Submitted:
17 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Primary Structural Features of Prions and Prion-like Proteins
2.1. Prnp, PrPC, and PrPSc
2.2. Amyloid and Hyperphosphorylated Tau
3. Structural Variation of Prion-like Proteins and their Association with Neurodegeneration
4. Mechanistic Insights into Structural Changes Driving Protein Aggregation and Neurodegeneration
4.1. Dysregulation of Protein Homeostasis Associated with Protein Aggregation
4.2. PTM Associated Structural Anomalies and Protein Aggregation
4.3. Regulation of Protein Structures by Environmental Factors: Chaperones, RNA, and Ions
5. Disease-Related Self-Replication and Aggregation Model
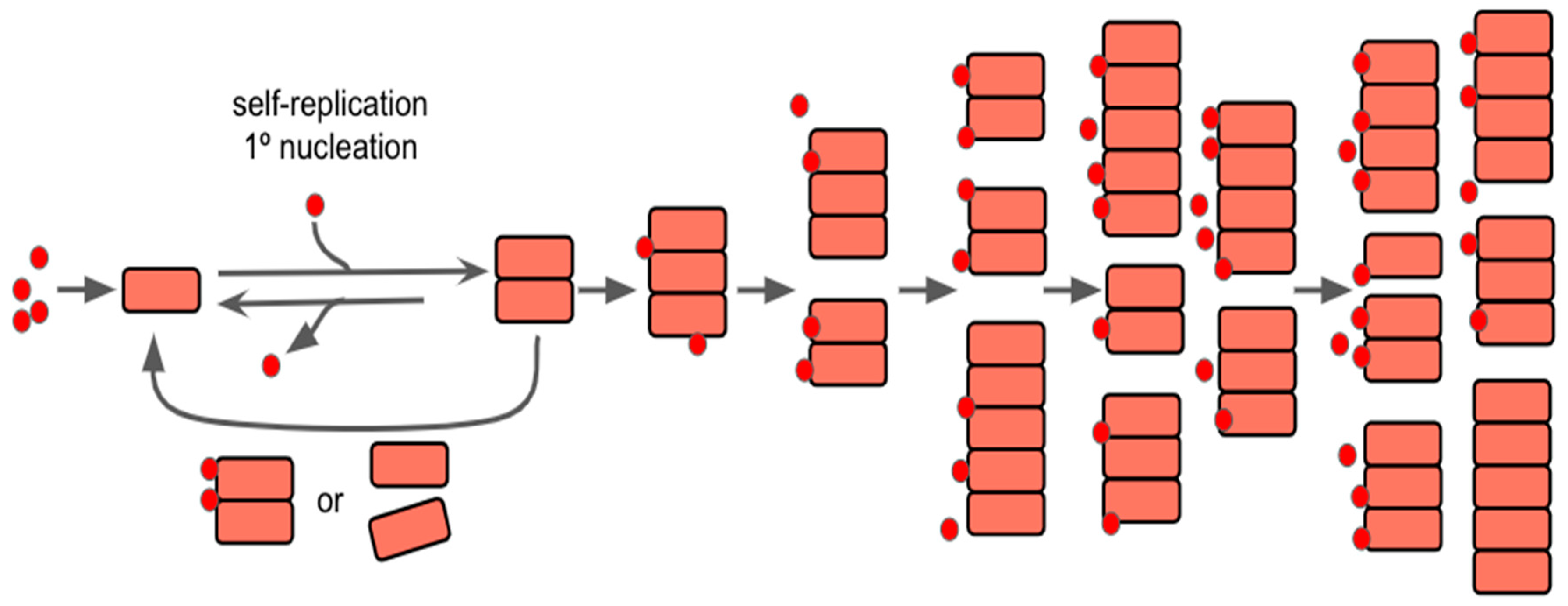
5.1. Self-Replication of Prions and Prion-Like Proteins
5.2. Neurological Inflammation
6. Perspectives in Prevention, Diagnosis, and Therapy
6.1. Pharmaceutical-Based and Therapeutic-Based Treatment Methods
6.2. Notable Advancements in Treatments in Recent Years
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prusiner, S.B. Novel Proteinaceous Infectious Particles Cause Scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.W. and Prusiner, S.B. Prions. Cold Spring Harbor Perspectives in Biology 2011, 3, a006833–a006833. [Google Scholar] [CrossRef]
- Legname, G. Elucidating the function of the prion protein. PLoS Pathog 2017, 13, e1006458. [Google Scholar] [CrossRef]
- Aoyagi, A.; Condello, C.; Stöhr, J.; Yue, W.; Rivera, B.M.; Lee, J.C.; Woerman, A.L.; Halliday, G.; Van Duinen, S.; Ingelsson, M.; et al. Aβ and tau prion-like activities decline with longevity in the Alzheimer’s disease human brain. Science Translational Medicine, 2019, 11, eaat8462. [Google Scholar] [CrossRef] [PubMed]
- Kostylev, M.A.; Tuttle, M.D.; Lee, S.; Klein, L.E.; Takahashi, H.; Cox, T.O.; Gunther, E.C.; Zilm, K.W. and Strittmatter, S.M. Liquid and Hydrogel Phases of PrPC Linked to Conformation Shifts and Triggered by Alzheimer’s Amyloid-β Oligomers. Molecular Cell, 2018, 72, 426–443. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A. and Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron, 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Valotassiou, V.; Malamitsi, J.; Papatriantafyllou, J.; Dardiotis, E.; Tsougos, I.; Psimadas, D.; Alexiou, S.; Hadjigeorgiou, G. and Georgoulias, P. SPECT and PET imaging in Alzheimer’s disease. Annals of Nuclear Medicine, 2018, 32, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Maundrell, K. and Soto, C. Is loss of function of the prion protein the cause of prion disorders? Trends in Molecular Medicine, 2003, 9, 237–243. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, S.E.; Jung, S.; et al. Discriminant analysis of prion sequences for prediction of susceptibility. Exp Mol Med, 2013, 45, e48. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N. Hot Spot of Structural Ambivalence in Prion Protein Revealed by Secondary Structure Principal Component Analysis. The Journal of Physical Chemistry B, 2014, 118, 9826–9833. [Google Scholar] [CrossRef] [PubMed]
- Angers, R.C.; Kang, H.E.; Napier, D.; Browning, S.; Seward, T.; Mathiason, C.; Balachandran, A.; Mckenzie, D.; Castilla, J.; Soto, C.; et al. Prion Strain Mutation Determined by Prion Protein Conformational Compatibility and Primary Structure. Science, 2010, 328, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B. [URE3] as an Altered URE2 Protein: Evidence for a Prion Analog in Saccharomyces Cerevisiae. Science, 1994, 264, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Xiang, Y.; Jin, W.S.; et al. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol Psychiatry, 2018, 23, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M. Structure of NFT: Biochemical Approach. Advances in Experimental Medicine and Biology, 2019, 1184, 23–34. [Google Scholar] [CrossRef] [PubMed]
