1. Introduction
Initially developed by Clausius and refined later by Boltzmann [
1], entropy expresses system order as measured by uncertainty or unpredictability. Young cells in ‘healthy order’ are expected to display low entropy, while tissue aging reflects deterioration of physical structures such as misfolded/deformed RNA, DNA, or proteins, with harmful downstream effects in tissues and organs [
2]. Entropy can be reversed during early development, growth, and under metabolic conditions where energy is expended to attenuate disorder [
3,
4,
5]. Common to other mammalian organ systems [
6], the adult human ovary demonstrates nonlinear dynamics characterized by multi-stability, hysteresis, and transitions across different metabolic states (
i.e., ovulatory vs non- ovulatory functions). The fertility/infertility divide may thus be classified within the ovarian field as a stochastic process, where reproductive potential is optimized at an entropy minimum while subfertility expands with a declining capacity to maintain orderliness over time.
Current models of cellular aging portray DNA damage accumulating with lost regenerative function, more genomic infidelity, and eventually, cell loss without replacement (death). For the ovary, early diminishments of reserve presage a final functional collapse at menopause. Of note, this process is only partially heritable (<50%) and underscores the importance of non-genetic factors in ovarian aging [
7]. For example, twins sharing the same household may acquire dissimilar age-related epigenetic changes, revealing an energy system not fully under genetic or environmental control [
8,
9]. If this paradigm is correct, it invites speculation on possible external inputs able to alter the functional dynamic and influence clinical outcomes (
i.e., recover reserve by reducing entropy).
2. Experimental Program
With IRB approval, written informed consent would be obtained from patients (age 20-55) already scheduled for standard GYN laparoscopy for benign indications [
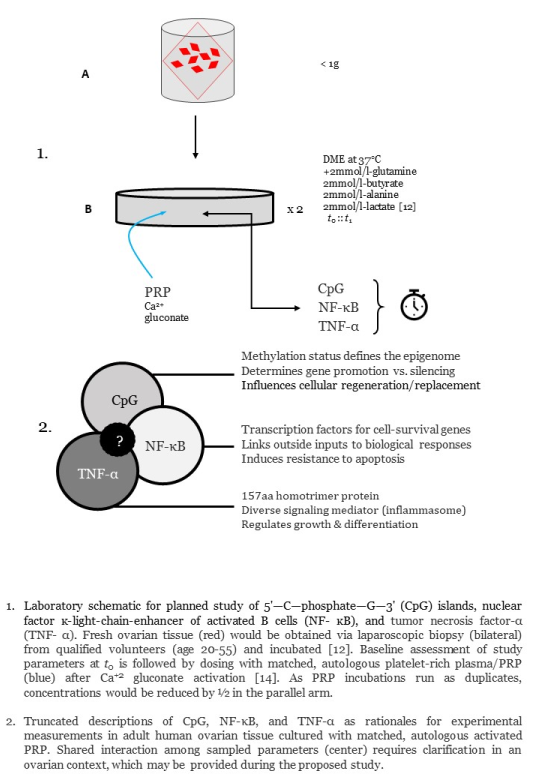
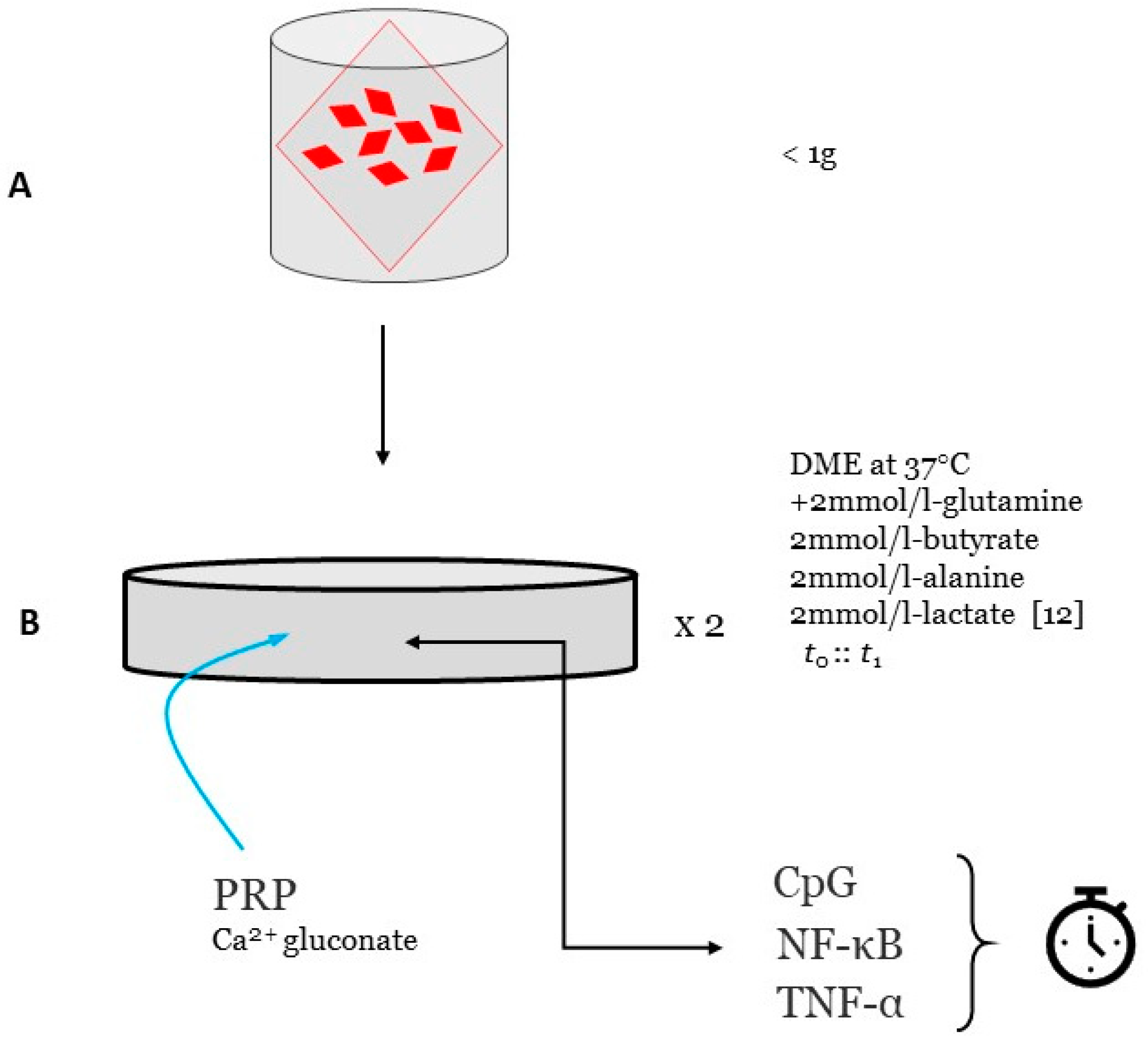
