Submitted:
09 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mathematical formulation
3. , K and p
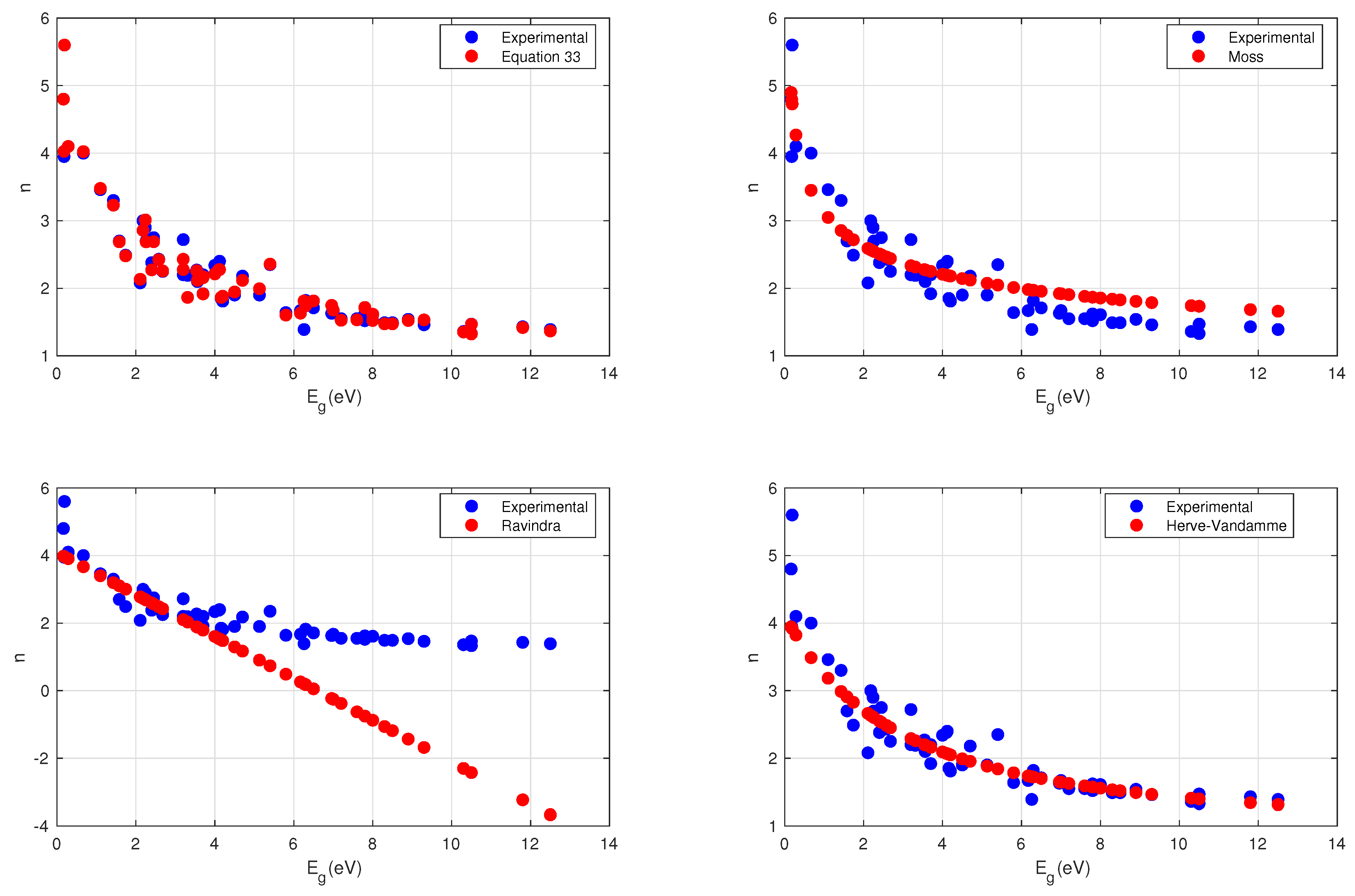
4. Results
4.1. Moss Relation
| : ; | : A; B | ||||||
|---|---|---|---|---|---|---|---|
| C | 5.4 | 10.9 | 49.7 | 3.32; 0.30 | 23.27; 5.5 | 166.91 | 2.35 |
| 1.1 | 4.0 | 44.4 | 4.08;0.70 | 13.32;2.9 | 161.06 | 3.46 | |
| 0.67 | 2.7 | 41.0 | 4.64;1.14 | 10.52;2.03 | 175.51 | 4.0 | |
| 1.43 | 3.55 | 33.5 | 4.18;0.98 | 10.90;2.12 | 155.76 | 3.3 | |
| 2.24 | 4.46 | 36.0 | 4.27;0.96 | 12.67;2.22 | 184.34 | 2.9 | |
| 0.18 | 2.3 | 35.0 | 4.19;0.99 | 8.97;2.12 | 47.34 | 3.95 | |
| 2.45 | 5.6 | 34.9 | 3.58;0.57 | 13.98;3.15 | 128.14 | 2.75 | |
| 2.18 | 4.7 | 33.7 | 3.90;0.77 | 12.58;2.52 | 145.52 | 3.0 | |
| 12.50 | 17.1 | 14.9 | 2.63;0.28 | 15.96;4.6 | 43.77 | 1.39 | |
| 10.5 | 15 | 11.3 | 2.42;0.27 | 13.01;4.5 | 32.28 | 1.33 | |
| 10.3 | 14.8 | 12.3 | 2.45;0.27 | 13.50;4.5 | 34.53 | 1.36 | |
| 8.9 | 10.3 | 13.6 | 4.13;1.47 | 11.83;1.40 | 47.92 | 1.54 | |
| 8.5 | 10.5 | 12.3 | 3.38;0.84 | 11.96;2 | 40.08 | 1.49 | |
| 8.3 | 10.4 | 12.2 | 3.28;0.78 | 11.26;2.1 | 39.19 | 1.49 | |
| 8 | 10.6 | 14 | 3.97;0.59 | 12.18;2.60 | 43.09 | 1.61 | |
| 7.2 | 9.1 | 12.1 | 3.34;0.88 | 10.54;1.90 | 39.08 | 1.55 | |
| 6.17 | 7.7 | 12.8 | 3.66;1.19 | 9.92;1.53 | 43.73 | 1.67 | |
| 5.8 | 7.7 | 12.1 | 3.23;0.85 | 9.65;1.90 | 38.35 | 1.64 | |
| 8.0 | 10.6 | 17.1 | 3.26;0.63 | 13.46;2.6 | 54.63 | 1.61 | |
| 7.0 | 9.4 | 17 | 3.32;0.69 | 12.64;2.40 | 55.21 | 1.67 | |
| 6.3 | 7.5 | 15.2 | 4.35;1.81 | 10.67;1.20 | 57.71 | 1.82 | |
| 2.11 | 5.8 | 20.6 | 2.67;0.36 | 10.93;3.69 | 43.71 | 2.08 | |
| 2.68 | 5.3 | 21.7 | 3.21;0.61 | 10.72;2.62 | 69.55 | 2.25 | |
| 11.8 | 15.7 | 15.9 | 2.84;0.36 | 15.80;3.90 | 47.80 | 1.43 | |
| 10.5 | 13.8 | 15.9 | 3.0;0.45 | 14.81;3.3 | 48.63 | 1.47 | |
| 5.13 | 7.4 | 22 | 3.6;0.80 | 12.76;2.27 | 80.97 | 1.9 | |
| 3.31 | 7.3 | 18.1 | 2.52;0.31 | 11.49;3.99 | 40.07 | 2.19 | |
| 3.7 | 6.4 | 17.1 | 2.95;0.55 | 10.46;2.70 | 49.88 | 1.92 | |
| 2.4 | 4.9 | 20.4 | 3.18;0.64 | 9.99;2.5 | 63.98 | 2.38 | |
