Submitted:
12 August 2023
Posted:
14 August 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Composition of CH-SA-HA Construction
2.2. Experimental Animals
2.3. Modeling Defects of the Critical Size of the Mandibular Angle in Rats
2.4. Morphological Analysis of Bone Tissue
2.5. Statistical Analysis
3. Results
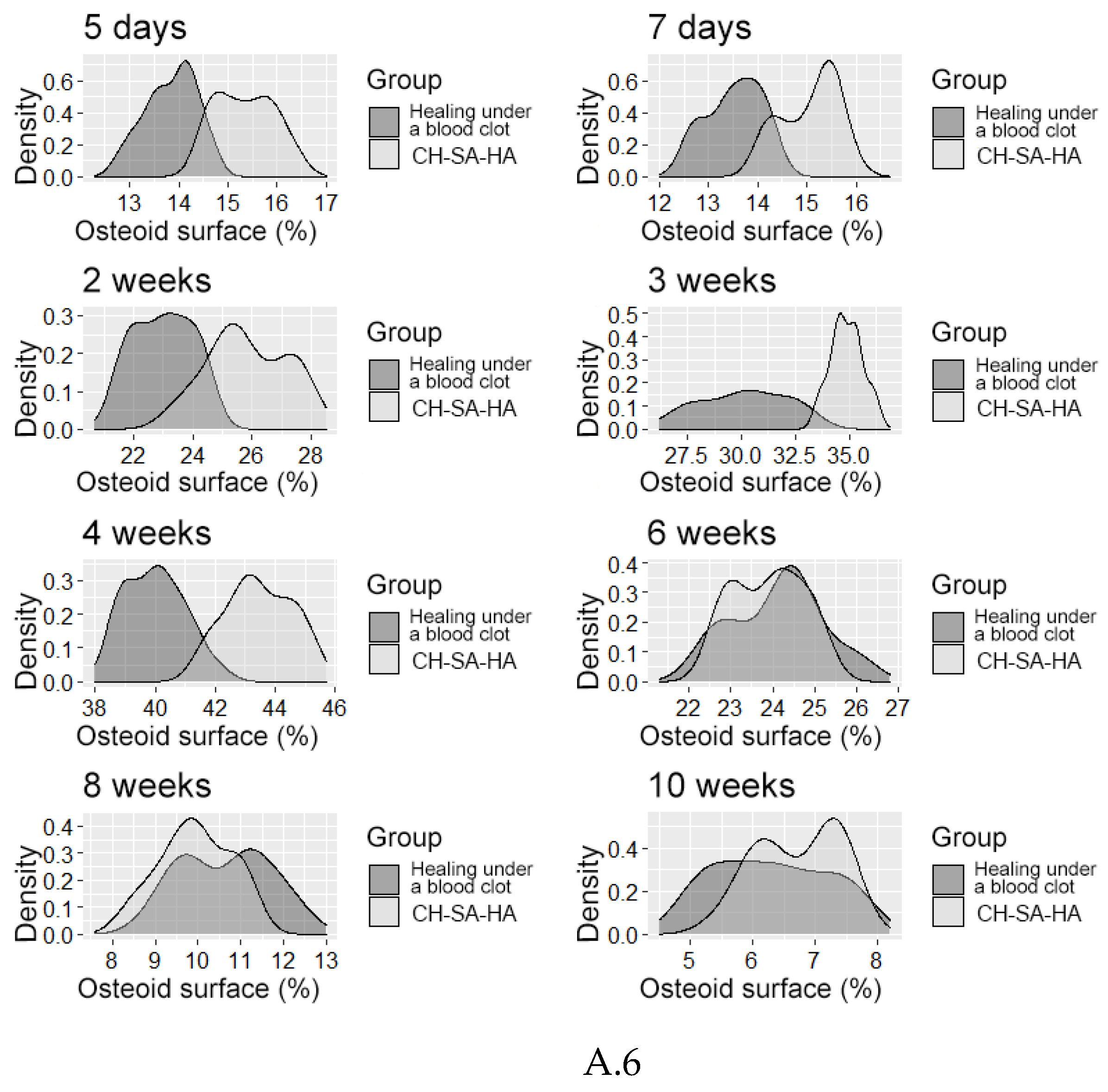
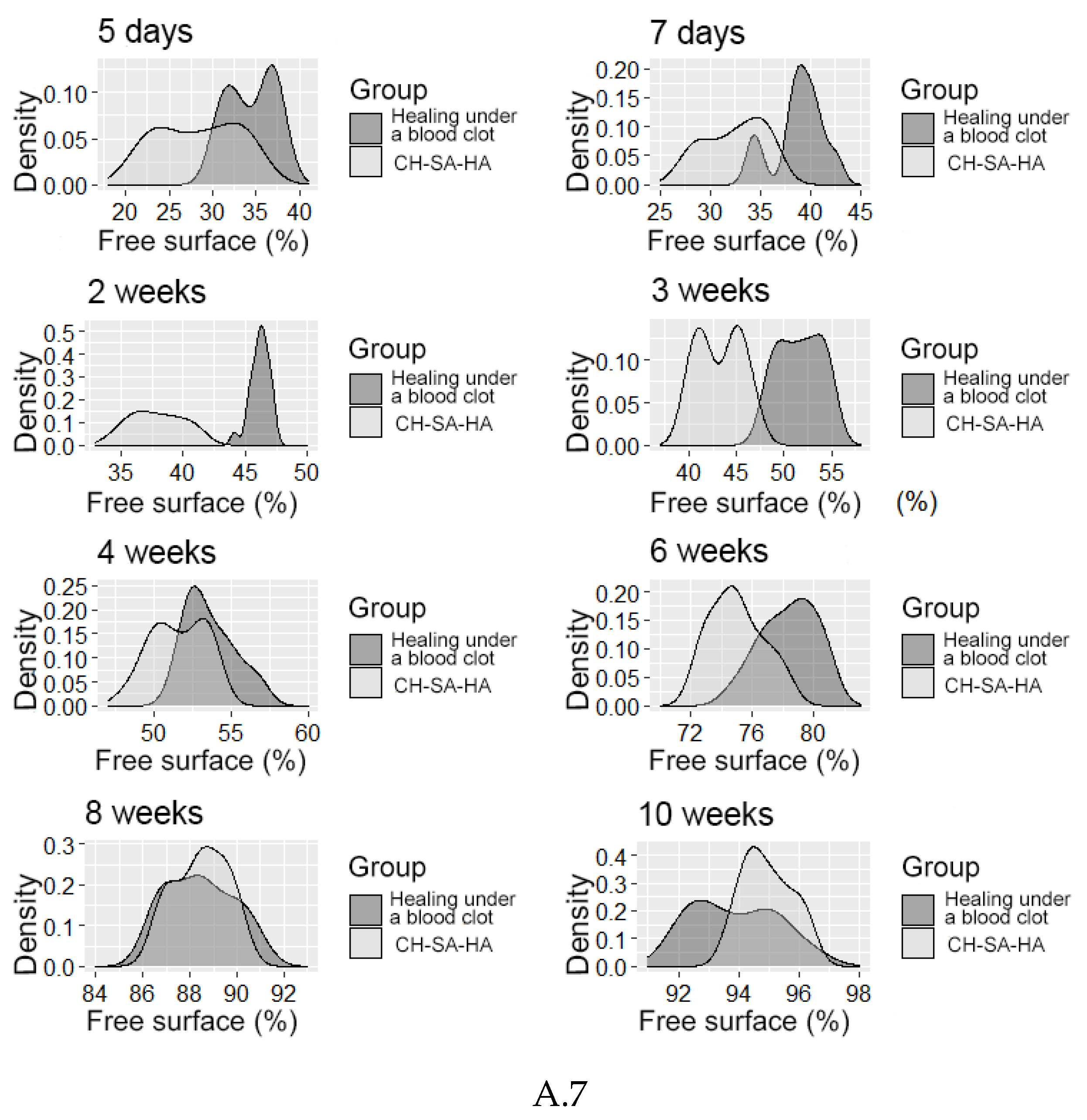
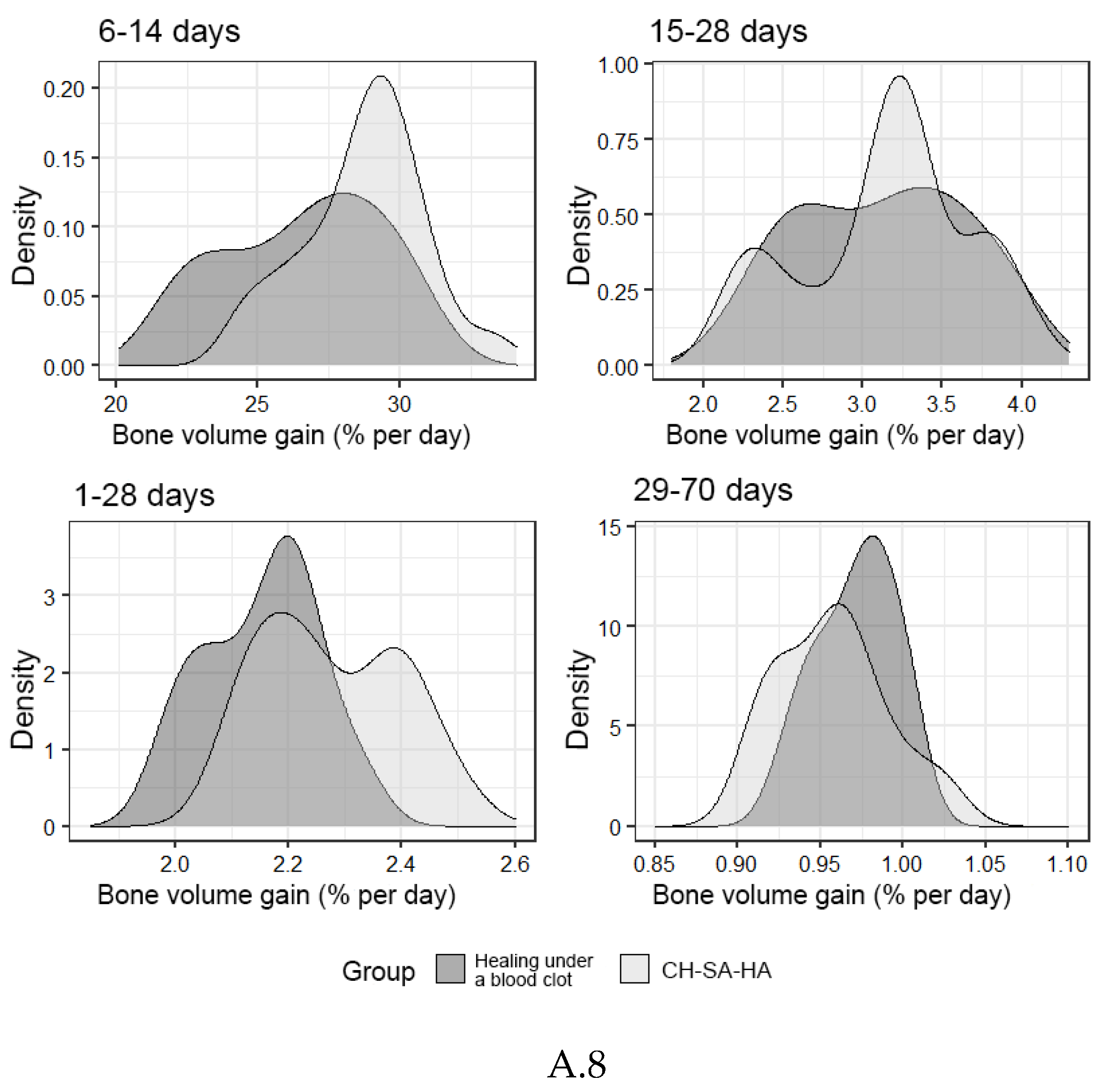
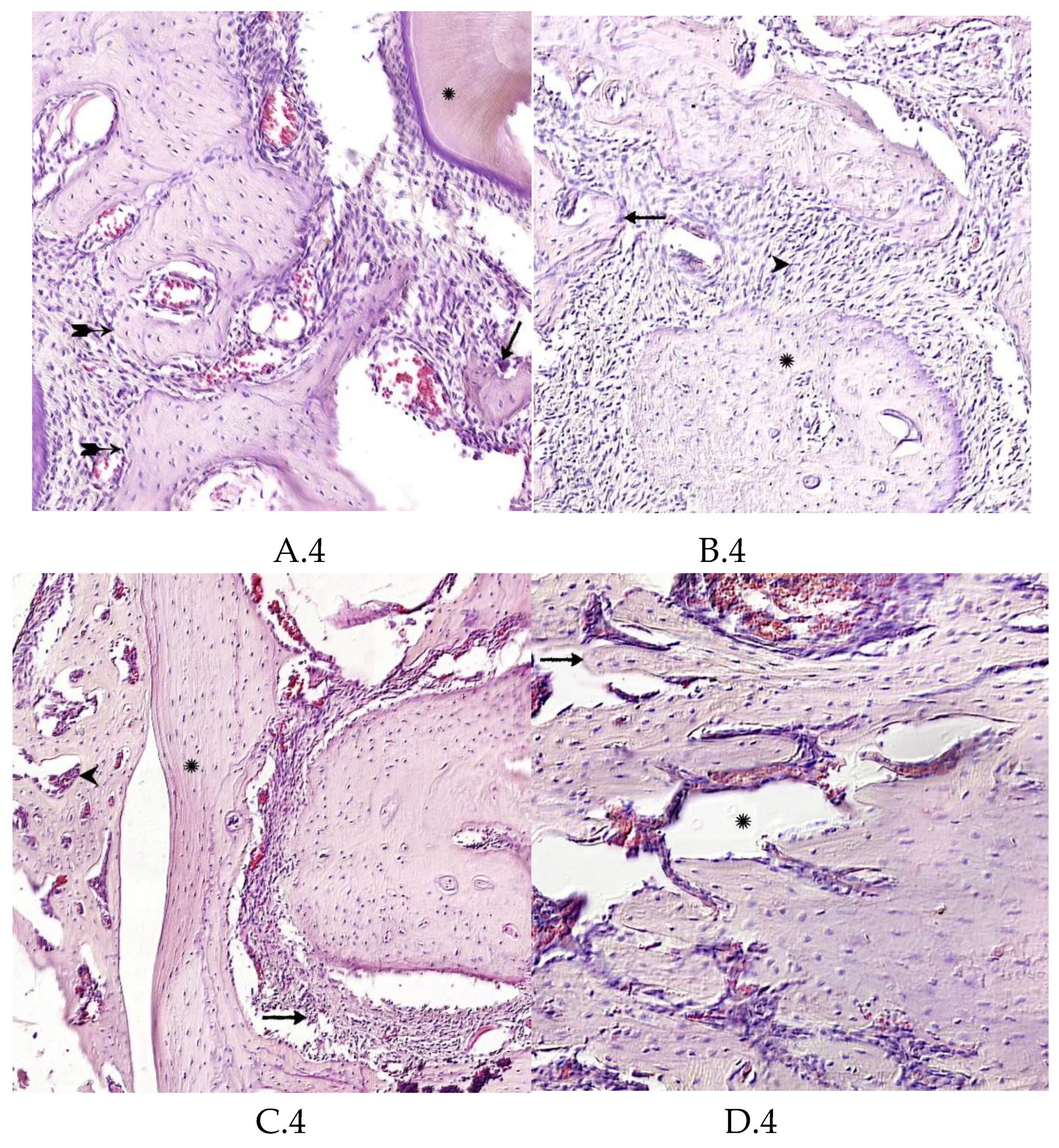
Morphological Analysis of Bone Tissue Restoration in the Defect Zone
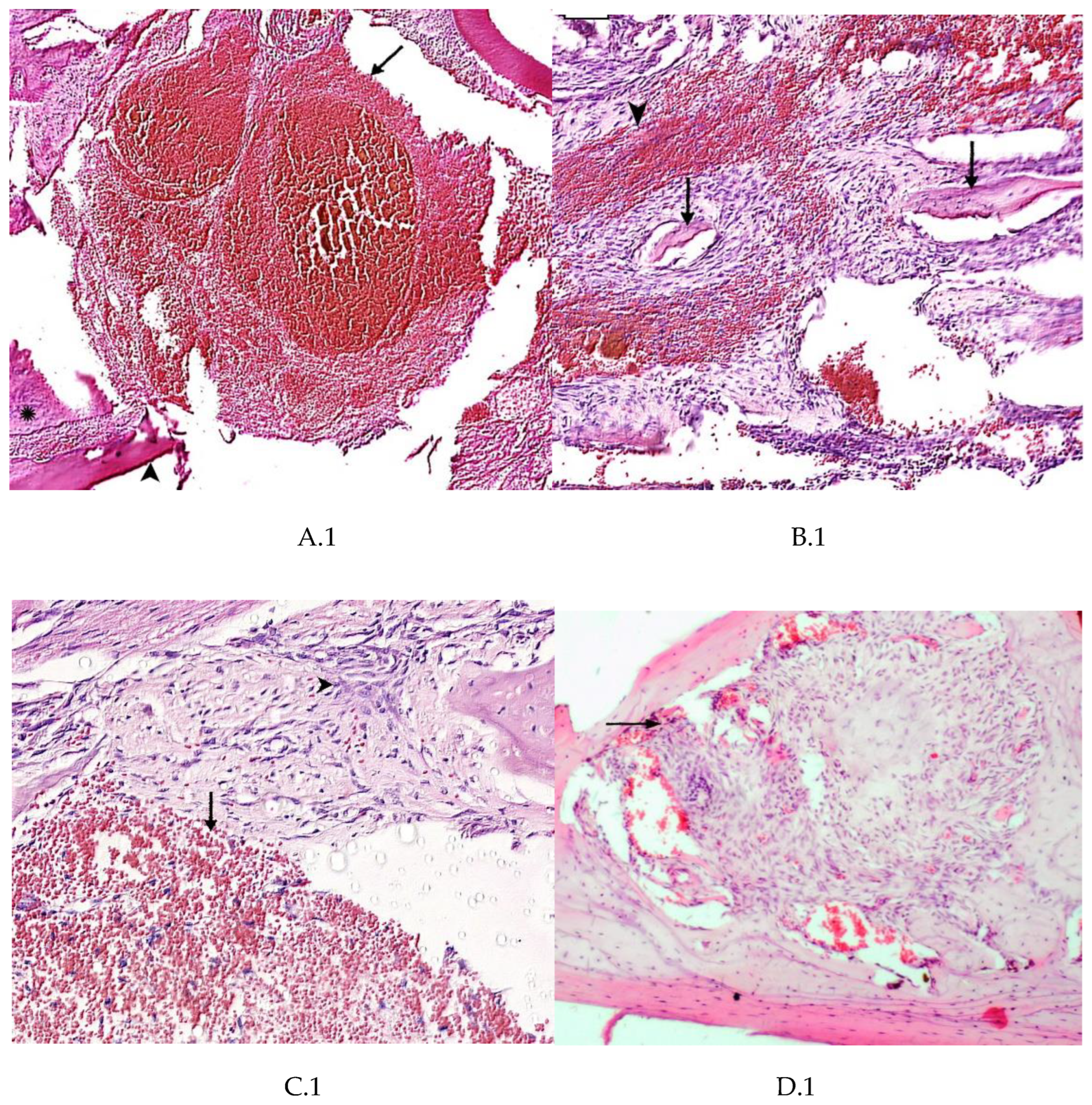
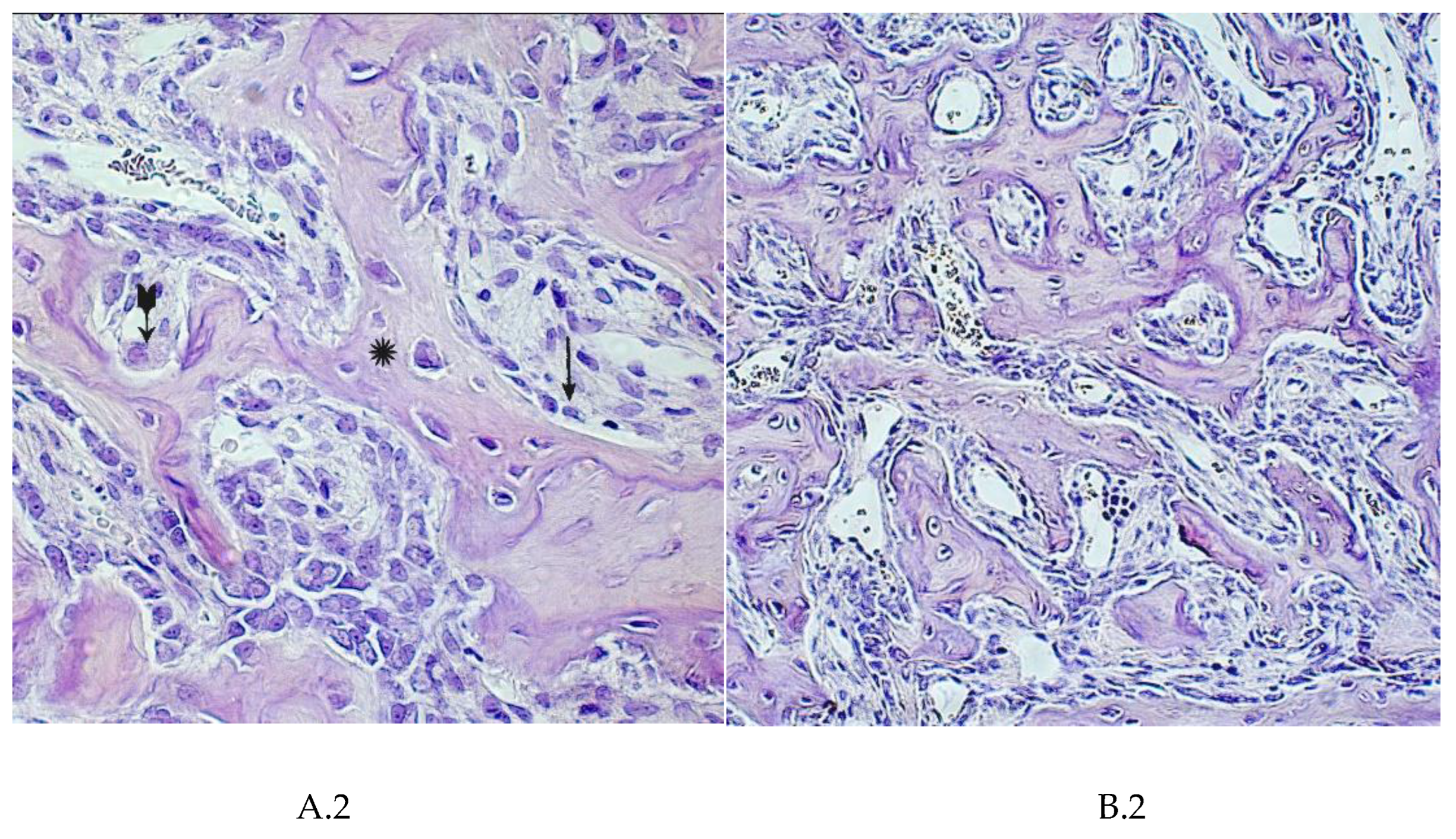
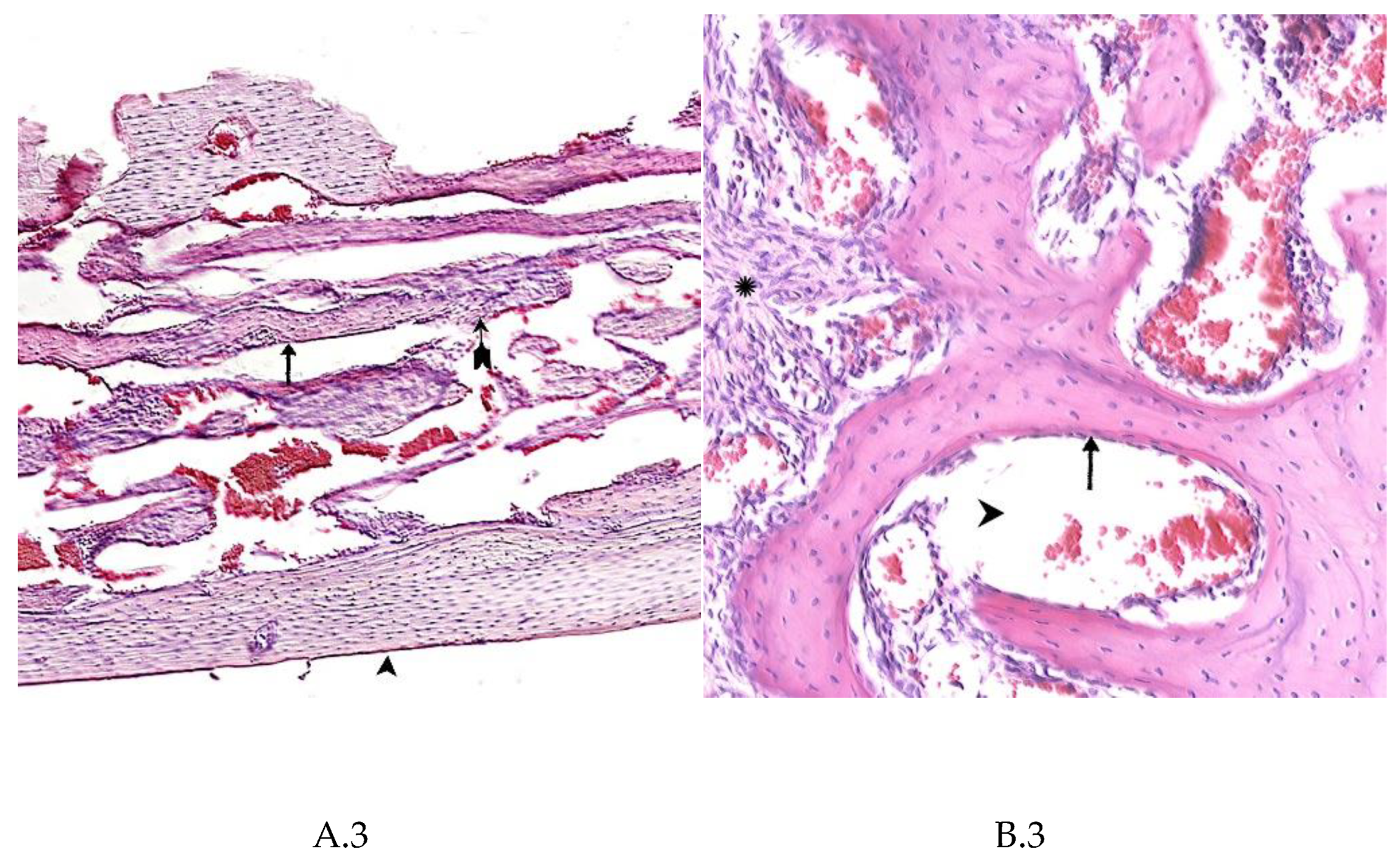
4. Summary
5. Discussion
Statement of Authorship
Acknowledgments
Conflicts of Interest
Protocol
Petition
References
- Shaykhaliev,A.I.; Stretskiy, G.M.; Krasnov, M.S.; Rybakova, E.Yu.; Tikhonov, V.E.; Arazashvili, L.D.; Gevorkov, G.L.; Yamskova, V.P.; Yamskov, I.A. Use of materials with bioregulatory peptide complex, affecting osteoreparation process (the results of preclinical tests). Russian Dental Journal. 