10]. This study is powered by <1g of tissue obtained from each ovary by standard punch biopsy (see
Figure 1). No hormone use within six months would be confirmed before enrollment [
11]. Deidentified ovarian samples are maintained fresh as the experimental substrate and cultured at 37 °C/5% CO
2, based on a previously published complex tissue protocol [
12]. While a signal multiplier array incorporated a protein-protein interaction model for platelet-derived bFGF and VEGF [
13], it failed to include guidance on methods or study design to audit cellular response. Here, the reagent used as external stimulus is matched-autologous PRP processed with calcium gluconate activation [
14].
Information on ovarian nuclear factor ĸ-light-chain-enhancer of activated B cells (NF-κB) dynamics is to be supplied from DNA binding at κB enhancer motif sequences found in NF-κB target genes [
15]. DNA-protein binding may be measured either by electro- mobility shift assay (EMSA) technique, or by quantitative ELISA [
16,
17]. Alternatively, a fluidic chip system could be used whereby DNA, histone/protamine, and transcription factor NF-κB are observed to record how PRP cytokine inputs alter DNA-protein configurations [
18]. Transcriptional activation data also can be quantified using a NF-κB consensus promoter sequence linked to a ‘reporter gene’ and luciferase assay [
19,
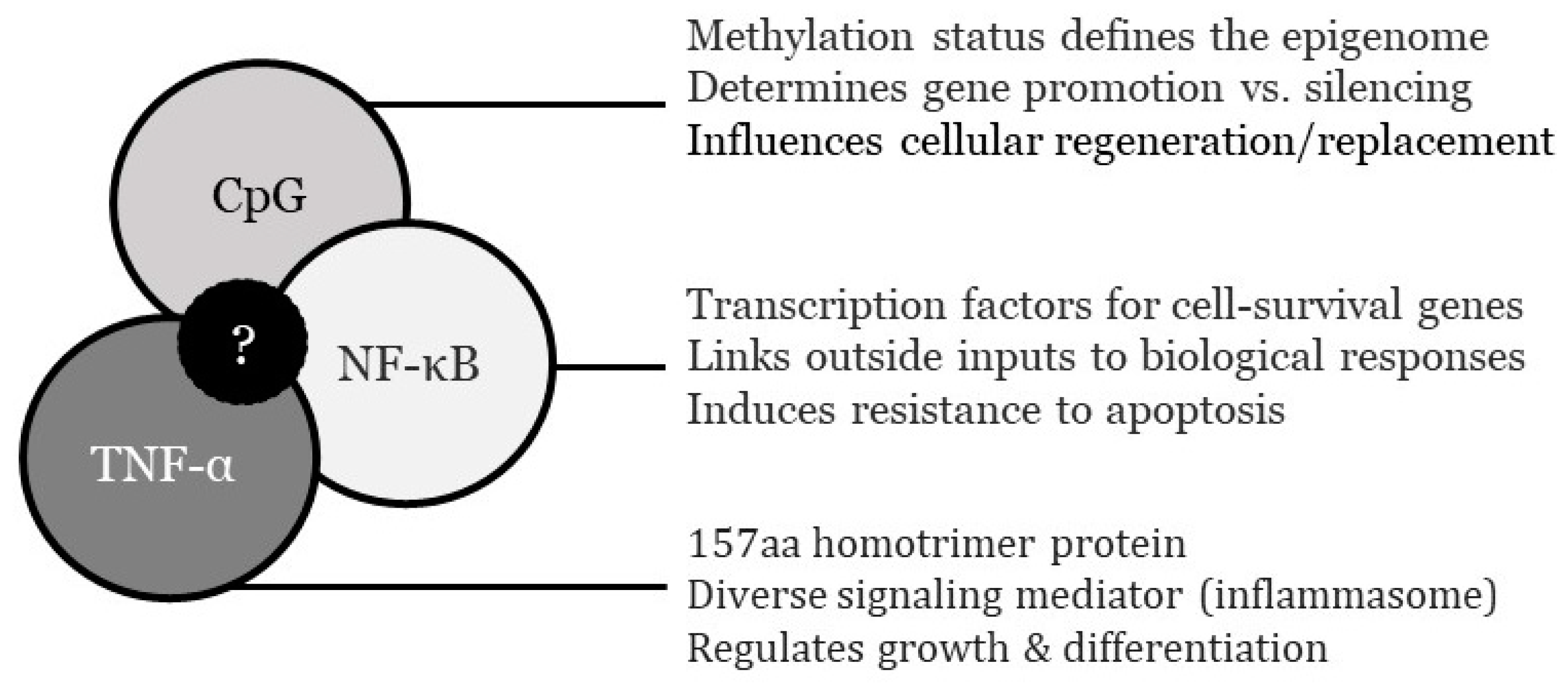
20]. Differences in tumor necrosis factor-α (TNF-α) levels pre- vs. post-PRP exposure would be marked by quantitative immunoassay (Promega; Madison WI). As an enhancement of NF-κB data, recording changes in TNF-α will improve knowledge of how this pleiotropic cytokine may impact differentiation, proliferation, and survival after PRP. Although unlikely, short-term PRP effects on 5'—C—phosphate—G—3' (CpG) islands also could be monitored to quantify expression noise attenuation post-stimulus [
21]. Since the duration of any PRP impact on NF-κB, TNF-α, or CpG islands is unknown, the end-point for experimental culture termination is recursive and will need to be delimited by data not currently available.
