1. Introduction
Presently, at least 190 countries commercially produce organic food which is due to the fast-expanding organic agriculture industry. Almost every nation in the world has started organic farming, and its proportion with respect to farms having commercial farming is increasing day by day [
1]. Mentha (Mentha arvensis), a well-known essential oil crop having commercial importance in the food, fragrance, and pharmaceutical industries is a perennial herb of the Lamiaceae family [
2]. India is the world's top producer and exporter of mentha oil and its by-products. Brazil and China were the first to cultivate mentha. India recently overtook other countries as the world leader in the cultivation of this essential oil-yielding plant. In India, Uttar Pradesh provides almost 90% of total mentha production, with the remaining 10% coming from Punjab and Rajasthan [
3]. However, in Punjab state, it is grown on a nearly 15,000-hectare area of land [
4]. India exports between 25,000 and 30,000 tonnes of menthol in a variety of forms (arvensis oil, dementholized mint oil, menthol crystals and powder, etc.) which along with other by-products of mentha contributes roughly to 80% of the global supply in terms of oil and by-products of mentha followed by China and Japan contributing about 10% to the global market. Intense care during the plant's growth and development is essential for the successful production of mentha plants [
5]. Depending on the agro-climatic conditions, soil properties, and nutrient availability result in the difference in the oil and herb yield of mentha plants [
6].
The majority of small and marginal mentha growers in the Indo-Gangetic plains of north India have discovered that transplanting mentha arvensis in the months of March and April using nursery-raised plants is very successful. But in today’s global agriculture production, chemical fertilizers are now widely used to increase agricultural productivity which has ultimately drained the soil in respect of nutrients which have caused a huge loss of soil productivity. On the other hand, the long-term use of organic fertilizers leads the way to restore the macro and micro-nutrients for better soil health and production of crops. Organic manures help in recycling the organic crop wastes and straw residue available in the field. To such a high advantage for farmers and to cope with the nutritional needs of the crop, the use of organic manures viz., farmyard manure (FYM), poultry manure, vermicompost, biofertilizers, neem cake, etc., has become essential in the cultivation of field crops especially medicinal and aromatic plants as these crops have huge potential in organic farming. The composition of organic fertilizers, cultivar type, agronomic practices, and environmental factors also influence the chemical composition of aromatic oils in organic farming [
7].
There are numerous eco-friendly pesticides available in the market which are prepared from mentha oil. This essential oil also has a wide range of industrial uses, including the use in cosmetic and therapeutic products. Therefore, Organic farming must be promoted in order to raise the mentha plant to boost the manufacturing of environmentally favourable by-products of Japanese mentha. Organic fertilizers have an enormous potential to supply nutrients and can help lessen the over-dependence on chemical fertilizers. The use of organic manure has been linked to increasing the soil qualities like increased plant-water holding capacity, cation exchange capacity, and lowered bulk density, and also it can promote healthy microbial activity in soil [
8]. Improved soil aggregation and pH stabilization are two advantages of adding well-decomposed organic manures to the soil [
9]. Therefore, organic farming helps to preserve the soil's health and is a crucial component of sustainable farming.
Keeping the views of the above aspects, the present research work was, therefore, undertaken to find out the response of organic nutrient management on the vegetative, yield parameters, and soil characteristics of Japanese mentha.
2. Materials and Methods
The study was conducted at the School of Organic Farming, Punjab Agricultural University, Ludhiana, Punjab, India from 2020 to 2022. The experiment was carried out in Randomized Complete Block Design with four replications (
Table 1) and eight treatments with different sources of organic manures/chemical fertilizers, on Typic Ustochrept soil of Research Farm of Punjab Agricultural University, Ludhiana which is situated at 30ᵒ56ʹ N latitude and 75◦52ʹ E longitude with a mean elevation of 247 m above mean sea level.
The experimental site is located in Trans-Gangetic Plains Region, which has scorching summer, cool winter, and high rainfall during the monsoon season. The experimental site experiences 705 millimeters of annual rainfall on average, with 80% of that falling during the monsoon season (June to September). Before sowing the experiment, a soil sample was analyzed for different physical and chemical properties of the soil (
Table 2). The physicochemical properties of the experimental site's soil are presented in
Table 2.
