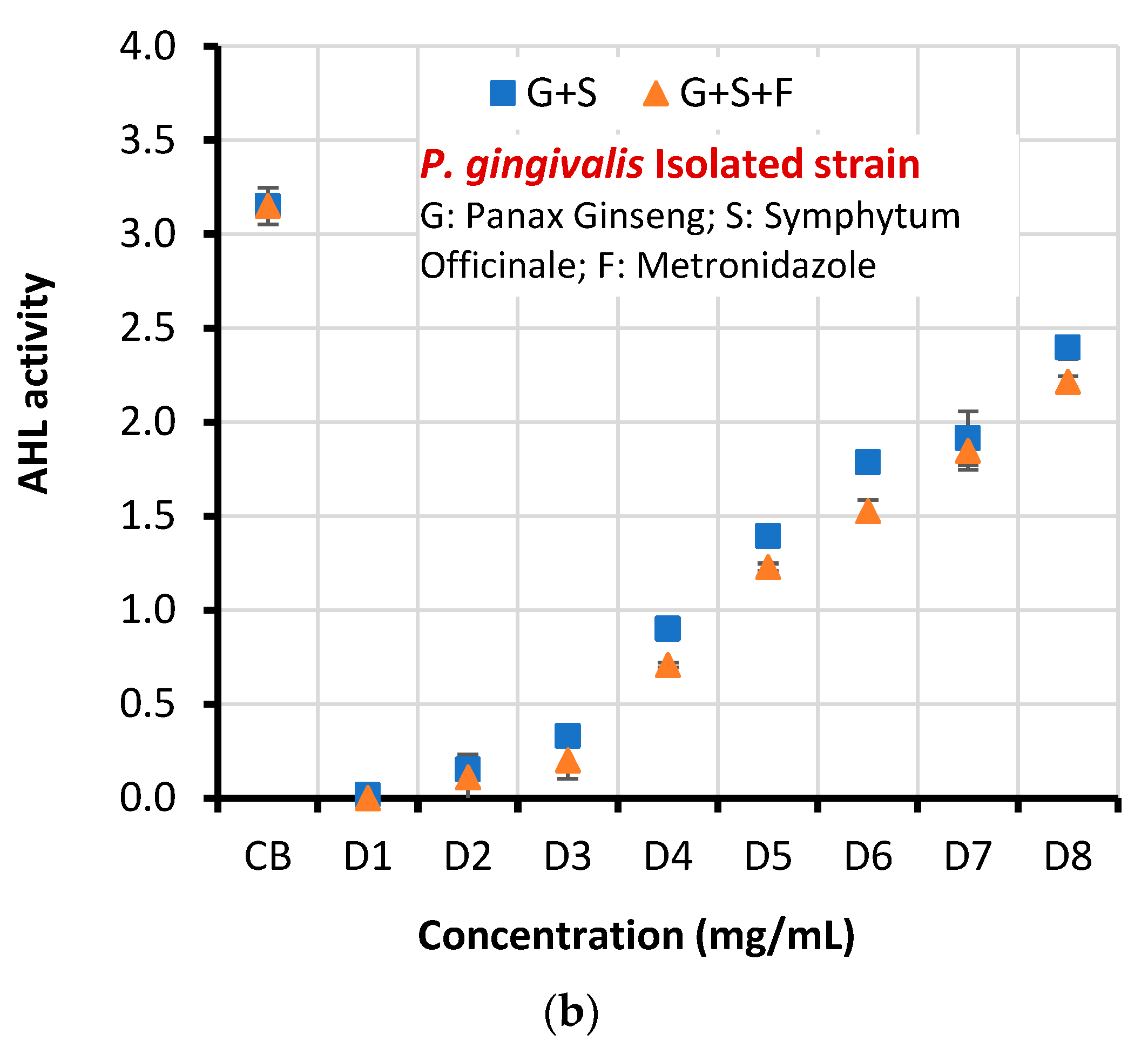
| Clinical isolate | CB | 3.151±0.098 | |||
| D1 | 0.017±0.002 | 0.000 | 3.204 | 0.013 | |
| D2 | 0.152±0.011 | 0.111±0.120 | |||
| D3 | 0.331±0.003 | 0.201±0.098 | |||
| D4 | 0.901±0.014 | 0.709±0.013 | |||
| D5 | 1.394±0.010 | 1.229±0.020 | |||
| D6 | 1.787±0.009 | 1.527±0.058 | |||
| D7 | 1.915±0.143 | 1.847±0.101 | |||
| D8 | 2.397±0.060 | 2.215±0.029 | |||
| Microorganism | Concentration |
Percentage inhibition of AHL activity [(1-(AHL with dilution/AHL with control))×100] |
|
| G+S | G+S+F | ||
| Standard strain (ATCC 33277) | D1 | 99.78 | 99.89 |
| D2 | 95.39 | 96.14 | |
| D3 | 89.73 | 92.88 | |
| D4 | 74.91 | 82.09 | |
| D5 | 67.55 | 68.22 | |
| D6 | 53.04 | 55.62 | |
| D7 | 46.07 | 52.86 | |
| D8 | 18.25 | 22.61 | |
| Clinical isolate | D1 | 99.46 | 100 |
| D2 | 95.17 | 96.47 | |
| D3 | 89.49 | 93.62 | |
| D4 | 71.40 | 77.49 | |
| D5 | 55.76 | 60.99 | |
| D6 | 43.28 | 51.53 | |
| D7 | 39.22 | 41.38 | |
| D8 | 23.92 | 29.70 | |
| X2 | 3.291 | ||
| p value | 0.00013 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