- 15. Falcon B, Zhang W, Murzin AG, et al. Structures of filaments from Pick's disease reveal a novel tau protein fold. Nature 2018, 561, 137–140. [CrossRef] [PubMed]
- Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature, 2017, 547, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang W, Falcon B, Murzin AG, et al. Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer's and Pick's diseases. Elife, 2020, 9, e53084. [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron, 2014, 82, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Burré J, Sharma M, Südhof TC. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A, 2018, 115, E4004–E4013. [Google Scholar]
- Guerrero-Ferreira R, Taylor NM, Mona D, et al. Cryo-EM structure of alpha-synuclein fibrils. Elife, 2018, 7, e36402. [Google Scholar] [CrossRef]
- Li B, Ge P, Murray KA, et al. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun., 2018, 9, 3609. [Google Scholar] [CrossRef]
- Peelaerts W, Bousset L, Van der Perren A, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature, 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Rey NL, George S, Steiner JA, et al. Spread of aggregates after olfactory bulb injection of α-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta Neuropathol., 2019, 138, 785–810. [Google Scholar]
- Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc Natl Acad Sci U S A, 2011, 108, 4194–4199. [Google Scholar] [CrossRef]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci., 2013, 14, 38–48. [Google Scholar] [CrossRef]
- 26. Sawaya MR, Sambashivan S, Nelson R, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 2007, 447, 453–457. [CrossRef] [PubMed]
- Watts JC, Condello C, Stöhr J, et al. Serial propagation ofprion strains in cultured cells. Proc Natl Acad Sci U S A, 2014, 111, E1453–1461. [Google Scholar]
- Wille H, Bian W, McDonald M, et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci U S A, 2009, 106, 16990–16995. [Google Scholar] [CrossRef] [PubMed]
- De Cecco E, Celauro L, Vanni S, et al. The uptake of tau amyloid fibrils is facilitated by the cellular prion protein and hampers prion propagation in cultured cells. J Neurochem., 2020, 155, 577–591. [Google Scholar] [CrossRef] [PubMed]
- La Vitola P, Beeg M, Balducci C, et al. Cellular prion protein neither binds to alpha-synuclein oligomers nor mediates their detrimental effects. Brain., 2019, 142, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Condello C, Lemmin T, Stohr J, et al. Structural het- erogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc Natl Acad Sci U S A, 2018, 115, E782–E791. [Google Scholar]
- Scialo C, De Cecco E, Manganotti P, et al. Prion and Prion-Like Protein Strains: deciphering the Molecular Basis of Heterogeneity in Neurodegeneration. Viruses, 2019, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Ondrejcak T, Hu NW, Qi Y, et al. Soluble tau aggregates inhibit synaptic long-term depression and amy- loid beta-facilitated LTD in vivo. Neurobiol Dis., 2019, 127, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature, 2006, 443, 780–6. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 1984, 122, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Goate, A.; Chartier-Harlin, M.-C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L. Segregation of a Missense Mutation in the Amyloid Precursor Protein Gene with Familial Alzheimer's Disease. Nature, 1991, 349, 704–706. [Google Scholar] [CrossRef]
