1. Introduction
Arterial hypertension is a well-known burden on global health care and the most significant risk factor leading to vascular disease [
1]. This systemic disease plays a critical role for around 8.5 million deaths yearly, caused by its negative effects, which manifest as stroke, ischemic heart disease, other vascular disease and renal disease [
2].
The PAMELA study demonstrated the value of ABPM (ambulatory blood pressure monitoring), as it provides nighttime values and better assessment of cardiovascular risk [
3]. Nighttime measurements give a complete picture of blood pressure (BP) profiles, dipping, and are the most important predictor of cardiovascular risk and long-term outcomes and therefore should be taken into account in terms of steering treatment [
4,
5,
6,
7]. Both home BP and ABPM are recommended to establish diagnosis and monitor treatment [
8]. Currently, ABPM has consistently proven to be the gold standard for the blood pressure measurement because of its many advantages and has even been shown to be superior than office and home blood pressure measurements [
9].
However, ABPM taken with standard cuff devices repeatedly inflate during sleep, are disruptive and could potentially influence the true representation of nighttime BP profiles [
10]. Not only is the disruption substantial to the patient’s compliance and willingness to repeat these tests in the future, but have been implicated in potential inaccurate measurements due to sympathetic arousal [
11]. Data shows that cuff inflations cause artefacts due to motor activity, cuff errors, arousals, arrhythmias and affect measurement outcomes [
10]. Cuff inflations have also been shown to specifically cause nighttime arousals, which are directly proportional to beat-to-beat elevations in BP [
11]. Historically, cuff based measurements have been used to look at long term cardiovascular outcomes and risk, however even this method only indirectly measures blood pressure through pulse wave detection and maximum volume changes which are prone to error [
12,
13].
Since 2015, a cuffless BP measurement device has been commercially available and in clinical use throughout Europe [
14]. This device uses, after an initial contralateral cuff-based calibration measurement, pulse transit time (PTT) to estimate BP via an algorithm patented by the manufacturer. This device has been validated according to the ESH International Protocol Revision 2010 for the Validation of Blood Pressure Measuring Devices in Adults (ESH IP 2010) [
15]. This non-inflating measurement technique could potentially benefit patients and clinicians alike. According to the manufacturers, this would enable acquisition of nighttime BP values without sympathetic arousal. The comfort of the device could improve compliance and allow physicians to more freely prescribe ABPM as needed [
16]. Nevertheless, previous analyses have shown that the cuffless BP measurement device measures higher nighttime BP values than the cuff based reference devices, and therefore need to be used with caution [
17,
18,
19].
The influence of the cuff on various nighttime measurements is unknown, and needs to be looked at further before trending towards cuffless devices. This is especially significant, as there may be an inherent bias in validation studies when comparing to cuff based measurements since the cuff inflations may lead to higher BP values when compared to measurements with only a cuffless device. The newly published 2023 ESH Guidelines for the management of arterial hypertension explicitly do not recommend cuffless devices for diagnosis or monitoring of BP [
20]. This stresses the point that in clinical practice and research, we need to better understand if cuff based devices are affecting BP measurements.
Therefore, the aim of this study was to gain further insight regarding the potential effect of cuff inflations on arousals, sleep efficiency, desaturations and mean nighttime BP.
2. Materials and Methods
2.1. Study Center
The study took place at the Hypertension Clinic of the Medical Outpatient Department of the University Hospital Basel, Basel Switzerland. This hypertension clinic is an approved ESH (European Society of Hypertension) Center of Excellence [
21].
2.2. Device Details and Familiarization
In April 2015, the non-invasive cuffless device was introduced at the Medical Outpatient and Hypertension Clinic at the University Hospital Basel. At the time of enrollment, our clinic had two years of experience with the correct application of this device with more than 500 measurements before the start of the study. Experienced cardiologists (TB, ASV), both ESH certified hypertension specialists, read all de-vice measurements.
The cuffless non-invasive system estimates BP based on the PTT technique, permits continuous beat-to-beat BP monitoring. This device consists of a finger photoplethysmograph, three ECG leads and a watch-like device with integrated actigraph. Transit time of a pulse wave from the corresponding ECG R-wave to the finger photoplethysmography signal is calculated [
18]. After a single-cuff-based calibration measurement on the opposite upper arm, systolic and diastolic BP levels are calculated using a non-linear model incorporating changes of the PTT and its relation to BP [
22]. Increased pulse wave propagation results in shorter PTT and is associated with higher BP and vice versa. Pulse wave propagation and thus PTT depends on arterial wall stiffness and tension, both of which vary according to BP differences [
23,
24]. An actigraph continuously measures activity or movement and is used to assess sleep disorders and generally, the time a patient is asleep.
In the current study, we analyzed BP, arousals, sleep efficiency, and desaturation. Asleep BP cut off values were adapted based on a previous publication from our research group, which listed J point values for detecting elevated BP using the cuffless device [
18]. The following cut-offs for the detection of mean asleep systolic and diastolic hypertension with the cuffless device was ≥ 136 mmHg and ≥ 83 mmHg respectively [
18]. Arousals, were defined as activity and position changes during the night. Sleep efficiency was defined as total sleep time (TST) divided by time in bed (TIB) taken from automatically generated reports [
25]. TIB was defined as documented by the patient in self-reported patient protocols. Oxygen desaturations were defined as number of desaturations during TIB with a minimum duration of 10 seconds and ≥4% oxygen desaturation, and were then indexed to time in bed [
26].
The standard cuff-based device used on the first day of measurements was Spacelabs 90217A (Spacelabs Healthcare, USA) 24-hour BP monitor [
18].