| 1.74 | 4.0 | 20.6 | 3.29;0.73 | 9.08;2.26 | 65.81 | 2.49 | |
| 3.54 | 6.15 | 25.2 | 3.46;0.66 | 12.45;2.61 | 91.98 | 2.27 | |
| 7.8 | 11.3 | 22.0 | 3.08;0.44 | 15.77;3.5 | 67.74 | 1.62 | |
| 6.26 | 9.9 | 22.6 | 2.98;0.41 | 14.96;3.64 | 67.46 | 1.39 | |
| 6.96 | 13.4 | 27.5 | 2.52;0.195 | 19.19;6.44 | 64.84 | 1.63 | |
| 6.5 | 11.1 | 25.4 | 2.82;0.31 | 16.80;4.60 | 70.28 | 1.71 | |
| 3.7 | 6.24 | 23.2 | 3.40;0.67 | 12.03;2.54 | 82.36 | 2.2 | |
| 4.10 | 5.68 | 23.7 | 4.31;1.36 | 11.60;1.58 | 109.70 | 2.38 | |
| 4.12 | 5.57 | 23.3 | 4.46;1.54 | 11.40;1.45 | 110.68 | 2.4 | |
| 3.70 | 6.50 | 23.7 | 3.28;0.58 | 12.41;2.8 | 79.87 | 2.2 | |
| 4.7 | 7.49 | 26.1 | 3.47;0.62 | 13.98;2.80 | 94.53 | 2.18 | |
| 4.0 | 6.65 | 25.9 | 3.50;0.66 | 13.12;2.65 | 95.83 | 2.34 | |
| 3.2 | 5.24 | 25.7 | 3.89;0.95 | 11.60;2.04 | 111.56 | 2.72 | |
| 7.80 | 12.1 | 23.3 | 2.87;0.33 | 16.80;4.3 | 66.76 | 1.52 | |
| 4.20 | 9.15 | 23.3 | 2.56;0.25 | 14.60;4.95 | 52.82 | 1.81 | |
| 3.56 | 7.46 | 26.0 | 2.93;0.37 | 13.93;3.9 | 71.62 | 2.1 | |
| 4.50 | 8.26 | 23.0 | 2.88;0.38 | 13.78;3.76 | 64.45 | 1.90 | |
| 3.2 | 5.40 | 22.60 | 3.56;0.81 | 11.04;2.2 | 86.03 | 2.2 | |
| 4.16 | 8.60 | 21.30 | 2.60;0.29 | 13.53;4.44 | 50.28 | 1.85 | |
| 9.3 | 13.6 | 18.3 | 2.72;0.32 | 15.77;4.30 | 51.16 | 1.46 | |
| 3.54 | 6.36 | 26.1 | 3.39;0.60 | 12.88;2.82 | 92.21 | 2.27 | |
| 2.58 | 5.54 | 27.0 | 3.31;0.56 | 12.23;2.96 | 89.0 | 2.43 | |
| 2.26 | 4.34 | 27.0 | 3.88;0.93 | 10.82;2.08 | 117.85 | 2.70 | |
| 1.58 | 4.13 | 25.7 | 3.42;0.67 | 10.30;2.55 | 82.42 | 2.7 | |
| 0.286 | 3.5 | 55.33 | 4.28;0.66 | 13.91;3.21 | 80.55 | 4.1 | |
| 0.165 | 3.0 | 66.12 | 4.94;0.87 | 14.08;2.83 | 87.58 | 4.8 | |
| 0.190 | 2.2 | 66.80 | 5.86;1.46 | 12.12;2.01 | 186.90 | 5.6 |
4.2. Ravindra Relation
4.3. Herve-Vandamme Relation
5. Discussion
5.1. Empirical fitting constants
5.2. Convergence criterion
5.3. Exceptional materials
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ou, Q.; Bao, X.; Zhang, Y.; Shao, H.; Xing, G.; Li, X.; Shao, L.; Bao, Q. Band structure engineering in metal halide perovskite nanostructures for optoelectronic applications. Nano Materials Science 2019, 1, 268–287. [Google Scholar] [CrossRef]