- Goedert, M.; Wischik, C.M.; Crowther, R.A.; Walker, J.E.; Klug, A. Cloning and sequencing of the cDHA-encoding a core protein of the paired helical filament of Alzheimer’s disease: Identification of the microtubule-associated protein tau. Proc Natl Acad Sci USA, 1988, 85, 4051–4055. [Google Scholar] [CrossRef]
- Goedert, M.; Clavaguera, F.; Tolnay, M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci, 2010, 33, 317–325. [Google Scholar] [CrossRef]
- McKinnon, C.; Goold, R.; Andre, R.; Devoy, A.; Ortega, Z.; Moonga, J.; Linehan, J.M.; Brandner, S.; Lucas, J.J.; Collinge, J.; Tabrizi, S.J. 2016. Prion-mediated neurodegeneration is associated with early impairment of the ubiquitin-proteasome system. Acta Neuropathol, 2016, 131, 411–25. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, C.; Wang, S.B.; Xie, W.L.; Guo, Y.; Zhang, J.; Shi, Q.; Chen, C.; Dong, X.P. 2012. Activation of the macroautophagic system in scrapie-infected experimental animals and human genetic prion diseases. Autophagy, 2012, 8, 1604–20. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.J.; Jang, B.; Kim, H.J.; Mostafa, M.N.; Park, S.J.; Kim, Y.S.; Choi, E.K. Impairment of Neuronal Mitochondrial Quality Control in Prion-Induced Neurodegeneration. Cells, 2022, 11, 2744. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain, 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, X.; Wu, X. and Zhang, M. Formation of biological condensates via phase separation: Characteristics, analytical methods, and physiological implications. Journal of Biological Chemistry, 2019, 294, 14823–14835. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Khanna, R.; Avni, A. and Mukhopadhyay, S. Heterotypic electrostatic interactions control complex phase separation of tau and prion into multiphasic condensates and co-aggregates. Proceedings of the National Academy of Sciences, 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.O.; Passos, Y.M.; Amaral, M.J.; Macedo, B.; Tempone, M.H.; Bezerra, O.C.L.; Moraes, M.O.; Almeida, M.S.; Weber, G.; Missailidis, S.; et al. Liquid-liquid phase separation and fibrillation of the prion protein modulated by a high-affinity DNA aptamer. The FASEB Journal, 2020, 34, 365–385. [Google Scholar] [CrossRef]
- Baskakov, I.; Katorcha, E. and Makarava, N. Prion Strain-Specific Structure and Pathology: A View from the Perspective of Glycobiology. Viruses, 2018, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- DeArmond, S.J.; Sánchez, H.; Yehiely, F.; Qiu, Y.; Ninchak-Casey, A.; Daggett, V.; Camerino, A.P.; Cayetano, J.; Rogers, M.; Groth, D.; et al. Selective Neuronal Targeting in Prion Disease. Neuron, 1997, 19, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Baskakov, I.V. From Posttranslational Modifications to Disease Phenotype: A Substrate Selection Hypothesis in Neurodegenerative Diseases. International Journal of Molecular Sciences, 2021, 22, 901. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Mandelkow, E.M.; Biernat, J.; Gustke, N.; Meyer, H.E.; Schmidt, B.; Mieskes, G.; Söling, H.D.; Drechsel, D. and Kirschner, M.W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. The EMBO Journal, 1990, 9, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X. and Iqbal, K. Hyperphosphorylation of Microtubule-Associated Protein Tau: A Promising Therapeutic Target for Alzheimer Disease. Current Medicinal Chemistry, 2008, 15, 2321–2328. [Google Scholar] [CrossRef]
