1. Introduction.
Arterial stiffness, generally measured using direct or indirect measurements of pulse wave velocity (PWV), is a strong risk factor for major adverse cardiovascular events (MACE) (1). Oxidative stress is an important contributor to arterial stiffness (2). However, studies aimed at using antioxidant therapy to reduce PWV have generally found only
small reductions in PWV (3,4). Most studies have investigated the effects of only one or two different antioxidants (3). The present study measured changes in arterial stiffness index (SI- a measure of PWV) using pulse wave analysis following two-weeks of treatment using an oral formulation of 4 potent antioxidants (l-cysteine, thiamine, pyridoxine and ascorbic acid) compared to pacebo.
2. Methods
2.1. Study Design and Subjects
The study followed a single blind, randomised, parallel group design of two weeks of treatment with either the antioxidant formulation or placebo in a diverse group of subjects. A single blind design was used because of difficulty obtaining matching placebos and because the outcomes assessed were measured by electronic devices which avoid the possibility for observer bias. The intention was to study 100 subjects with 50 subjects randomised to each treatment arm. The exclusion criteria were the presence of atrial fibrillation or medication with antioxidant supplements. A diverse group of subjects representative of the general population was chosen so that the results would be of relevance to all groups that may choose to take antioxidant supplements. Subjects were recruited by word of mouth, from patients attending a general practice clinic and from social groups at housing estates.
2.2. Study Procedures
The subjects attended on the first occasion to sign a consent form and for the recording of demographic data and vital signs. Baseline recordings of SI and reflection index (RI- a measure of small to medium size artery compliance) were performed after resting in a seated position for 10 minutes. The subjects were then given a bottle containing greater than two weeks supply of the antioxidant formulation or placebo according to a randomisation schedule. The schedule was created by an independent pharmacist who sealed the randomisation schedule in an envelope that remained sealed until the data collection had been completed. Randomisation was performed in blocks of four. The subjects were instructed not to change their medication and to maintain the usual diet, caffeine and alcohol intake and to continue their usual level of physical activity until the study was completed.
The subjects were instructed to take their study medication in the morning at the same time each day and to attend two weeks after the first visit at the same time and same day of the week as at the first visit. At the second visit recordings of SI and RI were again performed approximately 4 hours after taking their medication. Vital signs were recorded and they were questioned about the occurrence of possible adverse events. Their bottles of study medication were returned, and the remaining tablets counted to determine compliance. Each subject then received bottles of the antioxidant formulation for open label treatment to provide additional information on tolerability.
2.3. Study Formulations
The antioxidant treatment consisted of two tablets each containing l-cysteine hydrochloride monohydrate 322 mg, thiamine hydrochloride (vitamin B1) 80 mg, pyridoxine hydrochloride (vitamin B6) 10 mg and ascorbic acid (vitamin C) 150 mg (Bluegum Pharmaceuticals, Botany, NSW, Australia). The tablets were maintained at below 25 degrees Celcius. The formulation underwent regular testing to determine purity and stability. The ingredients and the amounts used were chosen because they provide a diversity of antioxidant mechanisms and therefore may have additive antioxidant effects, and because the formulation has previously been reported to significantly reduce hangover symptoms (5) which are believed to be largely due to oxidative stress (6).
Placebo capsules were filled with corn flour by an independent pharmacist who also filled dark brown glass bottles with the antioxidant and placebo formulations and labelled the bottles with numbers according to the randomisation schedule. As far as was possible volunteers were kept from conversing with each other to avoid discussions about the appearance of the formulations they were receiving.
2.4. Measurement of SI and RI
SI and RI were measured in the sitting position after 10 minutes of rest using a Pulse Trace recorder (Micro Medical, Gillingham, Kent, UK). Six initial recordings were made to ensure that a stable, good quality arterial waveform was displayed on the monitor after which three further recordings were made and the mean values of SI and RI from these three recordings were entered as the baseline and outcome measurements. Patients in whom the Pulse Trace recorder could not calculate SI results (usually because of the absence of a recognisable reflected pulse wave) could not be included in the efficacy analysis but were included in the safety assessment.