The mentha suckers are laid end to end, 4-5 cm deep in furrows, 45 cm apart, and are then covered with soil by planking lightly (
Table 3).
The gross plot size with dimensions of 3.0 m × 2.7 m having six rows is 8.1 m2. The plot was harvested leaving one border row. In the present study, a bio-fertilizer was used for evaluating the performance of the mentha plant. The recommended fertilizer has been added in the form of urea and single superphosphate. The organic source in the present study was farmyard manure from an organic farm. The bio-fertilizer contains bacillus, pseudomonas, and azotobacter. It is available for sale to the farmers by the Department of Microbiology, Punjab Agricultural University, Ludhiana, Punjab.
2.1. Biometrical studies
2.1.1. Emergence count
Two random spots of one-meter row length were selected in each plot and marked for taking an observation of emergence count. The number of emerged plants at both spots was counted at 10, 15, and 20 days after planting during both experimental years (during three successive cropping seasons). The mean of observations from both randomly selected spots was expressed as the number of plants per square meter.
2.1.2. The number of stools
Two random spots of one-metre row length were selected in each plot and marked for recording the number of stools. The number of stools was counted at 30 days intervals starting from 60 days after planting till harvesting during both experimental years (during three successive cropping seasons) and the mean was expressed as the number of stools per square meter.
2.1.3. Plant height
Five random plants were tagged in each plot excluding border rows to record the plant height. The height of the main shoot was measured starting from ground level up to the tip of the uppermost leaf at 30 days intervals starting from 60 days after planting till harvesting during the years of study (during three successive cropping seasons).
2.1.4. Dry Matter Accumulation (DMA)
Samples meant for DMA were taken by harvesting above ground plant portion of 50 cm row length in each plot at 30 days intervals starting from 60 days after planting till harvesting of the crop. Collected samples were sundried followed by oven drying at 60 ºC temperature till constant weight was obtained. Dry matter accumulation was expressed in terms of g plant-1.
2.1.5. Leaf Area Index (LAI)
The leaf area index was recorded at 30 days interval starting from 60 days after planting till harvesting with the help of a Sun scan canopy analyzer model named “Sun Scan Probe type SS1 by Delta-T devices USA” from each plot of the experimental study.
2.1.6. Leaf-to-stem ratio
A sample of 200 g herbage was collected from each plot at 30 days intervals starting from 60 days after planting of mentha suckers till harvesting. The leaves and stems of the above-collected samples were separated manually. Then these samples were sun-dried followed by oven drying at 60 ºC temperature till constant weight is obtained. The leaf-to-stem ratio on a dry weight basis was calculated as given below:
2.1.7. Fresh herb yield
The Crop harvesting was done manually with a sickle at about 5 cm above the ground level. After harvesting the net plot area (after the remaining two border lines of the mentha suckers), the fresh herbage was immediately weighed and expressed in quintals per hectare.
2.1.8. Dry herb yield
The plot was harvested leaving the border rows. The net area for harvesting was 3.0 m x 2.7 m =5.4 m2. A sample of 500 g fresh herb from each plot was taken for determining moisture content then sundried followed by oven drying at 60 ºC temperature till constant weight was obtained. Then dry herb yield was also expressed in quintals per hectare.
2.2. Laboratory studies
2.2.1. Oil content
A sample of 500 g fresh herb from each plot was taken after harvesting and then essential oil was extracted with Clevenger's apparatus. Oil content was worked out on a v/w basis from the fresh weight of the herb and expressed as a percentage. The oil samples were dried using anhydrous sodium sulphate and stored in sealed glass vials at 5 ºC prior to the analysis physicochemical properties of the oil.
2.2.2. Oil yield
It was calculated from essential oil content by multiplying it with fresh herb yield at harvest respectively in each plot. It was expressed as l ha-1.
2.3. Available and uptake N, P, and K in the soil
Soil samples were taken at a depth of 0 to 15 cm from each plot. The samples of the soil were air-dried, processed, and kept in polythene bags for further analysis. The amount of organic carbon [
10], available N [
11], available P [
12], and available K [
13] in the soil samples was examined.