2.3. Study Design
As reported previously, enrolment into the Somnotouch-NIBP Compared to Standard Ambulatory 24-hours Blood Pressure Measurement, VAST Study (VAST= Validation Somnotouch, NCT03054688) took place at the Medical Outpatient and Hypertension Clinic at the University Hospital Basel between May and December 2017 [
17,
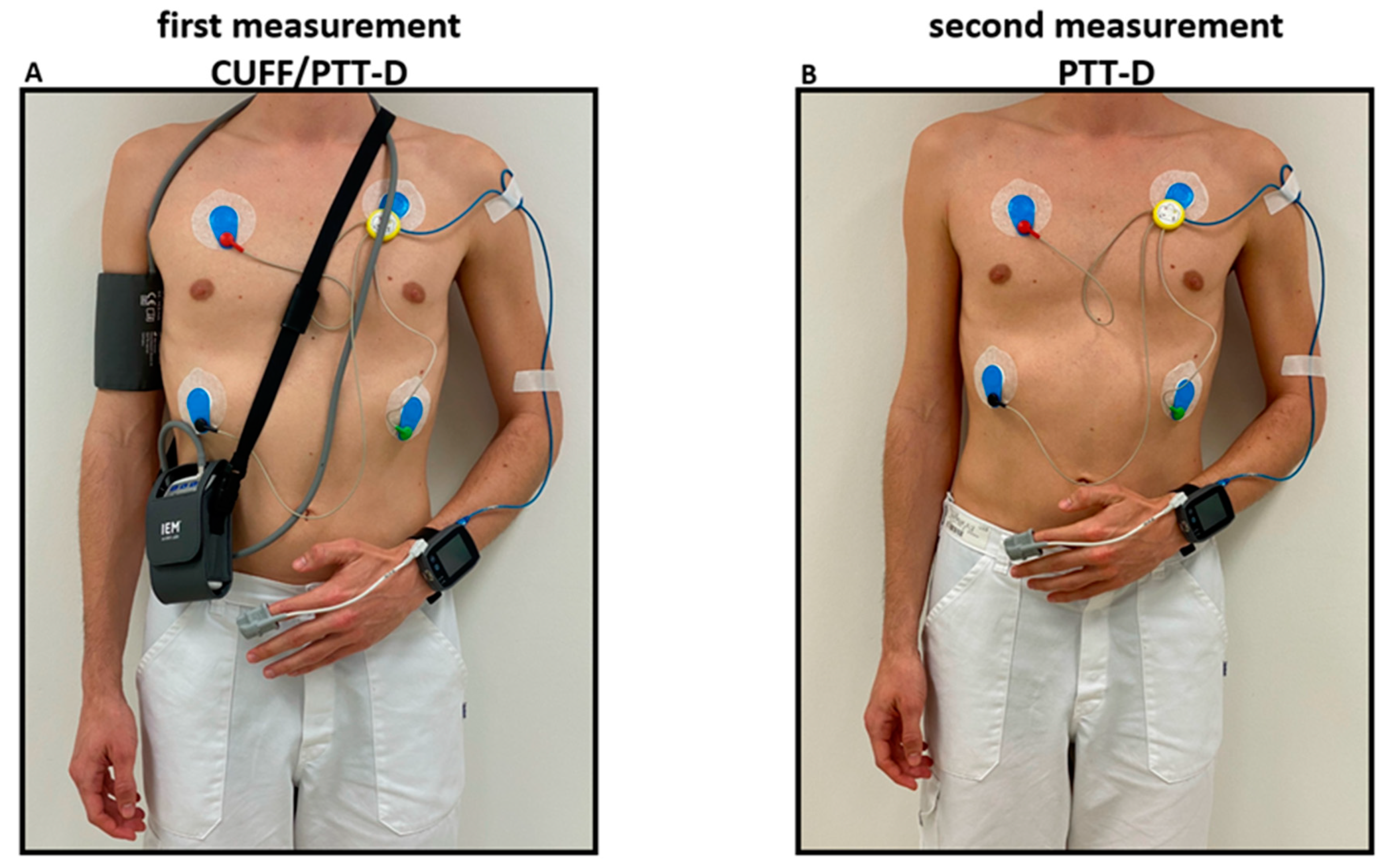
18]. Both devices were mounted on the participant, in a sitting upright position with legs uncrossed and back supported. An appropriately sized cuff was placed on the right arm and connected to the cuff based ambulatory BP measurement device. The cuffless device was placed on the left forearm and connected to the photoplethysmograph on the left index finger and the ECG electrodes according to manufacturer’s instructions (
Figure 1).
On the first day of measurement, a manually triggered cuff based measurement was taken with the Spacelabs 90217A after 5 min of rest. This measurement was used as a calibration measurement for the cuffless device [
27]. The cuff-based device was programmed for measurements every 20 minutes from 06:00 to 22:00 and every 30 minutes during the remaining period. Simultaneously, the cuffless device recorded beat-to-beat PTT according to the manufacturer’s standard programming. This part of the study is referred to as CUFF/PTT-D.
Participants of the VAST study were offered participation in the substudy, and were consecutively enrolled [
17]. Consent was given for a second 24h measurement with only the cuffless device within 4 days after the initial CUFF/PTT-D measurement. This substudy measurement is referred to as PTT-D. PTT-D consisted of only measurements taken with the cuffless device worn on the left arm and attached to the photoplethysmograph and ECG electrodes (
Figure 1). For the second measurement, the cuffless device was similarly calibrated with a validated Omron1300 device, as described above [
28]. Participants were given questionnaires to individually record their activities, sleep schedules, medications and biometrics.
2.4. Statistical Analysis
Distribution of continuous variables was determined using skewness, kurtosis and visual inspection of the histogram. The data were presented as medians (interquartile range) and means (±standard deviations). Comparisons were done using paired Wilcoxon Signed Rank Test. Categorical variables were described as counts (percentages) and compared using Fisher’s exact test.
Statistical analyses were performed using R Version 4.2.3 a p-value of <0.05 was pre-specified to indicate statistical significance [
29].
2.5. Ethical Approval and Trial Registration
The study protocol complies with the Declaration of Helsinki, was approved by the local ethics committee, Ethikkommission Nordwest- und Zentralschweiz (Ethics Commission Northwest and Central Switzerland), (EKNZ 2017-00323), registered (NCT 03054688) and externally monitored. Informed consent was obtained from all participants.
3. Results
3.1. Baseline Characteristics
Consecutive enrolment of 21 individuals took place from May to December 2017.
Table 1 shows baseline characteristics of the participants.
3.2. Blood Pressure Measurements
Table 2 outlines mean 24h, awake and asleep BP measurements on both days, with CUFF/PTT-D und PTT-D alone. CUFF/PTT-D had mean BP measurements of 134/83mmHg, 139/86mmHg, and 131/80mmHg, respectively. PTT-D had mean BP measurements of 136/88mmHg, 140/91mmHg, and 131/84mmHg respectively. PTT-D had higher means during all phases of BP measurement. However, there was no significant difference between the groups except for diastolic 24h (p=0.023) and diastolic awake (p=0.019) measurements.
