Submitted:
18 August 2023
Posted:
21 August 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Working Principles and Properties of TiO2-Based Photocatalytic Building Materials
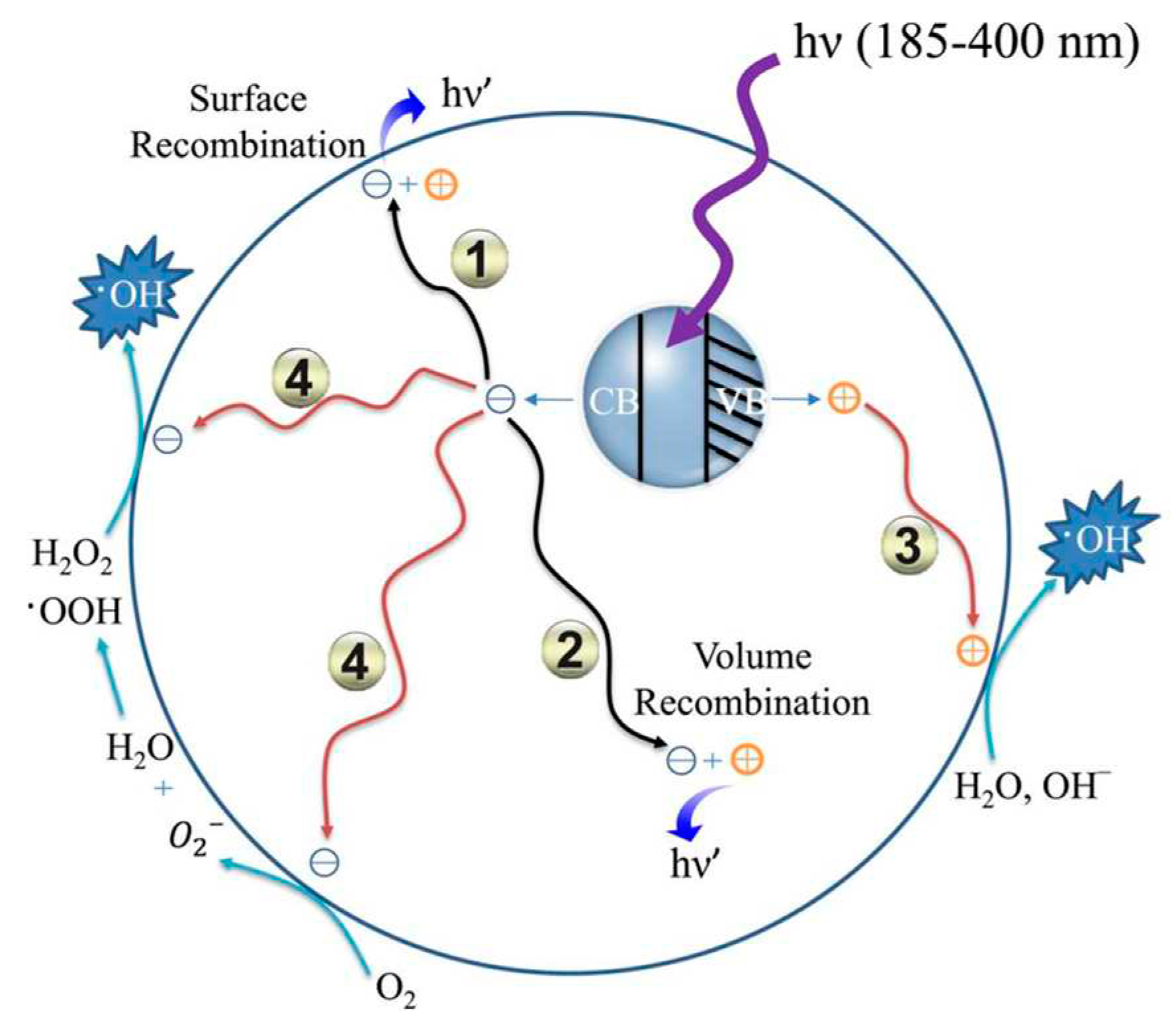
2.1. The Basic Principle Mechanism of Photocatalysts
2.2. The Mechanism of Photocatalysts for Air Purification and Deodorisation
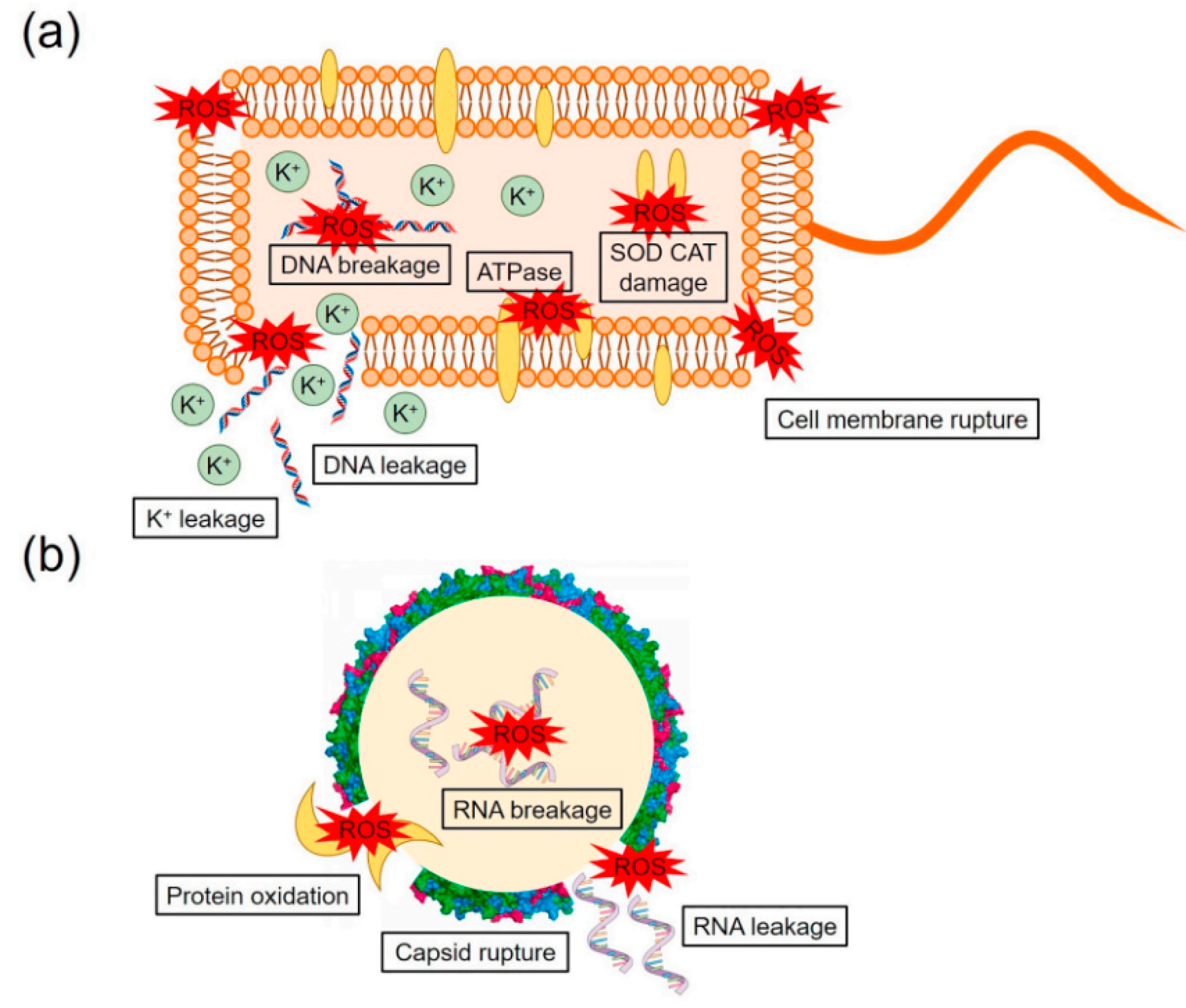
2.3. The Mechanism of Photocatalysts for Disinfection
3. The Study Status of TiO2-Based Photocatalytic Building Materials
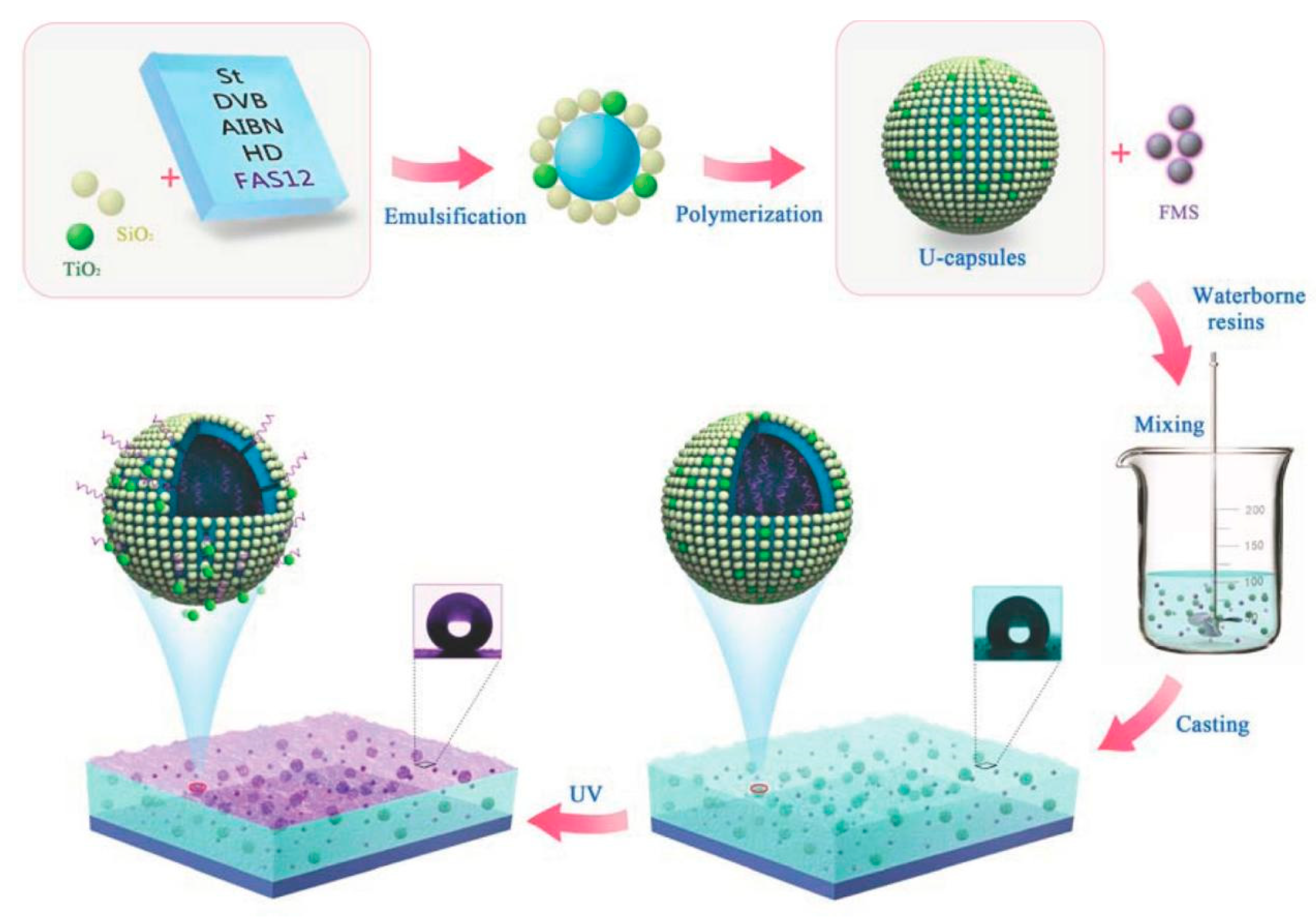
4. Preparation of TiO2 Photocatalytic Building Materials
4.1. Sol-Gel Method
4.2. Hydrothermal Method
4.3. Spray-Drying Method
- Production of nanoparticles with a narrow size distribution to enhance the photocatalytic activity of building materials.
- An efficient and scalable method for producing nanoparticles with a uniform distribution.
- Allows the phase composition, crystal size and surface area of nanoparticles to be adjusted.
4.4. Anodic Oxidation Method
4.5. Microwave-Assisted Method
5. Strategies for Improve TiO2 Photocatalytic Efficiency
5.1. Strategies for REDUCING aggregation of TiO2