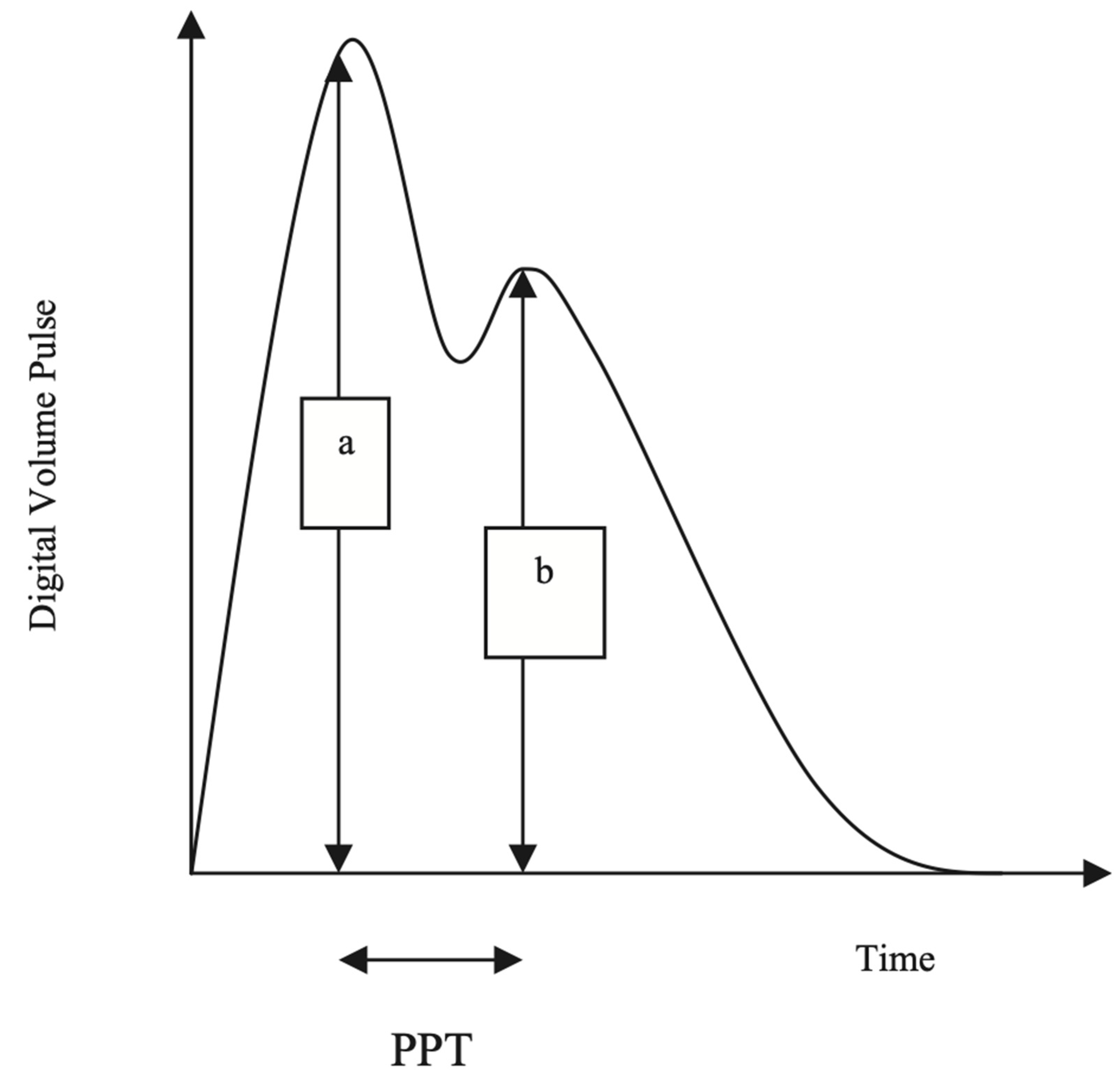
The Pulse Trace recorder uses a digital photoplethysmograph transmitting infrared light technique from a finger cuff applied to the left index finger. The amount of light transmitted through the finger varies proportionally to changes in its blood volume. The signal from the photoplethysmograph obtained over a 30-s period is averaged by the system, to produce a single digital volume pulse (DVP) waveform (
Figure 1). Chowienczyk et al. (7) demonstrated that the peripheral pressure pulse is related to the DVP by a single generalized transfer function, which is inbuilt in the Pulse Trace system.