- Geng, T.; Ma, Z.; Chen, Y.; Cao, Y.; Lv, P.; Li, N.; Xiao, G. Bandgap engineering in two-dimensional halide perovskite Cs 3 Sb 2 I 9 nanocrystals under pressure. Nanoscale 2020, 12, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Kuo, C.C.; Jhuang, L.S.; Chen, P.H.; Lai, Y.F.; Chen, F.C. Bandgap engineering enhances the performance of mixed-cation perovskite materials for indoor photovoltaic applications. Advanced Energy Materials 2019, 9, 1901863. [Google Scholar] [CrossRef]
- Nenkov, M.R.; Pencheva, T.G. Determination of thin film refractive index and thickness by means of film phase thickness. Central European Journal of Physics 2008, 6, 332–343. [Google Scholar] [CrossRef]
- Ono, M.; Aoyama, S.; Fujinami, M.; Ito, S. Significant suppression of Rayleigh scattering loss in silica glass formed by the compression of its melted phase. Optics Express 2018, 26, 7942–7948. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.; Dai, J.; Li, A.; Du, G.; Chang, R.; Ho, S. Effect of a microstructure on the formation of self-assembled laser cavities in polycrystalline ZnO. Journal of Applied Physics 2001, 90, 1663–1665. [Google Scholar] [CrossRef]
- Moss, T.S. A Relationship between the Refractive Index and the Infra-Red Threshold of Sensitivity for Photoconductors. Proceedings of the Physical Society. Section B 1950, 63, 167. [Google Scholar] [CrossRef]
- Moss, T.S. Photoconductivity in the Elements. Proceedings of the Physical Society. Section A 1951, 64, 590. [Google Scholar] [CrossRef]
- Ravindra, N.; Srivastava, V. Variation of refractive index with energy gap in semiconductors. Infrared Physics 1979, 19, 603–604. [Google Scholar] [CrossRef]
- Reddy, R.; Nazeer Ahammed, Y. A study on the Moss relation. Infrared Physics & Technology 1995, 36, 825–830. [Google Scholar] [CrossRef]
- Ravindra, N.; Auluck, S.; Srivastava, V. On the Penn Gap in Semiconductors. physica status solidi (b) 1979, 93, K155–K160. [Google Scholar] [CrossRef]