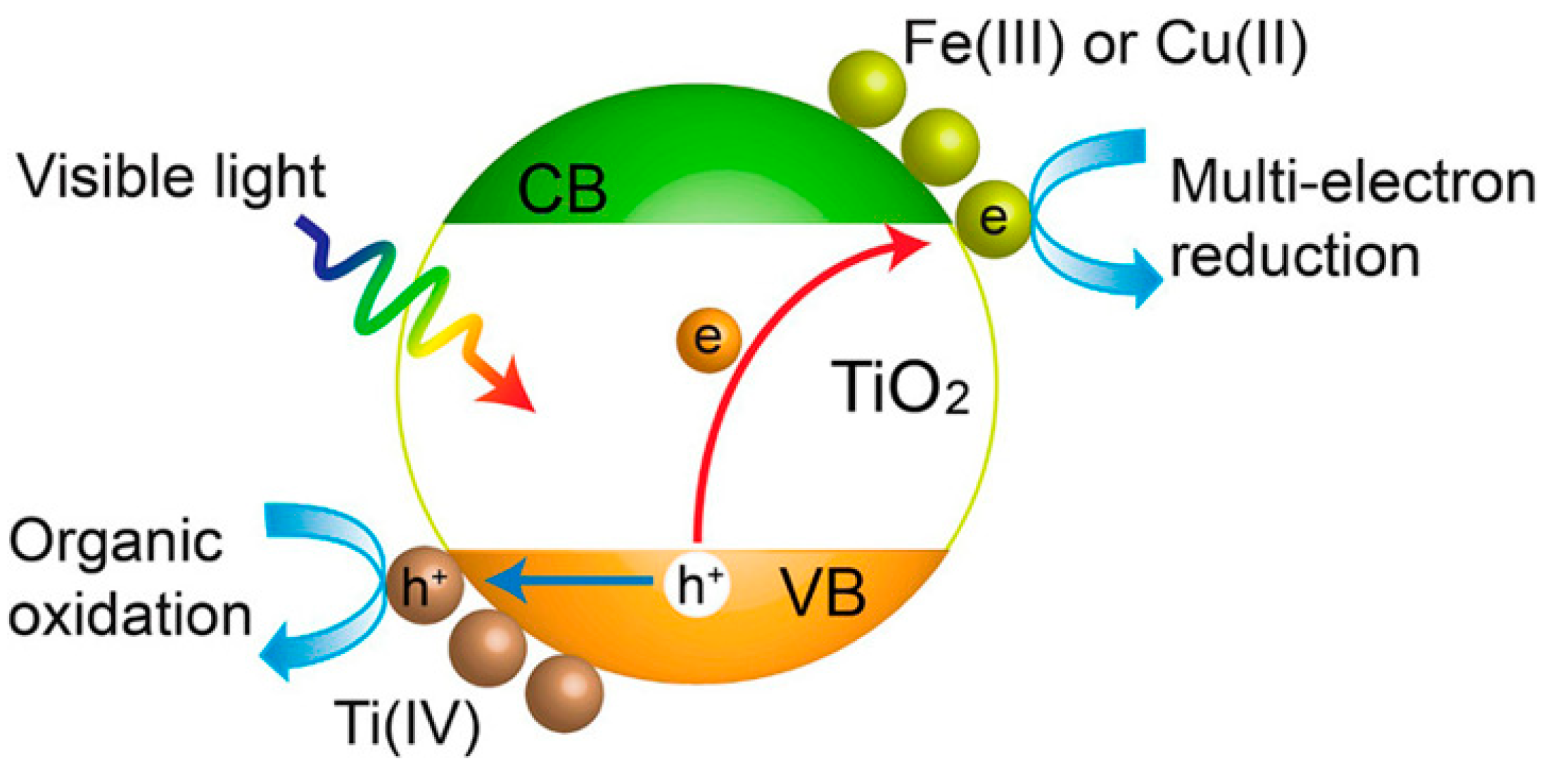
5.2. Strategies for Improving the Photocatalytic Efficiency of TiO2
6. Application Status and Future Prospects of TiO2-Based Building Materials
6.1. Application Status and Key Influencing Factors of Photocatalytic Building Materials
6.2. Future Perspective and Related Problem Discussions
- Stability of photocatalytic materials: The stability of photocatalytic materials is critical in practical applications. It is important to study the stability of these materials, which undergo prolonged exposure to light and environmental factors, to improve their lifetime. Some factors that may affect the stability of photocatalytic materials include humidity, temperature, pH, pollutants, and microorganisms. Moreover, the photocatalytic materials may also degrade the substrates or binders that they are attached to, resulting in a reduction of mechanical strength and durability [82]. Therefore, developing strategies to enhance the stability of photocatalytic materials and their substrates or binders is necessary for their long-term performance.