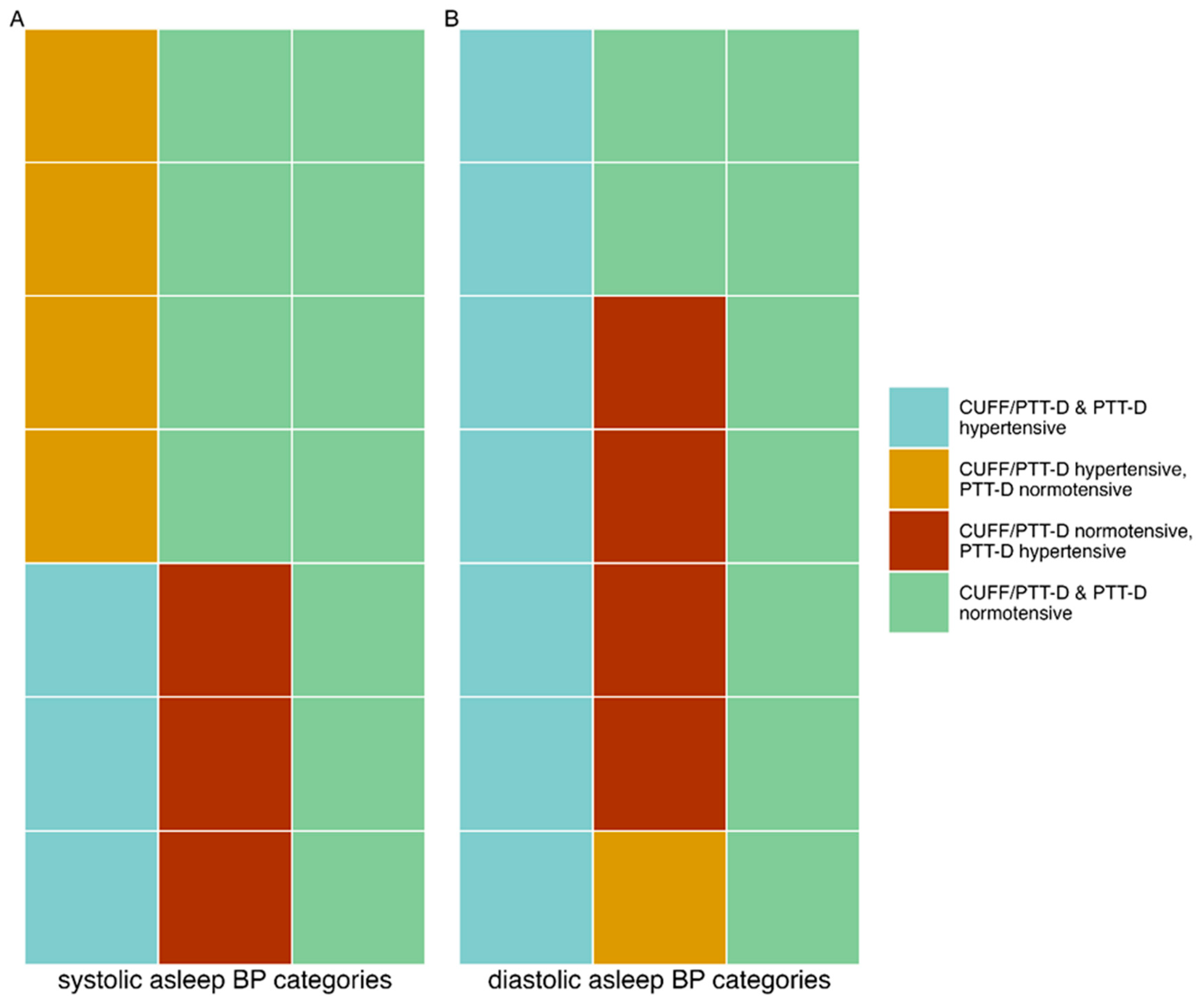
3.3. Asleep Hypertensive versus Normotensive Classification Differences between CUFF/PTT-D and PTT-D. Formatting of Mathematical Components
Asleep Systolic Hypertension:
14% (3) of patients had hypertensive values with CUFF/PTT-D and PTT-D. 19% (4) had hypertensive values with CUFF/PTT-D and normotensive values with PTT-D. 14% (3) had normotensive values with CUFF/PTT-D and hypertensive PTT-D values. Normotensive values were seen in 52% (11) with both the CUFF/PTT-D and PTT-D. 33% (7) had discrepancies with asleep systolic hypertensive categorizations (p=0.820) (see also
Figure 2, panel A).
Asleep Diastolic Hypertension:
14% (n=7) of patients had hypertensive values with CUFF/PTT-D and PTT-D. 5 % (n=1) were hypertensive with CUFF/PTT-D and normotensive with PTT-D. 19% (n=4) were normotensive with CUFF/PTT-D and hypertensive with PTT-D. 43 % (n=9) were normotensive in both measurements. 24% (n=5) had discrepancies with asleep diastolic hypertensive categorizations (p=0.310) (see also
Figure 2, panel B).