The DVP wave (
Figure 2) consists of an early systolic peak (a), which results from an increase in digital blood volume from a pressure wave transmitted from the left ventricle to the finger along a direct path. The second peak, (b), occurs in diastole, and is formed by pressure waves reflected up to the aorta and thence to the finger, from sites of impedance mismatch in the lower body. The time between the systolic and diastolic peaks (peak to peak time, PPT) can be used to infer the time taken for the pressure wave to travel from the aorta to the lower body, and thence as a reflected wave back up to the aorta to the finger. This path length is unknown but is proportional to the subject’s height (h). An index of large arterial stiffness (stiffness index, SI) can therefore be derived, like the calculation of pulse wave velocity (PWV) by the formula: h/PPT. SI has been shown to be strongly correlated to central (aortic and carotid femoral) PWV (7).
An index of small to medium-sized arterial stiffness can be derived from the magnitude of the reflected waves from the lower limbs to the aorta. The reflection index (RI) is measured using b/a X 100%.
A previous study in 115 subjects reported good intra-individual reproducibility, with a co-efficient of variation (CV) for SI of 8%, and for RI, 5% (8).
2.5. Statistical analysis
All results are expressed as the mean and standard deviation.
The change in SI, RI, blood pressures and heart rate from baseline following the two-week treatment period was calculated. Differences between antioxidant and placebo treatments for change from baseline were analysed using Student’s t test. Estimates of the effect size were made according to the method of Cohen (difference between means divided by the pooled standard deviation) (9), or if there was a substantial difference between the standard deviation of the change from baseline between antioxidant and placebo therapy, by the method of Glass (difference between the means divided by the standard deviation of the control group) (10). Effect sizes of 0.3 or less are weak, those greater than 0.8 are strong and those greater than 1.2 are very strong. Predictors of change of SI were analysed by linear regression (age, gender, body mass index, SBP and heart rate).
Baseline differences between the two-treatment group were made using Student’s t test or the Chi square test for categoric variables.
The number of subjects required to detect a difference in SI between baseline and the end of the 2 -week treatment period of 1.0 m/sec between antioxidant and placebo groups (with a power of 80%, assumed equal standard deviations of 1.6 m/sec and alpha = 0.05 two tailed) was calculated to be 42 subjects in each group. A standard deviation of 1.60 was calculated from previously collected data, and 1.00 m/sec was the smallest change from baseline in SI between groups considered to be clinically significant. A sample size of 50 subjects in each group was chosen to allow for dropouts and failure to obtain arterial waveforms suitable for analysis.
2.6. Ethics
The subjects signed a consent form before any research activities commenced. Study conformed with the declaration of Helsinki and the protocol was approved by the Gold Coast University Hospital Ethics Committee.