2.3.1. Available Nitrogen
Five grams of soil were weighed and added to the micro-Kjeldahl distillation assembly's distillation flask. A 25 ml of 32% KMnO4 solution was added to this, and the flask was placed in its proper position in the assembly. 10 ml of N/50 H2SO4 and two drops of methyl red indicator were added to another 150 ml conical flask. This conical flask was then put beneath the distillation apparatus' delivery tube, such that the delivery tube was thoroughly immersed in the contents of the conical flask. Then, through the inlet placed in the distillation tube, 25 ml of 2.5% NaOH solution was injected into the distillation flask (containing soil and KMnO4), and the inlet was immediately closed with the help of a stopcock. The distillation process started, and 30 ml of distillate was obtained. This distillate was then titrated with N/50 NaOH solution, and the quantity of available N in the given soil was estimated using the volume of NaOH needed to achieve the endpoint (pink to yellow) as follows:
Calculations
Weight of soil taken = 5.0 g
The volume of N/50 H2SO4 taken = 10 ml
The volume of N/50 NaOH used in titration = X ml
The volume of N/50 H2SO4 used for the absorption of ammonia = (10-X) ml
One ml of N/50 H2SO4 = 0.00028 g of N
Percent (%) available nitrogen in the sample = (10-X) x 0.00028 x 100/5 = A Nitrogen (kg ha-1) = A x 22,400
2.3.2. Soil Available phosphorus
A 0.05 M NaHCO3 extractant method was used to extract the available phosphorus in the soil. A pinch of Darco G 60 and 20 ml of 0.5 M NaHCO3 was added to 1 g of soil in a 150 ml conical flask. The suspension was filtered using Whatman No. 1 filter paper after being shaken for half an hour on an electric shaker. Five ml of extract were pipetted into a 25 ml volumetric flask. The flask was shaken for a while after adding 0.5 ml of 5 N H2SO4 to stop CO2 evolution. Four ml of ascorbic acid was added to this flask, and the final volume was prepared using distilled water. The flask was kept for half an hour, and the colour intensity developed was measured with a colorimeter at 760 nm.
Standard curve: Standard curve was prepared using a 5 ppm phosphate standard solution. In volumetric flasks with a capacity of 25 ml, different amounts of standard solution (0, 0.2, 0.4, 0.6, 0.8, and 1.0 ml) were taken. After adding 5 ml of the extract (0.05 M NaHCO3) and 0.5 ml of 0.5 N H2SO4 to each flask, shaking was done for a while until CO2 evolution stopped, and then the colour was developed in the same way as the samples.
Calculations:
Weight of soil taken = 1.0 g Volume of extractant added = 20 ml First dilution = 20 times
The volume of extractant taken for color development = 5 ml
Final volume made = 25 ml
Second dilution = 5 times
Total dilution = 20 x 5 = 100 times
% transmittance as read from colorimeter = T
ppm of P as read from standard curve against % T = A ppm of P in the given soil = A x 100 = B
Phosphorus (kg ha-1) in the given soil = B x 2.24
2.3.3. Soil Available phosphorus
The sum of exchangeable and water-soluble potassium is the most widely accepted method for determining potassium availability. The extractant used in this experiment is a neutral ammonium acetate solution. A 5 g soil and 25 ml of neutral ammonium acetate solution were added to a 150 ml conical flask and shaken for 5 minutes, the flask was shaken. Whatman No. 1 filter paper was used to filter the suspension. 5 ml of extract was placed in a 25 ml volumetric flask, and the final volume was made with distilled water. The readings indicated by the galvanometer needle were recorded after this solution was fed into the atomizer of the flame photometer (in which 100 of the galvanometer was calibrated by feeding a 10 ppm solution of K to the atomizer). The quantity of available potassium in the soil samples was then estimated using a standard curve.
Standard curve: The standard curve was prepared with a 10 ppm potassium (K) solution diluted to 100 ppm. For this, different amounts of 100 ppm K solution (0, 0.2, 0.4, 0.6, 0.8, and 1.0 ml) were placed in 25 ml volumetric flasks. Each flask received 5 ml of neutral normal ammonium acetate solution, followed by distilled water to make the final volume. The atomizer was supplied with these varied concentrations of solutions, and the readings were recorded. The relationship between potassium concentrations and galvanometer readings was plotted as a curve.