2014, 18(4), 12-16; [CrossRef]
- Lou, X. Induced pluripotent stem cells as a new strategy for osteogenesis and bone regeneration. Stem Cell Rev. Rep. 2015, 11(4), 645-651;. [CrossRef]
- Raposo-Amaral, C.E.; Bueno, D.F.; Almeida, A.B.; Jorgetti, V.; Costa, C.C.; Gouveia, C.H.; Vulcano, L.C.; Fanganiello, R.D.; Passos-Bueno, M.R.; Alonso, N. Is bone transplantation the gold standard for repair of alveolar bone defects? J. Tissue Eng. 2014, 5, 2041731413519352;. [CrossRef]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Alveolar bone tissue engineering in critical-size defects of experimental animal models: a systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2017, 11(10), 2935-2949;. [CrossRef]
- Khan,F.; Tanaka, M.; Rafi, S. Ahmad fabrication of polymeric biomaterials: a strategy for tissue engineering and medical devices. J. Mater. Chem. B. 2015, 3, 8224-8249;. [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions – a 21st century perspective. Bone Res. 2013, 3, 216-248;. [CrossRef]
- Bolshakov, I.N.; Levenetz, A.A.; Furtsev, T.V.; Kotikov, A.R.; Patlataya, N.N.; Ryaboshapko,E.I.; Dmitrienko, A.E.; Nikolaenko, M.M.; Matveeva, N.D.; Ibragimov, I.G. Experimental Reconstruction of Critical Size Defect of Bone Tissue in the Maxillofacial Region When Using Modified Chitosan. Biomed. Transl. Sci. 2022, l.2(1), 1-8;. [CrossRef]
- Bolshakov, I.N.; Gornostaev, L.M.; Fominykh, O.I.; Svetlakov, A.V. Synthesis, Chemical and Biomedical Aspects of the Use of Sulfated Chitosan. Polymers. 2022, 14(6), 1-27;. [CrossRef]
- Li, Y.; Kim, J.H.; Choi, E.H.; Han, I. Promotion of osteogenic differentiation by non-thermal biocompatible plasma treated chitosan scaffold. Sci. Rep. 2019, 9(1), 3712;. [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17-39;. [CrossRef]