- Photocatalytic reaction rate: The photocatalytic reaction rate is a key issue that affects the practical application of photocatalytic building materials. It is necessary to ensure that the reaction rate is fast enough to effectively degrade harmful substances in the air. Therefore, exploring different photocatalytic reaction mechanisms is necessary to increase the reaction rate. Some factors that may influence the reaction rate include light intensity, wavelength, catalyst loading, surface area, morphology, crystallinity, doping, and co-catalysts [4]. Moreover, the reaction rate may also depend on the type and concentration of pollutants, as well as the presence of other substances that may interfere with the photocatalysis [4]. Therefore, optimizing these factors to enhance the reaction rate is essential for achieving high efficiency and selectivity of photocatalysis.
- Selectivity of photocatalytic materials: The selectivity of photocatalytic materials refers to their ability to selectively oxidize or reduce specific pollutants in the presence of other substances [83]. Selectivity is important for achieving high efficiency and avoiding unwanted by-products or secondary pollution. However, most photocatalytic materials have low selectivity and tend to react with various organic and inorganic compounds in the air [84]. This may lead to a decrease in photocatalytic activity and an increase in energy consumption. Therefore, designing and modifying photocatalytic materials with high selectivity for target pollutants is a key challenge for their application in air pollution control.
- Economics of photocatalytic materials: The economics of photocatalytic materials involves the cost-effectiveness and feasibility of their production and application. The cost of photocatalytic materials depends on several factors, such as the type and amount of raw materials, the synthesis method, the fabrication process, the scale-up potential, and the maintenance cost. The benefits of photocatalytic materials depend on their performance, durability, environmental impact, and social acceptance [58]. Therefore, evaluating and optimizing the economics of photocatalytic materials is essential for their widespread adoption and implementation in air pollution prevention.