Calculations
Weight of soil taken = 5.0 g
Volume of extractant used = 25 ml First dilution = 5 times
Second dilution = 5 times Total dilution = 25 times
Reading indicated by galvanometer = X
ppm of K as read from standard curve against X = Y ppm of K in the given soil = Y x 25 = Z
Potassium (kg ha-1) in given soil = Z x 2.24
2.4. Soil chemical properties after harvesting the crop
2.4.1. Soil pH
A Beckman glass electrode pH meter was used to determine the pH of the soil. In a 150 ml beaker, a 12.5 g soil sample was weighed, then 25 ml of distilled water was added and stirred at least 4 times over the period of half an hour. It took this long for the soil and water to reach a state of equilibrium. The soil suspension was stirred again after an hour, and the pH was tested using buffer solutions of known pH. The pH meter was calibrated using a buffer solution and adjusted using the buffer set knob. The pH of the soil solution was measured after the electrode was washed with distilled water and cleaned with filter paper.
2.4.2. Soil Electrical Conductivity
In a 100 ml beaker, 20 g of soil was weighed and 40 ml distilled water was added. The soil was stirred for 4-5 minutes at a time and then left overnight to produce a clear supernatant solution. A conductivity meter was used to determine the electrical conductivity of this supernatant. Electrical current conductivity through soil suspension is related to the amount of soluble salts present. The electrical conductivity was expressed as deci Siemens per meter (dS/m).
2.4.3. Soil Organic Carbon
The rapid titration method (wet digestion) was used to determine organic carbon [
10]. In a 250 ml conical flask, 2 g of dry soil was treated with 100 ml of 1 N K
2Cr
2O
7 solution. The flask was then gradually filled with 20 ml of concentrated sulphuric acid. After 30 minutes, the flask was filled with 0.5 g NaF, 100 ml distilled water, and 10 drops of Diphenylamine indicator. A 10 ml solution was titrated against a ferrous ammonium sulphate solution (blank titration) (0.5 N FAS). The transformation from violet to light green to blue was the final step. The organic carbon was determined using the volume of FAS used for titration and represented as a percentage of 1 N K
2Cr
2O
7.
2.5. Statistical analysis
The CPCS1 software, which was developed by the Department of Statistics at Punjab Agricultural University, Ludhiana, India, was used to analyze the data. All comparisons were conducted at a significance level of 5%. One-way analysis of variance (ANOVA) was performed to determine the treatment effects at a 0.05 level of probability.
3. Results
The plant growth attributes play a major role in alleviating the crop yield but balanced fertilization is one of the main factors responsible for the growth and development. The organic sources of nutrients with microbial bio-fertilizer not only help in supplementing the nutrition but also maintain the favorable physicochemical and biological soil environment.
3.1. Growth and yield attributes
The data presented in
Table 4,
Table 5,
Table 6,
Table 7 and Table 8 revealed that the application of 100% N from FYM + bio-fertilizer (T8) and RDF + bio-fertilizer (T5) treated plots resulted in significantly higher emergence count, plant height, No. of stools, dry matter accumulation, leaf area index, herb (fresh and dry weight), and oil yield in mentha plants over the other treatment viz., T2 i.e., RDF (N75 kg ha-1, P2O5 20 kg ha-1) and T1 i.e., control (no manure or fertilizer). The emergence count with the application of 100% N from FYM + bio-fertilizer (T8) resulted in higher emergence to the tune of 27.1, 32.2, 37.3% at 10 DAS, and 11.9, 13.8, 23.0 % at 15 DAS and 35.2, 38.1, 52.7% at 20 DAS over the treatments T2 (RDF N 75 kg ha-1, P2O5 20 kg ha-1), T4 (RDF + root soaking with bio-fertilizer) and T5 (RDF + bio-fertilizer 10 kg ha-1), respectively. This higher emergence of suckers could be due to better soil conditions, better availability of both macro and micronutrients, and improved enzymatic activity in treated plots which is required for better growth. The study from the other scientists also recorded similar results and recorded higher emergence count with the combined application of chemical fertilizers [
15] and bio-fertilizer as compared to the application of sole inorganic fertilizers [
16].