- Jiang,T.; Khan, Y.; Nair, L.S.; Abdel-Fattah, W.I.; Laurencin, C.T. Functionalization of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds via surface heparinization for bone tissue engineering. J. Biomed. Mater. Res. A. 2010, 93(3), 1193-1208;. [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In vivo bone regeneration induced by a scaffold of chitosan/dicarboxylic acid seeded with human periodontal ligament cells. Int. J. Mol. Sci. 2019, 20(19), 4883;. [CrossRef]
- Dhandayuthapani, B.; Yoshida, Ya.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: a review. Int. J. Polymer Sci. 2011; Article ID 290602:1-19;. [CrossRef]
- Chatzipetros, E.; Christopoulos, P.; Donta, C.; Tosios, K.-I.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.-M.; Tsiklakis, K. Research application of nano-hydroxyapatite/chitosan scaffolds on rat calvarial critical-sized defects: a pilot study. Med. Oral Patol. Oral Cir. Bucal. 2018, 23(5), e625-e632;. [CrossRef]
- La, W.-G.; Jang, J.; Kim, B.S.; Lee, M.S.; Cho, D.-W.; Yang, H.S. Systemically replicated organic and inorganic bony microenvironment for new bone formation generated by a 3D printing technology. RSC Adv. 2016, 6, 11546-11553;. [CrossRef]
- Ko, H.F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 28, 368(1917), 1981-1997;. [CrossRef]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 955-967;. [CrossRef]
- Kirichenko, А.К.; Patlataya, N.N.; Sharkova, А.F.; Pevnev, А.А.; Kontorev, К.V.; Shapovalova, О.V.; Gorban, М.Е.; Bolshakov, I.N. Pathomorphism of Limb Major Vessels in Experimental Atherogenic Inflammation. The Role of Adventitial Intimal Relations. Review. Modern Technologies in Medicine. 2017, 9(3), 157-163; [CrossRef]
- Tumshevits, O.N.; Bolshakov, I.N.; Belousova, Yu.B.; Zykova, L.D.; Tumshevits, V.O. Method for treatment of periodontitis with insulin-dependent diabetes mellitus with "HAG-BOL" drugs. Patent RF No 2309748, 01/10/2006.