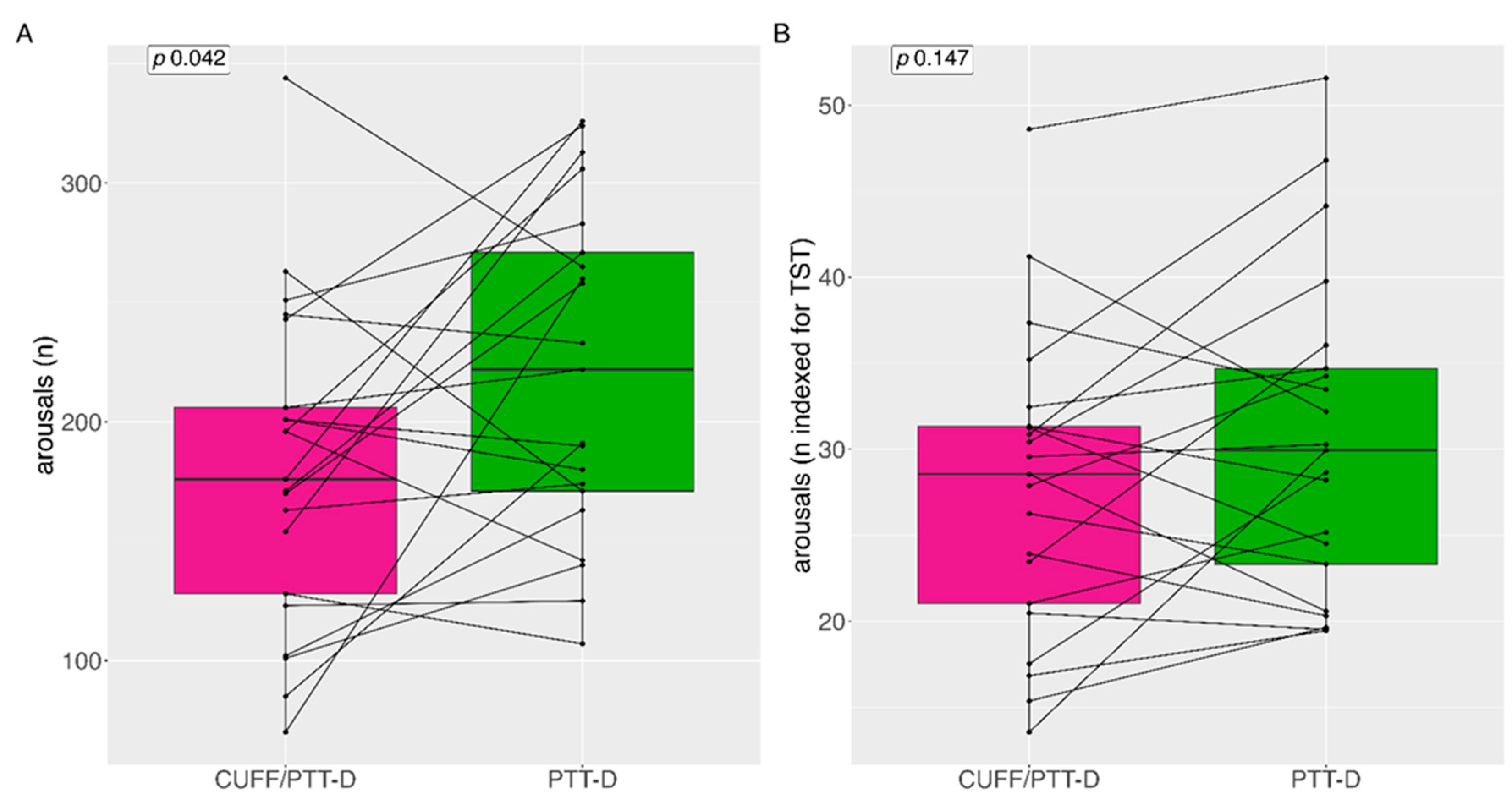
3.4. Arousal as a Potential effect of Cuff Based Measurements
Total median (IQR=interquartile range) number of arousals for CUFF/PTT-D and PTT-D at night were 222 (110) and 176 (99) respectively (p=0.042) (
Figure 3A). The number of CUFF/PTT-D and PTT-D total mean (SD) arousals at night were 221 (±67) and 180 (±65) respectively. Median arousals indexed during the total sleep time (TST) (in hours) were 29 (11) and 30 (13) for CUFF/PTT-D and PTT-D, respectively (p=0.147) (
Figure 3B).
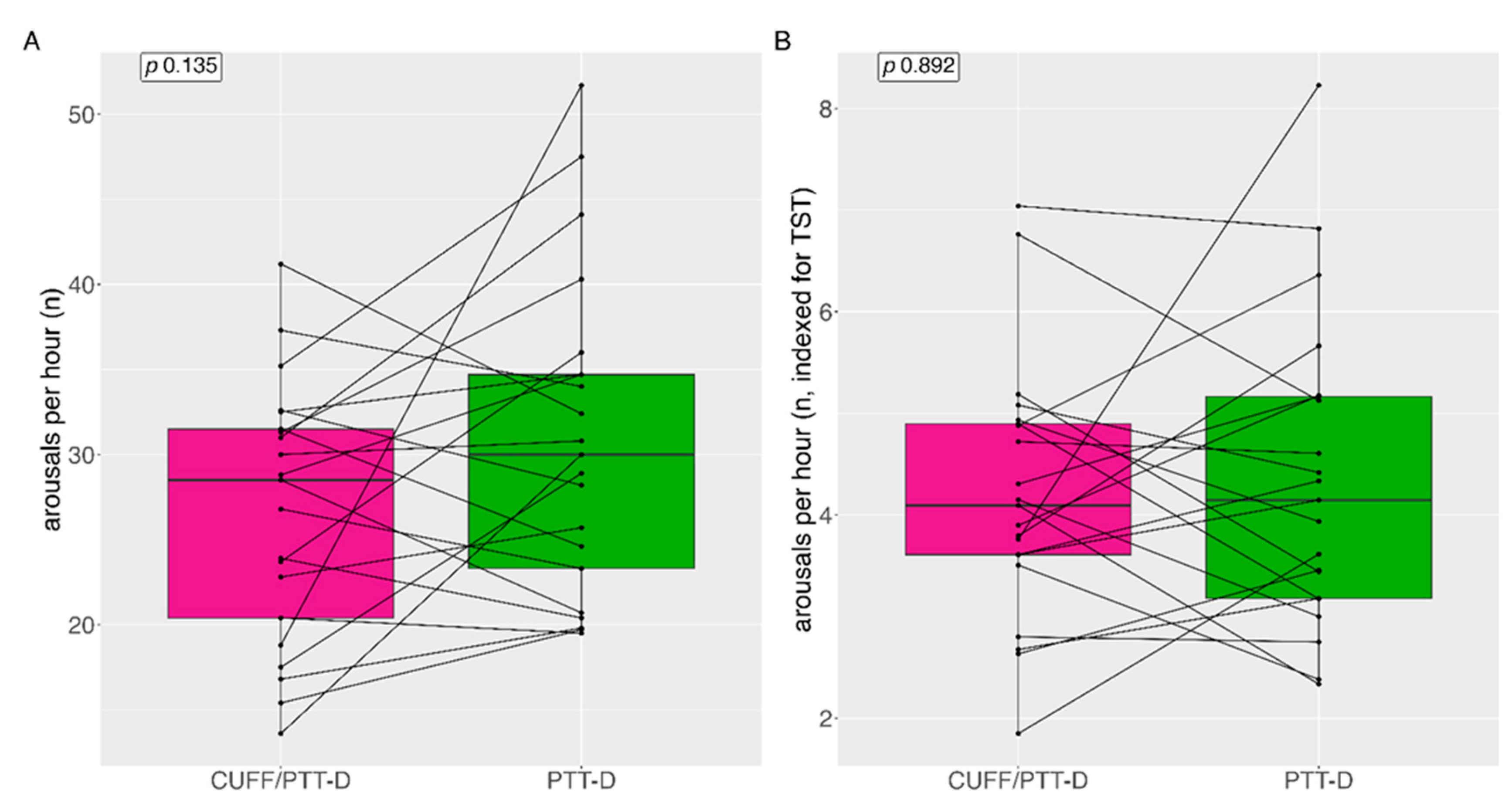
Median (IQR) arousals per hour with CUFF/PTT-D and PTT-D were 29 (12) and 30 (13) (p=0.135) respectively (
Figure 4A). Mean (SD) arousals per hour with CUFF/PTT-D and PTT-D were 27 (±7) and 31 (±9) (p=0.135) respectively. 14 patients (66%) showed increased arousals with PTT-D alone. (
Figure 4A).