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jason, K.M.; et al. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer's disease—Evidence from epidemiological and animal studies. Biomed. J. 2018, 41, 141–162. [Google Scholar]
- Stanaszek-Tomal, E. Anti-Smog Building and Civil Engineering Structures. Processes 2021, 9, 1446. [Google Scholar] [CrossRef]
- Majbauddin, A.; Onishi, K.; Otani, S.; et al. Association between Asian Dust-Borne Air Pollutants and Daily Symptoms on Healthy Subjects: A Web-Based Pilot Study in Yonago, Japan. J. Environ. Public Health 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- He, F.; Jeon, W.; Choi, W. Photocatalytic air purification mimicking the self-cleaning process of the atmosphere. Nat. Commun. 2021, 12, 2528. [Google Scholar] [CrossRef]
- Saputera, W.H.; Amri, A.F.; Daiyan, R.; et al. Photocatalytic Technology for Palm Oil Mill Effluent (POME) Wastewater Treatment: Current Progress and Future Perspective. Materials 2021, 14, 2846. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem Zvesti 2023, 77, 677–701. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Jenny, S.D.; et al. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar]
- Gopalan, A.I.; et al. Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; et al. One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4, 576–584. [Google Scholar] [CrossRef]
- Alonso-Tellez, A.; et al. Comparison of Hombikat UV100 and P25 TiO2 performance in gas-phase photocatalytic oxidation reactions. J. Photochem. Photobiol. A Chem. 2012, 250, 58–65. [Google Scholar] [CrossRef]
- Resende, S.F.; Nunes, E.H.M.; Houmard, M.; et al. Simple sol-gel process to obtain silica-coated anatase particles with enhanced TiO2-SiO2 interfacial area. J. Colloid Interface Sci. 2014, 433, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekar, M.; et al. Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater. Res. Bull. 2018, 97, 238–243. [Google Scholar] [CrossRef]
- Yuan, T.; Yao, W. Preparation and Properties of g-C3N4-TiO2 Cement-Based Materials Supported by Recycled Concrete Powder. Catalysts 2023, 13, 312. [Google Scholar] [CrossRef]
- Fang, Y.; et al. Preparation and Properties of Magnesium Cement-Based Photocatalytic Materials. Catalysts 2022, 12, 420. [Google Scholar] [CrossRef]
- Domínguez-Espíndola, R.B.; et al. A critical review on advances in TiO2-based photocatalytic systems for CO2 reduction. Appl. Therm. Eng. 2022, 216, 119009. [Google Scholar] [CrossRef]
- Kumer, A.; Chakma, U. Developing the amazing photocatalyst of ZnAg2GeSe4, ZnAg2Ge0.93Fe0.07Se4 and ZnAg2Ge0.86Fe0.14Se4 through the computational explorations by four DFT functionals. Heliyon 2021, 7, e07467. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies—Abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; et al. Volatile organic compounds (VOCs) removal by photocatalysts: A review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Bouwman, A.F.; Van der Hoek, K.W.; et al. Global air emission inventories for anthropogenic sources of NOx, NH3 and N2O in 1990. Environ. Pollut. 1998, 102, 135–148. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Zhang, W. Effects of substrates on N2O emissions in an anaerobic ammonium oxidation (anammox) reactor. Springerplus 2016, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- Razavi, Z.; et al. Adsorption and photocatalytic removal of SO2 using natural and synthetic zeolites-supported TiO2 in a solar parabolic trough collector. J. Clean. Prod. 2021, 310, 127376. [Google Scholar] [CrossRef]
- Mendoza, J.A.; et al. Photocatalytic performance of TiO2 and WO3/TiO2 nanoparticles coated on urban green infrastructure materials in removing nitrogen oxide. Int. J. Environ. Sci. Technol. 2017, 15, 581–592. [Google Scholar] [CrossRef]
- Chen, D.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Yousefi, A.; Allahverdi, A. Effective dispersion of nano-TiO2 powder for enhancement of photocatalytic properties in cement mixes. Constr. Build. Mater. 2013, 41, 224–230. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Shin, E.W. Morphological effect of TiO2 catalysts on photocatalytic degradation of methylene blue. J. Ind. Eng. Chem. 2011, 17, 397–400. [Google Scholar] [CrossRef]
- Igenepo John, K.; et al. Unravelling the effect of crystal dislocation density and microstrain of TiO2 nanoparticles on tetracycline removal performance. Chem. Phys. Lett. 2021, 776, 138725. [Google Scholar] [CrossRef]
- Liu, C.; et al. Applications and Advances in TiO2 Based Photocatalytic Building Materials. J. Phys. Conf. Ser. 2021, 2011, 012049. [Google Scholar] [CrossRef]