- Kovacech, B.; Skrabana, R.; and Novak, M. Transition of Tau Protein From Disordered to Misordered in Alzheimer’s Disease. Neurodegener Dis. [CrossRef]
- Ghetti, B.; Piccardo, P.; Frangione, B.; Bugiani, O.; Giaccone, G.; Young, K.; Prelli, F.; Farlow, M.R.; Dlouhy, S.R. and Tagliavini, F. Prion Protein Amyloidosis. Brain Pathology, 1996, 6, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J. L.; McIntire, T. M.; Milton, S. C.; Cotman, C. W.; Glabe, C. G. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science, 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.M.S.; Gomes, R.N.; Pedron, T.; et al. Cellular prion protein offers neuroprotection in astrocytes submitted to amyloid β oligomer toxicity. Mol Cell Biochem. 2022. [Google Scholar] [CrossRef]
- Pritzkow, S.; Morales, R.; Lyon, A.; Concha-Marambio, L.; Urayama, A. and Soto, C. Efficient prion disease transmission through common environmental materials. Journal of Biological Chemistry, 2018, 293, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Katorcha, E.; Gonzalez-Montalban, N.; Makarava, N.; Kovacs, G.G.; and Baskakov, I.V. Prion replication environment defines the fate of prion strain adaptation. PLOS Pathogens, 2018, 14, e1007093. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.K.; Kim, C.; Haldiman, T.; Kacirova, M.; Wang, B.; Bohon, J.; Chance, M.R.; Kiselar, J. and Safar, J.G. Structurally distinct external solvent-exposed domains drive replication of major human prions. PLOS Pathogens, 2021, 17, e1009642. [Google Scholar] [CrossRef] [PubMed]
- Roterman, I.; Stapor, K.; Gądek, K.; Gubała, T.; Nowakowski, P.; Fabian, P.; and Konieczny, L. On the Dependence of Prion and Amyloid Structure on the Folding Environment. International Journal of Molecular Sciences, 2021, 22, 13494. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Brennich, M.; Mancini, G.; Tran, T.H.; Legname, G.; D’Angelo, P. and Giachin, G. Deciphering Copper Coordination in the Mammalian Prion Protein Amyloidogenic Domain. Biophysical Journal, 2020, 118, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.M.; Treusch, S.; Ren, H.-Y.; Halfmann, R.; Duennwald, M.L.; Lindquist, S. and Cyr, D.M. Chaperone-dependent amyloid assembly protects cells from prion toxicity. Proceedings of the National Academy of Sciences, 2008, 105, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- King, C.-Y. The Mutability of Yeast Prions. Viruses, 2022, 14, 2337. [Google Scholar] [CrossRef] [PubMed]
- Meisl, G.; Xu, C. K.; Taylor, J. D.; T. Michaels, T. C.; Levin, A.; Otzen, D.; Klenerman, D.; Matthews, S.; Linse, S.; Andreasen, M.; J. Knowles, T. P. Uncovering the universality of self-replication in protein aggregation and its link to disease. Science Advances, 2022. [Google Scholar]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Borthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature, 1995, 373, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Sui, C.; Liu, F.; Chen, T.; Zhang, L.; Zheng, Y.; Liu, B.; Gao, C. The protein arginine methyltransferase PRMT9 attenuates MAVS activation through arginine methylation. Nat Commun, 2022, 13, 5016. [Google Scholar] [CrossRef] [PubMed]
- Peinado, J.R.; Chaplot, K.; Jarvela, T.S.; Barbieri, E.M.; Shorter, J. and Lindberg, I. Sequestration of TDP-43216-414 Aggregates by Cytoplasmic Expression of the proSAAS Chaperone. ACS Chemical Neuroscience, 2022, 13, 1651–1665. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, M. and Zhu, C. Neuroinflammation in Prion Disease. International Journal of Molecular Sciences, 2021, 22, 2196. [Google Scholar] [CrossRef]