The data presented in
Table 4 recorded the highest plant height of 80.8, 86.4, and 88.3 cm with the application of 100% N from FYM + bio-fertilizer @ 10 kg ha-1 (T8) during the three years of study, which was however statistically similar with the other treatments having RDF + bio-fertilizer @ 10 kg ha-1, treatment having 100% N from FYM + root soaking with bio-fertilizer and treatment RDF + root soaking with bio-fertilizer. However, control (T1) recorded the lowest plant height (56.4, 60.6, and 60.5 cm) at harvest during the three successive cropping seasons (2020 to 2022), which might be due to the role of FYM and bio-fertilizer that significantly increased the plant height in T8 treatment. The vital role of the beneficial bacteria present in the applied biofertilizer is capable of enhancing the level of various hormones substances viz., gibberellins, auxins, and cytokinins [
17]. Plant height was also significantly affected by adding organic manures especially which might be due to the slow release of organic manures. The combined application of organic manure and biofertilizer also improves the physicochemical and biological properties of soil by increasing soil organic matter, cation exchange capacity, water holding capacity, and availability of mineral nutrients that result in increased plant height [
18]. Organic manure, contains a variety of essential nutrients like nitrogen (N), phosphorus (P), and potassium (K), along with other major micronutrients. These nutrients are gradually released into the soil, ensuring a steady supply for plant uptake. Adequate nutrient availability might have resulted in the growth of mentha plants, which resulted in the higher plant height in the present study. It also acts as a food source for beneficial soil microorganisms. These microorganisms play a crucial role in decomposing organic matter and converting it into plant-available forms. The presence of a diverse and active microbial community in the soil enhances nutrient cycling, thus supporting the growth of mentha suckers in the rhizosphere [
19]. Therefore, the increase in plant height due to the application of organic manures and bio-fertilizers is probably due to the higher availability of nutrients in the present study [
20].
The maximum stools count per meter square (81.23, 87.83, 90.18 m2) at harvest was recorded in plots that received 100% N from FYM applied with bio-fertilizer @ 10 kg ha-1 during three successive years of study (2020 to 2022), which was however statistically similar with other treatment viz., T5 (RDF + bio-fertilizer 10 kg ha-1), T7 (100% N from FYM + root soaking with bio-fertilizer) and T4 (RDF + root soaking with bio-fertilizer). All the organic and inorganic treatments along with bio-fertilizer recorded a significantly higher stool count than the plots receiving the recommended dose of fertilizer only in the three cropping years. The higher number of stools count can be attributed to the augmented nitrogen release facilitated by growth-promoting substances produced by the microbial activity in organic manures treated plots. These substances were likely to play a role in inducing higher leaf length, leaf width, and overall leaf abundance. Organic manures release humic substances which may form complexes with mineral ions, potentially enhancing enzyme catalysis, stimulating respiration, photosynthesis, and nucleic acid metabolism, as well as exerting hormonal activity. In a similar study, the application of effective microbes (EM) to peppermint plants alone or in conjunction with other organic fertilizers enhanced different growth parameters of Peppermint plants also [
21].