In addition to functional immunohistochemical findings, ovarian cells are ideally imaged by stereological analysis [
22] to note potential differences in matrix/ cytoarchitectural elements by the Delesse principle [
23]. As each patient would contribute two biopsies, the investigation is run in duplicate to validate observed findings and to offer preliminary dose-finding data (see
Figure 2).
3. The Older Ovary: Special Features
Unlike most other adult endocrine organs, the human ovary begins to show functional decline relatively early (approx. 30-35yrs). It is generally agreed that this negative slope involves a follicular endowment which recedes soon after menarche [
24,
25]. Losses in ovarian reserve can bring adversity to those wishing to conceive as well as for patients disinterested in fertility (i.e., symptomatic menopause) [
26]. Against this background, if epigenetic changes act as a peri-menopause trigger [
27], one theory supports the concept of ovarian compromise as merely an early sign of accelerated aging [
28]. As such, the adult human ovary would be merely the first and most fragile unit evincing senescence traits, manifesting more broadly later [
29].
Fundamentally this is a degenerative and entropic sequence typified by poor tissue homeostasis [
30]. Primordial ovarian follicles first emerge during the fetal period and their programmed decline with age has been well documented [
31]. Indeed, early animal experiments with transplanted young ovaries into aged recipients fixed the central role of competent ovarian tissue [
26] with good order and minimal entropy. Follicular density loss is perhaps the most conspicuous structural feature of the aging ovary. Collagen gradually displaces hormonally active elements while fibrillin-1 and EMILIN-1 vanish over time. Such changes offer a substructural insight into tissue disorder, as the ovary becomes less pliable and more fibrotic with approaching menopause. Unsurprisingly, significantly more elastin is observed in menopausal ovary biopsies where HRT is used vs. no HRT [
32]. Rigidity of the granulosa compartment also resists follicle growth commensurate with progressive entropy, becoming more inclined to quiescence [
32].
The diversity of platelet cargo proteins [
33] implies that ovarian entropy is able to be addressed at several loci, where PRP might manipulate signal networks to drive improved perfusion, HOX regulation, N-glycan post-translational modification, adjustment of voltage-gated ion channels, telomere stabilization, optimization of SIRT3, and ribosome and mitochondria recovery [
34]. Another example is transcription factor FIGLA, which directs expression of Gdf9, Lhx8, Nobox, Sohlh1&2, and Taf4b as controllers of oocyte growth and differentiation [
35]. In a murine model, FIGLA knockdown severely squelches meiosis to cause oocyte apoptosis. Non-operation of any regulatory member yields follicles overtaken by fibrosis, with downregulation of genes preferentially expressed in oocytes [
36].
Since TNF-α is known to upregulate NF-κB [
37] and the NF-κB signaling system coordinates Gdf9 actions [
38], this draws notice to cytokines of PLT source either mimicking FIGLA or boosting its function. For the follicular unit and its local support matrix where remodeling, regeneration and/or proliferation effects are presumably induced by PRP [
39,
40], clarification of CpG, TNF-α, and NF-κB roles is required to provide important background detail. Most cell processes are not indifferent to such interstitial elements: Membrane tension and cytoskeletal focal adhesions [
41] set the stage for ovarian function, although these have yet to be specifically evaluated in human ovarian tissue pre- and post-PRP dosing.
In this regard, availing of computer-assisted 2D fluorescence imaging (Analytical Technologies; Singapore) of the ovarian cytoskeleton and juxta-follicular components can assist in documenting and classifying changes associated with local PRP injection, similar to techniques described recently [
41]. Others have detailed PRP-integrated alginate gelatin composites where PLT cytokines ‘seed’ cell behavior, form vascular endothelial cells, and order macrophage polarization in a paracrine manner [
42]. While activated PRP or its cytokine products may impact structures extraneous to the granulosa compartment, more research is needed to build on recently reported responses [
34,
41]. If parallel connective tissue responses are verified after ovarian PRP use as outlined here, this would help explain reports from its early clinical use [
33,
43,
44].