- Kwon, H.S. and Koh, S.H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration, 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, J.F.; Bendheim, P.E.; Kim, Y.S.; Carp, R.I.; Haase, A.T. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proceedings of the National Academy of Sciences of the United States of America, 1991, 88, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Raeber, A.J.; Race, R.E.; Brandner, S.; Priola, S.A.; Sailer, A.; Bessen, R.A.; Mucke, L.; Manson, J.; Aguzzi, A.; Oldstone, M.B.; Weissmann, C.; Chesebro, B. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. The EMBO Journal, 1997, 16, 6057–6065. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Takaomi Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.Y. Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron, 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M. and Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals, 2018, 11, 44. [Google Scholar] [CrossRef]
- Lopes, D.M.; Llewellyn, S.K. & Harrison, I.F. Propagation of tau and α-synuclein in the brain: therapeutic potential of the glymphatic system. Transl Neurodegener, 2022, 11, 19. [Google Scholar] [CrossRef]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiology of Disease, 2016, 93, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, Y.; Sekiya, M.; Saito, T. Amyloid-β plaque formation and reactive gliosis are required for induction of cognitive deficits in App knock-in mouse models of Alzheimer’s disease. BMC Neurosci, 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa-Muto, J.; Yamaguchi, K.; Kamatari, Y.O.; Kuwata, K. Synthesis of double-fluorescent labeled prion protein for FRET analysis. Bioscience, Biotechnology, and Biochemistry, 2015, 79, 11–1802. [Google Scholar] [CrossRef] [PubMed]
- Kostylev, M.A.; Kaufman, A.C.; Nygaard, H.B.; Patel, P.; Haas, L.T.; Gunther, E.C.; Vortmeyer, A. and Strittmatter, S.M. Prion-Protein-interacting Amyloid-β Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models. Journal of Biological Chemistry, 2015, 290, 17415–17438. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.C.; Kang, S.; Suh, J.; Park, Y.H.; Kang, M.J.; Pyun, J.M.; Choi, S.H.; Jeong, J.H.; Park, K.W.; Lee, H.W.; et al. (2019). Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer’s disease. Alzheimer's Research & Therapy, 2019, 11. [Google Scholar] [CrossRef]
- Koronyo, Y.; Rentsendorj, A.; Mirzaei, N.; Regis, G.C.; Sheyn, J.; Shi, H.; Barron, E.; Cook-Wiens, G.; Rodriguez, A.R.; Medeiros, R.; et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathologica, 2023, 145, 409–438. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Izuo, N. and Bitan, G. Aptamers targeting amyloidogenic proteins and their emerging role in neurodegenerative diseases. Journal of Biological Chemistry, 2022; 298, 101478. [Google Scholar] [CrossRef]
- Castle, A.R.; Wohlgemuth, S.; Arce, L. and Westaway, D. Investigating CRISPR/Cas9 gene drive for production of disease-preventing prion gene alleles. PLOS ONE, 2022, 17, e0269342. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, B.A.; Tahir, W.; Doh-Ura, K.; Gilch, S. and Schatzl, H.M. (2019). Combining autophagy stimulators and cellulose ethers for therapy against prion disease. Prion, 2019, 13, 185–196. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