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering, Tissue Eng. Part B Rev. 2009, 15(3), 353–370;. [CrossRef]
- Hasegawa, T.; Yamamoto, T.; Tsuchiya, E.; Hongo, H.; Tsuboi, K.; Kudo, A.; Abe, M.; Yoshida, T.; Nagai, T.; Khadiza, N.; Yokoyama, A.; Oda, K.; Ozawa, H.; de Freitas, P.H.L.; Li, M.; Amizuka, N. Ultrastructural and biochemical aspects of matrix vesiclemediated mineralization. Jpn. Dent. Sci. Rev. 2017, 53, 34–45;. [CrossRef]
- Thomas, A.M.; Gomez, A.J.; Palma, J.L.; Yap, W.T.; Shea, L.D. Heparin-chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials. 2014, 35(30), 8687-8693;. [CrossRef]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development. 2016, 143(15), 2706-2715;. [CrossRef]
- Li, H.; Chang, J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater. 2013, 9(2), 5379–5389;. [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A Review of in vitro and in vivo evidences, Tissue Eng. Part B Rev. 2010, 16(2), 199–207;. [CrossRef]
- Kuttappan, S.; Mathew, D.; Jo, J.-I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47;. [CrossRef]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone, Development. 2016, 143(15), 2706-2715;. [CrossRef]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized bone tissue engineering: approaches for potential improvement, Tissue Eng. Part B Rev. 2012, 18, 363–382;. [CrossRef]
- Sheridan, M.H.; Shea, L.D.; Peters, M.C.; Mooney, D.J. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control Release. 2000, 64(1-3), 91-102;. [CrossRef]
- Amaral, I.F.; Neiva, I.; da Silva, F.F.; Sousa, S.R., Piloto, A.M.; Lopes, C.D.F.; Barbosa, M.A.; Kirkpatrick, C.J.; Pego, A.P. Endothelialization of chitosan porous conduits via immobilization of a recombinant fibronectin fragment (rhFNIII(7-10)). Acta Biomaterialia. 2013, 9(3), 5643-5652;. [CrossRef]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and biocompatible macroporous scaffolds with tunable performances prepared based on 3D printing of the pre-crosslinked sodium alginate/hydroxyapatite hydrogel ink. Macromol. Mater. Eng. 2019, 304, 1800698; [CrossRef]
- Sancilio, S.; Marsich, E.; Schweikl, H.; Cataldi, A.; Gallorini, M. Redox control of IL-6-mediated dental pulp stem-cell differentiation on alginate/hydroxyapatite biocomposites for bone ingrowth. Nanomaterials (Basel). 2019, 9(12), 1656;. [CrossRef]
- Sumayya, A.S.; Muraleedhara Kurup, G. Marine macromolecules cross-linked hydrogel scaffolds as physiochemically and biologically favorable entities for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 807–825;. [CrossRef]
- Li, Z.; Liao, Y.; Li, D.; Li, D.; Wang, H.; Sun, X.; Chen, X.; Yan, H.; Lin, Q. Design and properties of alginate/gelatin/cellulose nanocrystals interpenetrating polymer network composite hydrogels based on in situ cross-linking. Research Square, 2022, 1-27, . [CrossRef]
- Filardo, G.; Perdisa, F.; Gelinsky, M.; Despang, F.; Fini, M.; Marcacci, M.; Parrilli, A.P.; Roffi, A.; Salamanna, F.; Sartori, M.; Schütz, K.; Kon, E. Novel alginate biphasic scaffold for osteochondral regeneration: An in vivo evaluation in rabbit and sheep models. J. Mater. Sci. Mater. Med. 2018, 29(6), 74;. [CrossRef]
- Torres, A.L.; Gaspar, V.M.; Serra, I.R.; Diogo, G.S.; Fradique, R.; Silva, A.P.; Correia, I.J. Bioactive polymeric–ceramic hybrid 3D scaffold for application in bone tissue regeneration. Mater. Sci. Eng. C 2013, 33, 4460–4469;. [CrossRef]
- Mahmoud, E.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360;. [CrossRef]
- Yeo, Y.J.; Jeon, D.W.; Kim, C.S.; Choi, S.H.; Cho, K.S.; Lee, Y.K.; Kim, C.-K. Effects of chitosan nonwoven membrane on periodontal healing of surgically created one-wall intrabony defects in beagle dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 86–93;. [CrossRef]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Ta, C.N.; Jackson, A.J.; Toney, M.F.; Frank, C.W. Structure and mechanism of strength enhancement in interpenetrating polymer network hydrogels. Macromolecules. 2011, 44, 5776-5787; dx.doi.org/10.1021/ma200693e.
