Submitted:
11 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
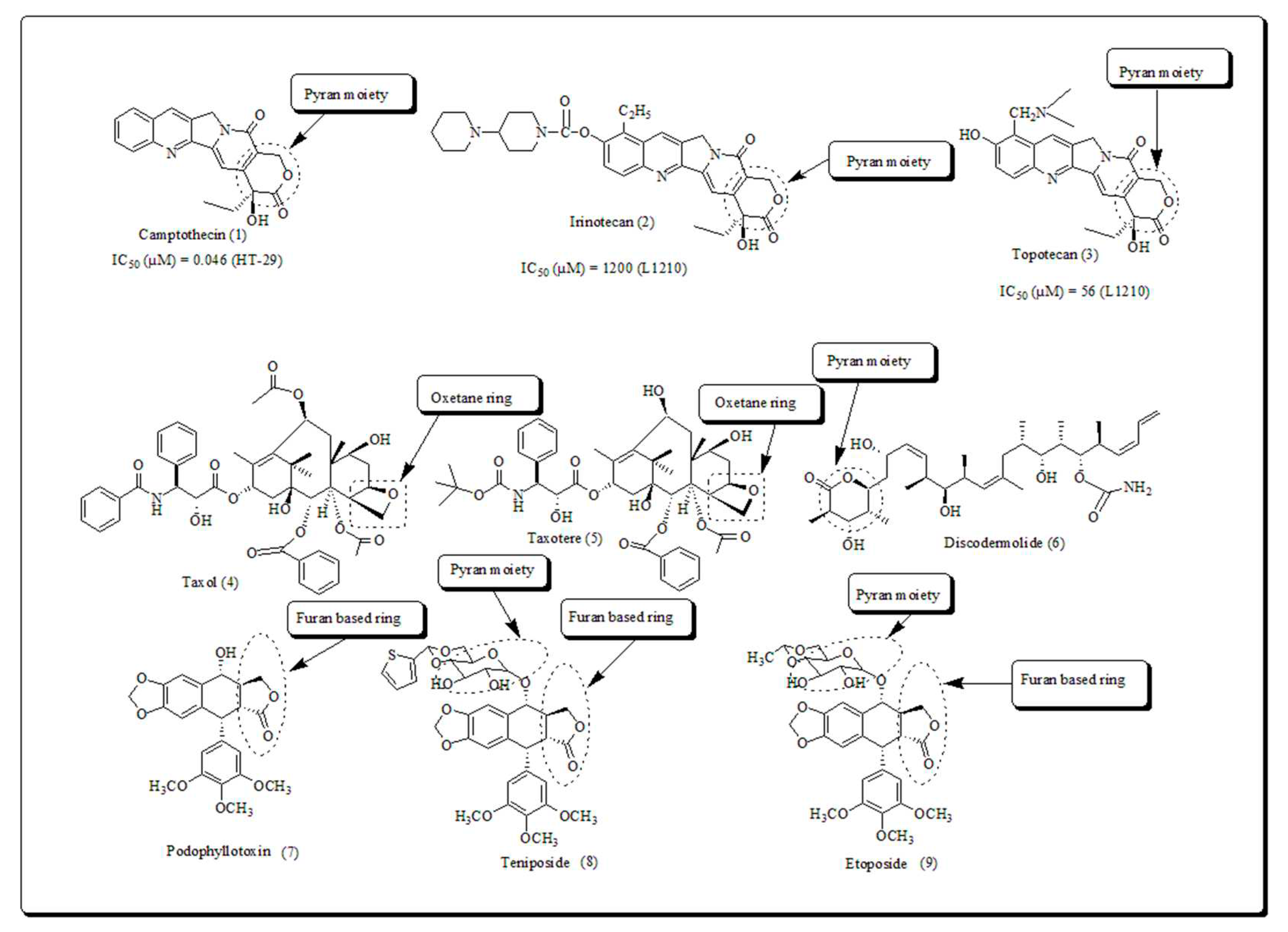
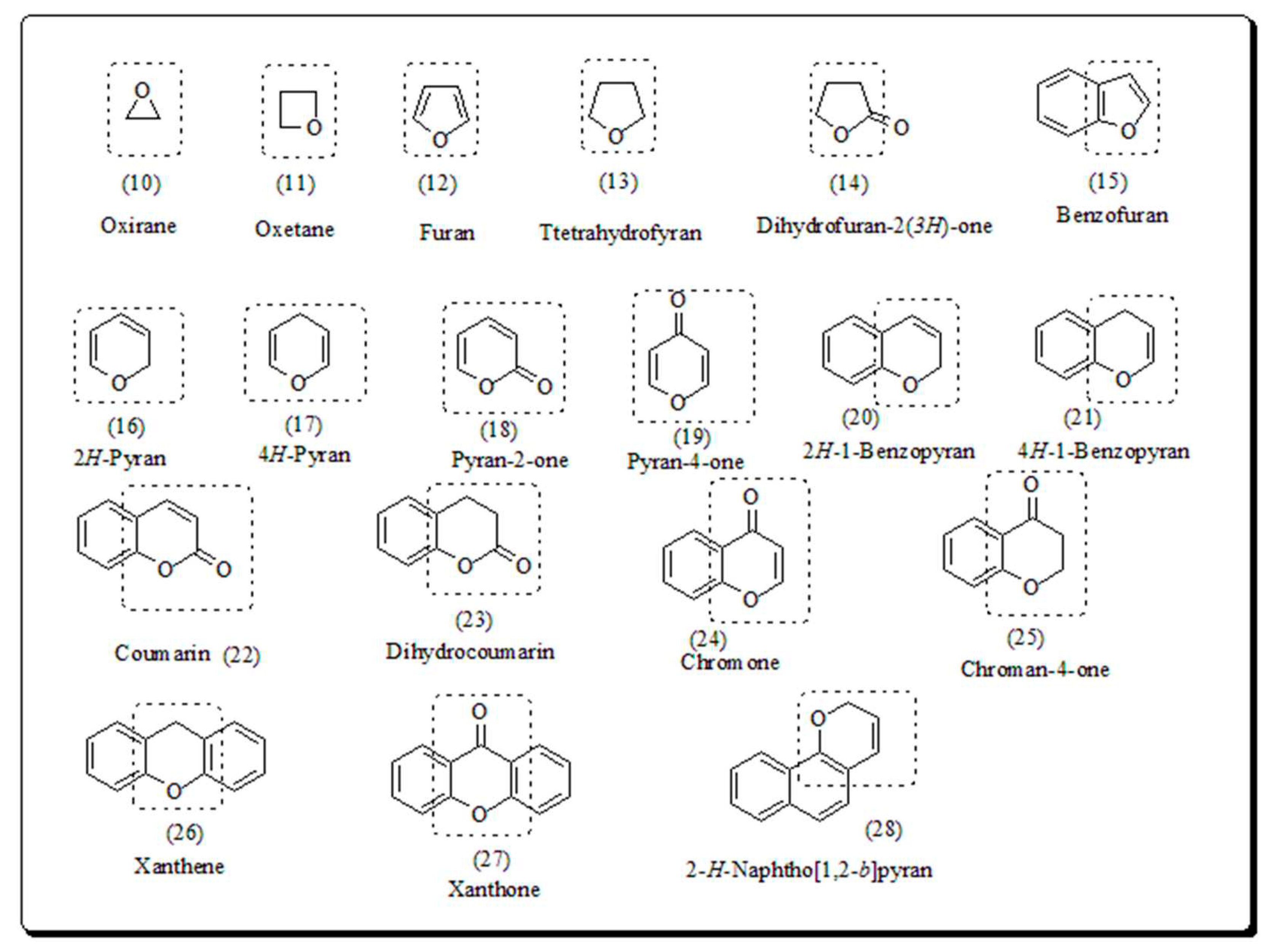
2. Pyran based oxygen containing heterocyclic scaffolds
- 1.
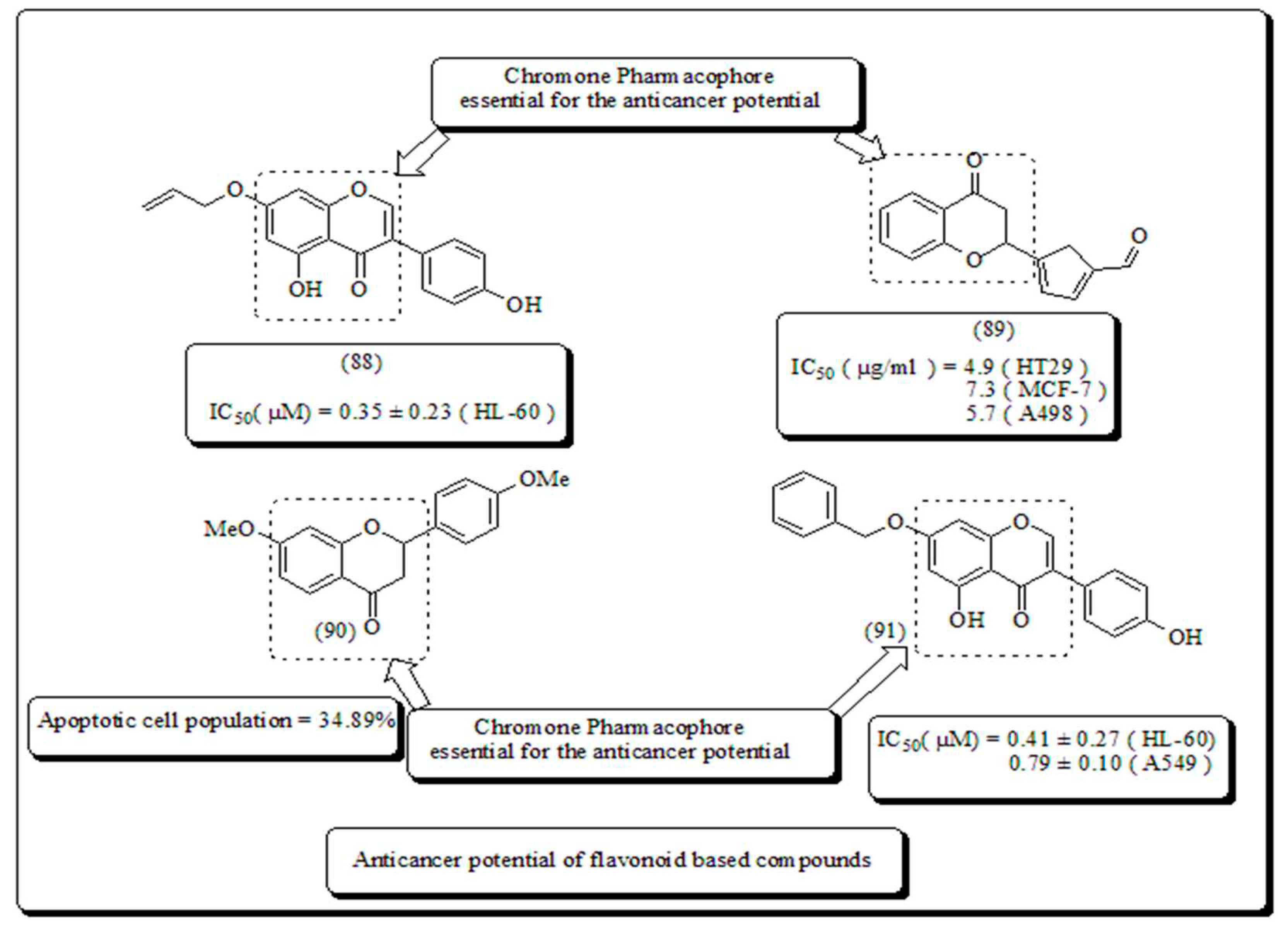
- Flavone-based oxygen containing heterocyclic scaffolds
- 2.
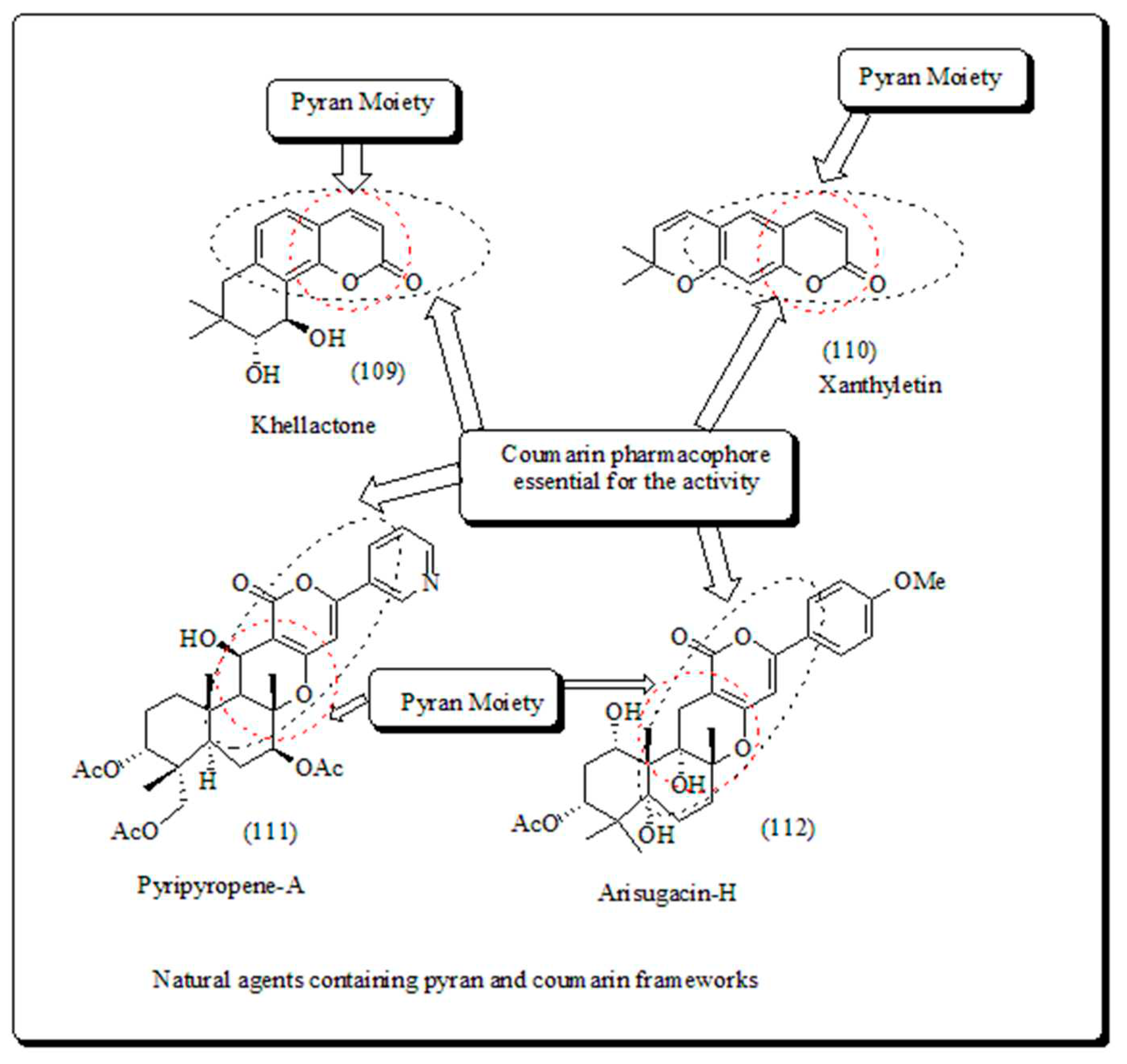
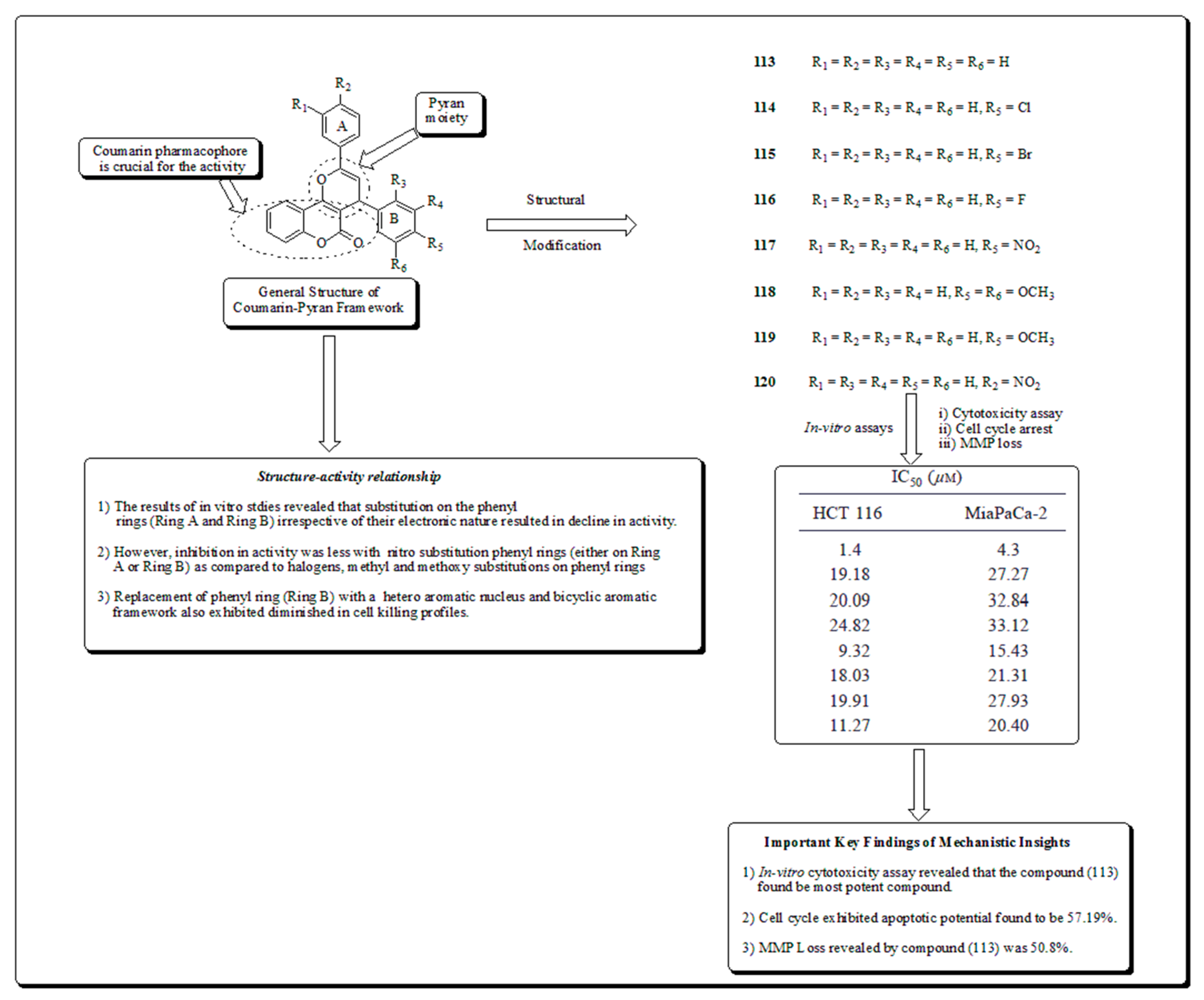