- Cárdenas, C.; et al. Functionalized building materials: Photocatalytic abatement of NOx by cement pastes blended with TiO2 nanoparticles. Constr. Build. Mater. 2012, 36, 820–825. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Demeestere, K.; et al. Heterogeneous photocatalytic removal of toluene from air on building materials enriched with TiO2. Build. Environ. 2008, 43, 406–414. [Google Scholar] [CrossRef]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.-C.; Poon, C.-S. Photocatalytic cement-based materials: Comparison of nitrogen oxides and toluene removal potentials and evaluation of self-cleaning performance. Build. Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Aïssa, A.H.; et al. Characterization and photocatalytic performance in air of cementitious materials containing TiO2. Case study of formaldehyde removal. Appl. Catal. B Environ. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Pirola, C.; et al. Photocatalytic coatings for building industry: Study of 1 year of activity in the NO x degradation. J. Coat. Technol. Res. 2011, 9, 453–458. [Google Scholar] [CrossRef]
- Karapati, S.; et al. TiO2 functionalization for efficient NOx removal in photoactive cement. Appl. Surf. Sci. 2014, 319, 29–36. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; et al. Photocatalytic NOx abatement by calcium aluminate cements modified with TiO2, Improved NO2 conversion. Cem. Concr. Res. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.S. Photocatalytic NO x degradation of concrete surface layers intermixed and spray-coated with nano-TiO2 : Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; et al. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef]
- Seo, D.; Yun, T.S. NOx removal rate of photocatalytic cementitious materials with TiO2 in wet condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Poon, C.S. Superior photocatalytic NOx removal of cementitious materials prepared with white cement over ordinary Portland cement and the underlying mechanisms. Cem. Concr. Compos. 2018, 90, 42–49. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Zanganeh, S.; et al. Hydrothermal synthesis and characterization of TiO2 nanostructures using LiOH as a solvent. Adv. Powder Technol. 2011, 22, 336–339. [Google Scholar] [CrossRef]
- Keerthana, B.G.T.; et al. Hydrothermal synthesis and characterization of TiO2 nanostructures prepared using different solvents. Mater. Lett. 2018, 220, 20–23. [Google Scholar] [CrossRef]
- Payan, A.; Fattahi, M.; Roozbehani, B. Synthesis, characterization and evaluations of TiO2 nanostructures prepared from different titania precursors for photocatalytic degradation of 4-chlorophenol in aqueous solution. J. Environ. Health Sci. Eng. 2018, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; et al. Hydrothermal synthesis of different TiO2 nanostructures: Structure, growth and gas sensor properties. J. Mater. Sci. Mater. Electron. 2012, 23, 2024–2029. [Google Scholar] [CrossRef]
- Carne-Sanchez, A.; et al. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Masahashi, N. Fabrication of a TiO2 photocatalyst by anodic oxidation of Ti in an acetic acid electrolyte. Surf. Coat. Technol. 2014, 240, 226–232. [Google Scholar] [CrossRef]
- Lai, L.; et al. Enhanced adhesive strength between SU-8 photoresist and titanium substrate by an improved anodic oxidation method for high aspect-ratio microstructures. J. Micromech. Microeng. 2019, 29, 047002. [Google Scholar] [CrossRef]
- Falk, G.S.; et al. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanoparticle Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Chen, J.; et al. Recent progress in enhancing photocatalytic efficiency of TiO2 -based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Liao, C.; Wu, Q.; Teng, S.; et al. Nanocomposite gels via in-situ photoinitiation and disassembly of TiO2-Clay composites with polymers applied as UV protective films. ACS Appl. Mater. Interfaces 2014, 6, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Ibusuki, T.; Takeuchi, K. Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis—Science Direct. J. Mol. Catal. 1994, 88, 93–102. [Google Scholar] [CrossRef]
- Ghedini, E.; et al. Multifunctional and Environmentally Friendly TiO2-SiO2 Mesoporous Materials for Sustainable Green Buildings. Molecules 2019, 24, 4226. [Google Scholar] [CrossRef]
- Zou, L.; et al. Removal of VOCs by photocatalysis process using adsorption enhanced TiO2–SiO2 catalyst. Chem. Eng. Process. Process Intensif. 2006, 45, 959–964. [Google Scholar] [CrossRef]
- Chen, K.; et al. Fabrication of All-Water-Based Self-Repairing Superhydrophobic Coatings Based on UV-Responsive Microcapsules. Adv. Funct. Mater. 2015, 25, 1035–1041. [Google Scholar] [CrossRef]