4. Ovarian Signaling Response after PRP
With attention to ovarian cellular entropy, experimental evidence exists [
45] that NF-ĸB oscillations are involved in governing immune responses, cellular growth, development, and apoptosis. These NF-ĸB actions are driven by inhibitory proteins ĸB and IĸB, where an inverse relation has been reported between pulse frequency and quantity of IĸB [
45,
46]. NF-κB actually embraces an entire transcription factor family, responsible for vital immune and inflammatory actions [
47]. This includes NF-κB1, NF-κB2, RelA, RelB, and c-Rel, as transcription mediators of key target genes [
48]. In general, NF-κB proteins are sequestered in the cytoplasm by inhibitory proteins typified by ankyrin repeats [
49,
50]. NF-κB enhances expression of pro-inflammatory genes including those for cytokines and chemokines, and also participates in inflammasome regulation. NF-κB also orchestrates survival, activation and differentiation of inflammatory T cells [
49]. In eukaryotes, the IĸB-NF-ĸB module ideally operates like a signal transduction unit, where inputs are external stimuli conducted by membrane receptors, and its outputs are signals channeled to the nucleus to regulate gene expression [
46]. NF-κB may convey specificity of contextual information via quantitative features of its signaling dynamics [
51], and greater metabolic noise correlates to unwanted entropy as seen with aging and disease.
The use of fluorescent tagged proteins would enable tracking of dynamic NF-κB traffic, where its nuclear localization has been confirmed along with an oscillation period near 90min–an observation in reasonably close alignment with results predicted by mathematical models of NF-κB signaling [
52,
53]. Subsequently, NF-κB has become perhaps the best-known exemplar of pulsing or oscillating genetic circuits mapped by active imaging [
54,
55,
56]. Pulsed TNF-α stimulation also affects gene expression in a target-specific way, providing another connection for signal dynamics and target gene expression [
57]. As expected, these ordered oscillatory patterns are largely squelched in high entropy states, although platelet cytokines can induce nuclear translocation of NF-κB and upregulate mRNA expression of NF-κB-dependent mediators outside the ovary [
58]. Direct measurement of these effects in human ovarian tissue awaits confirmation, and the present approach would help meet this need.
Several Kyoto Encyclopedia of Gene and Genome (KEGG) processes are known to be preferentially boosted in young mammalian follicles compared to aged ovaries, areas open to therapeutic enhancement. For example, leucocyte rich platelet rich fibrin (L-PRF) mediates NF-κB signaling, toll-like receptor signaling (TLR), and MAPK signaling via T-cell receptor signaling pathway, and other platelet derivatives are closely involved in the JAK-STAT signaling network to upregulate STAT1 [
59]. How platelet cargo proteins interact with follicular surface markers after PRP dosing intersects with entropy studies, as membrane order and structure directly impact homeostasis via ion transport and modulation of signaling pathways [
34,
60].
5. Cytokine Effects on Cellular Entropy
The biochemical work of the adult human ovary exhibits transitional fluctuations where a closed compartment should uniformly reach a stable state. Is not a violation of the 2
nd Law of Thermodynamics since dampened oscillations by intermediate species may form
en route to equilibrium, even as overall Gibbs free energy (G
0) decreases [
61,
62]. Here, G
0 unifies enthalpy and entropy with change in free energy, ΔG, being the sum of enthalpy plus the product of temperature and entropy:
where H is enthalpy, T is temperature, and S is entropy; heat is not involved/added in life models, thus ΔH
0 = 0. Protein synthesis lowers cellular entropy by amino acid polymerization (cell growth also reduces entropy) making G
0 for protein synthesis net positive [
63]. Although perhaps not applicable to every ovarian process, hydrophobic effects also contribute to decreased entropy of aqueous solutions [
64]. As this hypothesis for intraovarian PRP posits that activated PLT-derived signals operate as entropy modulators, any growth stimulated by this treatment would involve protein synthesis with augmented amino acid synthesis, where required components are actively assembled from the dispersed field (cytosol). Reestablishing ordered signaling, if confirmed here, would agree with earlier work connecting entropy (‘serial irregularity’ by mathematical network analysis) and reproductive fitness [
65].
Cell division in eukaryotes is also governed by M-phase Promoting Factor (MPF), a heterodimeric protein kinase with Cdk1 (kinase subunit) and cyclin B (regulatory- targeting subunit) constituents [
66]. Interestingly, MPF activity was first discovered in a reproductive context where (amphibian) oocytes and embryos were studied [
62,
67,
68]. While MPF and NF-κB share a common signaling coordinator in ‘wee1’ [
69], understanding the interplay among these mediators could be improved using the proposed study protocol.
There are innumerable nodes in regulatory networks where outside signaling could be relevant, especially NF-κB, which normally regulates biological processes as a function of dynamic oscillation [
46,
70]. If re-ordering of NF-κB (
i.e., nuclear sequestration) were documented in the experimental program after activated PRP, feedback on regulatory genes and NF-κB activation may be contingent on nuclear localization as facilitated by PLT cytokines. These signals, acting either individually or in concert, would tend to upset the prevailing energetics of protein synthesis soon after interfacing with ovarian tissue.
6. Intraovarian PRP—Epigenetic Impacts
Molecular processes essential to normal ovarian function are indexes of epigenomic competency as communicated via stochastic feedback signals [
71]. As menopause nears, noise and entropy gradually overtake these well-ordered oscillations. Low ovarian reserve is a clinical problem where no uniform initiating factor is likely causative, but oligoovulation and non-responsiveness to gonadotropins comprise a familiar presentation. How (or if) cytokines of PLT origin alter methylation status by CpG audits represents another technique to gauge intraovarian PRP actions.