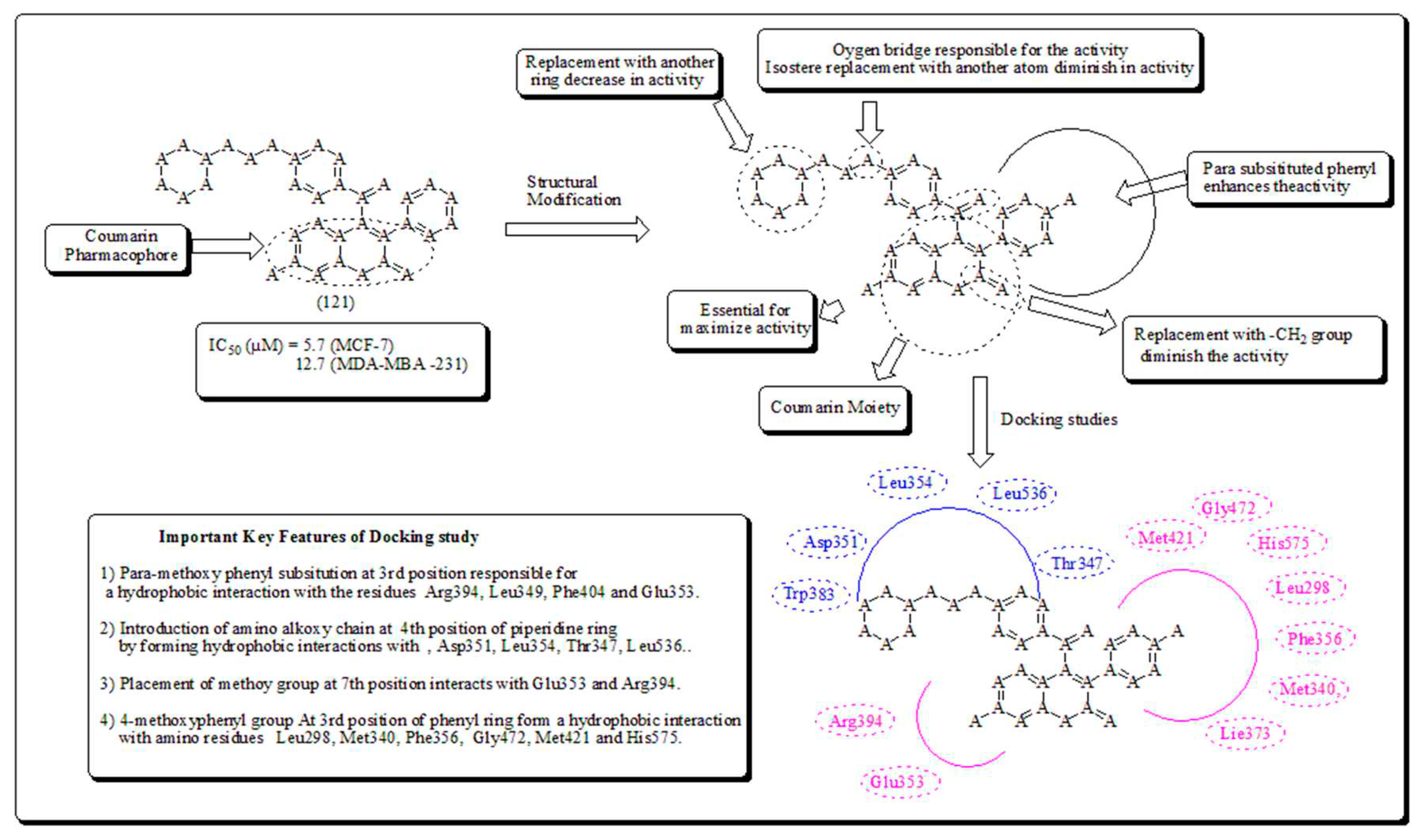
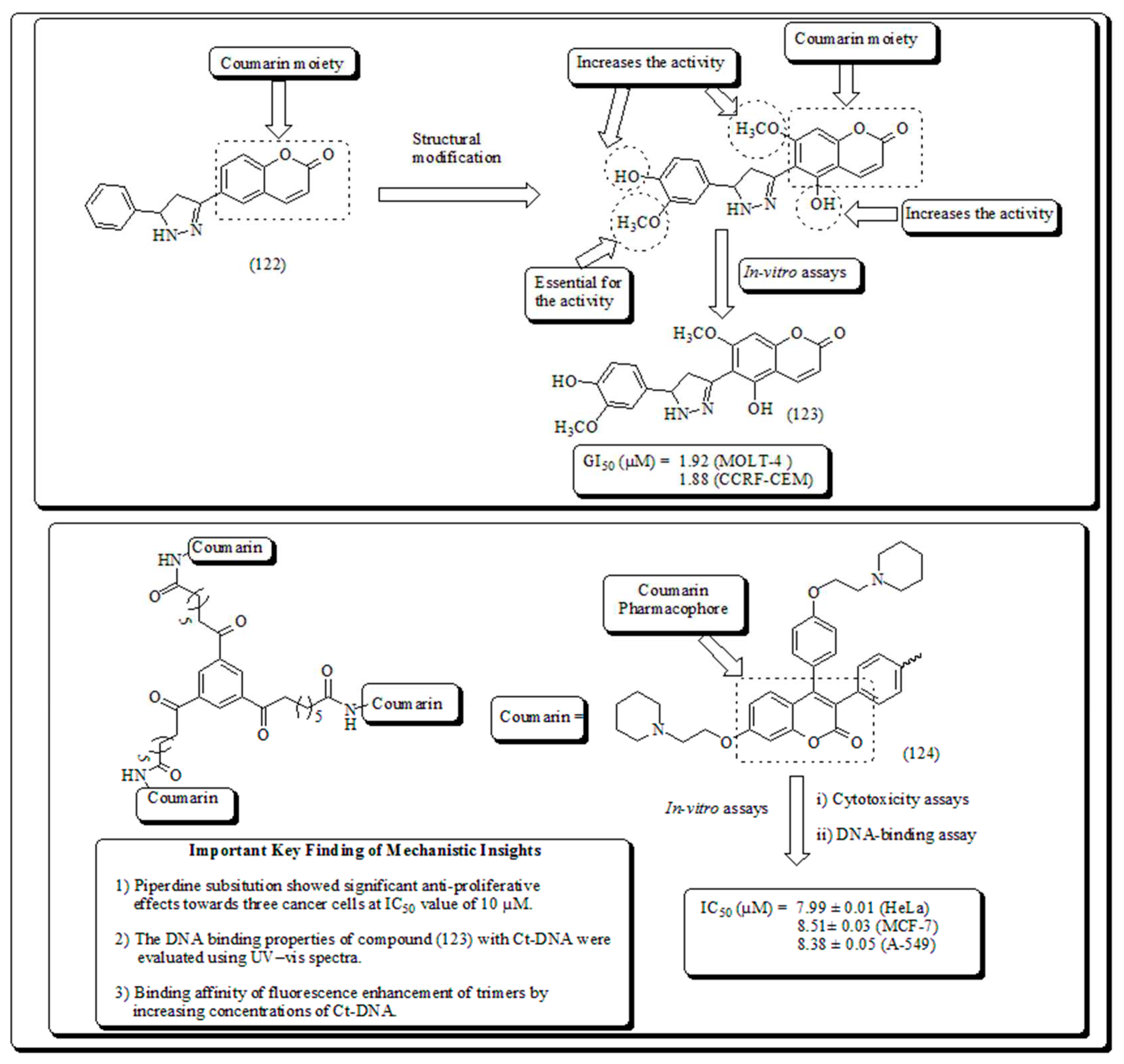
- Coumarin-based oxygen containing heterocyclic scaffolds
- 2.
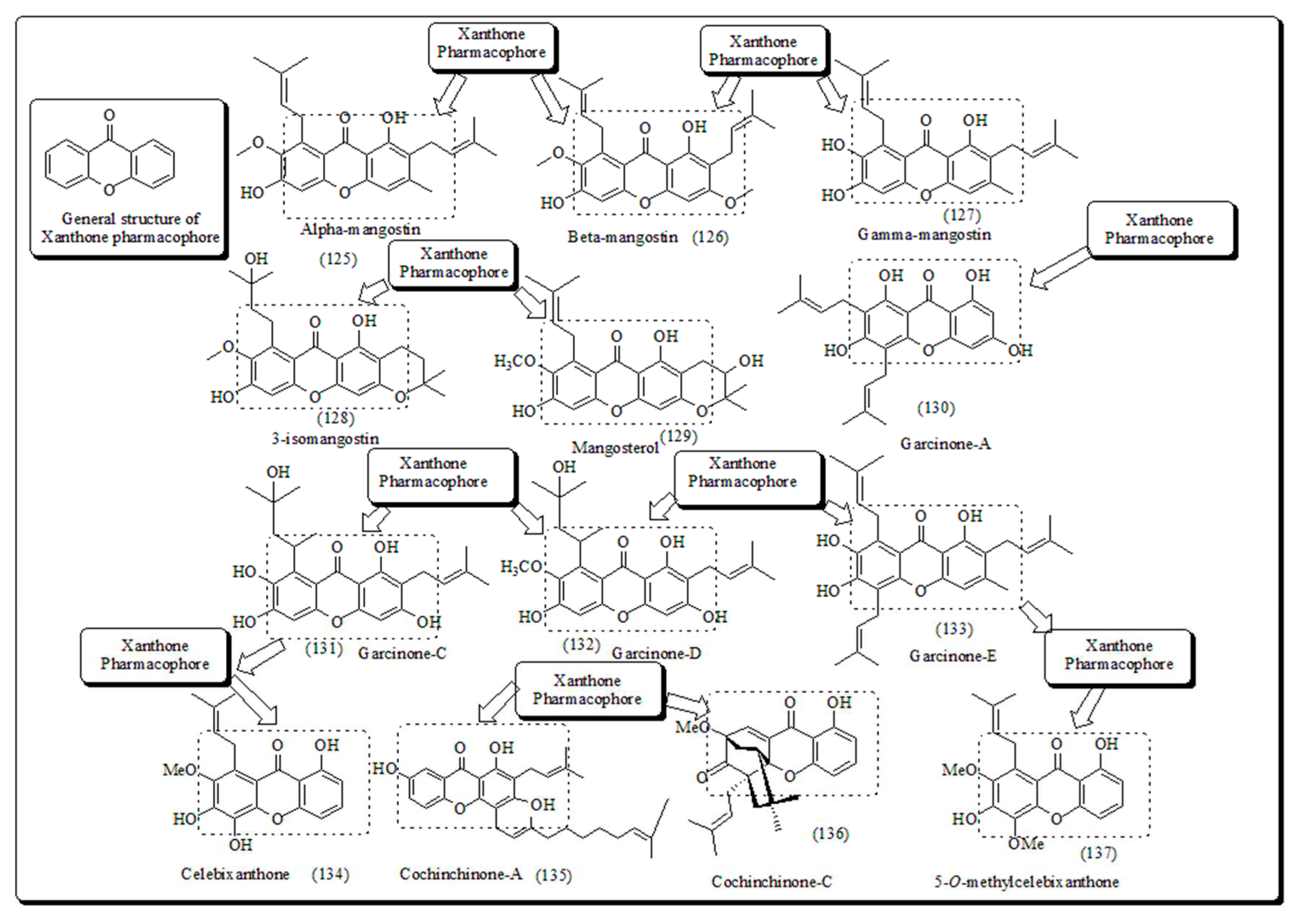
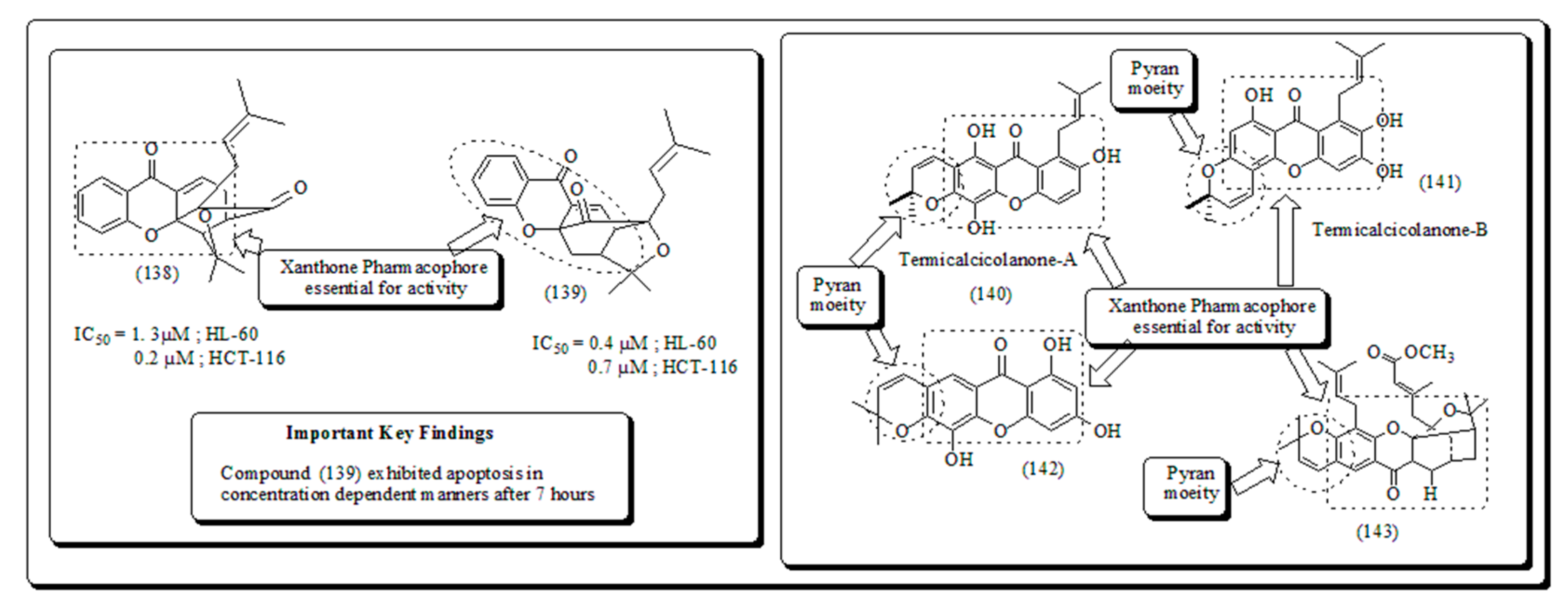
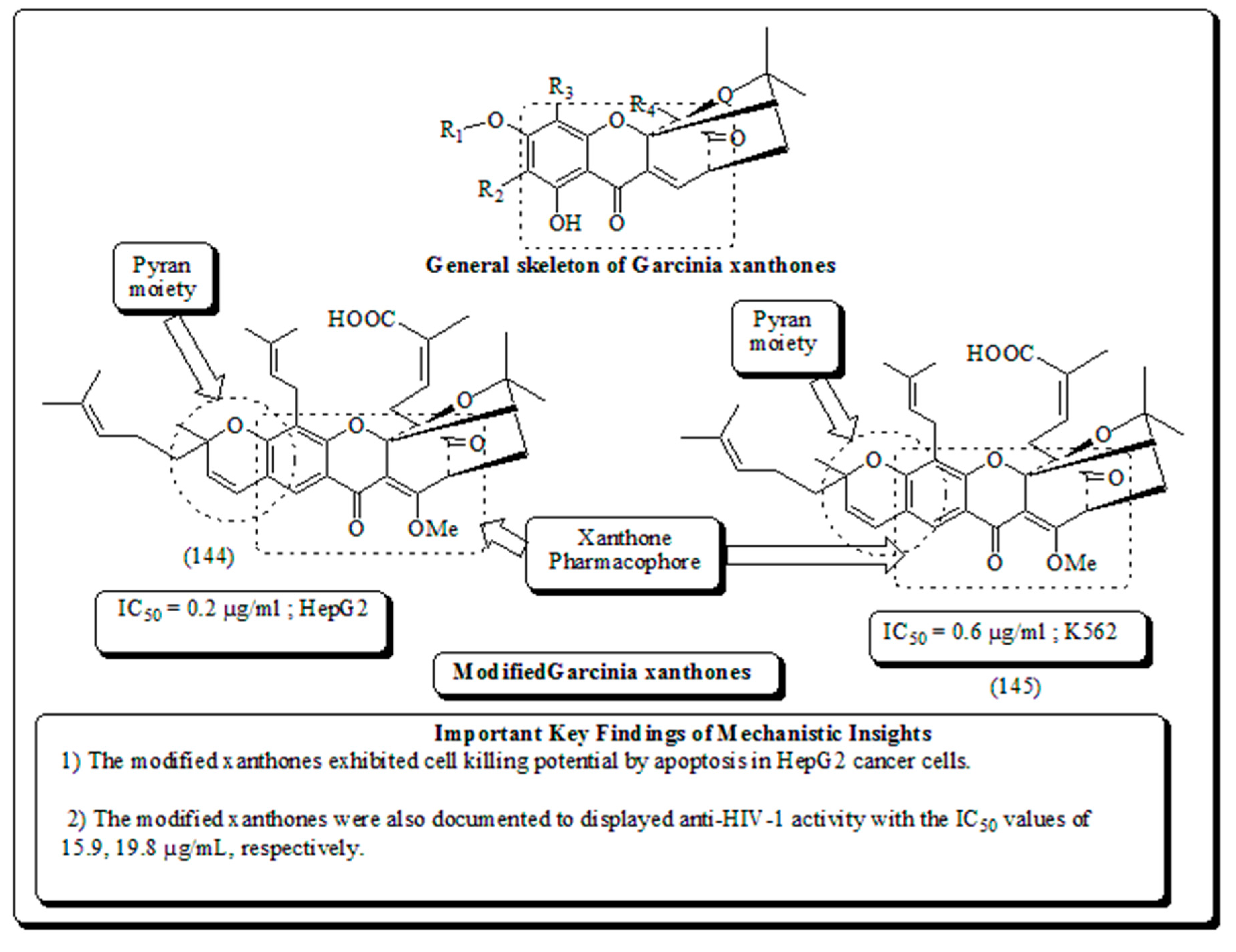
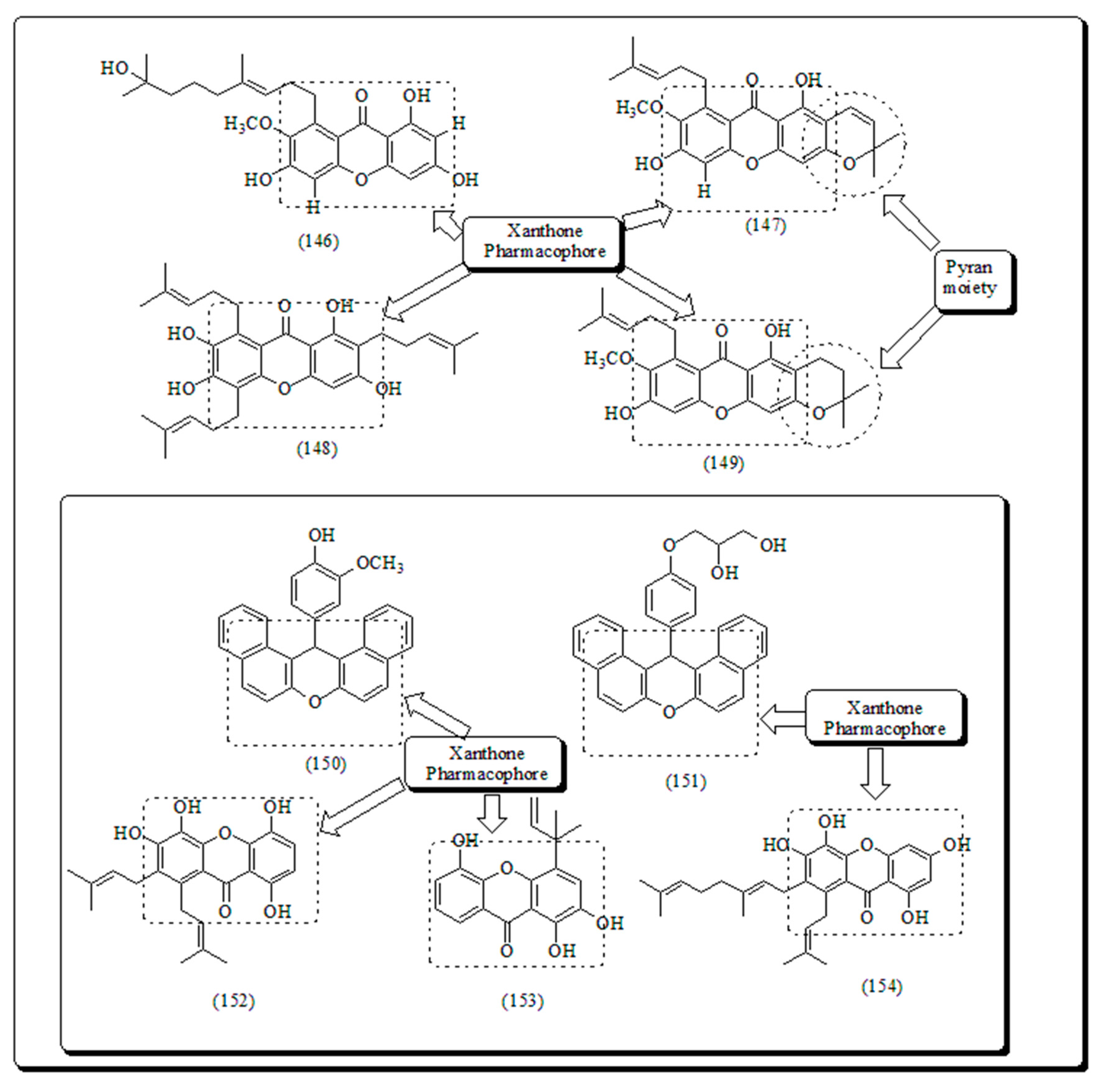
- Xanthone-based oxygen-containing heterocyclic scaffolds
- 4.