3. Results
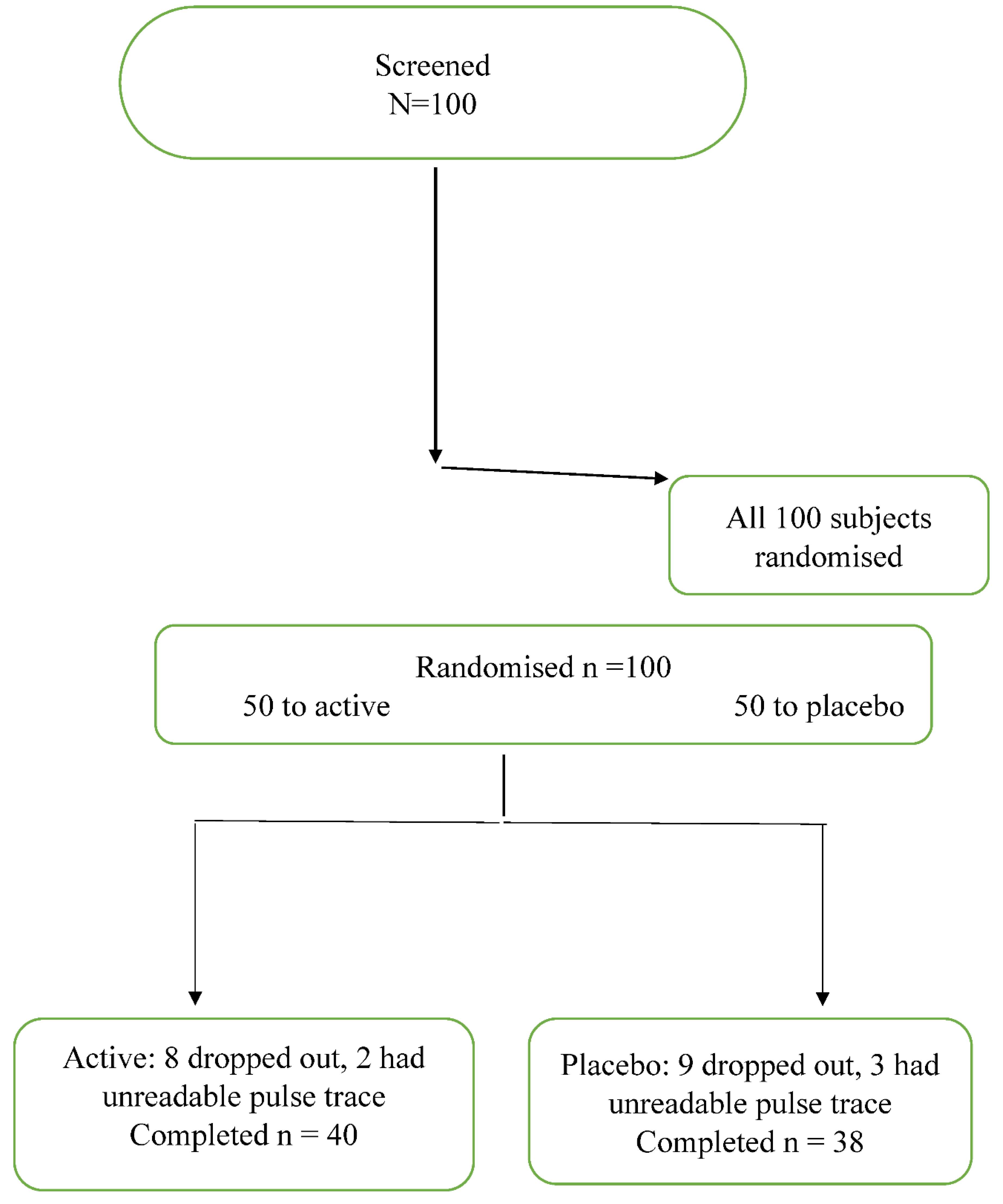
One hundred subjects were enrolled in the study and randomised to antioxidant or placebo therapy. Fifteen patients failed to attend for the second study day and 7 subjects had arterial waveforms that could not be analysed. A total of 40 subjects who were randomised to the antioxidant treatment and 38 patients randomised to placebo had complete data sets. A Consort flow chart is presented in figure 1.
The baseline characteristics and the changes from baseline to the end of study of SI, RI, blood pressures and heart rates are presented in
Table 1. The antioxidant and placebo groups were well matched apart from BMI.