Median (IQR) arousals per hour indexed to TST was 4 (1) and 4 (2) for CUFF/PTT-D and PTT-D respectively (p=0.892) (
Figure 4B).
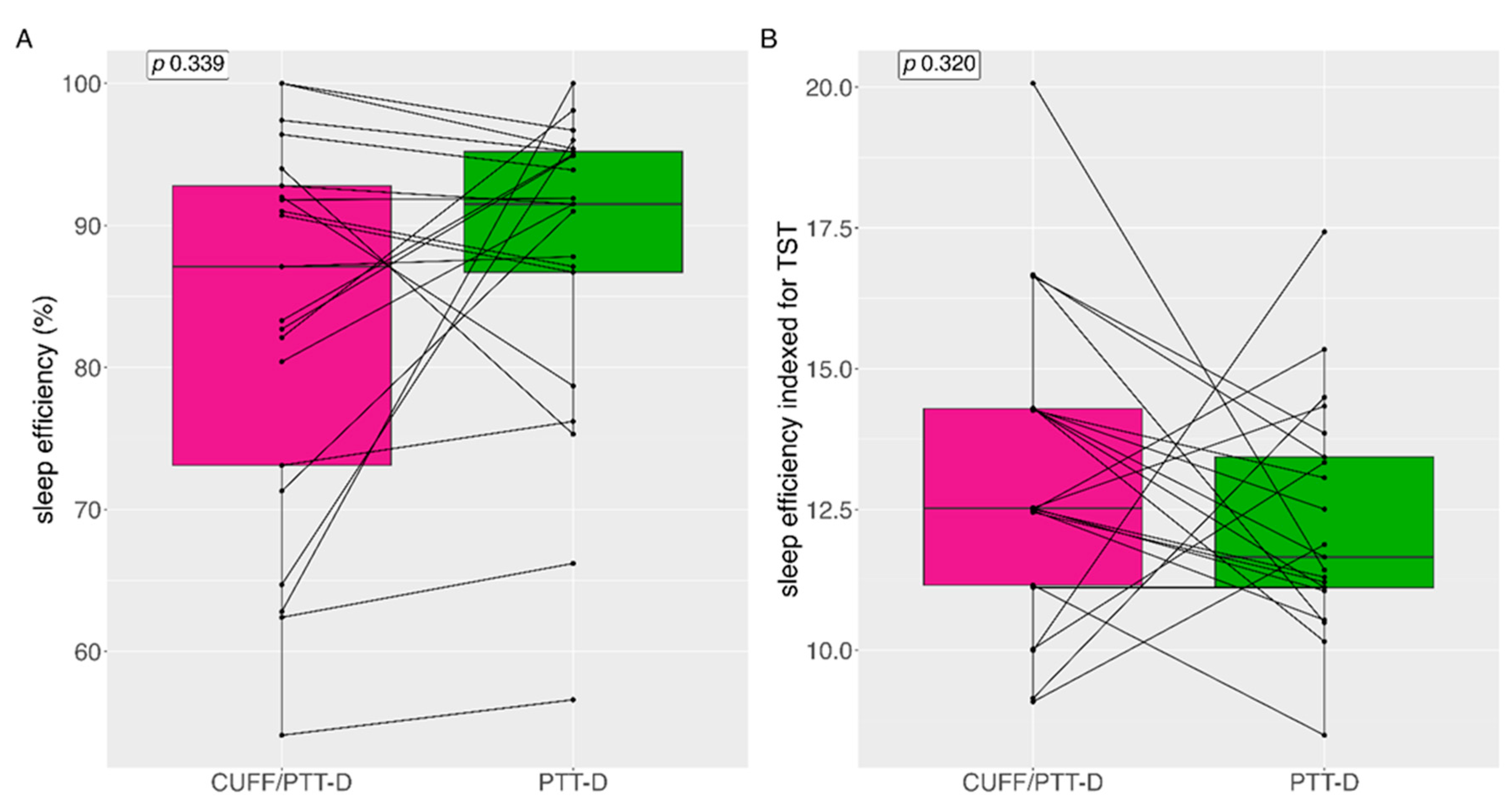
3.5. Sleep Efficiency
Median (IQR) sleep efficiency with CUFF/PTT-D and PTT-D was 87.1% (21) and 91.5% (12.6) (p=0.339) respectively (
Figure 5A). Mean (SD) sleep efficiency with CUFF/PTT-D and PTT-D was 83% (±13.6) 87.9% (±11) respectively. 9 (43%) patients showed reduced sleep efficiency with PTT-D alone (
Figure 5)
Median (IQR) sleep efficiency indexed during the total sleep time (TST) (in hours) was 12.5% (3.2) and 11.7% (2.6) for CUFF/PTT-D and PTT-D respectively (p=0.320) (
Figure 5B).