- Darnell, M.; Sun, J.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials. 2013, 34, 8042-8048;. [CrossRef]
- TIǧlI, R.S.; Gumüşderelioǧlu, M. Evaluation of alginate-chitosan semi IPNs as cartilage scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 699–709;. [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65,1172–1187;. [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K..-H.; Kim, S.-K.) Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269-281;. [CrossRef]
- Shchipunov, Y.A.; Postnova, I. Formation of calcium alginate-based macroporous materials comprising chitosan and hydroxyapatite. Colloid J. 2011, 73, 565–574;. [CrossRef]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2016, 64, 416–427;. [CrossRef]
- Liu, D.; Liu, Z.; Zou, J.; Li, L.; Sui, X.; Wang, B.; Yang, N.; Wang, B. Synthesis and characterization of a hydroxyapatite-sodium alginate-chitosan scaffold for bone regeneration. Front. Mater. 2021, 8, 69;. [CrossRef]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2016, 64, 416–427;. [CrossRef]
- Park, J.S.; Choi, S.H.; Moon, I.S.; Cho, K.S.; Chai, J.K.; Kim, C.K.. Eight-week histological analysis on the effect of chitosan on surgically created one-wall intrabony defects in beagle dogs. J. Clin. Periodontol. 2003, 30, 443–53;. [CrossRef]
- Lu, Q.; Li, M.Y.; Zou,; Cao, T. Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novo adipogenesis J. Control. Release. 2014, 174, 43-50; [CrossRef]
- Shen , H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Cell affinity for bFGF immobilized heparin-containing poly(lactide-co-glycolide) scaffolds. Biomaterials. 2011, 32(13), 3404-3412;. [CrossRef]
- Minardi, S.; Pandolfi, L.; Taraballi, F.; Wang, X.; De Rosa, E.; Mills, Z.D.; Liu, X.; Ferrari, M.; Tasciotti. E. Enhancing Vascularization through the Controlled Release of Platelet-Derived Growth Factor-BB. ACS Appl. Mater. Interfaces. 2017, 9(17),14566-14575;. [CrossRef]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23(9), 4486;. [CrossRef]
- Ho, Y.C.; Mi, F.L.; Sung, H.W.; Kuo, P.L. Heparin-functionalized chitosan-alginate scaffolds for controlled release of growth factor. Int. J. Pharm. 2009, 376(1-2), 69-75;. [CrossRef]
- Lazarous, D.F.; Unger, E.F.; Epstein, S.E.; Stine, A.; Arevalo, J.L.; Chew, E.Y.; Quyyumi. A.A. Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J. Am. Coll. Cardiol. 2000,36(4),1239-1244;. [CrossRef]
| Early dates | |||||
| Area of mandibular defect | |||||
| Experiment period | Experience CH-SA-HA1 | Control Healing under a blood clot1 | Defect periphery1Control + experience | ||
| Numerical density of inflammatory infiltrate (unit/0.043 mm2) | |||||
| 3 day1 | 56,1[46,4;67,6] | 67,4[57,6;72,1]** | 9,3[7,9;9,7]** | ||
| 5 day1 | 85,7[72,1;90,7] | 93,7[86,1;99,4]** | 14,6[13,4;15,7]** | ||
| 7 day1 | 88,1[74,6;91,9] | 91,4[81,5;99,4]** | 11,3[10,1;12,3]** | ||
| Volumetric density of bone tissue (BV (%) | |||||
| 5 day1 | 1,1[0,9;1,3] | 1,1[0,8;1,5] | 65,3[64,2;66,4]** | ||
| 7 day1 | 15,1[13,0;16,7] | 16,2[14,9;17,9] | 66,5[65,6;68,8]** | ||
| Trabeculae thickness (mm) | |||||
| 5 day1 | 0,04[0,03;0,05] | 0,04[0,04;0,05] | 0,15[0,15;0,16]** | ||
| 7 day1 | 0,06[0,05;0,07] | 0,06[0,05;0,06] | 0,15[0,15;0,16]** | ||
| Intertrabecular spaces (mm) | |||||
| 5 day1 | 0,61[0,60;0,63] | 0,61[0,59;0,63] | 0,21[0,21;0,23]** | ||
| 7 day1 | 0,43[0,42;0,45] | 0,43[0,43;0,45] | 0,20[0,19;0,21]** | ||
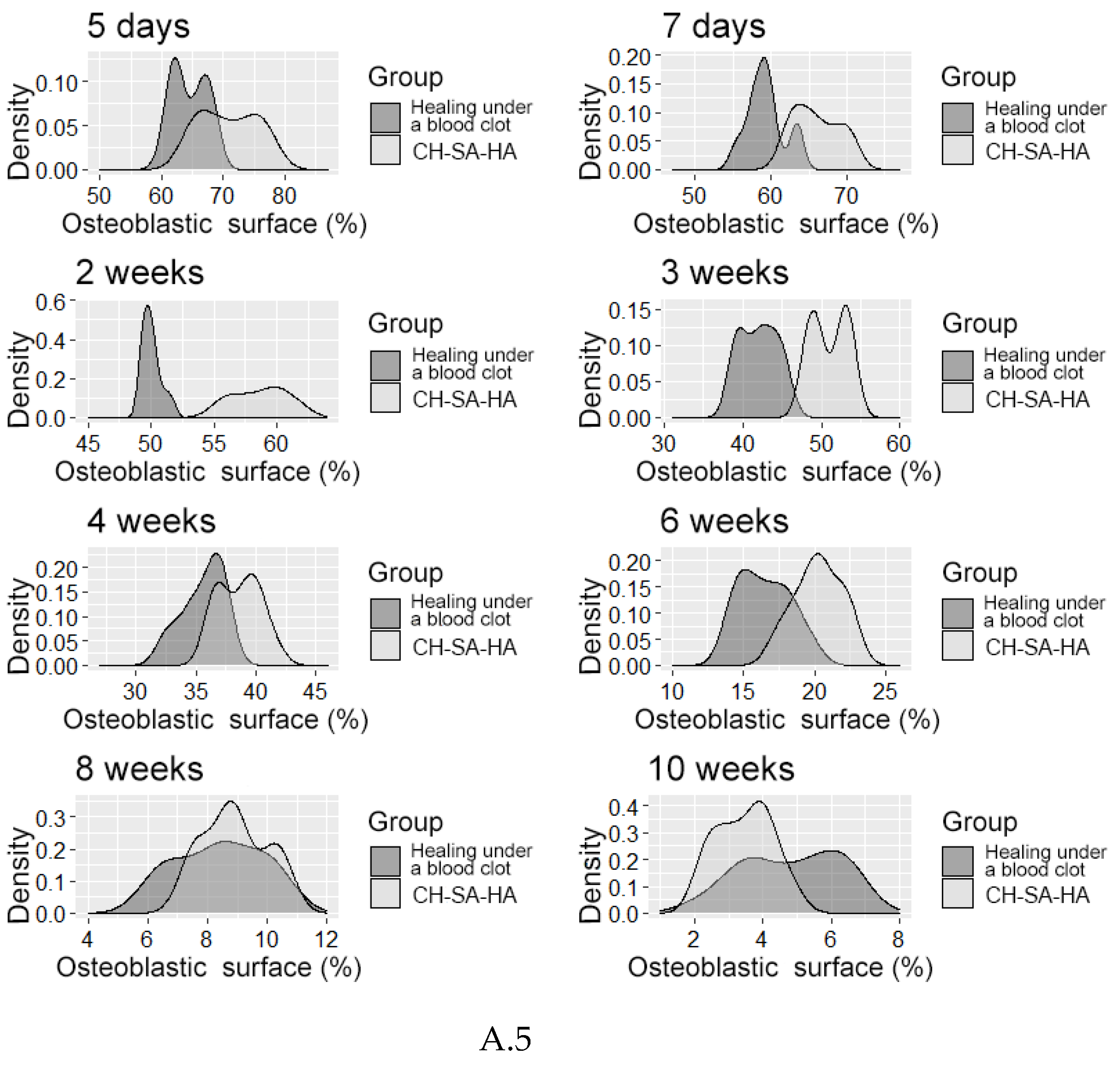
| Osteoblastic surface (%) | |||||