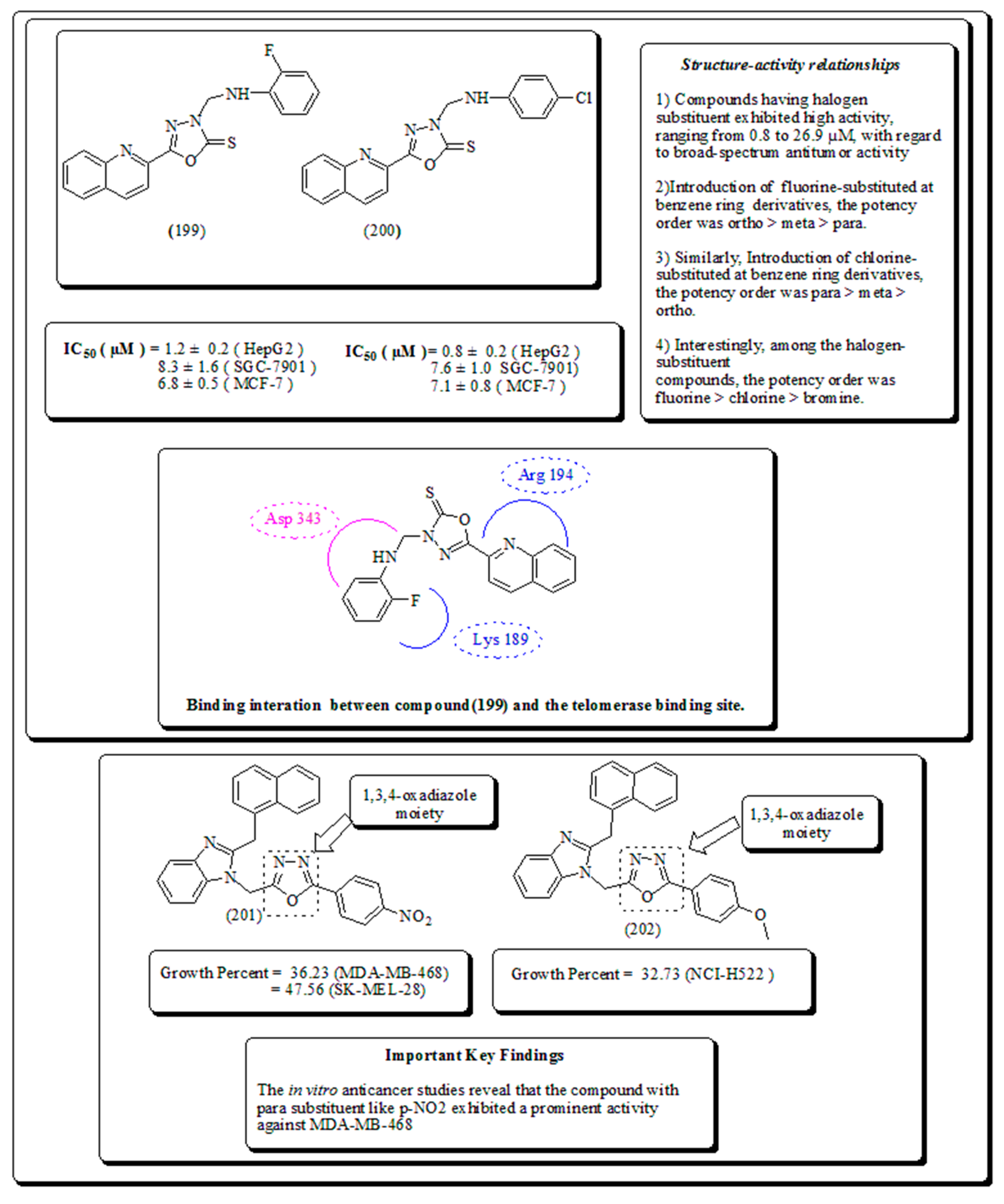
- Miscellaneous oxygen-containing heterocyclic scaffolds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chin, Y.W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, D.; Shri, R.; Kumar, S. Mechanistic insights and docking studies of phytomolecules as potential candidates in the management of cancer. Curr. Pharm. Des. 2022, 28, 2704–2724. [Google Scholar] [CrossRef] [PubMed]
- Dua, R.; Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K. Pharmacological significance of synthetic heterocycles scaffold: a review. Adv. Biol. Res. 2011, 5, 120–144. [Google Scholar]
- Sharma, P.; Shri, R.; Ntie-Kang, F.; Kumar, S. Phytochemical and Ethnopharmacological Perspectives of Ehretia laevis. Molecules, 2021, 26, 3489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shri, R.; Kumar, S. Phytochemical and In Vitro Cytotoxic Screening of Chloroform Extract of Ehretia Microphylla Lamk. Stresses, 2022, 2, 384–394. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, P.; Gupta, G.K.; Ntie-Kang, F.; Kumar, D. Structure Activity Relationship and Mechanistic Insights for Anti- HIV Natural Products. Molecules, 2020, 25, 2070. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Kaur, R.; Lobe, M.M.; Gupta, G.K.; Ntie-Kang, F. In search of therapeutic candidates for HIV/AIDS: Rational approaches, design strategies, structure-activity relationship and mechanistic insights. RSC Adv. 2021, 11, 17936–17964. [Google Scholar] [CrossRef]
- Singla, R.K.; Sharma, P.; Dubey, A.K.; Gundamaraju, R.; Kumar, D.; Kumar, S.; Madaan, R.; Shri, R.; Tsagkaris, C.; Parisi, S.; et al. Natural Product-Based Studies for the Management of Castration-Resistant Prostate Cancer: Computational to Clinical Studies. Front. Pharmacol. 2021, 12, 732266. [Google Scholar] [CrossRef]
- Singla, R.K; Sharma, P.; Kumar, D.; Gautam, R.K.; Goyal, R.; Tsagkaris, C.; Dubey, A.K.; Bansal, H.; Sharma, R.; Shen, B. The role of nanomaterials in enhancing natural product translational potential and modulating endoplasmic reticulum stress in the treatment of ovarian cancer. Front. Pharmacol. 2022, 13, 987088. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 7, 36977. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, S.K. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef] [PubMed]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem Heterocycle Compd 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Von Angerer, E.; Biberger, C.; Leichlt, S. Studies on Heterocycle-Based Pure Estrogen Antagonist. Ann. N. Y. Acad. Sci. 1995, 761, 176–191. [Google Scholar] [CrossRef]
- Kumar, D.; Nepali, K.; Bedi, P.M.S.; Kumar, S.; Malik, F.; Jain, S. 4,6-diaryl Pyrimidones as Constrained Chalcone Analogues: Design, Synthesis and Evaluation as Antiproliferative Agents, Anti-Cancer Agents Med. Chem. 2015, 15, 793–803. [Google Scholar]
- Kumar, D.; Singla, R.K.; Sharma, P.; Kumar, L.; Kaur, N.; Dhawan, R.K.; Sharma, S.; Dua, K.; Sharma, R. Phytochemistry and Polypharmacological Potential of Colebrookea Oppositifolia Smith, Curr. Top. Med. Chem. 2022. [Google Scholar]
- Kumar, D.; Singh, G.; Sharma, P.; Qayum, A.; Mahajan, G.; Mintoo, M.J.; Singh, S.K.; Mondhe, D.M.; Bedi, P.M.S.; Jain, S.K.; Gupta, G.K. 4-aryl/heteroaryl-4H-fused pyrans as Anti-proliferative Agents: Design, Synthesis and Biological Evaluation. Anti-Cancer Agents Med. 2018, 18, 57–73. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Nepali, K.; Mahajan, G.; Mintoo, M.J.; Singh, A.; Singh, G.; Mondhe, D.M.; Singh, G.; Jain, S.K. Gupta, G.K.; Ntie-Kang, F. Antitumour, acute toxicity and molecular modeling studies of 4-(pyridine-4-yl)-6-(thiophen-2-yl)pyrimidin-2(1H)–one against Ehrlich ascites Carcinoma and sarcoma-180. Heliyon 2018, 4, e0061. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R.; Rao, H.S.; Kumar, D. Phytochemistry and Medicinal Attributes of A. Scholaris: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 1000–10. [Google Scholar]
- Kaur, T.; Sharma, P.; Gupta, G.; Ntie-Kang, F.; Kumar, D. Treatment of tuberculosis by natural drugs: A review. Plant Archieves, 2019, 19, 2168–2176. [Google Scholar]
- Kumar, D.; Sharma, P.; Mahajan, A.; Dhawan, R.; Dua, K. Pharmaceutical interest of in-silico approaches, Phys. Sci. Rev. 2022. [Google Scholar]
- Damayanthi, Y.; Lown, J.W. Podophyllotoxins: Current status and recent developments. Curr. Med. Chem. 1998, 5, 205–252. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E. Plant antitumor agents. VI. The isolation and structure of Taxol, a novel anti-leukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Jordan, A.; Hadfield, J.A.; Lawrence, N.J.; McGown, A.T. Tubulin as a target for anticancer drugs: Agents which interact with the mitotic spindle. Med. Res. Rev. 1998, 18, 259–296. [Google Scholar] [CrossRef]
- Luduena, R.F.; Roach, M.C. Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacol. Ther. 1991, 49, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Podowyssotzki, V. Pharmacological studies of Podophyllum peltatum. Arch. Exp. Pathol. Pharmacol. 1880, 13, 29–52. [Google Scholar] [CrossRef]
- Dwyer, P.J.; Leyland-Jones, B.; Alonso, M.T.; Marsoni, S.; Wittes, R.E. Etoposide (VP-16-213): Current status of an active anticancer drug. N. Engl. J. Med. 1985, 312, 692–700. [Google Scholar] [CrossRef]
- Jin, Y.; Chena, S.W.; Tiana, X. Synthesis and biological evaluation of new spin-labeled derivatives of podophyllotoxin. Bioorg. Med. Chem. 2006, 14, 3062–3068. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhang, Z.W.; Hui, L.; Chen, S.W.; Tian, X. Novel semi synthetic spin-labeled derivatives of podophyllotoxin with cytotoxic and anti-oxidative activity. Bioorg. Med. Chem. 2010, 20, 983–986. [Google Scholar] [CrossRef]
- Wall, M.E. Camptothecin and taxol: discovery to clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirota, N.; Elfring, G.L.; Miller, L.L.N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. Engl. J. Med. 2000, 343, 905–914. [Google Scholar] [CrossRef]
- Gore, M.; Bokkel Huinink, W.; Carmichael, J.; Gordon, A.; Davidson, N.; Coleman, R.; Spaczynski, M.; Heron, J.F.; Bolis, G.; Malmstrom, H.; Malfetano, J.; Acarabelli, C.; Vennin, P.; Ross, G.; Fields, S.Z.J. Clinical evidence for topotecan-paclitaxel non--cross-resistance in ovarian cancer. Clin. Oncol. 2001, 19, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.H.; Nogales, E. Tubulin structure: Insights into microtubule properties and functions. Curr. Opin. Struct. Biol. 1998, 8, 785–91. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Basthians, H. Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug. Resist. Updat. 2007, 10, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Georg, G.I.; Harriman, G.C.B.; Himes, R.H.; Mejillano, M.R. 7(p- Azidobenzoyl)-taxol synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 1992, 2, 735–738. [Google Scholar] [CrossRef]
- Georg, G.I.; Cheruvallath, Z.S.; Himes, R.H.; Mejillano, M.R. Semi synthesis and biological activity of taxol analogs: Baccatin III 13-(Nbenzoyl-( 2′R,3′S)-3′-(p-tolyl)isoserinate), Baccatin III 13-(N-ptoluoyl)-( 2′R,3′S)-3′-phenylisoserinate), Baccatin III 13-(N-benzoyl- (2′R,3′S)-3′-(p- trifluoromethylphenyl)isoserinate), and Baccatin III 13-(N-(p trifluoromethylbenzoyl)-(2′R,3′S)-3′ phenylisoserinate). Bioorg. Med. Chem. Lett. 1992, 2, 1751–1754. [Google Scholar]
- Longley, R.E.; Caddigan, D.; Harmody, D.; Gunasekera, M.; Gunasekera, S.P. Discodermolide: A new, marine-derived immunosuppressive compound. I. In vitro studies. Transplantation, 1991, 52, 650–656. [Google Scholar] [CrossRef]
- Ter-Haar, E.; Kowalski, R.J.; Hamel, E.; Lin, C.M.; Longley, R.E.; Gunasekera, S.P. Discodermolide toxic marine agent that stabilizes microtubules more potently than taxol. Biochemistry 1996, 35, 243–250. [Google Scholar] [CrossRef]