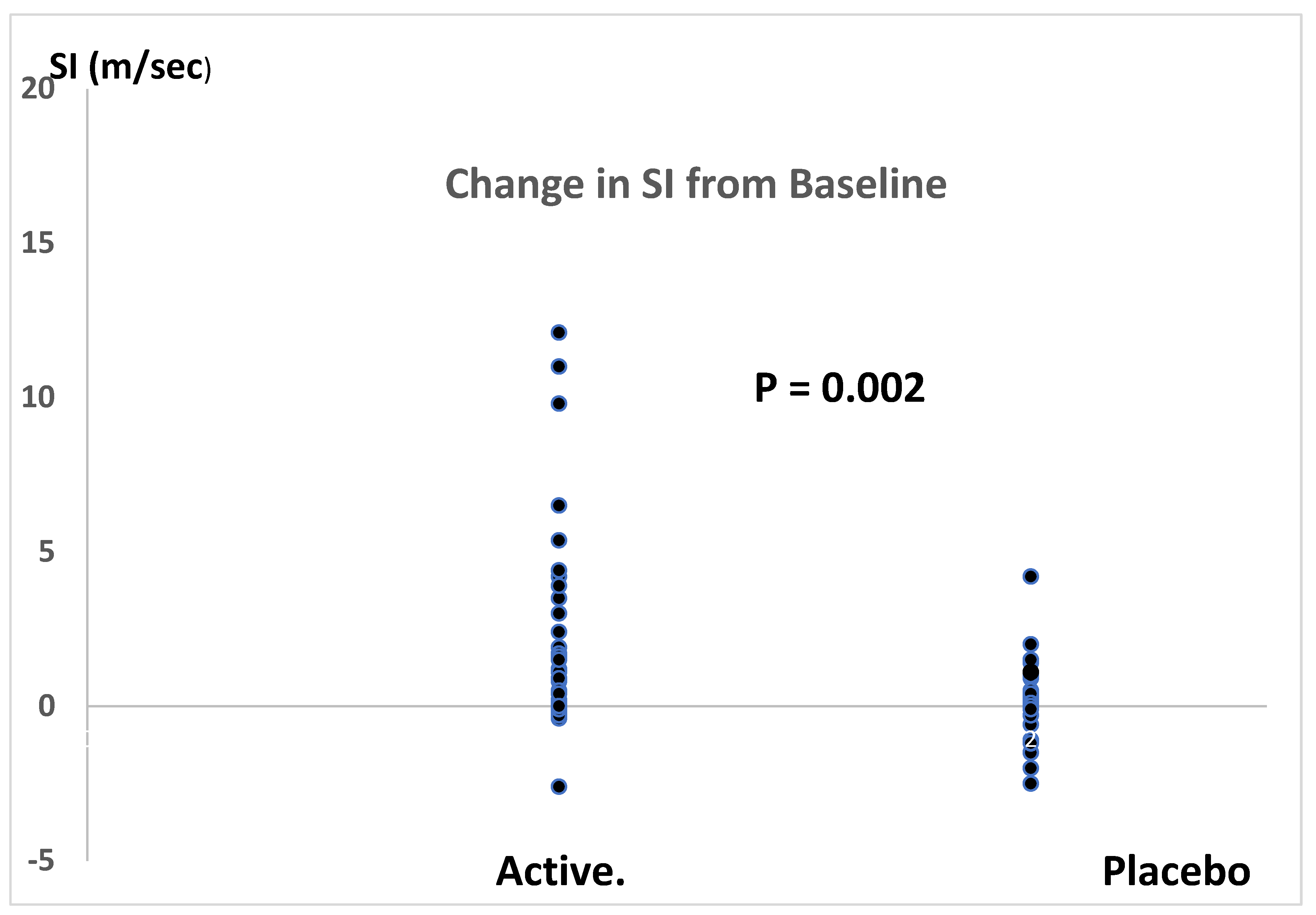
Antioxidant therapy significantly reduced SI by 1.7 m/sec (placebo corrected) with 95% confidence levels of 0.6 to 2.7 m/sec. This represented a 19% reduction in SI with 95% confidence levels of 9% to 31%. The effect size for the fall in SI, calculated by the method of Glass (because of unequal variances between antioxidant and placebo therapies), was 1.4.