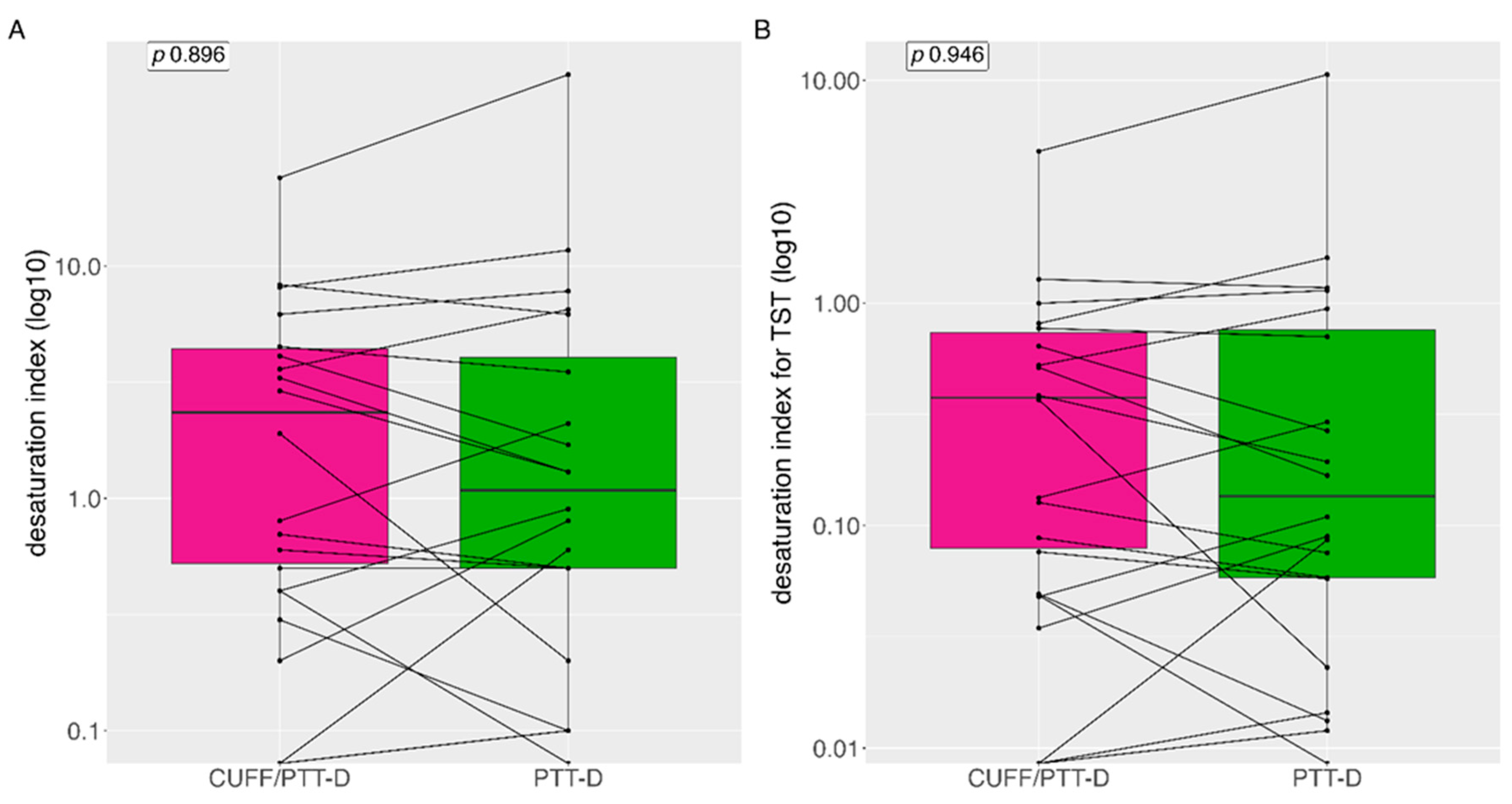
3.6. Desaturation Index
Median (IQR) desaturation index was 0.8 (3.95) and 0.9 (4.5) with CUFF/PTT-D and PTT-D respectively, no significant difference was seen between the groups (p=0.896) (
Figure 6A). Mean (SD) desaturation index was 3.4 (±5.4) and 5.4 (±14.4) with CUFF/PTT-D and PTT-D respectively. When indexed with TST median (IQR) desaturation index was 0.13 (0.65) and 11.65 (2.6) for CUFF/PTT-D and PTT-D respectively (p=0.95) (
Figure 6B).
3.7. Mean and Mean Differences of Sleep Parameters during the Two Measurement Days.
The mean (SD) differences regarding sleep paramters between the two measurement days are outlined in
Table 3. A significant difference in total sleep time was seen in with mean CUFF/PTT-D and PTT-D being 389 (±82) and 435 (±64), p= 0.048.
4. Discussion
To our knowledge, this is the first study that looks at the possible impact of cuff-based inflations on nighttime BP measurements. The cuff inflations seemed to have no impact on mean systolic BP, but a possible impact on mean diastolic BP. Of note, there was no significant difference regarding systolic and diastolic asleep values during CUFF/PTT-D and during PTT-D alone. We showed, however, that when classifying the two groups into hypertensive or normotensive, there were significantly more asleep systolic hypertensive classifications with simultaneous measurements of CUFF/PTT-D than with PTT-D alone. In this small cohort, we could demonstrate significantly more arousals during the PTT-D alone than during CUFF/PTT-D, however this difference was not statistically significant if indexed to the TST.
Increasing data shows that nocturnal measurements obtained by ABPM is a stronger predictor of cardiovascular outcomes than awake ambulatory measurements [
30]. However, convincing patients to undergo 24 hour ABPM is a challenge. Cuff based measurements and their inflations cannot be easily ignored, and are reported as being disruptive. Within hypertension circles, there are often debates regarding how cuff measurements influence sleep quality and thus nocturnal or asleep BP values [
31,
32]. In our cohort, we saw no unequivocal influence of cuff inflations on mean asleep cuffless device measurements. We concluded this by comparing the measurements from a cuffless device worn simultaneously with a cuff based device with the measurements from a cuffless device without simultaneous cuff based measurements.