DNA methylation plays a major role in gene expression and how this evolves with aging and disease has been well characterized. Most age-related DNA methylation drift is attributed to adult stem cell replication, yet there is controversy with respect to methylation changes being strictly from proliferation errors or due to other factors also relevant to non-proliferating cells [
9,
72]. Any net gain/loss here as methylation drift results in genomic instability. By example, CpG sites can experience methylation loss over time to activate retrotransposons. Conversely, hypermethylation with age can occur within or near unmethylated CpG islands [
9]. Age-related DNA methylation drift is highly conserved and inversely proportional to lifespan [
73]. Age-related DNA methylation entropy as measured by Jensen-Shannon distribution [
74] affects up to 25% of detectable CpG sites. This has been checked as a function of age in blood, heart, kidney, liver, lung, muscle (skeletal), and spleen [
9], and this experimental program would expand this to include ovary post-PRP dosing. Epigenetic clocks are often based on just one sample site, so accuracy is limited to the source on which it was trained [
29]. Epigenetic clocks developed from machine learning tools have reported informative CpGs by regressing a transformed version of chronological age on a set of CpGs [
75,
76]. While this may be large to provide high accuracy, even a limited number of CpGs may offer adequate robustness to depict methylome properties [
29]. DNA methylation drift and increased entropy with age are caused by—and are markers for—stem cell replication in adult tissues [
9]. The experimental program described here should generate new data, at least indirectly, on how PRP may influence markers of ovarian cellular entropy.
7. Platelet Factors as Activators & Transport Modulators
For the adult human ovary, its component cells appear to function against a background of
phenoptosis—cell death pre-programmed by the genome. Normal ovarian metabolism generates many toxic by-products and errors to amplify this, causing cellular damage as seen in other tissue systems. While damage accumulation is a spontaneous entropy-driven process, the kinetics are not necessarily irreversible and are subject to genetic and environmental modification [
77]. In a murine model, an epigenome/ metabolome/epigenome paradigm has proven useful to explain the functional crosstalk needed for cellular differentiation [
49]. Being the first term in the set, epigenome status is rightly cast as a key player in cell-fate decisions for both embryonic and adult tissues [
78]. While adjusting the epigenome via extracellular inputs is possible, the extent to which different stimuli can push this is unresolved. While NF-κB is activated by various stimuli and can reprogram the epigenome by promoting latent enhancers, this depends on whether NF-κB is oscillatory or not. Tonic (non-oscillatory) NF-κB signaling opens chromatin [
79] by sustained disruption of histone-DNA interactions, triggering latent enhancers to express immune response genes. Previously unknown temporal aspects may fix a transcription factor's capacity and range for epigenetic reprogramming [
80]. Environmental cues influencing the epigenome include nutritional inputs, as a high-fat/high-carbohydrate diet was recently found to prompt nuclear translocation of NF-κB p65 factor transcription in surface epithelium cells of rabbit ovary, an intervention with harmful ovarian reserve results [
81].
PRP is a known suppressor of inflammatory NF-κB, where reduced doxorubicin- induced phosphorylation of IκB and NF-κB has been described [
42]. Models correlating nuclear NF-κB with mRNA expression offer predictions of high accuracy at single-cell resolution [
82]. Specific to its intraovarian use, autologous PRP does appear to foster improved tissue response although further clarification is required to define local signal modulation effects.
8. Discussion
Stimuli overlapping with PLT cytokines have been reported to enable recovery of ovarian tissue post-injury [
83]. For example, when paeoniflorin (a bioactive glucoside) was used after ischemia-reperfusion injury, protective ovary tissue effects were accompanied by increased levels of TNF-α, IL-1-β, IL-6 and NF-κB p65 [
83]. A brief TNF flash would be akin to burst exposure after intraovarian PRP, which has been shown to ‘jump start’ expression of NF-κB target pro-survival genes in other contexts [
84]. As a controversial intervention, intraovarian PRP has proven difficult to manage in multicenter clinical trials given nonuniform patient screening, sample preparation, and injection technique. As activated PRP puts a small cytokine bolus into an inactive or senescent tissue field where metabolic or endocrine responses—if any—await characterization, the proposed study aims to address these deficiencies.
If a PRP contribution to reduced ovarian entropy were to be convincingly proven using this experimental design, it would join existing feedback and oscillatory research where similar conclusions were advanced. For example, bolus estradiol (E2) increases growth hormone (GH) release substantially, while an abrupt GH spike stimulates fast internalization of its receptor to evoke second-messenger nuclear signaling [
85]. So, both magnitude and pattern are critical for physiologic signaling as the nonresponsive (older) ovary is characterized not by highly-ordered, information-rich pulsation, but rather a high entropy, non-cyclic, monotone field. Just as supplementary E2 can improve receptiveness, at least temporarily, to signaling elsewhere [
86], a yet-to-be defined PRP component may likewise restore ovarian reproductive capacity by effects within its local regulatory network after injection.
There are potential limitations with this design which warrant comment. First, while collapse of ovarian function with advanced maternal age may reflect slow replacement of normal follicular processes with (inactive) fibrosis, the problem of why this happens remains unsolved. If it occurs due to subtle dampening of molecular signaling, then the two markers proposed here (NF-κB and TNF-α) may be inferior to different, unchosen mediators. We do not have a full picture of which feedback circuits fail first in diminished ovarian reserve. The notion that ovarian decline is a critical, irreversible indicator serving to flag an entropy threshold also may be incomplete. Indeed, this protocol is shared before launch intending to gain from early input where others may use or improve the design. As data become available from our centers and elsewhere, further investigation will better define how to optimize intraovarian PRP (e.g., methodology comparisons or dose-finding studies).
In the meantime, as an adjunct to in vitro ovarian cell imaging, fully utilized mathematical models should help settle the problem of how activated PRP affects cellular entropy or local microarchitecture. The method can estimate concentrations/copy numbers of NF-κB to define how it binds to inhibitors, as well as clarify its effects on target gene mRNA levels [
55] secondary to PLT releasate. Because the NF-κB regulatory circuit is complex [
71], providing data on the dynamic evolution of the local ovarian NF-κB system has great investigational and clinical value. Given the clinical responses reported from independent, multicenter experience with intraovarian PRP [
33,
43,
44], the proposed study design can be a first step to elucidate a relevant mechanism.