- Song, W.; Lei, M,; Zhao, K. ; Hu, L.; Meng, Y.; Guo, D. Ceric ammonium nitrate-promoted oxidative coupling reaction for the synthesis and evaluation of a series of anti-tumor amide anhydro vinblastine analogs. Bioorg. Med. Chem. Lett. 2012, 22, 387–390. [Google Scholar] [CrossRef]
- Kowalski, R.J.; Giannakakou, P.; Gunasekera, S.P.; Longley, R.E.; Day, B.W.; Hamel, E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paxlitaxel-resistant cells. Mol. Pharmacol. 1997, 52, 613–22. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Roshangar, F.; Vourlounis, D. Chemical biology of the epothilones. Angew. Chem. Int. Ed. 1998, 37, 2014–2045. [Google Scholar] [CrossRef]
- Sasse, F. Microtubule binding. Curr. Biol. 2000, 10, R469–R469. [Google Scholar]
- Nakhi, A.; Adepu, R.; Rambabu, D.; Kishore, R.; Vanaja, G.R.; Kalle, A.M.; Pal, M. Thieno [3, 2-c] pyran-4-one based novel small molecules: Their synthesis, crystal structure analysis and in vitro evaluation as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 4418–4427. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Grondal, C.; Huttl, M.R.M. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, B.; Javanshir, S.; Maleki, A.; Safari, M.; Khavasi, H.R. An efficient synthesis of 4H-chromene, 4H-pyran, and oxepine derivatives via one-pot three-component tandem reactions. Tetrahedron Lett. 2012, 53, 6977–6981. [Google Scholar] [CrossRef]
- Moumou, Y.; Vasseur, J.; Trotin, F.; Dubois, J. ; Catechin production by callus cultures of Fagopyrum esculentum. Phytochemistry 1992, 31, 1239–1241. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W.J. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J. Nat. Prod. 2005, 68, 904–910. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Cao, G.Q. Selenium-based solid-phase synthesis of benzopyrans I: applications to combinatorial synthesis of natural products. Angew. Chem. Int. Ed. 2000, 39, 734–739. [Google Scholar] [CrossRef]
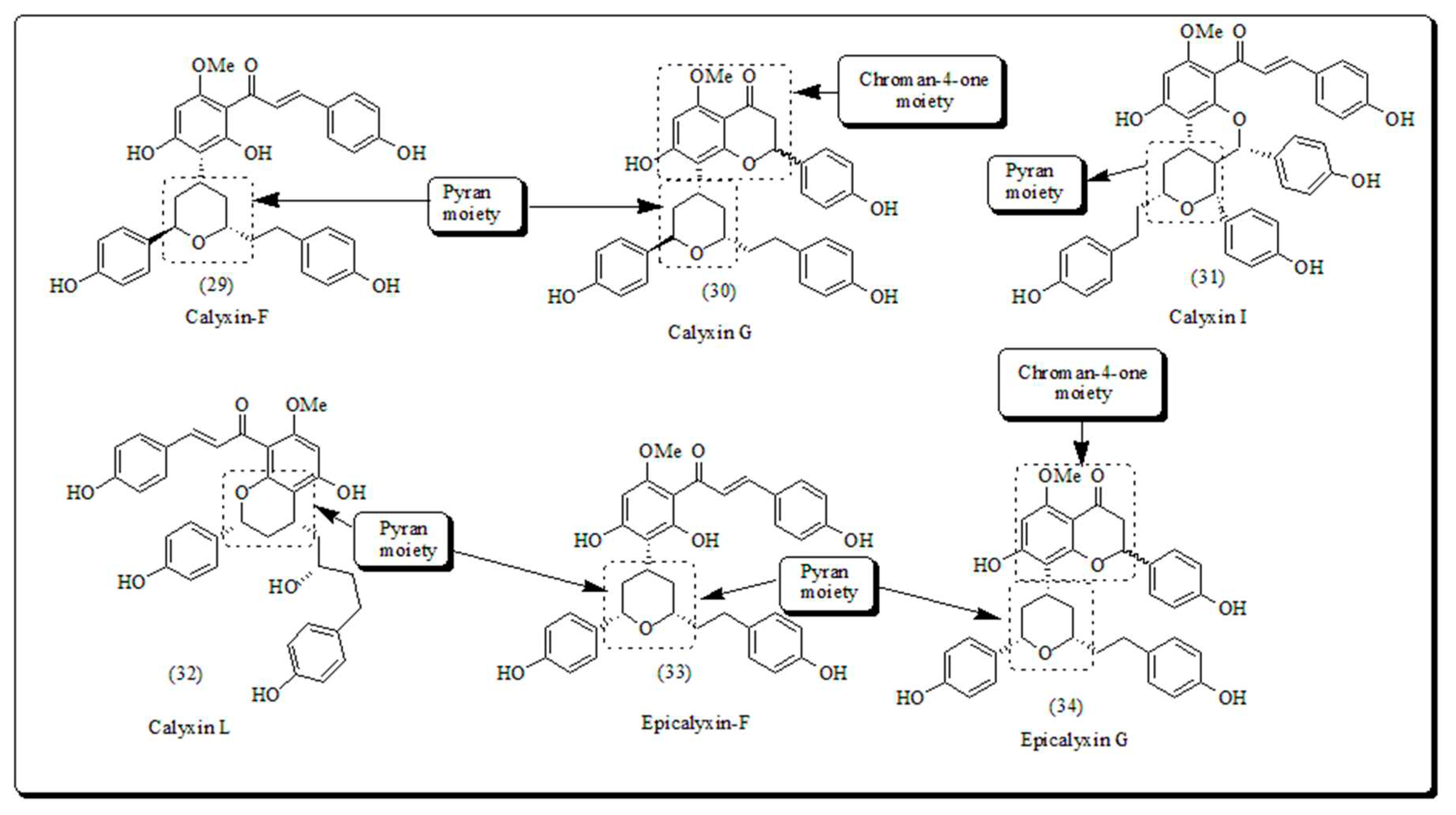
- Gewali, M.B.; Tezuka, Y.; Banskota, A.H.; Ali, M.S.; Saiki, I.; Dong, H.; Kadota, S. Epicalyxin F and calyxin I: two novel antiproliferative diarylheptanoids from the seeds of Alpinia blepharocalyx. Org. Lett. 1999, 1, 1733–1736. [Google Scholar] [CrossRef]
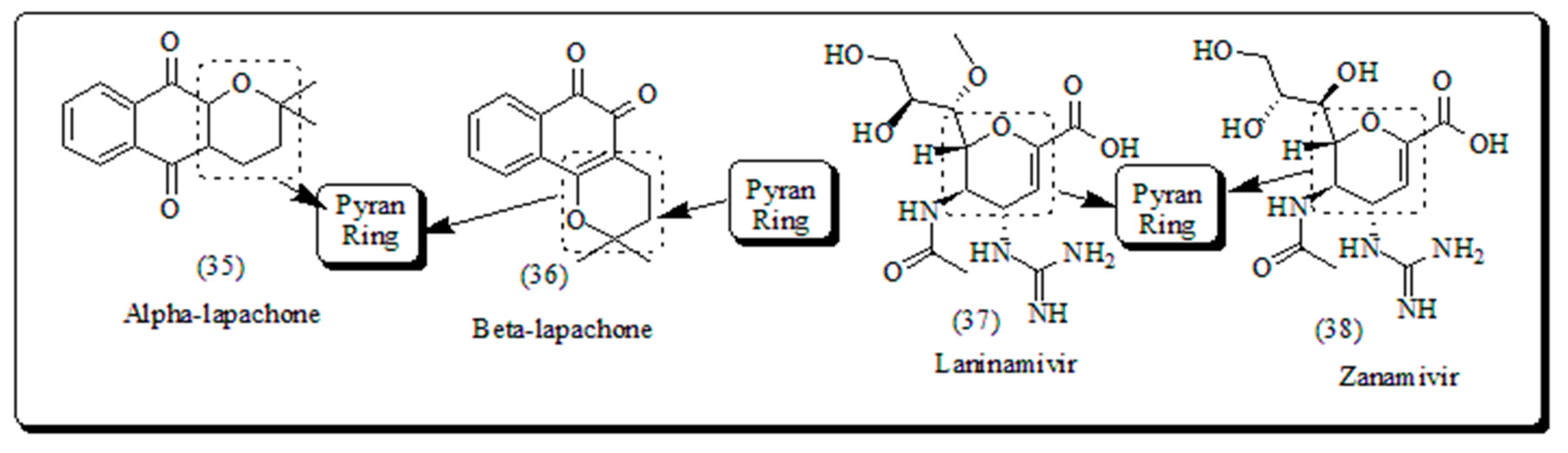
- Koyama, K.; Takahashi, M.; Oitate, M.; Nakai, N.; Takakusa, H.; Miura, S.; Okazaki, O. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favourable long-retention pro file in the mouse respiratory tract. Antimicrob. Agents Chemother. 2009, 53, 4845–4851. [Google Scholar] [CrossRef]
- Kiso, M.; Kubo, S.; Ozawa, M.; Le, Q.M.; Nidom, C.A.; Yamashita, M.; Kawaoka, Y. Efficacy of the new neuraminidase inhibitor CS-8958 against H5N1 influenza viruses. PLoS Pathog. 2010, 6, 1000786. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.; da Silva, F.C.; Bezerra, F.A.; Lourenco, M.; Kaiser, C.R.; Pinto, A.C.; Ferreira, V.F. Synthesis of α-and β-pyran naphthoquinones as a new class of antitubercular agents. Arch. Pharm. 2010, 343, 81–90. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, D.R.; de Souza, A.C.; Resende, J.A.; Santos, W.C.; dos Santos, E.A. ; C. Pessoa, M.O. de Moraes, L.V. Costa-Lotufo, R.C. Montenegro, V.F. Ferreira, Synthesis of new 9-hydroxy-α-and 7-hydroxy-β-pyran naphthoquinones and cytotoxicity against cancer cell lines, Org. Biomol. Chem. 2011, 9, 4315–4322. [Google Scholar]
- Dong, Y.; Shi, Q.; Nakagawa-Goto, K.; Wu, P.C.; Morris-Natschke, S.L.; Brossi, A.; Bastow, K.F.; Lang, J.Y.; Hung, M.C.; Lee, K.H. Antitumor agents 270. Novel substituted 6-Phenyl-4H-furo[3,2-c]pyran-4-one derivatives as potent and highly selective anti-breast cancer agents. Bioorg. Med. Chem. 2010, 18, 803–808. [Google Scholar] [CrossRef]
- He, M.Z.; Yang, N.; Sun, C.L.; Yao, X.J.; Yang, M. Modification and biological evaluation of novel 4-hydroxy-pyrone derivatives as non-peptidic HIV-1 protease inhibitors. Med. Chem. Res. 2010, 20, 200–209. [Google Scholar] [CrossRef]
- Schiller, R.; Tichotova, L.; Pavlik, J.; Buchta, V.; Melichar, B.; Votruba, I.; Kunes, J.; Spulak, M.; Pour, M. 3, 5-Disubstituted pyranone analogues of highly antifungally active furanones: Conversion of biological effect from antifungal to cytostatic. Bioorg. Med. Chem. Lett. 2010, 20, 7358–7360. [Google Scholar] [CrossRef]
- Hussain, H.; Aziz, S.; Schulz, B.; Krohn, K. Synthesis of a 4H-anthra [1, 2-b] pyran derivative and its antimicrobial activity. Nat. Prod. Commun. 2011, 6, 841–843. [Google Scholar] [CrossRef]
- Shahrisa, A.; Zirak, M.; Mehdipour, A.R.; Miri, R. Synthesis and calcium channel antagonist activity of new symmetrical and asymmetrical 4-[2-chloro-2-(4-chloro-6-methyl-2-oxo-2H-pyran-3-yl) vinyl]-substituted 1, 4-dihydropyridines. Chem. Heterocycl. Compd. 2011, 46, 1354–1363. [Google Scholar] [CrossRef]
- Bisht, S.S.; Jaiswal, N.; Sharma, A.; Fatima, S.; Sharma, R.; Rahuja, N.; Srivastava, A.K.; Bajpai, V.; Kumar, B.; Tripathi, R.P. A convenient synthesis of novel pyranosyl homo-C-nucleosides and their antidiabetic activities. Carbohydrates Res. 2011, 346, 1191–1201. [Google Scholar] [CrossRef]
- Wang, S.M. , Milne, G.W.A.; Yan, X.J.; Posey, I.J.; Nicklaus, M.C.; Graham, L.; Rice, W.G. Discovery of novel, non-peptide HIV-1 protease inhibitors by pharmacophore searching. J. Med. Chem. 1996, 39, 2047–2054. [Google Scholar] [CrossRef]
- Madda, J.; Venkatesham, A.; Kumar, B.N.; Nagaiah, K.; Sujitha, P.; Kumar, C.G.; Rao, T.P.; Babu, N.J. Synthesis of novel chromeno-annulated cis-fused pyrano[3,4-c]benzopyran and naphtho pyran derivatives via domino aldol-type/hetero Diels–Alder reaction and their cytotoxicity Evaluation. Bioorg. Med. Chem. Lett. 2014, 24, 4428–4434. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.A. , Garlich, J.R.; Su, J.; Peng, X.; Newblom, J.; Weber, K.; Durden, D.L. Synthesis and cancer stem cell-based activity of substituted 5-morpholino-7H-thieno[3,2-b]pyran-7-ones designed as next generation PI3K inhibitors. J. Med. Chem. 2013, 56, 1922–1939. [Google Scholar] [CrossRef] [PubMed]
- Siripong, P.; Kanokmedakul, K.; Piyaviriyagul, S.; Yahuafai, J.; Chanpai, R.; Ruchirawat, S.; Oku, N. Antiproliferative naphthoquinone esters from Rhinacanthus nasutus Kurz. roots on various cancer cells. J. Trad. Med. 2006, 23, 166–172. [Google Scholar]
- Frolova, L.V.; Magedov, I.V.; Romero, A.E.; Karki, M.; Otero, I.; Hayden, K.; Evdokimov, N.M.; Banuls, L.M.Y.; Rastogi, S.K.; Smith, W.R.; Lu, S.L. Structural simplification of bioactive natural products with multicomponent synthesis. 4. 4H-Pyrano-[2,3-b] naphthoquinones with anticancer activity. Bioorg. Med. Chem. Lett. 2012, 22, 5195–5198. [Google Scholar]
- Thi, T.A.D.; Thi, T.H.V.; Phuong, H.T.; Nguyen, T.H.; Duc, C.V.; Depetter, Y.; Van Nguyen, T.; D’hooghe, M. Synthesis and anticancer properties of new (dihydro) pyranonaphthoquinones and their epoxy analogs. Bioorg. Med. Chem. Lett. 2015, 25, 3355–3358. [Google Scholar]
- Kowalski, K.; Chyła, A.K.; Szczupak, L.; Hikisz, P.; Bernasińska, J.; Rajnisz, A.; Solecka, J.; Therrien, B. Ferrocenylvinyl-flavones: Synthesis, structure, anticancer and antibacterial activity studies. J. Organomet. Chem. 2013, 741, 153–161. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- De Groot, H.; Rauen, U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam. Clin. Pharmacol. 1998, 12, 249–255. [Google Scholar] [CrossRef]
- Middleton, J.E. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2- (2 phenylethyl) chromones of Imperata cylindrical. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Min, L.W. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar]