RI at baseline and the change in RI from baseline at the end of the study did not differ between the antioxidant and placebo groups.
The change in SI on antioxidant treatment was significantly and positively related to age (r = 0.362, P = 0.02). There were no other significant predictors of change in SI or RI and there were no significant differences between antioxidant therapy and placebo on changes in blood pressure or heart rate.
Compliance with medication was greater than 80% in all patients and there were no reported adverse events.
4. Discussion
The combination therapy of four potent antioxidants reduced SI by 1.7 m/sec (placebo corrected) with an effect size of 1.4 using the method of Glass (10), indicating a very strong effect in reducing PWV. The placebo corrected percent fall in SI (19% with 95% confidence limits of 7% and 31%) was impressive. This is a substantially greater reduction in PWV than has been reported for antioxidant therapies in previous studies. A meta-analysis of these studies found only a small improvement with antioxidant therapy with a weak effect size of 0.20 (3).
The results of the present study suggest that the antioxidant therapy used in the present study may be of value in the management of cardiovascular diseases. However, reductions in PWV during antioxidant therapy cannot be assumed to lead to a reduction in MACE. Studies of the effects of antioxidant supplementation in improving clinical outcomes have been disappointing (12). Outcome studies assessing the impact of potent antioxidant therapy with similar compositions to that used in the present studies are needed.
We studied a diverse population and found that, while the overall effect on PWV was statistically significant and of a substantial magnitude, the variability of PWV responses was greater in the subjects that received antioxidants than in the subjects that received placebo. This suggests that there are individuals or groups that respond to antioxidant therapy greater than others. Any benefits in reduction of MACE that may occur with the formulation used in the present study may depend upon the characteristics of the population that is treated. The meta- analysis of previous studies of the effects of antioxidants on PWV found that the greatest effects were in younger women who were not taking additional antioxidants from other sources (3). The present study found a significant relationship between the subjects’ age and the magnitude of change in SI during antioxidant therapy. Older subjects had a greater fall in SI. Further studies in population subgroups are needed to identify which populations are likely to respond the most to treatment and to perhaps experience the greatest clinical benefit.
The optimal duration of treatment with antioxidants to achieve improvements in PWV and the duration of these effects could not be established from the present study. The meta-analysis of previous studies of antioxidants found that the reduction in PWV was similar during short term and long-term therapy (3). The present study aimed to assess the efficacy of combining several potent antioxidants. The study could not define the relative importance of the individual antioxidants on the results achieved, and it is possible that most of the potentially beneficial effects were predominantly due to only one or two of the ingredients. Further studies of the individual components are required to clarify this matter.
The results of the present study compare favourably with studies of therapies other than oxidants on PWV. Several antihypertensive drugs including ACE inhibitors and calcium channel blockers reduce PWV with an effect size of around 0.66 (13). However, it likely that a considerable portion in the reduction in PWV is secondary to the fall in BP associated with antihypertensive therapy, as PWV has been reported to be closely related to SBP (14). Nitrates reduce PWV and SBP to a similar extent as antihypertensives (15). A meta-analysis of the effects of statins found a reduction in PWV with an effect size of 0.37 (16). Topical, but not oral, estrogens have been reported to reduce PWV with an effect size of approximately 0.33 (17). A meta-analysis of studies of the effects of isoflavones, which have antioxidant activity, found a reduction in PWV with an effect size of 0.38 (18). All these reported effect sizes are weak compared to the effect size for the change in SI in the present study.
It has been estimated that for each increase in PWV of 1.0 m/sec there is an increase in MACE of approximately 15% (19). If an intervention that lowers PWV by 1.0 m/sec reduces MACE by about 15%. the 1.7 m/sec reduction in PWV achieved in the present study suggests that therapy with the antioxidant formulation we used may be of value in clinical practice.
The single blinded design of the present study and the use of placebo which were different in appearance to the antioxidant formulation may be a possible limitation of the study. However, the major parameters of interest (SI, RI BPs and heart rate) were all calculated by electronic devices which protect against observer bias.