- Joo, J.B.; et al. Mesoporous Anatase Titania Hollow Nanostructures though Silica-Protected Calcination. Adv. Funct. Mater. 2012, 22, 166–174. [Google Scholar] [CrossRef]
- Yang, C.C.; et al. Using C-doped TiO2 Nanoparticles as a Novel Sonosensitizer for Cancer Treatment. Antioxidants 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Yi, X.; Liu, B.; Zhang, J.; Yi, X. Mesoporous TiO2/SiO2/Ag ternary composite aerogels for high photocatalysis. New J. Chem. 2019, 43, 6234–6241. [Google Scholar] [CrossRef]
- Nadeem Raza, W.R.; Gul, H.; Azam, M.; Lee, J.; Vikrant, K.; Kim, K.-H. Solar-light-active silver phosphate/titanium dioxide/silica heterostructures for photocatalytic removal of organic dye. J. Clean. Prod. 2020, 254, 120031. [Google Scholar] [CrossRef]
- Zhang, W.; et al. Micro/nano-bubble assisted synthesis of Au/TiO2@CNTs composite photocatalyst for photocatalytic degradation of gaseous styrene and its enhanced catalytic mechanism. Environ. Sci. Nano 2019, 6, 948–958. [Google Scholar] [CrossRef]
- Liang, Z.; et al. Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B Environ. 2019, 243, 711–720. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Ngoc, M.; Nguyen, M.V. Enhanced photocatalytic activity of nanohybrids TiO2 /CNTs materials. Mater. Lett. 2016, 165, 247–251. [Google Scholar] [CrossRef]
- Wang, S.G.; et al. Photocatalytic properties of TiO2/cnts films with different morphology on stainless steel substrates. Nano 2014, 9, 1450003. [Google Scholar] [CrossRef]
- Olana, M.H.; et al. Citrus sinensis and Musa acuminata Peel Waste Extract Mediated Synthesis of TiO2/rGO Nanocomposites for Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Bioinorg. Chem. Appl. 2022, 2022, 5978707. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, Y.; Liu, S.; et al. Comparing Graphene-TiO2 Nanowire and Graphene-TiO2 Nanoparticle Composite Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, S.; Teng, Y.; et al. Efficient removal of herbicide 2,4-dichloropheNOxyacetic acid from water using Ag/reduced graphene oxide co-decorated TiO2 nanotube arrays. J. Hazard. Mater. 2012, 241–242, 323–330. [Google Scholar] [CrossRef]
- Dong, W.; et al. Synchronous role of coupled adsorption and photocatalytic oxidation on ordered mesoporous anatase TiO2–SiO2 nanocomposites generating excellent degradation activity of RhB dye. Appl. Catal. B Environ. 2010, 95, 197–207. [Google Scholar] [CrossRef]
- Dholam, R.; et al. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; et al. Energy-level matching of Fe(III) ions grafted at surface and doped in bulk for efficient visible-light photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064. [Google Scholar] [CrossRef] [PubMed]
- Min Liu, R.I.M.N.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced Photoactivity with Nanocluster-Grafted Titanium Dioxide Photocatalysts. ACS NANO 2014, 8, 7229–7238. [Google Scholar]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; et al. A Study on Doped Heterojunctions in TiO2 Nanotubes: An Efficient Photocatalyst for Solar Water Splitting. Sci. Rep. 2017, 7, 14314. [Google Scholar] [CrossRef]
- Chen, M.; et al. Photocatalytic Oxidation of NOx under Visible Light on Asphalt-Pavement Surface. J. Mater. Civ. Eng. 2017, 29. [Google Scholar] [CrossRef]
- Khan, T.; et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Barolo, G.; et al. Mechanism of the Photoactivity under Visible Light of N-Doped Titanium Dioxide. Charge Carriers Migration in Irradiated N-TiO2 Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2012, 116, 20887–20894. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Xing, M.; Leghari, S.A.K. ; Sajjad, S, Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Mancuso, A.; et al. Enhanced visible-light-driven photodegradation of Acid Orange 7 azo dye in aqueous solution using Fe-N co-doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360. [Google Scholar] [CrossRef]
- Weerasinghe, R.; et al. Study on the effect of Fe and N co-doped supported TiO2/GF photocatalytic oxidation of nitrobenzene wastewater. E3S Web Conf. 2021, 237, 01036. [Google Scholar]
- Liua, Y.; Zhu, S.; Li, D. TiN nanoparticles hybridized with Fe, N co-doped carbon nanosheets composites as highly efficient electrocatalyst for oxygen reduction reaction. Chem. Eng. J. 2020, 400, 125968. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Moradi, S.; Kakavandi, B. LED-assisted sonocatalysis of sulfathiazole and pharmaceutical wastewater using N,Fe co-doped TiO2@SWCNT: Optimization, performance and reaction mechanism studies. J. Water Process Eng. 2020, 38, 101693. [Google Scholar] [CrossRef]