- Akao, Y.; Itoh, T.; Ohguchi, K.; Iinuma, M.; Nozawa, Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg. Med. Chem. 2008, 16, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Mays, J.R.; Hill, S.A.; Moyers, J.T.; Blagg, B.S. The synthesis and evaluation of flavone and isoflavone chimeras of novobiocin and derrubone. Bioorg. Med. Chem. 2010, 18, 249–266. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Hsieh, Y.S.; Kuo, W.H.; Chiou, H.L.; Yang, S.F.; Chiang, W.L.; Chu, S.C. ; The tumor-growth inhibitory activity of flavanone and 2'-OH flavanone in vitro and in vivo through induction of cell cycle arrest and suppression of cyclins and CDKs. J. Biomed. Sci. 2007, 14, 107–119. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, J.I.; Kim, G.H. Anti-carcinogenic effect of a new analogue 4'-chloroflavanone from flavanone in human breast cancer cells. Int. J. Mol. Med. 2010, 25, 293–298. [Google Scholar]
- Choi, E.J.; Lee, J.I. , Kim, G.H. Effects of 4',7-dimethoxyflavanone on cell cycle arrest and apoptosis in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2011, 34, 2125–2130. [Google Scholar] [CrossRef]
- Usman, H.; Hakim, E.H.; Harlim, T.; Jalaluddin, M.N.; Syah, Y.M.; Achmad, S.A.; Takayama, H. Cytotoxic chalcones and flavanones from the tree bark of Cryptocarya costata. Naturforsch., C. J. Biosci. 2006, 61, 184–188. [Google Scholar] [CrossRef]
- Shen, S.C.; Ko, C.H.; Tseng, S.W.; Tsai, S.H.; Chen, Y.C. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004, 197, 84–95. [Google Scholar] [CrossRef]
- Liao, S.Y.; Chen, J.C.; Qian, L.; Shen, Y.; Zheng, K.C. QSAR, action mechanism and molecular design of flavone and isoflavone derivatives with cytotoxicity against HeLa. Eur. J. Med. Chem. 2008, 43, 2159–70. [Google Scholar] [CrossRef]
- Hirunuma, M.; Shoyama, Y.; Sasaki, K.; Sakamoto, S.; Taura, F.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Flavone-catalyzed apoptosis in Scutellaria baicalensis. Phytochemistry 2011, 72, 752–60. [Google Scholar] [CrossRef] [PubMed]
- Switalska, M.; Grynkiewicz, G.; Strzadala, L.; Wietrzyk, J. Novel genistein derivatives induce cell death and cell cycle arrest through different mechanisms. Nutr. Cancer 2013, 65, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Murti, Y.; Mishra, P. Synthesis and evaluation of flavanones as anticancer agents. Indian J. Pharm. Sci. 2014, 76, 163–166. [Google Scholar] [PubMed]
- Kumar, D.; Singh, O.; Nepali, K.; Bedi, P.M.S.; Qayum, A.; Singh, S.; Jain, S.K. Naphthoflavones as antiproliferative agents: design, synthesis and biological evaluation. Anti-Cancer Agent Med. Chem. 2016, 16, 881–890. [Google Scholar] [CrossRef]
- Aghdassi, A.; Phillips, P.; Dudeja, V.; Dhaulakhandi, D.; Sharif, R.; Dawra, R.; Lerch, M.M.; Saluja, S. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007, 67, 616–625. [Google Scholar] [CrossRef]
- Mouria, M.; Gukovskaya, A.S.; Jung, Y.; Buechler, P.; Hines, O.J.; Reber, H.A.; Pandol, S.J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int. J. Canc. 2002, 98, 761–769. [Google Scholar] [CrossRef]
- Xue, W.; Song, B.A.; Zhao, H.J.; Qi, X.B.; Huang, Y.J.; Liu, X.H. , Novel myricetin derivatives: Design, synthesis and anticancer activity Eur. J. Med. Chem. 2015, 97, 155–163. [Google Scholar] [CrossRef]
- Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S.; Ajdari, S.; Davoodi, J.; Shafiee, A.; Foroumadi, A. Halogenated flavanones as potential apoptosis-inducing agents: synthesis and biological activity evaluation. Eur. J. Med. Chem. 2012, 58, 573–80. [Google Scholar] [CrossRef]
- Al-Kawkabani, A.; Boutemeur-Kheddis, B.; Makhloufi-Chebli, M.; Hamdi, M.; Talhi, O.; Silva, A.M. ; Synthesis of novel 2H,8H-pyrano[2,3 f]chromene-2,8-diones from 8-formyl-7-hydroxy-4-methylcoumarin. Tetrahedron Lett. 2013, 54, 5111–5114. [Google Scholar] [CrossRef]
- Shi, Y. , Zhou, C. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 956–960. [Google Scholar] [CrossRef]
- Hur, S.Y.; Kim, T.E.; Park, Y.G.; Kim, J.R.; Kim, J.W. Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp. Mol. Med. 2005, 37, 436–446. [Google Scholar]
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg. Med. Chem. Lett. 2011, 21, 5770–5773. [Google Scholar] [CrossRef]
- Reddy, N.S.; Mallireddigari, M.R.; Cosenza, S.; Gumireddy, K.; Bell, S.C. Reddy, E.P.; Reddy, M.V. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg. Med. Chem. Lett. 2004, 14, 4093–4097. [Google Scholar] [CrossRef] [PubMed]
- Manvar, A.; Bavishi, A.; Radadiya, A.; Patel, J.; Vora, V.; Dodia, N.; Rawal, K.; Shah, A. Diversity oriented design of various hydrazides and their in vitro evaluation against Mycobacterium tuberculosis H37 Rv strains. Bioorg. Med. Chem. Lett. 2011, 21, 4728–4731. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Lu, X.; Zheng, P.; Liu, L.; Han, C.; Hu, J.; Liu, Z.; Ma, T.; Li, Y.; Wang, L.; Chen, Z.; Liu, G. Highly suppressing wild-type HIV-1 and Y181C mutant HIV-1 strains by 10-chloromethyl-11-demethyl-12-oxo-calanolide A with druggable profile. J. Med. Chem. 2010, 53, 1397–1401. [Google Scholar] [CrossRef]
- Yeh, J.Y.; Coumar, M.S.; Horng, J.T.; Shiao, H.Y.; Kuo, F.M.; Lee, H.L.; Chen, I.C.; Chang, C.W.; Tang, W.F.; Tseng, S.N.; Chen, C.J. Anti-Influenza drug discovery: structure−activity relationship and mechanistic insight into novel angelicin derivatives. J. Med. Chem. 2010, 53, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, B.S.P.; Rodriguez, B.J.C. Synthesis of collinin, an antiviral coumarin. Aust. J. Chem. 2003, 56, 59–60. [Google Scholar]
- Anand, P.; Singh, B.; Singh, N. review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Cavalli, A.; Colizzi, F.; Belluti, F.; Bartolini, M.; Mancini, F.; Recanatini, M.; Andrisano, V.; Rampa, A. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg. Med. Chem. Lett. 2008, 18, 423–426. [Google Scholar] [CrossRef]
- Shi, Y. , Zhou, C. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 956–960. [Google Scholar] [CrossRef]
- Lin, C.M.; Huang, S.T.; Lee, F.W.; Kuo, H.S.; Lin, M.H. 6-Acyl-4-aryl/alkyl-5, 7-dihydroxycoumarins as anti-inflammatory agents. Bioorg. Med. Chem. 2006, 14, 4402–4409. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, S.; Kumar, D.; Budhiraja, A.; Dhar, K.L. Anticancer Hybrids- A Patent Survey. Recent Pat. Anticancer Drug Discov. 2014, 9, 303–339. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.M.; Eissa, A.A.; Abou-Seri, S.M.; Awadallah, F.M.; Hassan, G.S. Synthesis and biological evaluation of novel coumarin–pyrazoline hybrids endowed with phenylsulfonyl moiety as antitumor agents. Eur. J. Med. Chem. 2013, 60, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Bindal, S.; Luxami, V. Synthesis of new conjugated coumarin–benzimidazole hybrids and their anticancer activity. Bioorg. Med. Chem. 2013, 23, 3667–3672. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Sarkar, J.; Sinha, S. Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. 2010, 20, 7205–7211. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Avula, S.R.; Sharma, K. Palnati, G.R.; Bathula, S.R. Discovery of coumarin–monastrol hybrid as potential antibreast tumor-specific agent. Eur. J. Med. Chem. 2013, 60, 120–127. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Majee, A.; Hajra, A. Regioselective synthesis of pyrano[3,2-c]coumarins via Cu(II)-catalyzed tandem reaction. Tetrahedron Lett. 2013, 54, 3892–3895. [Google Scholar] [CrossRef]
- Kumar, D.; Malik, F.; Bedi, P.M.S.; Jain, S. 2,4-Diarylpyrano[3,2-c]chromen-5(4H)-ones as antiproliferative agents: design, synthesis and biological evaluation. Chem. Pharm. Bull. 2016, 64, 399–409. [Google Scholar] [CrossRef]
- Hussain, M.K.; Ansari, M.I.; Yadav, N.; Gupta, P.K.; Gupta, A.K.; Saxena, R.; Fatima, I.; Manohar, M.; Kushwaha, P.; Khedgikar, V. Design and synthesis of ERa/ERb selective coumarin and chromene derivatives as potential antibreast cancer and anti-osteoporotic agents. RSC Adv. 2014, 4, 8828–8845. [Google Scholar] [CrossRef]