The increment in the dry matter accumulation of mentha was very slow up to 60 DAP and thereafter it increased at a faster rate during the current study. The highest dry matter accumulation at harvest in three years study was recorded to the tune of 34.69, 38.06, and 40.70 g plant-1, where plots received 100% N from FYM + bio-fertilizer @ 10 kg ha-1 (T8), and it was at statistically at par with the T5 (RDF + bio-fertilizer 10 kg ha-1), T7 (100% N from FYM + root soaking with bio-fertilizer) and T4 (RDF + root soaking with bio-fertilizer) over the RDF (T2). However, control treatment (no manure or fertilizer) recorded the lowest dry matter accumulation (19.94, 21.39, and 21.58 g plant-1) at harvest during the three successive seasons, which might be due to the combined application of inorganic fertilizer or organic manure with bio-fertilizer which results in improved availability of macro and micronutrients as well as high biological nitrogen fixation and solubilization of fixed phosphate through phosphate solubilizing bacteria (PSB). Organic manures are well known for playing a significant role in improving soil physical properties and fostering organic matter accumulation which leads to the production of organic acids that inhibit the activity of the IAA oxidase enzyme. As a result, the presence of these organic acids enhances the promotive effect of auxin-IAA, a plant growth hormone, thereby directly influencing plant growth and herb yield. Similarly, humic acid released by organic manures might have a positive impact on the growth of both foliage and roots of mentha plants in the present study. It achieves this by stimulating cell elongation, improving water uptake by plant roots, and enhancing the development of root systems. This increased root growth contributes to improved nutrient uptake and nutrient availability. Furthermore, organic manures also promote an increased leaf surface area, allowing for greater photosynthetic activity and overall plant productivity.
The leaf area index was increased with the increase in the age of the plant and its highest value was recorded at harvest (150 DAP) to the tune of 9.20, 9.83, and 10.20 with the application of treatment T8 (100% N from FYM + bio-fertilizer 10 kg ha-1) during the three years of study (2020 to 2022) as presented in
Table 5. During all three years of study, similar trends were recorded, where plants received 100% N from FYM + bio-fertilizer @ 10 kg ha-1 (T8) recorded statistically similar leaf area index as obtained with the application of RDF + bio-fertilizer @10 kg ha
-1 (T5). The highest leaf area index value was recorded at harvest (150 DAP), which might be due to the continuous and long-term availability of nutrients through FYM during the cropping seasons.
The differential practices dose of organic manures and RDF with bio-fertilizer application did not significantly influence the leaf-to-stem ratio at the harvesting stage during the three cropping seasons. A similar result was reported in Mentha spicata with the application of different doses of nitrogen leading to a non-significant effect on leaf to stem ratio [
22].
3.2. Mentha herb yield, oil content, and oil yield
The data pertaining to fresh, dry herb yield, and oil yield is presented in
Table 5 revealing that the application of 100% N from FYM + bio-fertilizer @ 10 kg ha-1 treated plots recorded the fresh herb yield to the tune of 259.9, 268.0, and 276.8 q ha-1 and dry herb yield to the tune of 84.5, 88.6, 91.1 q ha-1 in three respective years. In the present study, fresh and dry herb yield recorded with the 100% N from FYM + bio-fertilizer @ 10 kg ha-1 (T8) was statistically similar with the other treatment viz. T5 (RDF + bio-fertilizer 10 kg ha-1), T4 (RDF + root soaking with bio-fertilizer), and T7 (100% N from FYM + root soaking with bio-fertilizer). While, the control recorded the lowest fresh herb yield (121.0, 123.2, 120.5 q ha-1) and dry herb yield (39.3, 40.5, and 40.8 q ha-1) during three seasons (2020 to 2022). The increase in the fresh weight in the current study might be due to higher metabolic activities of the plant arising due to the interaction between the biofertilizers and organic fertilizers that resulted in higher fresh herb yield in the three successive seasons. The enhanced fresh yield of Mentha arvensis in the present study when using organic manure as an application can also be attributed to the immediate availability of macro and micro-nutrients throughout the plant's extended growth period, as well as the increased cation exchange capacity that leads to the improvement in physical and chemical properties of the soil. Our finding clearly indicates that the combined use of organic manures has a significant impact on both biomass production and the yield of essential oil of mentha plants. The application of organic matter via manures enhances the release of diverse organic acids that aid in the solubilization of native soil nutrients, making them available for plant uptake. Furthermore, the utilization of organic manures alongside chemical fertilizers also addresses the supplementation of secondary and micronutrients, which are typically not supplied by inorganic fertilizers [
23]. Consequently, this combined approach improves nutrient bioavailability in the soil and brings about alterations in soil physical properties, resulting in enhanced root growth and water absorption capacity [
24]. Nitrogen plays a pivotal role in fresh herb yield by establishing a quicker source-sink relationship. It not only promotes increased production of photosynthates but also facilitates their translocation to the same leaves. A similar study was reported with the use of vermicompost, sludge, and microbial inoculants which results in enhanced yield and oil quality of Plants [
25]. In another study, the application of organic manures before planting to mentha plants favours its plant growth, due to the availability of sufficient macro and micronutrients in available forms for the long term and improved soil structure and microbial biomass [
26,
27]. The increment in fresh weight in the mentha plant might be attributed to the increase in both plant height and number of branches [
28]. A similar trend was observed in dry herb yield during three successive seasons. The increment in plant dry weight may be attributed to the increase in fresh weight. Similar results were recorded on marjoram [
18], Mentha arvensis [
20], and Thymus vulgaris [
29] also.