| 5 day1 | 70,5[66,4;74,8] | 64,3[62,0;67,1]** | 25,3[24,0;26,4]** | ||
| 7 day1 | 65,6[63,2;68,5] | 59,2[57,9;60,2]** | 21,8[20,2;23,5]** | ||
| Erosed bone surface (%) | |||||
| 5 day1 | 0,8[0,7;0,9] | 1,0[0,9;1,1]** | 12,0[11,3;13,3]** | ||
| 7 day1 | 1,5[1,4;1,8] | 1,9[1,6;2,1]** | 7,9[7,7;8,0]** | ||
| Interim dates | |||||
| Numerical density of inflammatory infiltrate (unit/0.043 mm2) | |||||
| 2 weeks1 | 26,8[24,2;28,5] | 31,5[28,8;33,7]** | 13,2[11,3;14,4]** | ||
| 3 weeks1 | 17,2[15,8;19,9] | 17,5[15,7;22,6] | 6,2[5,7;7,1]** | ||
| Volumetric density of bone tissue (BV %) | |||||
| 2 weeks1 | 30,0[28,8;31,0] | 28,3[25,8;29,9]** | 65,0[63,8;66,5]** | ||
| 3 weeks1 | 41,3[39,6;43,6] | 38,6[36,1;40,2]** | 67,8[67,0;69,3]** | ||
| 4 weeks1 | 62,9[60,7;66,8] | 60,9[58,0;62,0]** | 66,5[64,3;68,1]** | ||
| Trabeculae thickness (TT mm) | |||||
| 2 weeks1 | 0,12[0,11;0,13] | 0,11[0,10;0,12]* | 0,16[0,16;0,17]** | ||
| 3 weeks1 | 0,13[0,11;0,14] | 0,12[0,12;0,13]* | 0,15[0,14;0,16]** | ||
| 4 weeks1 | 0,16[0,15;0,17] | 0,14[0,13;0,15]* | 0,16[0,15;0,17]** | ||
| Intertrabecular spaces (ITS mm) | |||||
| 2 weeks1 | 0,37[0,36;0,37] | 0,39[0,38;0,40]** | 0,22[0,22;0,24]** | ||
| 3 weeks1 | 0,29[0,28;0,30] | 0,30[0,29;0,31]** | 0,24[0,23;0,25]** | ||
| 4 weeks1 | 0,21[0,21;0,22] | 0,22[0,21;0,22]** | 0,21[0,20;0,22] | ||
| Osteoblastic surface (OS %) | |||||
| 2 weeks1 | 58,9[57,0;60,2] | 49,9[49,5;50,4]** | 13,1[12,4;13,7]** | ||
| 3 weeks1 | 50,8[49,0;53,1] | 42,0[39,6;43,9]** | 9,1[8,4;9,8]** | ||
| 4 weeks1 | 38,9[37,1;40,1] | 36,0[34,7;37,0]** | 7,2[6,3;7,8]** | ||
| Erosed bone surface (ES %) | |||||
| 2 weeks1 | 3,5[3,3;3,6] | 3,9[3,7;3,9]** | 6,1[5,5;6,9]** | ||
| 3 weeks1 | 5,9[5,6;6,1] | 6,4[6,1;6,6]** | 2,9[2,7;3,0]** | ||
| 4 weeks1 | 9,9[9,7;10,0] | 10,7[10,5;11,1]** | 1,3[1,2;1,4]** | ||
| Late dates | |||||
| Volumetric density of bone tissue (BV %) | |||||
| 6 weeks1 | 67,5[65,4;68,6] | 62,6[60,0;64,2]** | 68,7[67,2;69,8]* | ||
| 8 weeks | 67,1[63,2;69,1] | 65,2[63,4;69,2] | 67,0[65,4;68,5] | ||
| 10 weeks | 67,3[65,1;68,2] | 68,3[66,6;69,2] | 67,8[66,3;69,2] | ||
| Trabeculae thickness (TT mm) | |||||
| 6 weeks | 0,16[0,15;0,17] | 0,16[0,15;0,16] | 0,15[0,14;0,17] | ||
| 8 weeks1 | 0,16[0,15;0,16] | 0,15[0,14;0,16]* | 0,15[0,14;0,15] | ||
| 10 weeks | 0,16[0,16;0,17] | 0,16[0,15;0,17] | 0,16[0,16;0,17] | ||
| Intertrabecular spaces (ITS mm) | |||||
| 6 weeks1 | 0,21[0,19;0,22] | 0,21[0,20;0,22] | 0,19[0,18;0,20]** | ||
| 8 weeks1 | 0,20[0,19;0,20] | 0,21[0,19;0,21] | 0,19[0,18;0,19]** | ||
| 10 weeks1 | 0,20[0,19;0,20] | 0,22[0,21;0,23] | 0,18[0,17;0,20]** | ||
| Osteoblastic surface (OS %) | |||||
| 6 weeks1 | 20,2[19,0;21,5] | 16,4[14,9;18,0]** | 4,0[2,8;4,7]** | ||
| 8 weeks1 | 8,7[8,1;9,9] | 8,4[7,1;9,6] | 1,5[1,3;1,8]* | ||
| 10 weeks1 | 3,6[2,8;4,0] | 5,0[3,6;6,1]** | 1,6[1,1;1,9]** | ||
| Erosed bone surface (ES %) | |||||
| 6 weeks1 | 4,8[4,6;4,9] | 5,1[4,7;5,6] | 0,8[0,7;0,9]** | ||
| 8 weeks1 | 2,6[2,5;2,8] | 3,1[3,0;3,4]** | 0,7[0,5;0,9]** | ||
| 10 weeks1 | 1,6[1,5;1,7] | 1,3[1,2;1,4]** | 0,9[0,8;0,9]** | ||
| Bone growth rate (% per day) | |||||
| 1-28 days | 2,24[2,17;2,39] | 2,17[2,07;2,21]** | |||
| 6-14 days | 28,9 [26,3;30,4] | 27,2 [25,2;29,0]** | |||
| 15-28 days | 3,23[2,85;3,36] | 3,15[2,64;3,52] | |||
| 29-70 days | 0,96[0,92;0,97] | 0,97[0,95;0,99] | |||
| 1-70 days (total) | 0,94[0,82;1,25] | 0,96[0,85;1,15] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