Author Contributions
ESS developed the project as Principal Investigator; he read and approved the final manuscript.
Funding
This project received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
ESS has been awarded U.S. Trademark #88505430 for specified process and method using autologous platelet cytokines.
References
- Gandrillon O, Gaillard M, Espinasse T, Garnier NB, Dussiau C, Kosmider O, et al. Entropy as a measure of variability and stemness in single-cell transcriptomics. Curr Opin Syst Biol 2021;27:100348. [CrossRef]
- Garcia-Martin JA, Clote P. RNA thermodynamic structural entropy. PLoS One 2015;10(11):e0137859. [CrossRef]
- Wand AJ, Sharp KA. Measuring entropy in molecular recognition by proteins. Annu Rev Biophys 2018;47:41-61. [CrossRef]
- Wallace ZS, Rosenthal SB, Fisch KM, Ideker T, Sasik R. On entropy and information in gene interaction networks. Bioinformatics 2019;35(5):815-22. [CrossRef]
- Wang Z. The entropy perspective on human illness and aging. Engineering 2021;9:22-6. [CrossRef]
- Fernandez-de-Cossio-Diaz J, Mulet R. Maximum entropy and population heterogeneity in continuous cell cultures. PLoS Comput Biol 2019;15(2):e1006823. [CrossRef]
- Bacon ER, Mishra A, Wang Y, Desai MK, Yin F, Brinton RD. Neuroendocrine aging precedes perimenopause and is regulated by DNA methylation. Neurobiol Aging 2019;74:213-24. [CrossRef]
- Föhr T, Waller K, Viljanen A, Sanchez R, Ollikainen M, Rantanen T, et al. Does the epigenetic clock GrimAge predict mortality independent of genetic influences: An 18 year follow-up study in older female twin pairs. Clin Epigenetics 2021;13(1):128. [CrossRef]
- Vaidya H, Jeong HS, Keith K, Maegawa S, Calendo G, Madzo J, et al. DNA methylation entropy as a measure of stem cell replication and aging. Genome Biol 2023;24(1):27. [CrossRef]
- Sills ES, Palermo GD. Combined hysteroscopy-laparoscopy approach for excision of pelvic nitinol fragment from Essure contraceptive device: Role of intraoperative fluoroscopy for uterine conservation. Obstet Gynecol Sci 2016;59(4):337-41. [CrossRef]
- Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol 2010;184(9):5029-37. [CrossRef]
- Sills ES, Wood SH. Experimental embryo recovery, ex vivo support and facilitated immunosurgical transposition for ectopic-to-eutopic pregnancy conversion. J Surg Transplant Sci 2020;7(1):1072. [CrossRef]
- Sills ES, Wood SH, Walsh APH. Intraovarian condensed platelet cytokines for infertility and menopause—Mirage or miracle? Biochimie 2023;204:41-7. [CrossRef]
- Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol 2018;34(9):756-60. [CrossRef]
- Chen S, Ji X, Dedkova LM, Potuganti GR, Hecht SM. Site-selective tyrosine phosphorylation in the activation of the p50 subunit of NF-κB for DNA binding and transcription. ACS Chem Biol 2023;18(1):59-69. [CrossRef]
- Wang Z, Potoyan DA, Wolynes PG. Stochastic resonances in a distributed genetic broadcasting system: the NFκB/IκB paradigm. J R Soc Interface 2018;15(138):20170809. [CrossRef]
- Chramiec-Głąbik A, Rawski M, Glatt S, Lin TY. Electrophoretic mobility shift assay (EMSA) and microscale thermophoresis (MST) methods to measure interactions between tRNAs and their modifying enzymes. Methods Mol Biol 2023;2666:29-53. [CrossRef]
- Che B, Sun D, Zhang C, Hou J, Zhao W, Jing G, et al. Gradient nanoconfinement facilitates binding of transcriptional factor NF-κB to histone- and protamine-DNA complexes. Nano Lett 2023;23(6):2388-96. [CrossRef]
- Nass N, Bayreuther K, Simm A. Systemic activation of NF-κB driven luciferase activity in transgenic mice fed advanced glycation end products modified albumin. Glycoconj J 2017;34(2):157-61. [CrossRef]
- Ernst O, Vayttaden SJ, Fraser IDC. Measurement of NF-κB activation in TLR-activated macrophages. Methods Mol Biol 2018;1714:67-78. [CrossRef]
- Morgan MD, Marioni JC. CpG island composition differences are a source of gene expression noise indicative of promoter responsiveness. Genome Biol 2018;19(1):81. [CrossRef]
- Paul-Gilloteaux P. Bioimage informatics: Investing in software usability is essential. PLoS Biol 2023;21(7):e3002213. [CrossRef]
- Pennarossa G, De Iorio T, Gandolfi F, Brevini TAL. Impact of aging on the ovarian extracellular matrix and derived 3D scaffolds. Nanomaterials (Basel) 2022;12(3):345. [CrossRef]
- Sills ES, Alper MM, Walsh AP. Ovarian reserve screening in infertility: Practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol 2009;146(1):30-6. [CrossRef]
- Desai S, Rajkovic A. Genetics of reproductive aging from gonadal dysgenesis through menopause. Semin Reprod Med 2017;35(2):147-59. [CrossRef]
- Wang X, Wang L, Xiang W. Mechanisms of ovarian aging in women: A review. J Ovarian Res 2023;16(1):67. [CrossRef]
- Das A, Destouni A. Novel insights into reproductive ageing and menopause from genomics. Hum Reprod 2023;38(2):195-203. [CrossRef]
- Loose JA, Amrit FRG, Patil T, Yanowitz JL, Ghazi A. Meiotic dysfunction accelerates somatic aging in Caenorhabditis elegans. Aging Cell 2022;21(11):e13716. [CrossRef]
- Li Piani L, Vigano' P, Somigliana E. Epigenetic clocks and female fertility timeline: A new approach to an old issue? Front Cell Dev Biol 2023;11:1121231. [CrossRef]
- Valtetsiotis K, Valsamakis G, Charmandari E, Vlahos NF. Metabolic mechanisms and potential therapeutic targets for prevention of ovarian aging: Data from up-to-date experimental studies. Int J Mol Sci 2023;24(12):9828. [CrossRef]