- Chew, M.Y.L.; Conejos, S.; Law, J.S.L. Green maintainability design criteria for nanostructured titanium dioxide (TiO2) façade coatings. Int. J. Build. Pathol. Adapt. 2017, 35, 139–158. [Google Scholar] [CrossRef]
- Tsukamoto, D.; et al. Selective photocatalytic oxidation of alcohols to aldehydes in water by TiO2 partially coated with WO3. Chemistry 2011, 17, 9816–9824. [Google Scholar] [CrossRef]
- Pastor, A.; et al. ZnO on rice husk: A sustainable photocatalyst for urban air purification. Chem. Eng. J. 2019, 368, 659–667. [Google Scholar] [CrossRef]
| Building material | Method | Light source | Efficiency | Reference/Year |
|---|---|---|---|---|
| cement mortar | Mixing with cement mortar | UV | The degradation rate of NOx can reach 40.0% | [32]2009 |
| cement mortar | Mix with mortar (2 and 5 wt%) | UV | NO (400 ppb) removal rate: 90 μ mol/(m2⋅h); Toluene (200ppb) removal: 100% | [33]2011 |
| ceramic tiles | Photocatalyst brushing on the top surface of tiles | UV | Toluene (17-35 ppbv) removal rate up to 512 μ g/(m2⋅h) | [31]2008 |
| cement mortar | Mix with mortar (1-10% wt%) | UV | Formaldehyde (20 ppm) removal rate up to 65% | [34]2011 |
| portland cement | Mix with cement slurry (0.5-5 wt%.) | UV | NOx (1 ppmv) removal amount:120 μmol/m2, 65 h | [29]2012 |
| Wall paint and plaster | Mixing with 2 wt% TiO2 | UV | NOx (400 ppb) conversion range ranges from 80% of 50 days samples to 30% of 1 year samples | [35]2011 |
| cement mortar | Mixed cement (0.5-2.5wt%) | Simulated sunlight | The removal rate of NO (1 ppm) can reach 15% | [36]2014 |
| cement mortar | Mixing with cement mortar | UV | The degradation efficiency of NOx (1000 ppb) can reach 60.4% | [37]2015 |
| cement mortar | Combine photocatalytic materials with building materials using mixing and spraying methods respectively | UV | NO (1000 ppb) removal condition: Material for spraying method: 220 μ Mol/(m2 Å h), mixed material: 80 μ mol/ (m2.h) | [38]2017 |
| cement mortar | Mix with cement mortar (0.5~2.5 wt%) | UVSunlightVisible light | The highest conversion rates of NO (500 ppb) are 38% (P25), 15% (P25), and 5.5% (Fe TiO2 and V-TiO2), respectively | [39]2017 |
| cement mortar | Mixing with cement mortar (1-10wt%) | UV | NO (1ppm) removal rate: 72% | [40]2017 |
| Concrete and gypsum | Coating deposited on the .test concrete wall | Sunlight | Efficient removal of NOx from polluted air. | [41]2017 |
| White cement (WC) and ordinary Portland cement paste | Mixed cement slurry (2-5wt%) | UV | NO (1000ppb) removal condition: OPC is 380 μ Mol/(m2. h) and WC at 500 μ mol/(m2⋅h) | [42]2018 |
| Building Name | Location | Building Materical | Benefits | Difficulties |
|---|---|---|---|---|
| Palazzo Italia | Milan, Italy | TiO2-based photocatalytic coating on façade | Purifies air, reduces carbon emissions, energy-efficient design, use of renewable energy sources | Cost of installation and maintenance |
| Jubilee Church | Rome, Italy | TiO2-coated façade | Reduces air pollution, improves air quality by breaking down harmful pollutants | Limited effectiveness in high-traffic areas |
| Palazzo Lombardia | Milan, Italy | TiO2-coated façade | Purifies air, reduces energy consumption by reflecting sunlight and reducing need for air conditioning | Cost of installation and maintenance |
| Bullitt Center | Seattle, USA | TiO2-coated roof | Purifies air, reduces air pollution by breaking down harmful pollutants | Limited effectiveness in high-traffic areas |
| Denby Dale Passivhaus | Yorkshire, UK | TiO2-coated façade | Purifies air, reduces air pollution, reduces energy consumption for heating and cooling | Cost of installation and maintenance |
| Edificio Malecon | Mexico City, Mexico | TiO2-coated façade | Reduces air pollution, improves air quality, self-cleaning properties, reduces energy consumption | Cost of installation and maintenance |
| Haze-Free Tower | Beijing, China | TiO2-coated façade | Reduces air pollution, improves air quality, enhances aesthetics, self-cleaning properties | Limited effectiveness in high-traffic areas |
| Queen's Building | Bristol, UK | TiO2-coated façade | Purifies air, reduces air pollution, self-cleaning properties | Limited effectiveness in shaded areas |
| Nanjing Green Lighthouse | Nanjing, China | TiO2-coated façade | Purifies air, reduces energy consumption, improves air quality, self-cleaning properties | Cost of installation and maintenance |
| LaFargeHolcim Headquarters | Switzerland | TiO2-coated façade | Reduces air pollution, self-cleaning properties, improves energy efficiency | Limited effectiveness in high-pollution areas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