The data pertaining to the oil content in the fresh herb of mentha plant presented in
Table 5, showed that no significant differences in the oil content were recorded due to the application of various nutrition treatments during the three successive cropping seasons. However, a slightly higher value of oil content percentage was recorded with treatments that involved either root soaking with bio-fertilizer or application of bio-fertilizer @ 10 kg ha-1 with organic and inorganic sources.
The oil yield of mentha showed significant variation due to the effect of different nutrient treatments. In the three successive cropping seasons, 100% N from FYM + bio-fertilizer @ 10 kg ha-1 recorded the highest oil yield (162.6, 176.2 and 177.3 kg ha-1) which was statistically at par with the treatment T5 i.e., RDF + bio-fertilizer @ 10 kg ha-1 (172.4, 170.8 and 169.8 kg ha-1), T7 i.e., 100% N from FYM + root soaking with bio-fertilizer (155.0, 167.5 and 172.6 kg ha-1) and T4 i.e., (RDF + root soaking with bio-fertilizer (167.2, 163.5 and 163.6 kg ha-1). During all the years of study, control was recorded with the lowest oil yield (68.2, 72.7, and 75.7 kg ha-1). The higher essential oil yield of mentha in organic manure-treated plots might be due to higher growth attributes (
Table 4) which leads to higher above-ground herb biomass accumulation and higher essential oil yield. In the present study, a positive and significant correlation was observed between the number of stools count and fresh yield with the oil percentage and essential oil yield. These findings indicate that mentha species exhibit a higher production of leaf material. In mentha plants, oil synthesis and accumulation primarily occur in the leaves, making them crucial for improving the oil percentage and yield. This could explain the higher oil percentage and essential oil yield observed in our study. The other benefits of organic manure on oil yield could be attributed to modifications in soil chemical, physical, and microbial properties, all of which can influence yield and its components. Organic manure known for its large particulate surface area, facilitates the effective retention of nutrients. Thus, plants receiving organic fertilizers tend to receive a greater nutrient supply compared to those receiving chemical fertilizers. The readily available nutrients, such as soluble potassium, nitrate, and phosphate, promote plant growth and crop yield. This result further confirms the advantages of integrated fertilization for enhancing essential oil yield, as observed in related studies. Similar results of organic manure and biofertilizer were registered in sweet marjoram [
30], lettuce [
31],
Rosmarinus officinalis [
32], marjoram [
33], peppermint [
34], and
Echinacea purpurea [
35].
3.3. Nutrient content in mentha
The data presented in
Table 6, revealed that the nitrogen content was the highest with the 100% N from FYM + bio-fertilizer @ 10 kg ha-1 (T8) which was about 1.68, 1.73 and 1.71% higher than RDF (T2), however statistically at par with RDF + bio-fertilizer @ 10 kg ha-1 (T5). The control recorded the lowest nitrogen content (1.41, 1.45, and 1.43%) during all three seasons. Nitrogen content in herbs was significantly influenced by the application of organic manure or inorganic fertilizer along with bio-fertilizer due to the availability of more nutrients during the crop growth season. But phosphorous and potassium content in herbs was not significantly influenced by the application of organic manure or inorganic fertilizer along with bio-fertilizer during the three cropping seasons The slightly higher P and K content in herbs (0.31, 0.32, 0.32%, and 1.56, 1.59 and 1.58%) was recorded with the 100% N from FYM + bio-fertilizer @ 10 kg ha-1 (T8). In a similar study, the combination of organic and inorganic sources of nutrition did not differ significantly in regard to phosphorous and potassium content in mentha herb [
36].