- Sills ES, Wood SH. Epigenetics, ovarian cell plasticity, and platelet-rich plasma: Mechanistic theories. Reprod Fertil 2022;3(4):C44-C51. [CrossRef]
- Ouni E, Bouzin C, Dolmans MM, Marbaix E, Pyr Dit Ruys S, Vertommen D, et al. Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum Reprod 2020;35(6):1391-410. [CrossRef]
- Merhi Z, Seckin S, Mouanness M. Intraovarian platelet-rich plasma administration could improve blastocyst euploidy rates in women undergoing in vitro fertilization. Clin Exp Reprod Med 2022;49(3):210-4. [CrossRef]
- Sills ES, Wood SH. Discussion of field effects after intraovarian injection of autologous platelet-rich plasma. Bull Natl Res Cent 2023;47:52. [CrossRef]
- Wang Z, Liu CY, Zhao Y, Dean J. FIGLA, LHX8 and SOHLH1 transcription factor networks regulate mouse oocyte growth and differentiation. Nucleic Acids Res 2020;48(7):3525-41. [CrossRef]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004;305(5687):1157-9. [CrossRef]
- Abdelnaser M, Alaaeldin R, Attya ME, Fathy M. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn Schmiedebergs Arch Pharmacol 2023: in press. [CrossRef]
- Reader KL, Mottershead DG, Martin GA, Gilchrist RB, Heath DA, McNatty KP, et al. Signaling pathways involved in the synergistic effects of human growth differentiation factor 9 and bone morphogenetic protein 15. Reprod Fertil Dev 2016;28(4):491-8. [CrossRef]
- Hajipour H, Farzadi L, Latifi Z, Keyhanvar N, Navali N, Fattahi A, et al. An update on platelet-rich plasma (PRP) therapy in endometrium and ovary related infertilities: Clinical and molecular aspects. Syst Biol Reprod Med 2021;67(3):177-88. [CrossRef]
- Zhang J, Zhang J, Zhang N, Li T, Zhou X, Jia J, et al. The effects of platelet-rich and platelet-poor plasma on biological characteristics of BM-MSCs in vitro. Anal Cell Pathol (Amst) 2020;2020:8546231. [CrossRef]
- Wang X, Li N, Zhang Z, Qin K, Zhang H, Shao S, Liu B. Visualization of cell membrane tension regulated by the microfilaments as a "shock absorber" in micropatterned cells. Biology (Basel) 2023;12(6):889. [CrossRef]
- Zhao H, Zhu W, Mao W, Shen C. Platelet-rich plasma inhibits Adriamycin-induced inflammation via blocking the NF-kappaB pathway in articular chondrocytes. Mol Med 2021;27(1):66. [CrossRef]
- Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk SY, Korun ZEU, Herlihy N, et al. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging (Albany NY) 2022;14(6):2513-23. [CrossRef]
- Garavelas A, Mallis P, Michalopoulos E, Nikitos E. Clinical benefit of autologous platelet-rich plasma infusion in ovarian function rejuvenation: Evidence from a before-after prospective pilot study. Medicines (Basel) 2023;10(3):19. [CrossRef]
- Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing. Nature 2010;466:267-71. [CrossRef]
- González-Miranda JM. On the effect of circadian oscillations on biochemical cell signaling by NF-κB. J Theor Biol 2013;335:283-94. [CrossRef]
- Mitchell S, Tsui R, Tan ZC, Pack A, Hoffmann A. The NF-κB multidimer system model: A knowledge base to explore diverse biological contexts. Sci Signal 2023;16(776):eabo2838. [CrossRef]
- Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol 2022;43(9):757-75. [CrossRef]
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. [CrossRef]
- Pflug KM, Lee DW, Keeney JN, Sitcheran R. NF-κB-inducing kinase maintains mitochondrial efficiency and systemic metabolic homeostasis. Biochim Biophys Acta Mol Basis Dis 2023;1869(5):166682. [CrossRef]
- Aqdas M, Sung MH. NF-κB dynamics in the language of immune cells. Trends Immunol 2023;44(1):32-43. [CrossRef]
- Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 2004;306(5696):704-8. [CrossRef]
- Hoffmann A, Levchencko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: Temporal control and selective gene activation. Science 2002;298:1241-5. [CrossRef]
- Levine JH, Lin Y, Elowitz MB. Functional roles of pulsing in genetic circuits. Science 2013;342(6163):1193-200. [CrossRef]
- Kizilirmak C, Bianchi ME, Zambrano S. Insights on the NF-κB system using live cell imaging: Recent developments and future perspectives. Front Immunol 2022;13:886127. [CrossRef]
- Ravindran PT, McFann S, Thornton RH, Toettcher JE. A synthetic gene circuit for imaging-free detection of signaling pulses. Cell Syst 2022;13(2):131-42.e13. [CrossRef]
- Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 2009;324(5924):242-6. [CrossRef]
- Yin W, Xu H, Sheng J, Xu Z, Xie X, Zhang C. Comparative evaluation of the effects of platelet-rich plasma formulations on extracellular matrix formation and the NF-κB signaling pathway in human articular chondrocytes. Mol Med Rep 2017;15(5):2940-8. [CrossRef]
- Jasmine S, Thangavelu A, Krishnamoorthy R, Alshuniaber MA, Alshatwi AA. Cytokine expression pattern and protein-protein interaction network analysis of leucocyte rich platelet rich fibrin and injectable form of platelet rich fibrin. Oral Maxillofac Surg 2021;25(2):223-9. [CrossRef]
- Gupta K, Toombes GE, Swartz KJ. Exploring structural dynamics of a membrane protein by combining bioorthogonal chemistry and cysteine mutagenesis. eLife 2019;8:e50776. [CrossRef]
- Zhabotinsky AM. Periodical process of oxidation of malonic acid solution. Biofizika 1964;9:306-11.