In the past few decades, new ways to measure BP have become available and easily implementable. However, the question remains if we should continue to only use cuff based measurements or if other measurement modalities could reduce the potential influence of cuff inflations. New methods for measuring BP without repeated cuff-inflations are interesting for clinicians and patients alike. Techniques using PTT seem to be a promising step in the right direction because of their comfort and increased number of measurements during the testing period. On the other hand, data and recent publication have shown that the PTT devices are not directly comparable in terms of measuring blood pressure and result in higher BP values when validated against a gold standard cuff based 24h blood pressure measurement [
18,
19]. Additionally indirect vs direct measurement and beat to beat vs mean blood values are not interchangeable clinically because outcome studies have never used cuffless blood pressure measurement data [
3,
5,
20].
The results of the current analysis show that there are no significant differences between the nighttime BP values when both devices are worn together vs only one device. Mean asleep systolic blood pressure measurements were the same during both measurement days and diastolic values were higher on the day when only wearing the PTT-D. 33% had discrepancies in their classification of nocturnal systolic hypertension, and 25% nocturnal diastolic hypertension classification. The cuffless device cutoff for nocturnal diastolic hypertension was 83 mmHg, based on our previous publication, which was also our mean value during the second day of measurement, causing more reclassifications [
22]. This could have potential therapeutic consequences.
Interestingly, 66% of patients had more arousals when wearing only the cuffless device. This needs to be studied further. A possible explanation that they do not reach the sleep depth/stages for arousals to be detected by the cuffless device when wearing both devices. In addition, reported sleep time or time in bed was shorter during the first set of measurements when wearing both devices. This could mean that the absolute number of arousals is not the correct comparison. We therefore indexed the arousals to the TIB and per hour total sleep time. After this, the number of arousals was still higher in the CUFF/PTT-D phase; however, it did not reach statistical significance. Sleep efficiency, the ratio of TST/TIB, in general varies based on age. According to the National Sleep Foundation, ≥ 85% sleep efficiency is considered an indicator of good sleep quality, however many publication use the cutoff of ≥80% [
33,
34]. Although sleep efficiency was above 80% in both groups it was 88% during the PTT-D measurements compared to 83% during the CUFF/PTT-D phase, demonstrating that cuff inflations may have an impact on this sleep parameter.
According to the manufacturer’s compendium the cuffless device has the advantage of less sleep disturbances [
25]. We did not find this to be the case. Our data indicates that the presumption that a cuff based devices may cause arousals, sleep disturbances and eventually higher mean BP may be incorrect especially in terms of mean BP values.
5. Strengths and Limitations
The strength of this study was the measurement of BP over a 24h period on two closely spaced days. Once using two separate devices simultaneously to record measurements and once with only one device for comparison on the same person.
The main limitation of this analysis was the limited number of patients. Also, two different calibration devices were uses on the two days of measurements. In addition, there were possible discrepancies between the two days when the measurements were taken; especially in terms of activity, stress and other possible influences on blood pressure. We used mean asleep values, which although clinically important does not elucidate the effect of individual cuff inflations on corresponding BP measurements. Most importantly, although this analysis was restricted to nighttime readings, we know according to total sleep time that both asleep periods were significantly different. In contrast to other cuffbased vs cuffless comparison studies we did not have access to the additional Y-connection device to compare simultaneously measured cuffbased measurements beat-to-beat cuffless measurements [
10]. Therefore, we could only used mean values.
6. Conclusions
Cuff inflations did not seem to influence all of the analyzed nighttime sleep parameters and mean BP values, but might lead to reclassification of nighttime hypertension.
Author Contributions
Conceptualization: A.V. T.B. P.K. ; Methodology: T.B.,A.V.; Validation: T.B., A.V. ; Formal Analysis: T.S.,A.V. Resources: T.B., M.M.; Data Curation: T.S., P.K., A.V., A.M., T.B.; Writing: Original Draft Preparation, T.S.; Writing – Review & Editing, A.V., T.B., M.M..; Supervision T.B., A.V., M.M..; Project Administration, T.S., P.K., T.B. ; Funding Acquisition, T.B., M.M.
Funding
This research was fully funded by the Medical Outpatient and Hypertension Clinic, ESH Hypertension Centre of Excellence, University Hospital Basel, Basel, Switzerland. This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. Dr. Thenral Socrates received funding from the University of Basel Research Fund for Excellent Junior Researchers and from the Department of Internal Medicine, University Hospital Basel. PD Dr. Annina Vischer received funding from the Department of Internal Medicine, University Hospital Basel. Dr. Philipp Krisai reports speaker fees from BMS/Pfizer. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- F. D. Fuchs and P. K. Whelton, “High Blood Pressure and Cardiovascular Disease,” Hypertension, vol. 75, no. 2, pp. 285-292, Feb 2020. [CrossRef]
- “Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants,” (in eng), Lancet, vol. 398, no. 10304, pp. 957-980, Sep 11 2021. [CrossRef]
- G. Mancia et al., “Ambulatory blood pressure normality: results from the PAMELA study,” (in eng), J Hypertens, vol. 13, no. 12 Pt 1, pp. 1377-90, Dec 1995.
- M. Tadic, C. Cuspidi, G. Grassi, and G. Mancia, “Isolated Nocturnal Hypertension: What Do We Know and What Can We Do?,” Integr Blood Press Control, vol. 13, pp. 63-69, 2020. [CrossRef]
- D. L. Clement et al., “Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension,” N Engl J Med, vol. 348, no. 24, pp. 2407-15, Jun 12 2003. [CrossRef]
- E. Dolan et al., “Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study,” (in eng), Hypertension, vol. 46, no. 1, pp. 156-61, Jul 2005. [CrossRef]
- V. Gaborieau, N. Delarche, and P. Gosse, “Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage,” (in eng), J Hypertens, vol. 26, no. 10, pp. 1919-27, Oct 2008. [CrossRef]
- S. Vischer and T. Burkard, “Principles of Blood Pressure Measurement – Current Techniques, Office vs Ambulatory Blood Pressure Measurement,” in Hypertension: from basic research to clinical practice, M. S. Islam Ed. Cham: Springer International Publishing, 2017, pp. 85-96.