- Gupta, V.; Ravindra, N. Comments on the Moss Formula. physica status solidi (b) 1980, 100, 715–719. [Google Scholar] [CrossRef]
- Penn, D.R. Wave-Number-Dependent Dielectric Function of Semiconductors. Phys. Rev. 1962, 128, 2093–2097. [Google Scholar] [CrossRef]
- Wemple, S.; DiDomenico Jr, M. Behavior of the electronic dielectric constant in covalent and ionic materials. Physical Review B 1971, 3, 1338. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico, M. Optical Dispersion and the Structure of Solids. Phys. Rev. Lett. 1969, 23, 1156–1160. [Google Scholar] [CrossRef]
- Hervé, P.; Vandamme, L. General relation between refractive index and energy gap in semiconductors. Infrared physics & technology 1994, 35, 609–615. [Google Scholar]
- Finkenrath, H. The Moss rule and the influence of doping on the optical dielectric constant of semiconductors—I. Infrared Physics 1988, 28, 327–332. [Google Scholar] [CrossRef]
- Sellmeier. Zur Erklärung der abnormen Farbenfolge im Spectrum einiger Substanzen. Annalen der Physik, 219, 272–282.
- Levi, A.F.J. The Lorentz oscillator model. In Essential Classical Mechanics for Device Physics; 2053-2571, Morgan & Claypool Publishers, 2016; pp. 5–1 to 5–21. [CrossRef]
- Gomaa, H.M.; Yahia, I.; Zahran, H. Correlation between the static refractive index and the optical bandgap: Review and new empirical approach. Physica B: Condensed Matter 2021, 620, 413246. [Google Scholar] [CrossRef]
- Moss, T.S. Relations between the Refractive Index and Energy Gap of Semiconductors. physica status solidi (b) 1985, 131, 415–427. [Google Scholar] [CrossRef]
- Ravindra, N.; Ganapathy, P.; Choi, J. Energy gap–refractive index relations in semiconductors – An overview. Infrared Physics & Technology 2007, 50, 21–29. [Google Scholar] [CrossRef]
| (n) | 33 (n) | |||||
|---|---|---|---|---|---|---|
| 0.67 | 3.45 | 3.66 | 3.48 | 4.02 | 4.0 | |
| 0.18 | 4.79 | 3.97 | 3.93 | 4.02 | 3.95 | |
| 0.286 | 4.27 | 3.90 | 3.94 | 4.09 | 4.1 | |
| 0.165 | 4.89 | 3.98 | 3.94 | 4.79 | 4.8 | |
| 0.190 | 4.73 | 3.96 | 3.92 | 5.51 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