- Tyson JJ. From the Belousov-Zhabotinsky reaction to biochemical clocks, traveling waves and cell cycle regulation. Biochem J 2022;479(2):185-206. [CrossRef]
- Razavi M, Saberi Fathi SM, Tuszynski JA. The effect of protein synthesis entropy reduction on cell size regulation and division size of unicellular organisms. Entropy (Basel) 2022;24(1):94. [CrossRef]
- Izato YI, Matsugi A, Koshi M, Miyake A. Computation of entropy values for non-electrolyte solute molecules in solution based on semi-empirical corrections to a polarized continuum model. Phys Chem Chem Phys 2023;25(11):8082-9. [CrossRef]
- Pincus SM, Padmanabhan V, Lemon W, Randolph J, Rees Midgley A. Follicle-stimulating hormone is secreted more irregularly than luteinizing hormone in both humans and sheep. J Clin Invest 1998;101(6):1318-24. [CrossRef]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature 1990;344:503-8 . [CrossRef]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 1971;177:129-45 . [CrossRef]
- Gerhart J, Wu M, Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol 1984;98:1247-55 . [CrossRef]
- Hu Z, Li L, Lan W, Wei X, Wen X, Wu P, et al. Enrichment of Wee1/CDC2 and NF-κB signaling pathway constituents mutually contributes to CDDP resistance in human osteosarcoma. Cancer Res Treat 2022;54(1):277-93. [CrossRef]
- Lee TK, Denny EM, Sanghvi JC, Gaston JE, Maynard ND, Hughey JJ, et al. A noisy paracrine signal determines cellular NF-kappaB response to lipopolysaccharide. Sci Signal 2009;2(93):ra65. [CrossRef]
- Karamched BR, Miles CE. Stochastic switching of delayed feedback suppresses oscillations in genetic regulatory systems. J R Soc Interface 2023;20(203):20230059. [CrossRef]
- Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol 2019;20(1):249. [CrossRef]
- Mendelsohn AR, Larrick JW. Epigenetic drift is a determinant of mammalian lifespan. Rejuvenation Res 2017;20(5):430-6. [CrossRef]
- Nielsen F. On the Jensen-Shannon symmetrization of distances relying on abstract means. Entropy (Basel) 2019;21(5):485. [CrossRef]
- Mao S, Su J, Wang L, Bo X, Li C, Chen H. A transcriptome-based single-cell biological age model and resource for tissue-specific aging measures. Genome Res 2023:gr.277491.122. [CrossRef]
- Rutledge J, Oh H, Wyss-Coray T. Measuring biological age using omics data. Nat Rev Genet 2022;23(12):715-27. [CrossRef]
- Aledo JC, Blanco JM. Aging is neither a failure nor an achievement of natural selection. Curr Aging Sci 2015;8(1):4-10.
- Aloia L. Epigenetic regulation of cell-fate changes that determine adult liver regeneration after injury. Front Cell Dev Biol 2021;9:643055. [CrossRef]
- Schächner C, Merkl PE, Pilsl M, Schwank K, Hergert K, Kruse S, et al. Establishment and maintenance of open ribosomal RNA gene chromatin states in eukaryotes. Methods Mol Biol 2022;2533:25-38. [CrossRef]
- Cheng QJ, Ohta S, Sheu KM, Spreafico R, Adelaja A, Taylor B, et al. NF-κB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science 2021;372(6548):1349-53. [CrossRef]
- Díaz-Hernández V, Montaño LM, Caldelas I, Marmolejo-Valencia A. A high-fat and high-carbohydrate diet promotes reminiscent hallmarks of an aging ovary in the rabbit model. Biomedicines 2022;10(12):3068. [CrossRef]
- Wong VC, Bass VL, Bullock ME, Chavali AK, Lee REC, Mothes W, et al. NF-κB-chromatin interactions drive diverse phenotypes by modulating transcriptional noise. Cell Rep 2018;22(3):585-99. [CrossRef]
- Bayram P, Karamese SA, Erol HS, Ozdemir B, Toktay E, Salum C. Protective effects of a natural product, paeoniflorin, on ischemia reperfusion injury on rat ovary tissue: Histopathological, immunohistochemical, and biochemical study. J Histotechnol 2023:1-14. [CrossRef]
- Chatterjee B, Roy P, Sarkar UA, Zhao M, Ratra Y, Singh A, et al. Immune differentiation regulator p100 tunes NF-κB responses to TNF. Front Immunol 2019;10:997. [CrossRef]
- Veldhuis JD, Anderson SM, Shah N, Bray M, Vick T, Gentili A, et al. Neurophysiological regulation and target-tissue impact of the pulsatile mode of growth hormone secretion in the human. Growth Horm IGF Res 2001;11 Suppl A:S25-37. [CrossRef]
- Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab 2001;86(2):551-60. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).