We did not obtain information about the subjects’ medical conditions or drug therapy to investigate whether these factors influenced the effect of the antioxidant therapy on SI. An analysis of the effects of medical disorders and therapy was beyond the scope of the present study which was intended to determine the effects of the antioxidant therapy on SI in a broad cross section of the community.
While a strong difference was found between the antioxidant formulation and placebo, no difference was found between treatments for the change in RI. This indicates that the change in arterial stiffness with the antioxidant therapy occurred in large arteries but not in the small and medium size arteries.
In conclusion, supplementation with a mixture of several potent antioxidants improves SI to an extent that may be of therapeutic value. Further studies in patients with a high risk of MACE should now be performed.
References
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Med Assoc 2014, 63, 636–646.
- Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011, 218, 90–95.
- Ashor AW, Siervo M, Lara J, Oggioni C, Mathers JC. Antioxidant vitamin supplementation reduces arterial stiffness in adults: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2014, 144, 1594–1602. [CrossRef] [PubMed]
- Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am Clin Nutr 2011, 93, 446–454. [CrossRef] [PubMed]
- Quinton JB, Kemm RM. Howes LG. A post marketing evaluation of Rapid Recovery in relieving symptoms of hangover. Clin Res Trials 2018, 4, 1–3.
- Mackus M, Loo AJ, Garssen J, Kraneveld AD, Scholey A, Verster JC. The role of alcohol metabolism in the pathology of alcohol hangover. J Clin Med. 2020, 9, 3421. [CrossRef] [PubMed]
- Millasseau SC, Guigui FG, Kelly RP, Prasad K, Cockcroft JR, Ritter JM, Chowienczyk PJ. Noninvasive assessment of the digital volume pulse: comparison with the peripheral pressure pulse. Hypertension. 2000, 36, 952–956. [CrossRef] [PubMed]
- Brillante DG, O'Sullivan AJ, Howes LG. Arterial stiffness indices in healthy volunteers using non-invasive digital photoplethysmography. Blood Pressure. 2008, 17, 116–123. [CrossRef] [PubMed]
- Sawilowsky, SS. New effect size rules of thumb. J Mod Appl Stat Meth 2009, 8, 26. [Google Scholar] [CrossRef]
- Ledesma RD, Macbeth G, Cortada de Kohan NU. Computing effect size measures with ViSta-the visual statistics system. Tut Quantit Meth Psychol. 2009, 5, 25–34. [CrossRef]
- Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Ed. 2012, 4, 279–282. [CrossRef] [PubMed]
- Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PloS one. 2013, 8, e56803.
- Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson I. Comparison of the Effects of Antihypertensive Agents on Central Blood Pressure and Arterial Stiffness in Isolated Systolic Hypertension. Hypertension. 2009, 54, 409–413. [CrossRef] [PubMed]
- Schiffrin, EL. Vascular stiffening and arterial compliance: Implications for systolic blood pressure. Am J Hypertens. 2004, 17, 39S–48S. [Google Scholar] [CrossRef] [PubMed]
- Nichols WW, Harripersaud K, Petersen JW. Nitrates and arterial function. Curr Cardiovasc Risk Rep. 2013, 7, 224–232. [CrossRef]
- D’elia L, La Fata E, Iannuzzi A, Rubba PO. Effect of statin therapy on pulse wave velocity: A meta-analysis of randomized controlled trials. Clin Exp Hypertension. 2018, 40, 601–608. [CrossRef] [PubMed]
- Kalenga CZ, Hay JL, Boreskie KF, Duhamel TA, MacRae JM, Metcalfe A, Nerenberg KA, Robert M, Ahmed SB. The association between route of post-menopausal estrogen administration and blood pressure and arterial stiffness in community-dwelling women. Front Cardiovasc Med. 2022, 9, 913609. [CrossRef] [PubMed]
- Man B, Cui C, Zhang X, Sugiyama D, Barinas-Mitchell E, Sekikawa A. The effect of soy isoflavones on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr 2021, 60, 60314.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010, 55, 1318–1327. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).