- T. J. Niiranen, J. Mäki, P. Puukka, H. Karanko, and A. M. Jula, “Office, home, and ambulatory blood pressures as predictors of cardiovascular risk,” (in eng), Hypertension, vol. 64, no. 2, pp. 281-6, Aug 2014. [CrossRef]
- T. L. Bothe, G. Bilo, G. Parati, R. Haberl, N. Pilz, and A. Patzak, “Impact of oscillometric measurement artefacts in ambulatory blood pressure monitoring on estimates of average blood pressure and of its variability: a pilot study,” (in eng), J Hypertens, vol. 41, no. 1, pp. 140-149, Jan 1 2023. [CrossRef]
- R. J. Davies, N. E. Jenkins, and J. R. Stradling, “Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep,” (in eng), Bmj, vol. 308, no. 6932, pp. 820-3, Mar 26 1994. [CrossRef]
- S. Lewington, R. Clarke, N. Qizilbash, R. Peto, and R. Collins, “Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies,” (in eng), Lancet, vol. 360, no. 9349, pp. 1903-13, Dec 14 2002. [CrossRef]
- P. S. Lewis et al., “Oscillometric measurement of blood pressure: a simplified explanation. A technical note on behalf of the British and Irish Hypertension Society,” Journal of Human Hypertension, vol. 33, no. 5, pp. 349-351, 2019/05/01 2019. [CrossRef]
- G. Bilo, C. Zorzi, J. E. O. Munera, C. Torlasco, V. Giuli, and G. Parati, “Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010,” Blood pressure monitoring, vol. 20, no. 5, p. 291, 2015. [CrossRef]
- E. O’Brien et al., “European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults,” (in eng), Blood Press Monit, vol. 15, no. 1, pp. 23-38, Feb 2010. [CrossRef]
- J. Gehring, H. Gesche, G. Drewniok, G. Küchler, and A. Patzak, “Nocturnal blood pressure fluctuations measured by using pulse transit time in patients with severe obstructive sleep apnea syndrome,” (in eng), Sleep Breath, vol. 22, no. 2, pp. 337-343, May 2018. [CrossRef]
- P. Krisai, A. S. Vischer, L. Kilian, A. Meienberg, M. Mayr, and T. Burkard, “Accuracy of 24-hour ambulatory blood pressure monitoring by a novel cuffless device in clinical practice,” (in eng), Heart, vol. 105, no. 5, pp. 399-405, Mar 2019. [CrossRef]
- T. Socrates, P. Krisai, A. S. Vischer, A. Meienberg, M. Mayr, and T. Burkard, “Improved agreement and diagnostic accuracy of a cuffless 24-h blood pressure measurement device in clinical practice,” (in eng), Sci Rep, vol. 11, no. 1, p. 1143, Jan 13 2021. [CrossRef]
- J. Nyvad, K. L. Christensen, N. H. Buus, and M. Reinhard, “The cuffless SOMNOtouch NIBP device shows poor agreement with a validated oscillometric device during 24-h ambulatory blood pressure monitoring,” (in eng), J Clin Hypertens (Greenwich), vol. 23, no. 1, pp. 61-70, Jan 2021. [CrossRef]
- G. Mancia Chairperson et al., “2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH),” (in eng), J Hypertens, Jun 21 2023. [CrossRef]
- E. S. O. Hypertension, “ESH Excellence Centers “ (in eng), PDF 2023. [Online]. Available: https://www.eshonline.org/esh-content/uploads/2018/02/ESH-EXCELLENCE-CENTRES-MAP-1.pdf.
- H. Gesche, D. Grosskurth, G. Küchler, and A. Patzak, “Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method,” (in eng), Eur J Appl Physiol, vol. 112, no. 1, pp. 309-15, Jan 2012. [CrossRef]
- E. L. Schiffrin, “Vascular stiffening and arterial compliance. Implications for systolic blood pressure,” (in eng), Am J Hypertens, vol. 17, no. 12 Pt 2, pp. 39s-48s, Dec 2004. [CrossRef]
- F. J. Callaghan, C. F. Babbs, J. D. Bourland, and L. A. Geddes, “The relationship between arterial pulse-wave velocity and pulse frequency at different pressures,” (in eng), J Med Eng Technol, vol. 8, no. 1, pp. 15-8, Jan-Feb 1984. [CrossRef]
- S. GmbH. Instruction Manuel SOMNOtouch™ NIBP. (2017). [Online]. Available: https://www.manualslib.com/manual/2079153/Somnomedics-Somnotouch-Nibp.html?page=7.
- D. Temirbekov, S. Güneş, Z. M. Yazıcı, and İ. Sayın, “The Ignored Parameter in the Diagnosis of Obstructive Sleep Apnea Syndrome: The Oxygen Desaturation Index,” (in eng), Turk Arch Otorhinolaryngol, vol. 56, no. 1, pp. 1-6, Mar 2018. [CrossRef]
- P. Baumgart and J. Kamp, “Accuracy of the SpaceLabs Medical 90217 ambulatory blood pressure monitor,” (in eng), Blood pressure monitoring, vol. 3, no. 5, pp. 303-307, 1998/10// 1998. [Online]. Available: http://europepmc.org/abstract/MED/10212370.
- H. Takahashi, T. Yokoi, and M. Yoshika, “Validation of the OMRON HBP-1300 upper arm blood pressure monitor, in oscillometry mode, for clinic use in a general population, according to the European Society of Hypertension International Protocol revision 2010,” Dublin: Dabl Educational Trust, 2014.
- R: A Language and Environment for Statistical Computing (2019). R Foundation for Statistical Computing, Vienna, Austria [Online]. Available: www.R-project.org.
- J. Boggia et al., “Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study,” (in eng), Lancet, vol. 370, no. 9594, pp. 1219-29, Oct 6 2007. [CrossRef]
- M. Middeke, “Effect of nocturnal blood pressure measurement on sleep and blood pressure during sleep,” (in eng), Z Kardiol, vol. 85 Suppl 3, pp. 99-105, 1996.
- J. E. Dimsdale, T. V. Coy, S. Ancoli-Israel, J. Clausen, and C. C. Berry, “The effect of blood pressure cuff inflation on sleep. A polysomnographic examination,” (in eng), Am J Hypertens, vol. 6, no. 10, pp. 888-91, Oct 1993. [CrossRef]
- S. Desjardins, S. Lapierre, C. Hudon, and A. Desgagné, “Factors involved in sleep efficiency: a population-based study of community-dwelling elderly persons,” (in eng), Sleep, vol. 42, no. 5, May 1 2019. [CrossRef]
- M. Ohayon et al., “National Sleep Foundation’s sleep quality recommendations: first report,” Sleep health, vol. 3, no. 1, pp. 6-19, 2017. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).