- Ganina, O.G.; Daras, E.; Bourgarel-Rey, V.; Peyrot, V.; Andresyuk, A.N.; Finet, J.P.; Fedorov, A.Y.; Beletskaya, I.P.; Combes, S. Synthesis and biological evaluation of polymethoxylated 4- heteroarylcoumarins as tubulin assembly inhibitor. Bioorg. Med. Chem. 2008, 16, 8806–8812. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Osman, H.; Bagley, M.C.; Lam, C.K.; Mohamad, S.; Zahariluddin, A.S.M. ; Synthesis and antimicrobial properties of some new thiazolylcoumarin derivatives. Eur. J. Med. Chem. 2011, 46, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Gali, R.; Banothu, J.; Gondru, R.; Bavantula, R.; Velivela, Y.; Crooks, P.A. One-pot multicomponent synthesis of indole incorporated thiazolylcoumarins and their antibacterial, anticancer and DNA cleavage studies. Bioorg. Med. Chem. Lett. 2015, 25, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.Z.; Gazioglu, I.; Sonmez, F.; Kucukislamoglu, M. Synthesis, antioxidant and anticholinesterase activities of novel coumaryl thiazole derivatives. Bioorg. Chem. 2015, 59, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.; Picciau, C.; Ferino, G.; Quezada, E.; Podda, G.; Uriarte, E.; Vina, D. Synthesis, human monoamine oxidase inhibitory activity and molecular docking studies of 3-heteroarylcoumarin derivatives. Eur. J. Med. Chem. 2011, 46, 1147–1152. [Google Scholar] [CrossRef]
- Garazd, Y.; Garazd, M.; Lesyk, R. Synthesis and evaluation of anticancer activity of 6-pyrazolinylcoumarin derivatives. Saudi Pharm J 2017, 25, 214–223. [Google Scholar] [CrossRef]
- Zhao, H.; Donnelly, A.C.; Kusuma, B.R.; Brandt, G.E.; Brown, D.; Rajewski, R.A.; Blagg, B.S. Engineering an antibiotic to fight cancer: optimization of the novobiocin scaffold to produce anti-proliferative agents. J. Med. Chem. 2011, 54, 3839–3853. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; TN, M.M.; Ahmad, A.; Khan, A.U. Synthesis of 4-Hydroxycoumarin Heteroarylhybrids as Potential Antimicrobial Agents. Arch. Pharm. 2011, 344, 394–401. [Google Scholar] [CrossRef]
- Kusuma, B.R.; Peterson, L.B.; Zhao, H.; Vielhauer, G. Holzbeierlein, J.; Blagg, B.S. Targeting the heat shock protein 90 dimer with dimeric inhibitors. J. Med. Chem. 2011, 54, 6234–6253. [Google Scholar] [CrossRef]
- Burlison, J.A.; Blagg, B.S.J. Synthesis and evaluation of coumermycin A1 analogues that inhibit the Hsp90 protein folding machinery. Org. Lett. 2006, 8, 4855–4858. [Google Scholar] [CrossRef]
- Tan, G.; Yao, Y.; Gu, Y.; Li, S.; Lv, M.; Wang, K.; Li, X. Cytotoxicity and DNA binding property of the dimers of triphenylethylene–coumarin hybrid with one amino side chain. Bioorg. Med. Chem. Lett. 2014, 24, 2825–2830. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, L.K.; Yao, Y.C.; Li, S.; Lv, M.; Wang, K.; Li, X.; Chen, H. Anticancer activity and DNA binding property of the dimers of triphenylethylene–coumarin hybrid with two amino side chains. Med. Chem. Res. 2015, 24, 2314–2324. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, Y.C.; Lv, S.M.; Chen, H.; Li, X. Cytotoxicity and DNA binding property of triphenylethylene-coumarin hybrids with two amino side chains. Bioorg. Med. Chem. Lett. 2014, 24, 900–904. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Yao, Y.; Zhou, L.; Zhao, J.; Gu, Y.; Li, X. Design, synthesis, and anti-tumor activities of novel triphenylethylene–coumarin hybrids, and their interactions with Ct-DNA. Bioorg. Med. Chem. Lett. 2013, 23, 4785–4789. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Y.C.; Gao, M.Y.; Rong, R.X.; Wang, K.R.; Li, X.L.; Chen, H. Anticancer activity and DNA binding property of the trimers of triphenylethylene–coumarin hybrids. Chin. Chem. Lett. 2016, 27(11), 1708–1716. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Streelman, D.R.; Sneden, A.T. Psorospermin, a new antileukemic xanthone from Psorospermum febrifugum. J. Nat. Pro. 1980, 43, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.K.; Sloman, D.L.; Porco Jr, J.A. Polycyclic xanthone natural products: structure, biological activity and chemical synthesis. J. Nat. Pro. Rep. 2013, 30, 382–391. [Google Scholar] [CrossRef]
- Lin, C.N.; Liou, S.J.; Lee, T.H.; Chuang, Y.C.; Won, S.J. Xanthone derivatives as potential anti-cancer drugs. J. Pharm. Pharmacol. 1996, 48, 539–544. [Google Scholar] [CrossRef]
- Chen, C.A.; Yeh, R.H.; Lawrence, D.S. Design and synthesis of a fluorescent reporter of protein kinase activity. J. Am. Chem. Soc. 2002, 124, 3840–3841. [Google Scholar] [CrossRef]
- Steiner, L.F.; Summerland, S.A. ; Xanthone as an ovicide and larvicide for the codling moth. J. Econ. Entomol. 1943, 36, 435–439. [Google Scholar] [CrossRef]
- Cheng, J.H.; Huang, A.M.; Hour, T.C.; Yang, S.C.; Pu, Y.S.; Lin, C.N. Antioxidant xanthone derivatives induce cell cycle arrest and apoptosis and enhance cell death induced by cisplatin in NTUB1 cells associated with ROS. Eur. J. Med. Chem. 2011, 46, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Chase, M. Angiosperm Phylogeny Group. Bot. J. Lin. Soc. 2003, 141, 399–436. [Google Scholar]
- Lee, K.H.; Chai, H.B.; Tamez, P.A.; Pezzuto, J.M.; Cordell, G.A.; Win, K.K.; Tin Wa, M. Biologically active alkylated coumarins from Kayea assamica. Phytochem. 2003, 64, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Laphookhieo, S.; Syers, J.K.; Kiattansakul, R.; Chantrapromma, K. Cytotoxic and antimalarial prenylated xanthones from Cratoxylum cochinchinense. Chem. Pharm. Bull. 2006, 54, 745–747. [Google Scholar] [CrossRef]
- Wu, C.P.; Van Schalkwyk, D.A.; Taylor, D.; Smith, P.J.; Chibale, K. ; Reversal of chloroquine resistance in Plasmodium falciparum by 9H-xanthene derivatives. Int. J. Antimicrob. Agent 2005, 26, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Campos-Esparza, M.R.; Sanchez-Gomez, M.V.; Matute, C. ; Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Cal. 2009, 45, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Chibale, K.; Visser, M.; Schalkwyk, D.; Smith, P.J.; Saravanamuthu, A.; Fairlamb, A.H. Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents. Tetrahedron, 2003, 59, 2289–2296. [Google Scholar] [CrossRef]
- Martinez, A.; Galano, A.; Vargas, R. Free radical scavenger properties of α-mangostin: thermodynamics and kinetics of HAT and RAF mechanisms. J. Phys. Chem. 2011, 115, 12591–12598. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Reyes-Fermin, L.M.; Nolasco-Amaya, E.G.; Orozco-Ibarra, M.; Medina-Campos, O.N.; Gonzalez-Cuahutencos, O.; Rivero-Cruz, I.; Mata, R. ROS scavenging capacity and neuroprotective effect of α-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp. Toxicol. Pathol. 2009, 61, 491–501. [Google Scholar] [CrossRef]
- Buelna-Chontal, M.; Correa, F.; Hernandez-Resendiz, S.; Zazueta, C.; Pedraza-Chaverri, J. Protective effect of α-mangostin on cardiac reperfusion damage by attenuation of oxidative stress. J. Med. Food 2011, 14, 1370–1374. [Google Scholar] [CrossRef]
- Reyes-Fermín, L.M.; González-Reyes, S.; Tarco-Álvarez, N.G.; Hernández-Nava, M.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Neuroprotective effect of α-mangostin and curcumin against iodoacetate-induced cell death. Nutr. Neurosci. 2012, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Schwaebe, M.K.; Moran, T.J.; Whitten, J.P. Total synthesis of psorospermin. Tetrahedron Lett. 2005, 46, 827–829. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Yi, H.; Ohguchi, K.; Ito, T.; Tanaka, T.; Kobayashi, E.; Iinuma, M.; Nozawa, Y. Preferential target is mitochondria in α-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorg. Med. Chem. 2004, 12, 5799–5806. [Google Scholar] [CrossRef] [PubMed]
- Pedro, M.; Cerqueira, F.; Sousa, M.E.; Nascimento, M.S.J.; Pinto, M. Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorg. Med. Chem. 2002, 10, 3725–3730. [Google Scholar] [CrossRef]
- Gnerre, C.; Thull, U.; Gailland, P.; Carrupt, P.A.; Testa, B.; Fernandes, E.; Silva, F.; Pinto, M.; Wolfender, J.L.; Hostettmann, K.; Cruciani, G. Natural and synthetic xanthones as monoamine oxidase inhibitors: Biological assay and 3D-QSAR. Helv. Chim. Acta 2001, 84, 552–570. [Google Scholar] [CrossRef]
- Poondru, S.; Zhou, S.; Rake, J.; Shackleton, G.; Corbett, T.H.; Parchment, R.E.; Jasti, B.R. ; High-performance liquid chromatographic method for the estimation of the novel investigational anti-cancer agent SR271425 and its metabolites in mouse plasma. J. Chromatogr. B. Biomed. Sci. Appl. 2001, 759, 175–178. [Google Scholar] [CrossRef]
- Chantarasriwong, O.; Cho, W.C.; Batova, A.; Chavasiri, W.; Moore, C.; Rheingold, A.L.; Theodorakis, E.A. Evaluation of the pharmacophoric motif of the caged Garcinia xanthones. Org. Biomol. Chem. 2009, 7, 4886–4894. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 2003, 66, 1124–1127. [Google Scholar] [CrossRef]
- Chiang, L.C.; Cheng, H.Y.; Liu, M.C.; Chiang, W.; Lin, C.C. In vitro evaluation of antileukemic activity of 17 commonly used fruits and vegetables in Taiwan. Lebensm Wiss Technol. 2004, 37, 539–544. [Google Scholar] [CrossRef]
- Balunas, M.J.; Su, B.; Brueggemeier, R.W.; Kinghorn, A.D. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J. Nat. Prod. 2008, 71, 1161–1166. [Google Scholar] [CrossRef]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 54, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Watanapokasin, R.; Jarinthanan, F.; Jerusalmi, A.; Suksamrarn, S.; Nakamura, Y.; Sukseree, S.; Uthaisang-Tanethpongtamb, W.; Ratananukul, P.; Sano, T. Potential of xanthones from tropical fruit mangosteen as anti-cancer agents: caspase-dependent apoptosis induction in vitro and in mice. Appl. Biochem. Biotechnol. 2010, 162, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complementary Altern. Med. 2012, 12, 1–10. [Google Scholar] [CrossRef]
- Kosem, N.; Ichikawa, K.; Utsumi, H.; Moongkarndi, P. In vivo toxicity and antitumor activity of mangosteen extract. J. Nat. Med. 2013, 67, 255–263. [Google Scholar] [CrossRef]
- Kim, S.J.; Hong, E.H.; Lee, B.R.; Park, M.H.; Kim, J.W.; Pyun, A.R.; Kim, Y.J.; Chang, S.Y.; Chin, Y.W.; Ko, H.J. α-Mangostin reduced ER stress-mediated tumor growth through autophagy activation. Immune Netw. 2012, 12, 253–260. [Google Scholar] [CrossRef]
- Cao, S.; Brodie, P.J.; Miller, J.S.; Randrianaivo, R.; Ratovoson, F.; Birkinshaw, C.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G. ; Antiproliferative xanthones of Terminalia calcicola from the Madagascar Rain Forest. J. Nat. Prod. 2007, 70, 679–681. [Google Scholar] [CrossRef]
- Han, Q.B.; Tian, H.L.; Yang, N.Y.; Qiao, C.F.; Song, J.Z.; Chang, D.C.; Luo, K.Q.; Xu, H.X. Polyprenylated xanthones from Garcinia lancilimba showing apoptotic effects against HeLa-C3 Cells. Chem. Biodiver. 2008, 5, 2710–2717. [Google Scholar] [CrossRef]
- Tao, S.J.; Guan, S.H.; Wang, W.; Lu, Z.Q.; Chen, G.T.; Sha, N.; Yue, Q.X.; Liu, X.; Guo, D.A. Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. J. Nat. Prod. 2009, 72, 117–124. [Google Scholar] [CrossRef]
- Han, Q.B.; Xu, H.X. Caged Garcinia xanthones: development since 1937. Curr. Med. Chem. 2009, 16, 3775–3796. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Lu, N.; Wang, J.; Yin, Y.; Ding, Y.; Zhang, X.; Gui, H.; Sun, Q.; Duan, H.; Zhang, L.; Zhang, Y. An oxidative analogue of gambogic acid-induced apoptosis of human hepatocellular carcinoma cell line HepG2 is involved in its anticancer activity in vitro. Eur. J. Canc. Prev. 2010, 19, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cai, B.; Shan, J.; Wang, S.; Di, L. Discovery and current status of evaluation system of bioavailability and related pharmaceutical technologies for Traditional Chinese Medicines—Flos Lonicerae Japonicae Fructus Forsythiae herb couples as an example. AAPS Pharm. Sci. Tech. 2015, 16, 28812–28840. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Okada, M.; Sayeed, I.; Xiao, G.; Stein, D.; Jin, P.; Ye, K. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc. Natl. Acad. Sci. 2007, 104, 16329–16334. [Google Scholar] [CrossRef]
- Zelefack, F.; Guilet, D.; Fabre, N.; Bayet, C.; Chevalley, S.; Ngouela, S.; Lenta, B.N.; Valentin, A.; Tsamo, E.; Dijoux-Franca, M.G. Cytotoxic and antiplasmodial xanthones from Pentadesma butyracea. J. Nat. Prod. 2009, 72, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Moosophon, P.; Kanokmedhakul, S.; Kanokmedhakul, K.; Soytong, K. Prenylxanthones and a bicyclo [3.3. 1] nona-2, 6-diene derivative from the fungus Emericella rugulosa. J. Nat. Prod. 2009, 72, 1442–1446. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Rana, K.C.; Mujahid, M.; Sehar, I.; Saxena, A.K.; Synthesis and in vitro study of 14-aryl-14H-dibenzo[a. j]xanthenes as cytotoxic agents. Bioorg. Med. Chem. Lett. 2009, 19, 5590–5593. [Google Scholar] [CrossRef]
- Niu, S.L.; Li, Z.L.; Ji, F.; Liu, G.Y.; Zhao, N.; Liu, X.Q.; Jing, Y.K. , Hua, Y.M. Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry, 2012, 77, 280–286. [Google Scholar] [CrossRef]
- Loetchutinat, C.; Chau, F.; Mankhetkorn, S. Synthesis and evaluation of 5-Aryl-3-(4-hydroxyphenyl)-1,3,4-oxadiazole-2-(3H)-thiones as P-glycoprotein inhibitors. Chem. Pharm. Bull. 2003, 51, 728–730. [Google Scholar] [CrossRef]
- Abadi, A.H.; Eissa, A.A.; Hassan, G.S. Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bull. 2003, 51, 838–844. [Google Scholar] [CrossRef]
- Szczepankiewicz, B.G.; Liu, G.; Jae,H. S.; Tasker, A.S.; Gunawardana, I.W.; Geldern, T.W.V.; Gwaltney, S.L.; Wu-Wong, J.R.; Gehrke, L.; Chiou, W.J.; Credo, R.B.; Alder, J.D.; Nukkala, M.A.; Zielinski, N.A.; Jarvis, K.; Mollison, K.W.; Frost, D.J.; Bauch, J.L.; Hui, Y.H.; Claiborne, AK.; Li, Q.; Rosenberg, S.H. New antimitotic agents with activity in multi-drug-resistant cell lines and in vivo efficacy in murine tumor models. J. Med. Chem. 2001, 44, 4416–4430. [Google Scholar] [PubMed]
- Kumar, D.; Sundaree, S. , Johnson, E.O.; K. Shah. An efficient synthesis and biological study of novel indolyl-1,3,4-oxadiazoles as potent anticancer agents. Bioorg. Med. Chem. Lett. 2009, 19, 4492–4494. [Google Scholar] [CrossRef] [PubMed]
- Aboraia, A.S.; Abdel-Rahman, H.M.; Mahfouz, N.M.; El-Gendy, M.A. Novel 5-(2-hydroxyphenyl)-3-substituted-2,3-dihydro-1,3,4-oxadiazole-2-thione derivatives: promising anticancer agents. Bioorg. Med. Chem. 2006, 14, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Choudhary, M.I.; Khan, K.M.; Rani, M.; Rahman, A.U. ; Structure-activity relationships of tyrosinase inhibitory combinatorial library of 2,5-disubstituted-1,3,4-oxadiazole analogues. Bioorg. Med. Chem. 2005, 13, 3385–3395. [Google Scholar] [CrossRef]
- Tan, T.M.C.; Chen, Y.; Kong, K.H.; Bai, J.; Li, Y.; Lim, S.G.; Ang, T.H.; Lam, Y. Synthesis and the biological evaluation of 2-benzenesulfonylalkyl-5-substituted-sulfanyl-[1,3,4]-oxadiazoles as potential anti-hepatitis B virus agents. Antiviral Res. 2006, 71, 7–14. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, H.; Yang, X.; Liu, Z. Stereo selective synthesis and fungicidal activities of (E)-alpha-(methoxyimino)-benzene acetate derivatives containing 1,3,4-oxadiazole ring. Bioorg. Med. Chem. Lett. 2006, 16, 2278–2282. [Google Scholar] [CrossRef]
- Warener, R.N. New Adventures in the Synthesis of Hetero-Bridged syn-Facially Fused Norbornadienes (“[n]Polynorbornadienes”) and Their Topological Diversity. Eur. J. Org. Chem. 2000, 65, 3363–3380. [Google Scholar] [CrossRef]
- Guan, M.; Bian, Z.Q.; Zhou, Y.F; Li, F.Y.; Li, Z.L; Huang, C.H. High-performance blue electroluminescent devices based on 2-(4-biphenylyl)-5-(4-carbazole-9-yl)phenyl-1,3,4-oxadiazole. Chem. Commun. 2003, 9, 2708–2709. [Google Scholar] [CrossRef]
- Guimaraes, C.R.W.; Boger, D.L.; Jorgensen, W.L. Elucidation of fatty acid amide hydrolase inhibition by potent α-ketoheterocycle derivatives from Monte Carlo simulations. J. Am. Chem. Soc. 2005, 127(49), 17377–17384. [Google Scholar] [CrossRef]
- Sankhe, N.M.; Durgashivaprasad, E.; Kutty, N.G. Rao, J.V.; Narayanan, K.; Kumar, N.; Raj, P.V. Novel 2, 5-disubstituted-1, 3, 4-oxadiazole derivatives induce apoptosis in HepG2 cells through p53 mediated intrinsic pathway. Arab. J. Chem. 2019, 12, 2548–2555. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gamal, E.D.; Mohammed, I.E.G.; Mohammed, S.; Abdel, M.; Kyung, H. Y.; Chang-Hyun, O. Synthesis and in vitro anti-proliferative activity of new 1,3,4-oxadiazole derivatives possessing sulfonamide moiety. Eur. J. Med. Chem. 2015, 90, 45–52. [Google Scholar]
- Qian-Ru, D.; Dong-Dong, L.; Ya-Zhou, P.; Jing-Ran, L.; Jian, S.; Fei, F.; Wei-Qing, Z,; Gong, H. B.; Zhu, H.L. Novel 1,3,4-oxadiazole thioether derivatives targeting thymidylate synthase as dual anticancer/antimicrobial agents. Bioorg. Med. Chem. 2013, 21, 2286–2297. [Google Scholar]
- Dalip, K.; Swapna, S.; Johnson, E.O.; Kavita, S. An efficient synthesis and biological study of novel indolyl-1,3,4-oxadiazoles as potent anticancer agents. Bioorg. Med. Chem. Lett. 2009, 19, 4492–4494. [Google Scholar]
- Ahmed, S.; Aboraia, H.M.; Rahman, A.; Nadia, M.; Mahmoud, A.; Gendy, E.L. Novel 5-(2-hydroxyphenyl)-3-substituted-2,3-dihydro-1,3,4-oxadiazole-2-thione derivatives: Promising anticancer agents. Bioorg. Med. Chem. 2006, 14, 1236–1246. [Google Scholar]
- Samir, B.; Shymaa, A.; Hassan, A.; Etman, F.; Badria, A. Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur. J. Med. Chem. 2012, 48, 192–199. [Google Scholar]
- Clitherow, J.W.; Beswick, P.; Irving, W.J.; Scopes, D.I.C.; Barnes, J.C.; Clapham, J.; Brown, J.D.; Evans, D.J.; Hayes, A.G. Novel 1, 2, 4-oxadiazoles as potent and selective histamine H3 receptor antagonists. Bioorg. Med. Chem. Lett. 1996, 6, 833–838. [Google Scholar] [CrossRef]
- Acri, J.B.; Wong, G.; Witkin, J.M. Stereospecific transduction of behavioral effects via diazepam-insensitive GABAA receptors. Eur. J. Pharmacol. 1995, 278, 213–223. [Google Scholar] [CrossRef]
- Gaster, L.M.; Blaney, F.E.; Davies, S.; Duckworth, D.M.; Ham, P.; Jenkins, S.; Jennings, A.J.; Joiner, G.F.; King, F.D.; Mulholland, K.R. The selective 5-HT1B receptor inverse agonist 10-Methyl-5-[[20-methyl-40- (5-methyl- 1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro- spiro[furo [2,3-f]indole-3, 40-piperidine] potently blocks terminal 5-HT auto receptor function both in vitro and in vivo. J. Med. Chem. 1998, 41, 1218–1235. [Google Scholar]
- Selkirk, J.V.; Scott, C.; Ho, M.; Burton, M.J.; Watson, J.; Gaster, L.M.; Collin, L.; Jones, B.J.; Middlemiss, D.N.; Price, G.W. ; SB-224289 novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. J. Pharmacol. 1998, 125, 202–208. [Google Scholar] [CrossRef]
- Macor, J.E.; Ordway, T.; Smith, R.L.; Verhoest, P.R.; Mack, R.A. Synthesis and use of 5-vinyl-1,2,4-oxadiazoles as michael acceptors. A rapid synthesis of the potent muscarinic agonist L-670, 548, J. Org. Chem. 1996, 61, 3228–3229. [Google Scholar]
- Suzuki, T.; Iwaoka, K.; Imanishi, N. , Nagakura, Y.; Miyata, K.; Nakahara, H.; Ohta, M.; Mase, T. Synthesis of the Selective 5-Hydroxytryptamine 4 (5-HT4) Receptor Agonist (þ)-(S)-2-Chloro-5-methoxy-4-[5-(2-piperidylmethyl)-1, 2, 4-oxadiazol- 3-yl] aniline. Chem. Pharm. Bull. 1999, 47, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, S.; Lampronti, I.; Vertuani, S.; Solaroli, N.; Recanatini, M.; Bryan, D.; McKinney, M. Design, synthesis and binding at cloned muscarinic receptors of N-[5-(10-substituted-acetoxymethyl)-3-oxadiazolyl] and N-[4-(10-substitutedacetoxymethyl)- 2-dioxolanyl] dialkyl amines. Bioorg. Med. Chem. 2000, 8, 1559–1566. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Fylaktakidou, K.C.; Litinas, K.E.; Hadjipavlou-Litina, D. Synthesis and biological evaluation of several coumarin-4-carboxamidoxime and 3- (coumarin-4-yl)-1,2,4-oxadiazole derivatives. Eur. J. Med. Chem. 1998, 33, 715–724. [Google Scholar] [CrossRef]
- Matsumoto, J.; Takahashi, T.; Agata, M.; Toyofuku, H.; Sasada, N. A study of the biological pharmacology of IFO, a new selective and reversible monoamine oxidase-B inhibitor. J. Pharmacol. 1994, 65, 51–57. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Kasibhatla, S.; Kuemmerle, J.; Kemnitzer, W.; Ollis-Mason, K.; Qiu, L.; Crogan-Grundy, C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery and structure-activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J. Med. Chem. 2005, 48, 5215–5223. [Google Scholar] [CrossRef] [PubMed]
- Anjos, J.V.; Neves, Filho, R. A.W.; Nascimento, S.C.; Srivastava, R.M.; Melo, S.J.; Sinou, D. Synthesis and cytotoxic profile of glycosyl-triazole linked to 1,2,4-oxadiazole moiety at C-5 through a straight-chain carbon and oxygen atoms. Eur. J. Med. Chem. 2009, 44, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Patel, G.; Chavers, A.K.; Chang, K.H.; Shah, K. Synthesis of novel 1,2,4-oxadiazoles and analogues as potential anticancer agents. Eur. J. Med. Chem. 2011, 46, 3085–3092. [Google Scholar] [CrossRef]
- Maftei, C.V.; Fodor, E.; Jones, P.G.; Daniliuc, C.G.; Franz, M.H. Kelter, G.; Neda, I. Novel 1, 2, 4-oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 2016, 72(9), 1185–1199. [Google Scholar] [CrossRef]
- Kemnitzer, W.; Kuemmerle, J.; Zhang, H.Z.; Kasibhatla, S.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers. 2. Identification of more aqueous soluble analogs as potential anticancer agents. Bioorg. Med. Chem. Lett. 2009, 19, 4410–4415. [Google Scholar] [CrossRef]
- Tohid, S.F.M.; Ziedan, N.I.; Stefanelli, F.; Fogli, S.; Westwell, A.D. Synthesis and evaluation of indole-containing 3,5-diarylisoxazoles as potential pro-apoptotic antitumour agents. Eur. J. Med. Chem. 2012, 56, 263–270. [Google Scholar] [CrossRef]
- Kumar, D.; Maruthi, N.; Sundaree, S.; Johnson, E.O.; Shah, K. An expeditious synthesis and anticancer activity of novel 4-(3′-indolyl)oxazoles. Eur. J. Med. Chem. 2010, 45, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Puthiyapurayil, P.; Poojary, B.; Chikkanna, C.; Kumar, B.S. Design, synthesis and biological evaluation of a novel series of 1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl)phenyl pyrazole moiety as cytotoxic agents. Eur. J. Med. Chem. 2012, 53, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Juan, S.; Hui, Z.; Zhong-Ming, Y.; Hai-Liang, Z. Synthesis, molecular modeling and biological evaluation of 2-aminomethyl-5- (quinolin-2-yl)-1,3,4-oxadiazole-2(3H)-thione quinolone derivatives as novel anticancer agent. Eur. J. Med. Chem. 2013, 60, 23–28. [Google Scholar]
- Mohammad, S.; Avijit, M.; Mohamed, J.A. Synthesis, characterization and anticancer evaluation of 2-(naphthalen-1-ylmethyl/naphthalen-2-yloxymethyl)-1-[5-(substitutedphenyl)-[1,3,4]oxadiazol-2-ylmethyl]-1H-benzimidazole. Arab. J. Chem. 2014, 7, 418–424. [Google Scholar]
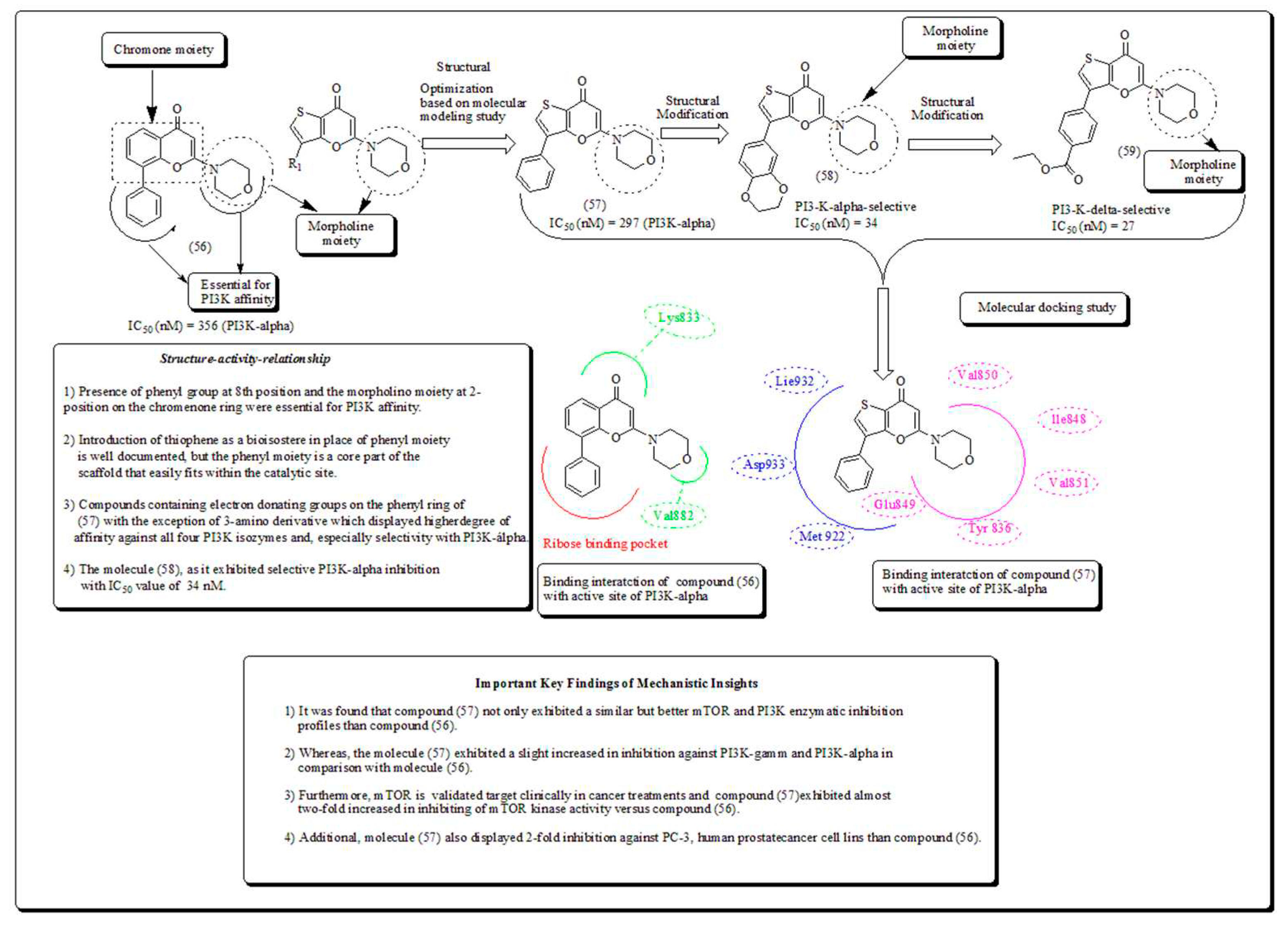
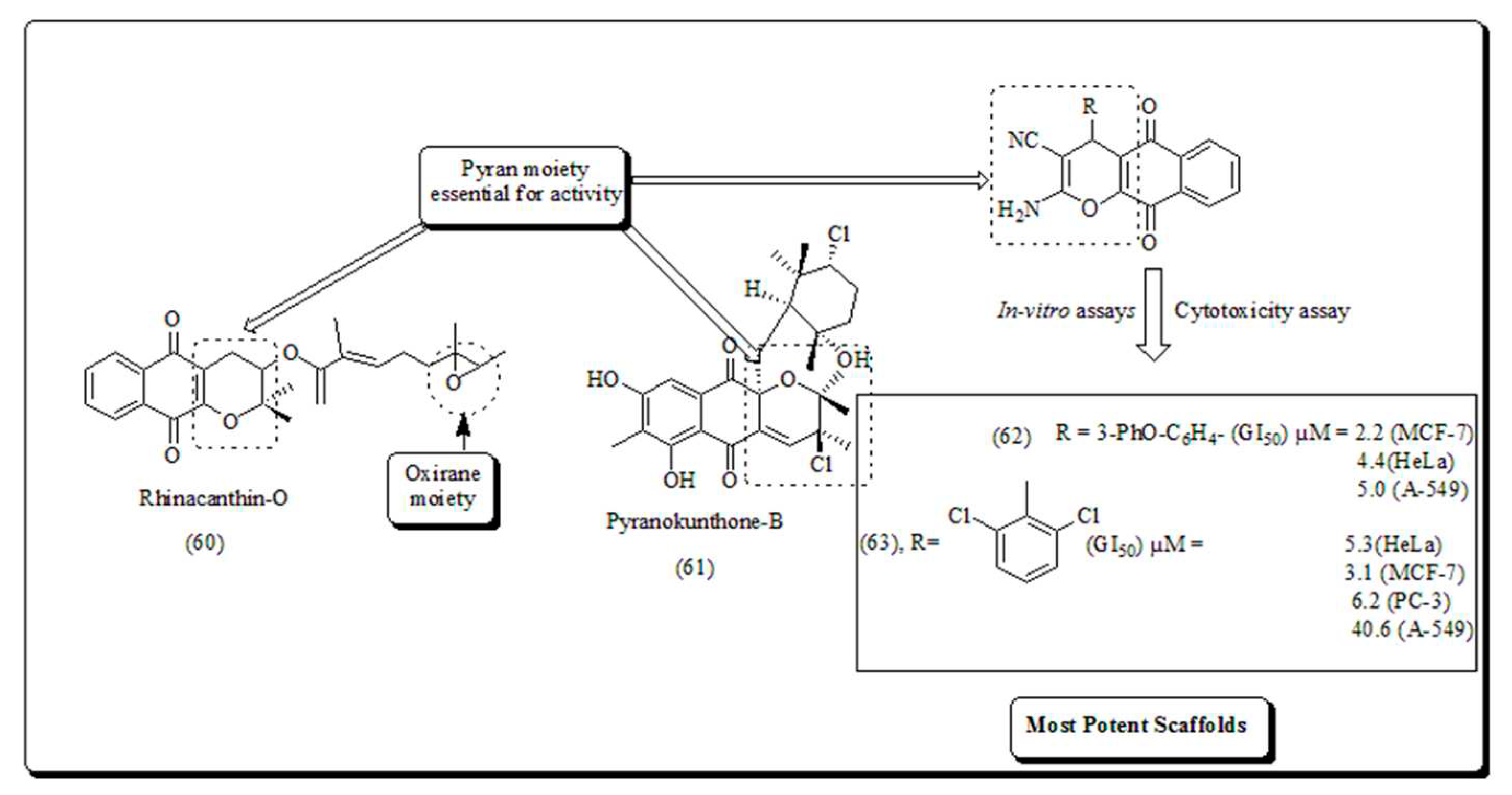
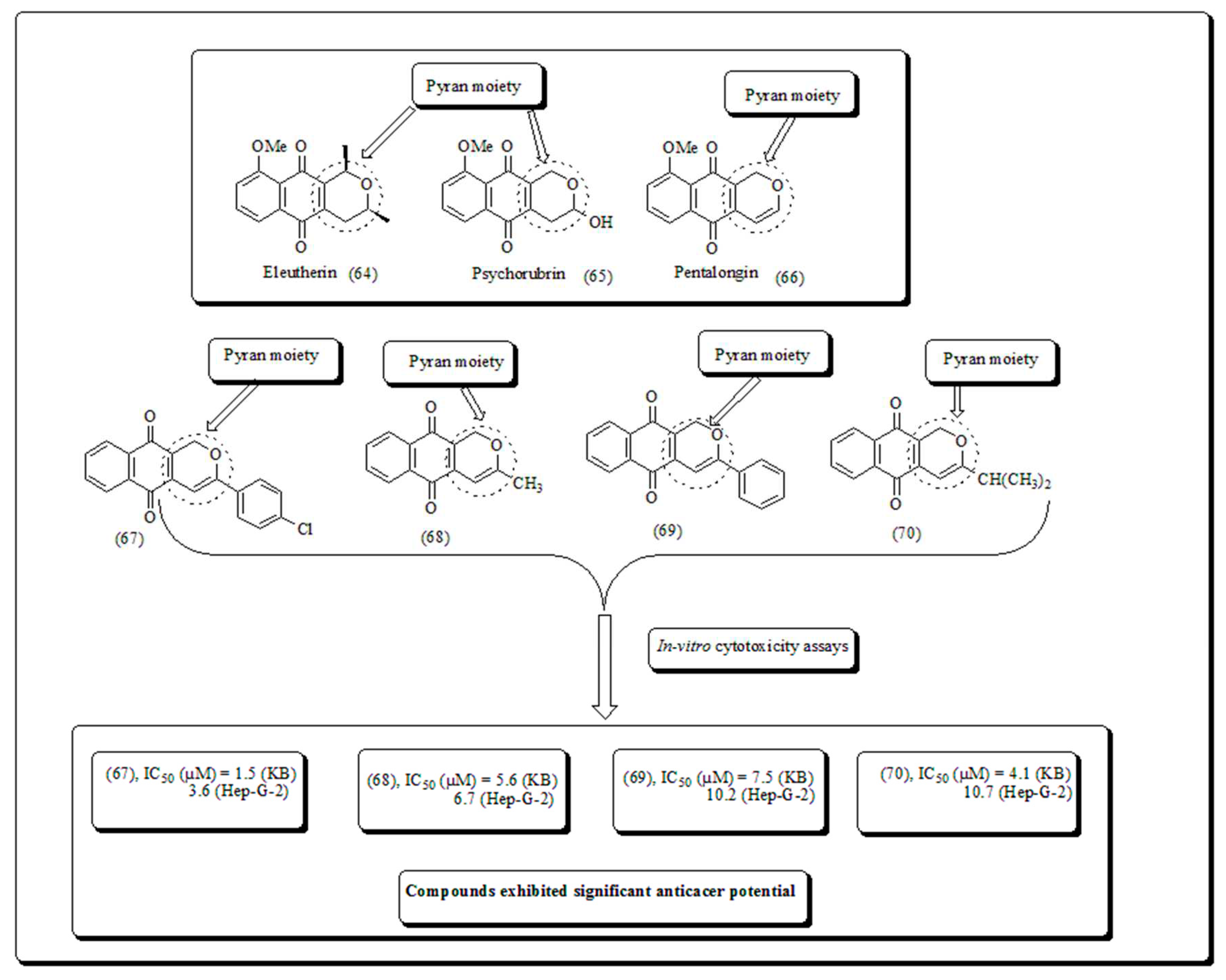
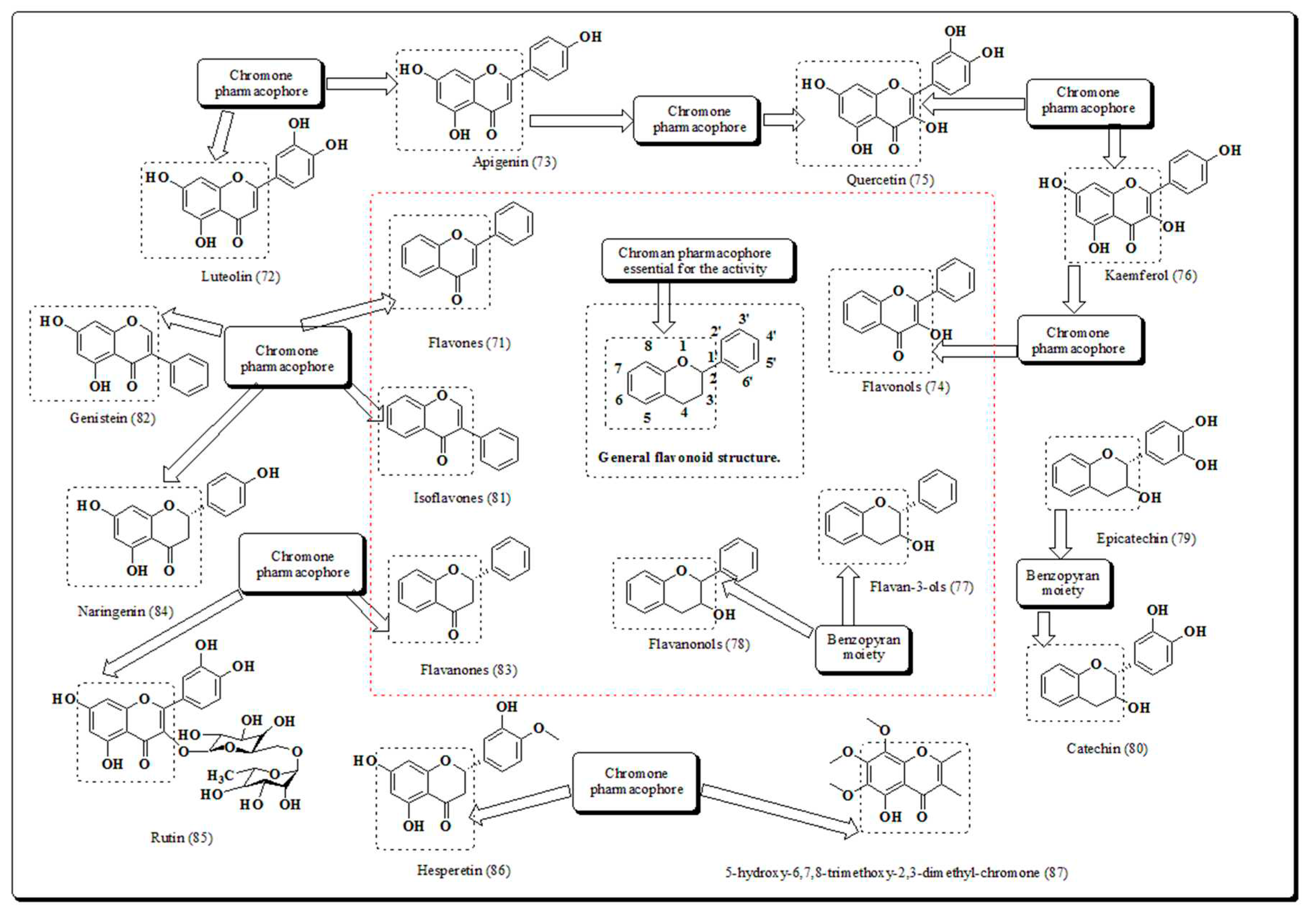
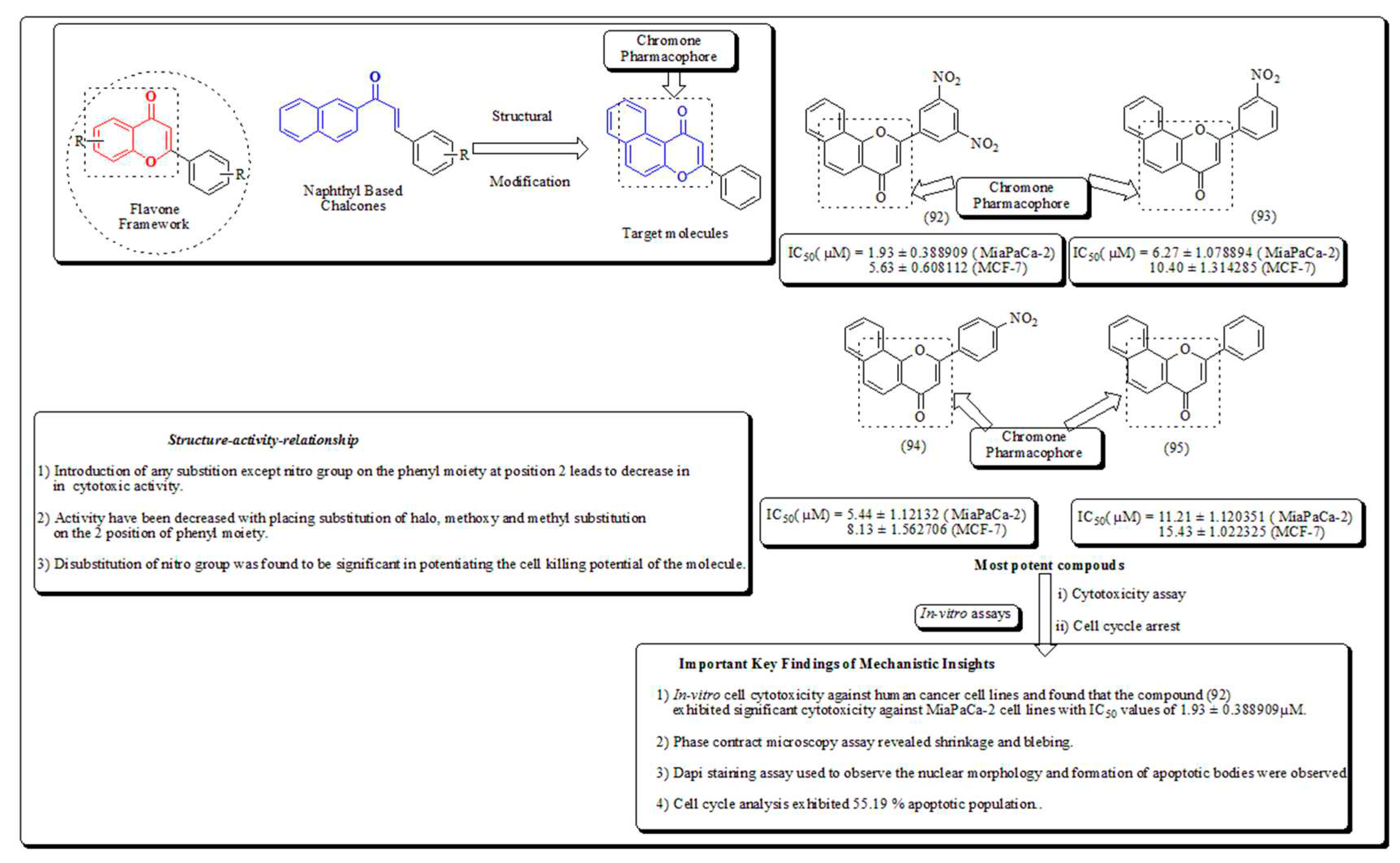
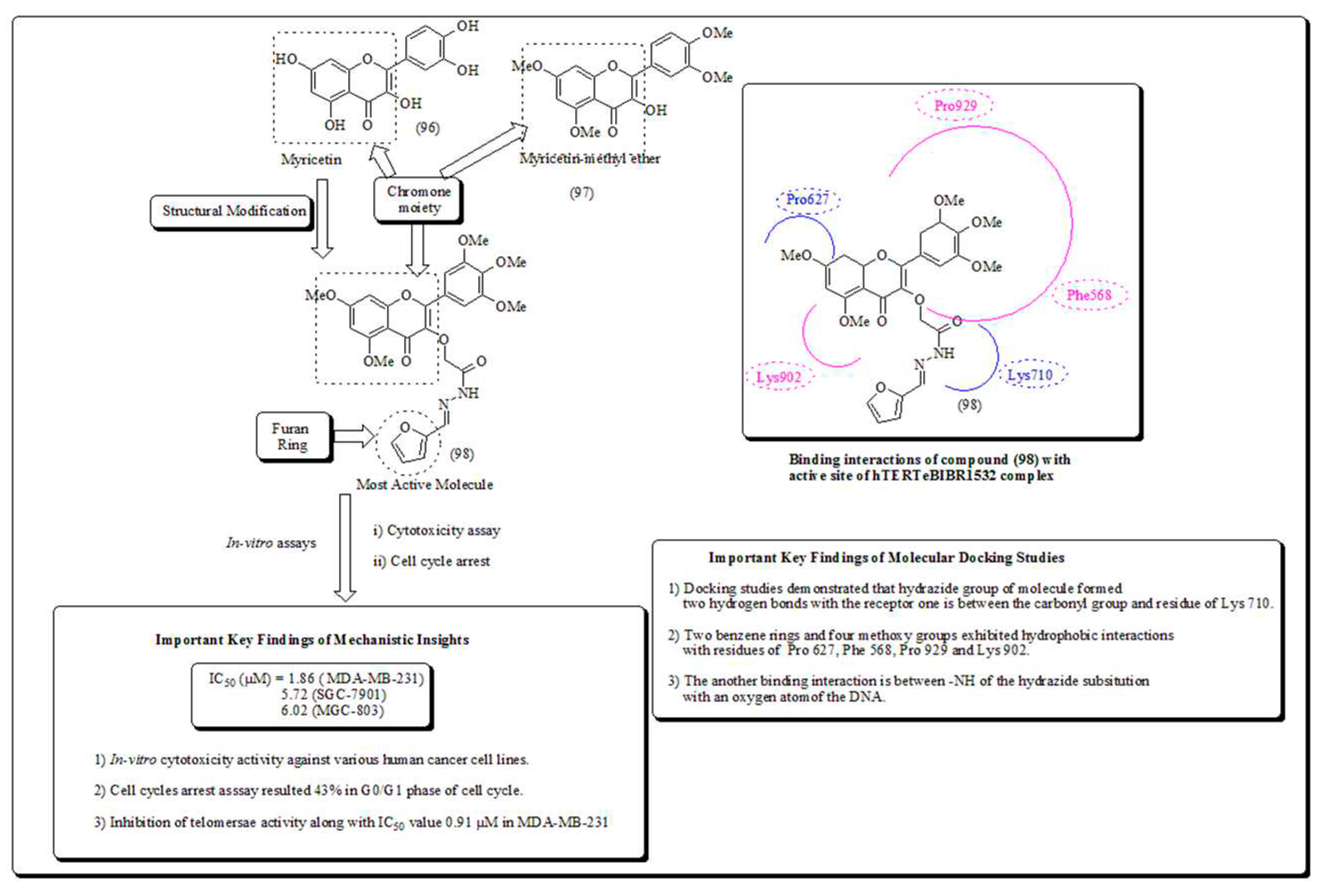
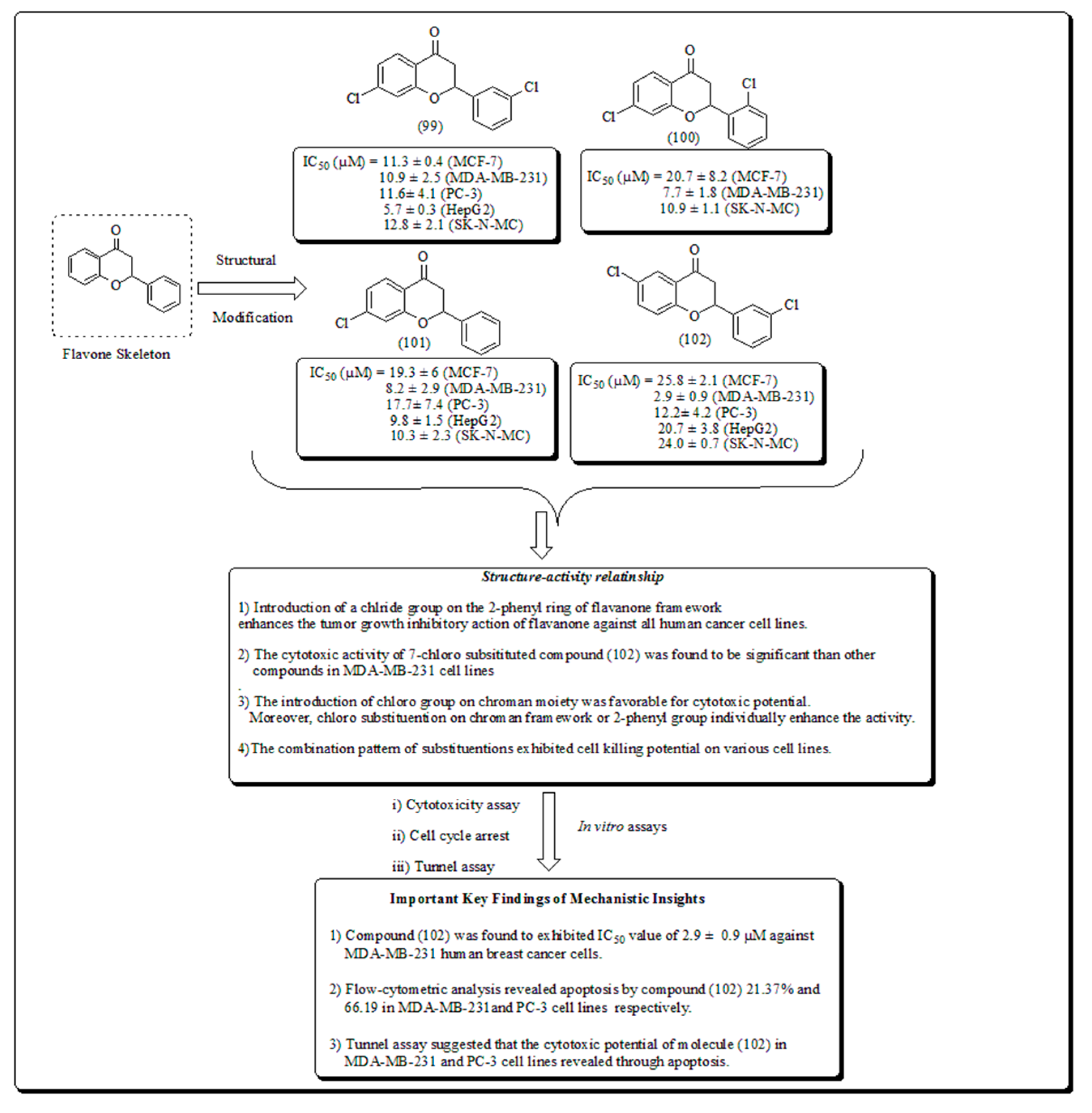
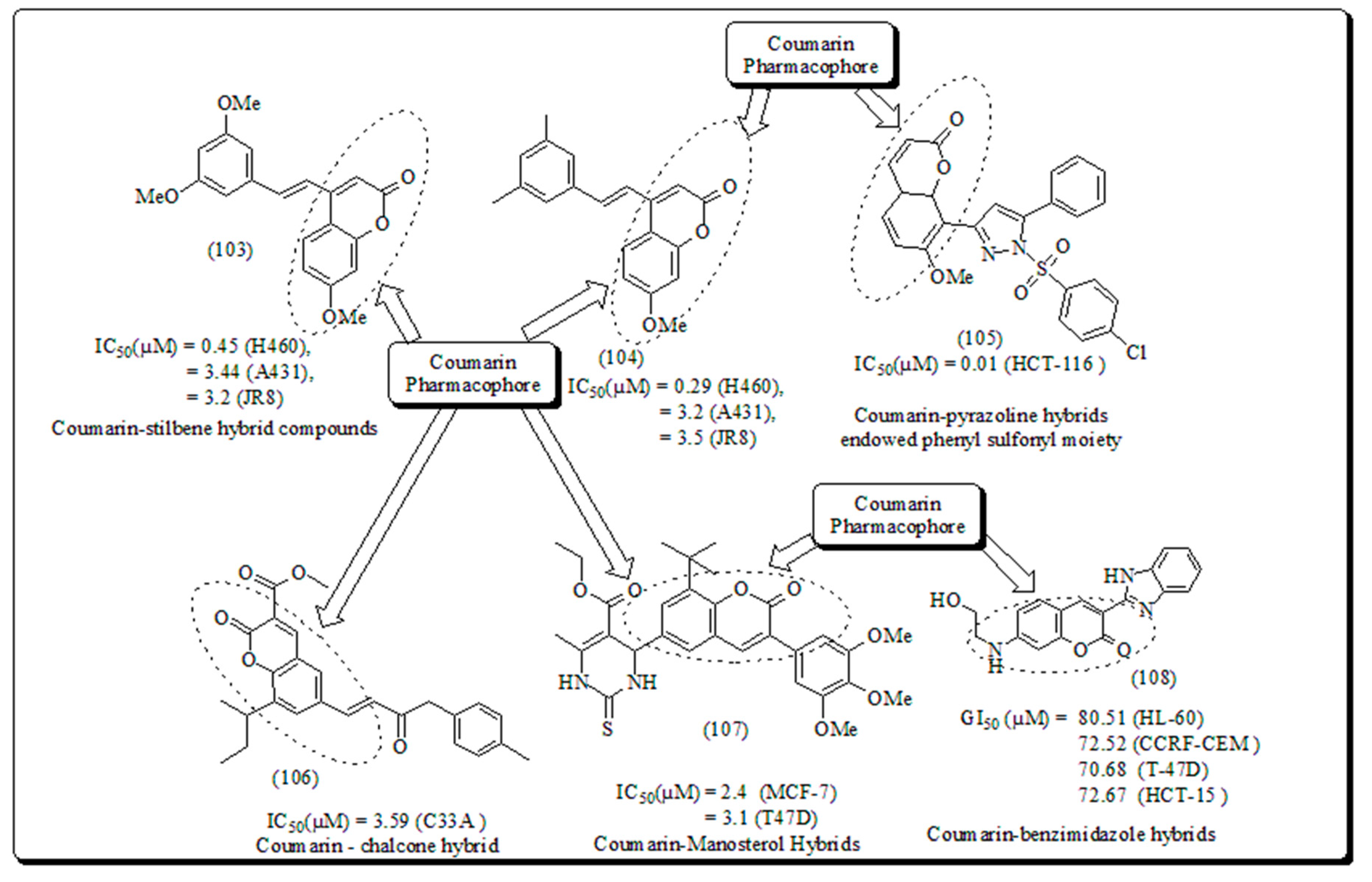
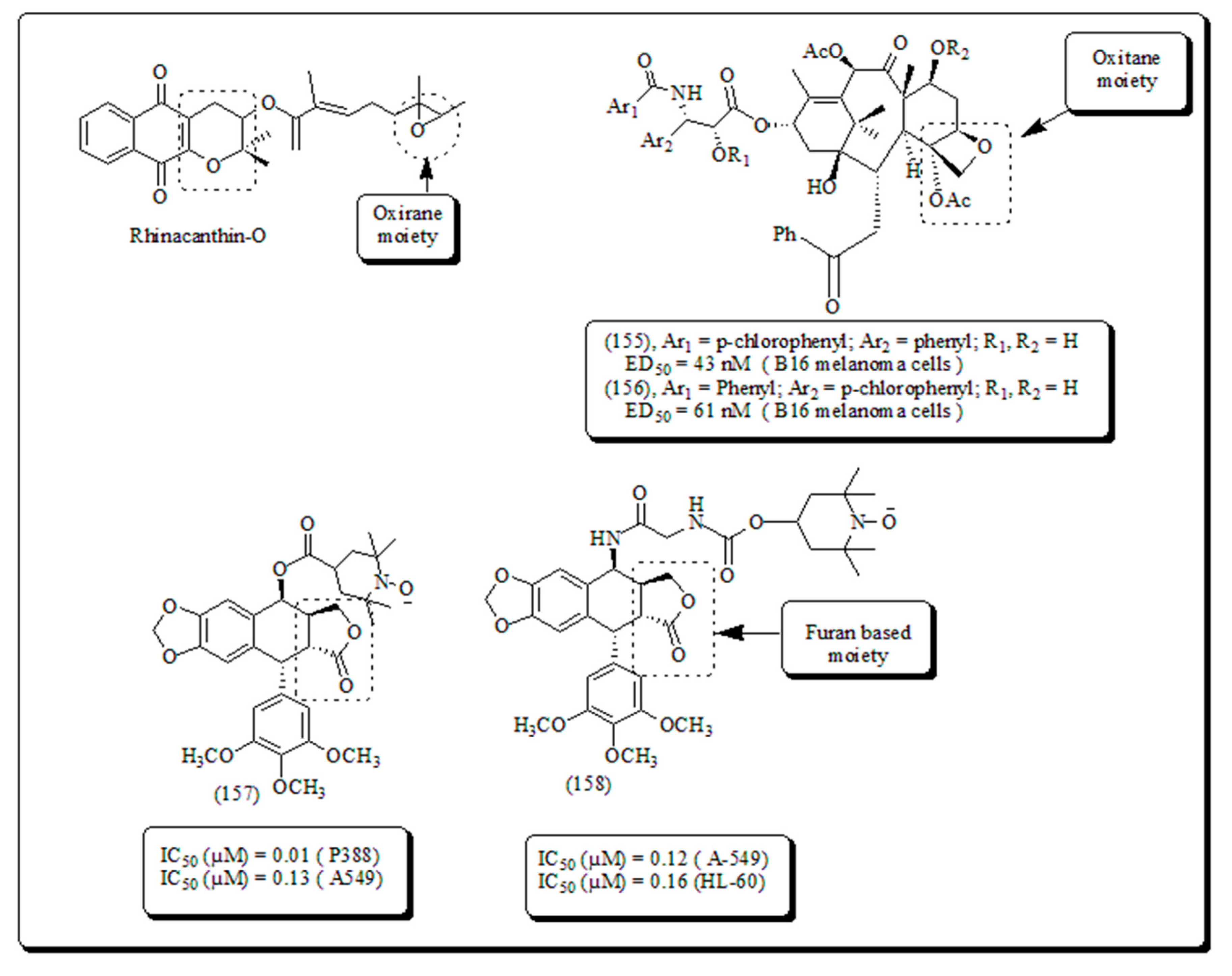
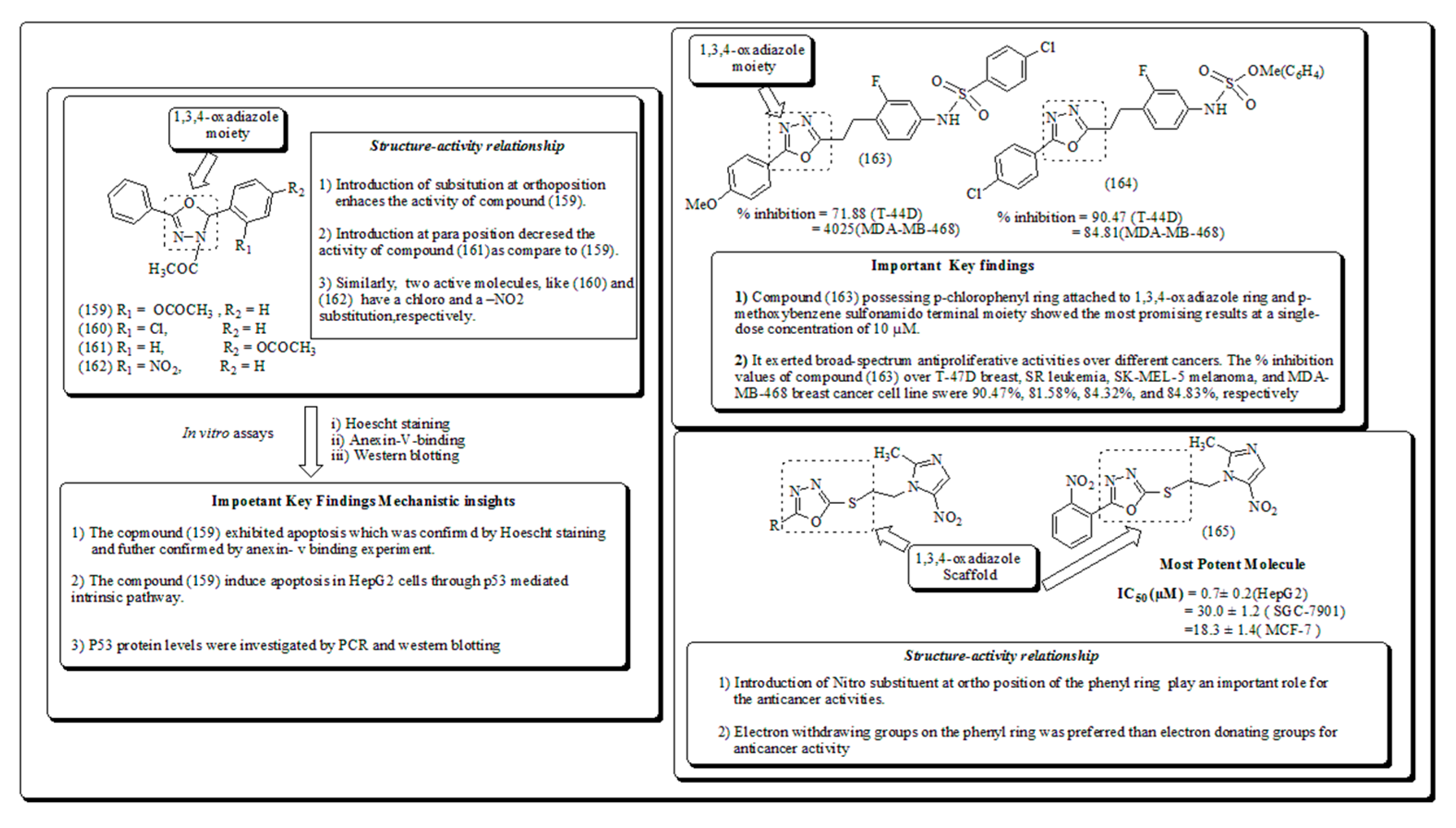
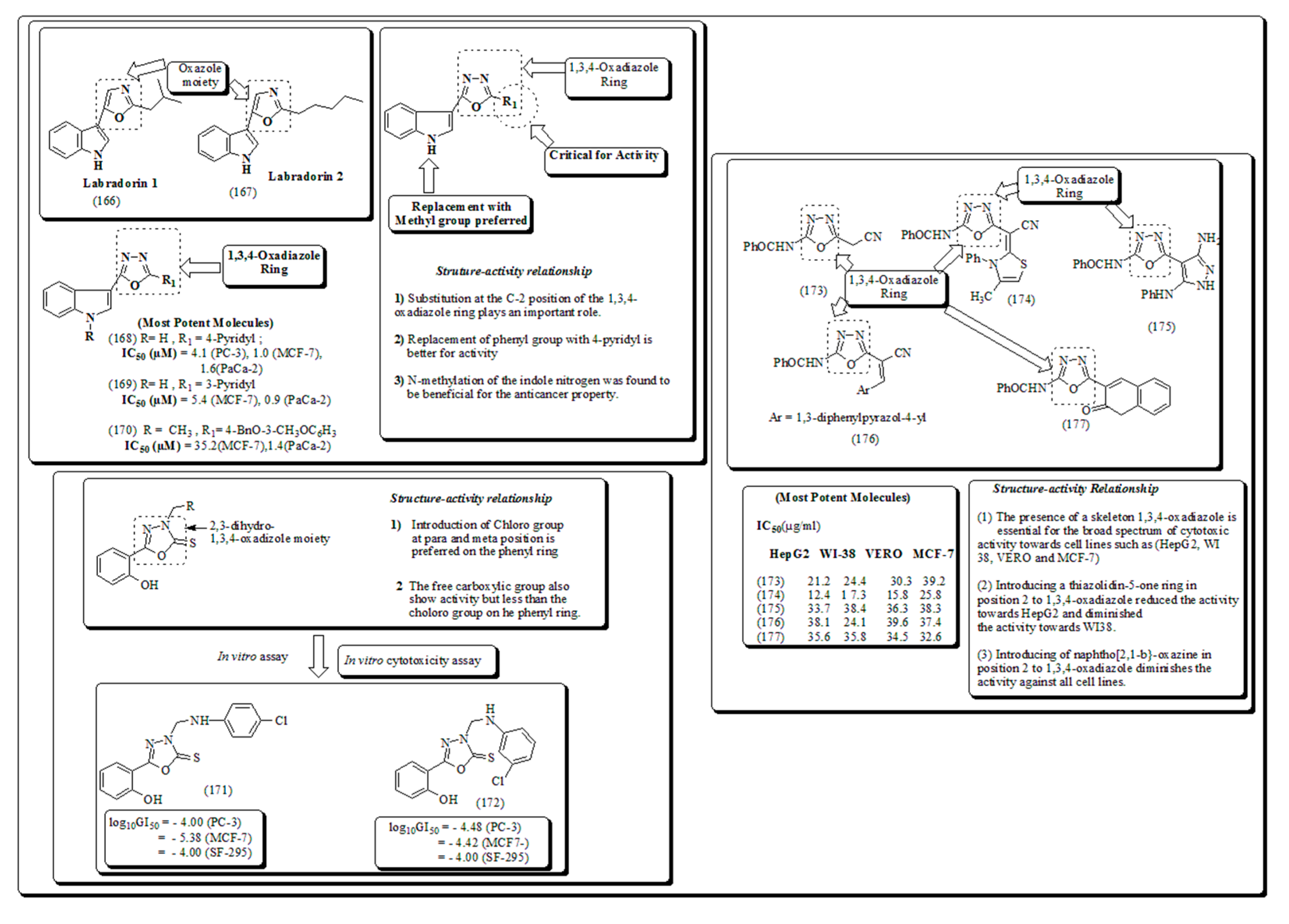
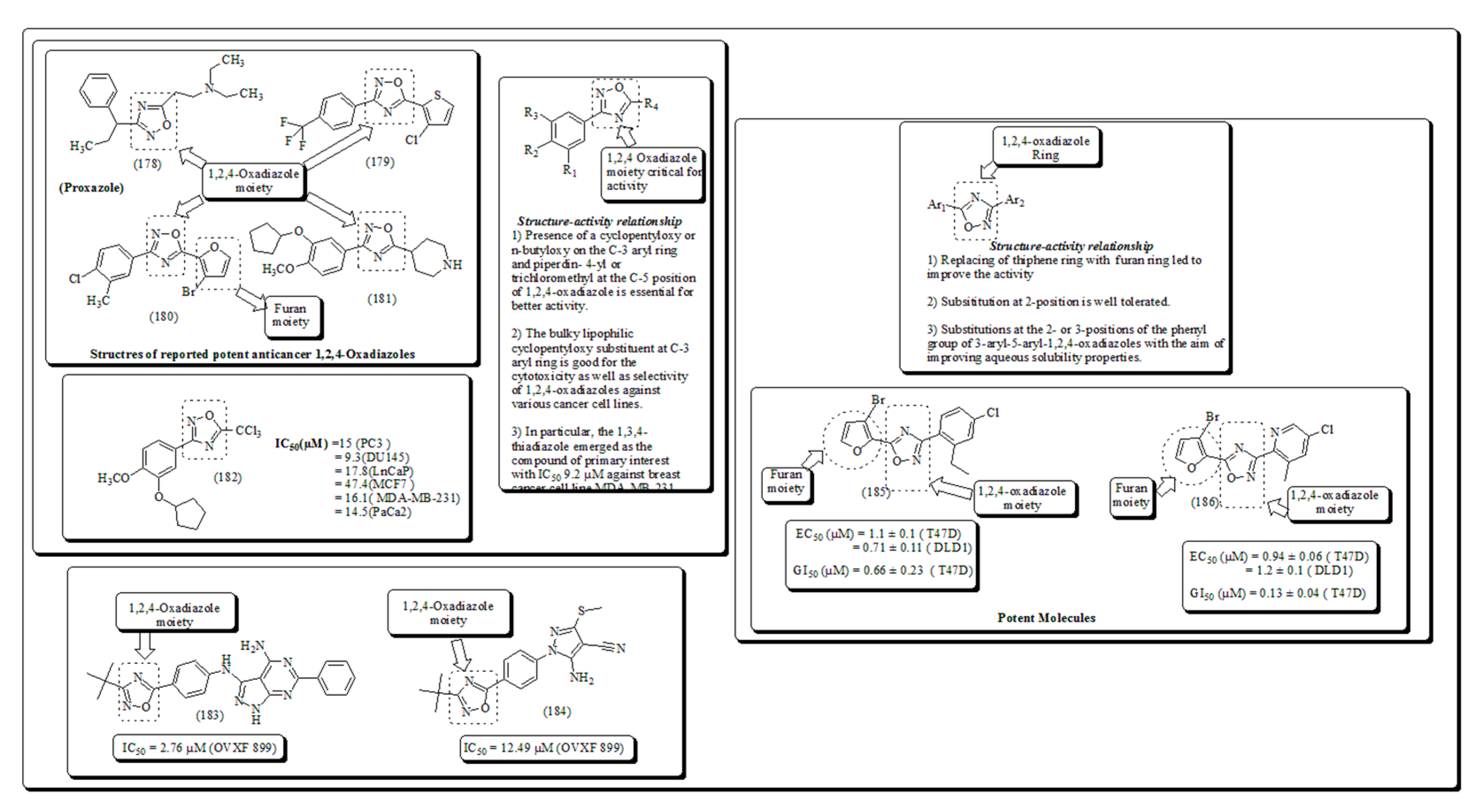
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).