3.4. Soil physicochemical properties after harvest of the crop
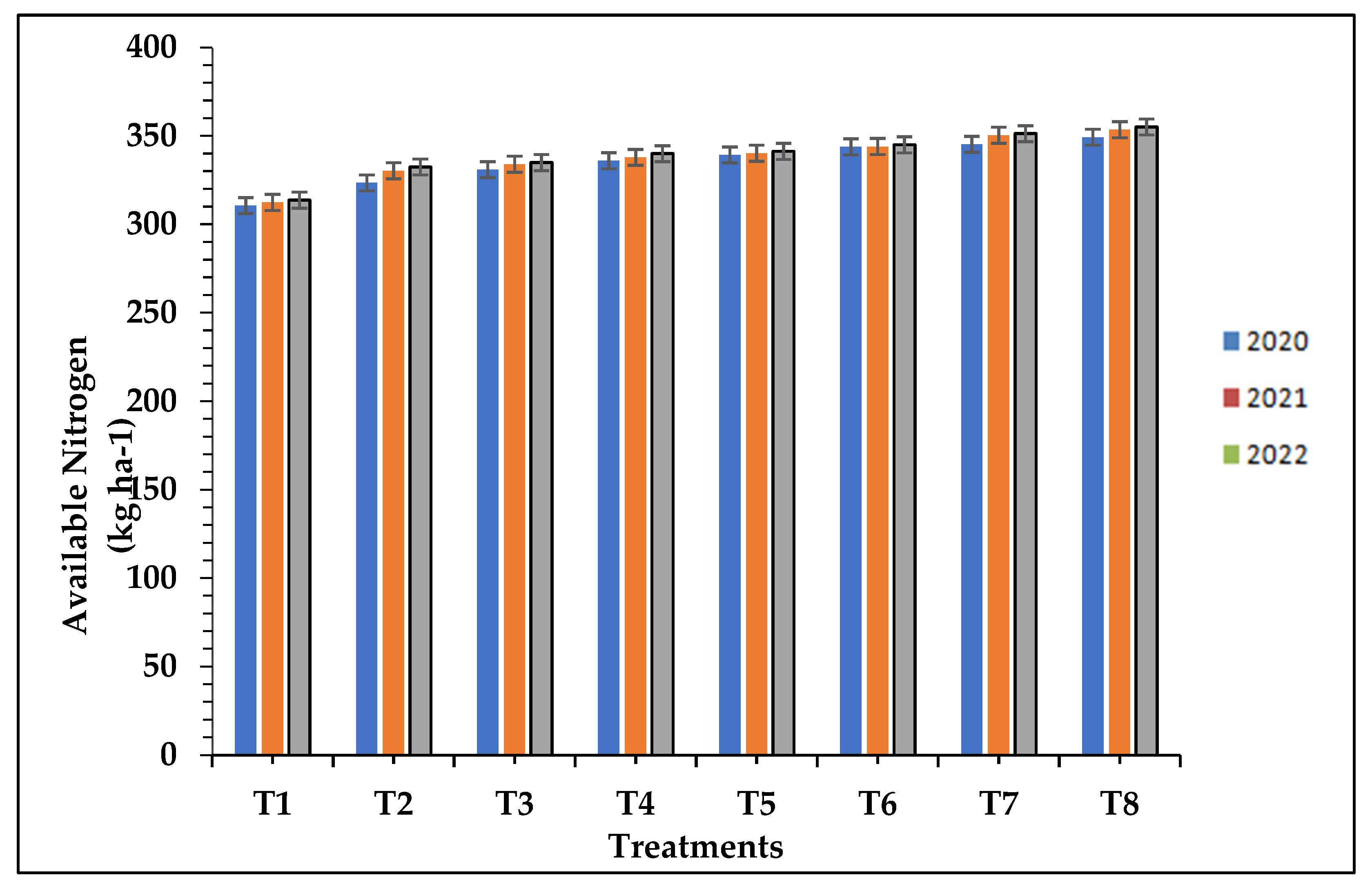
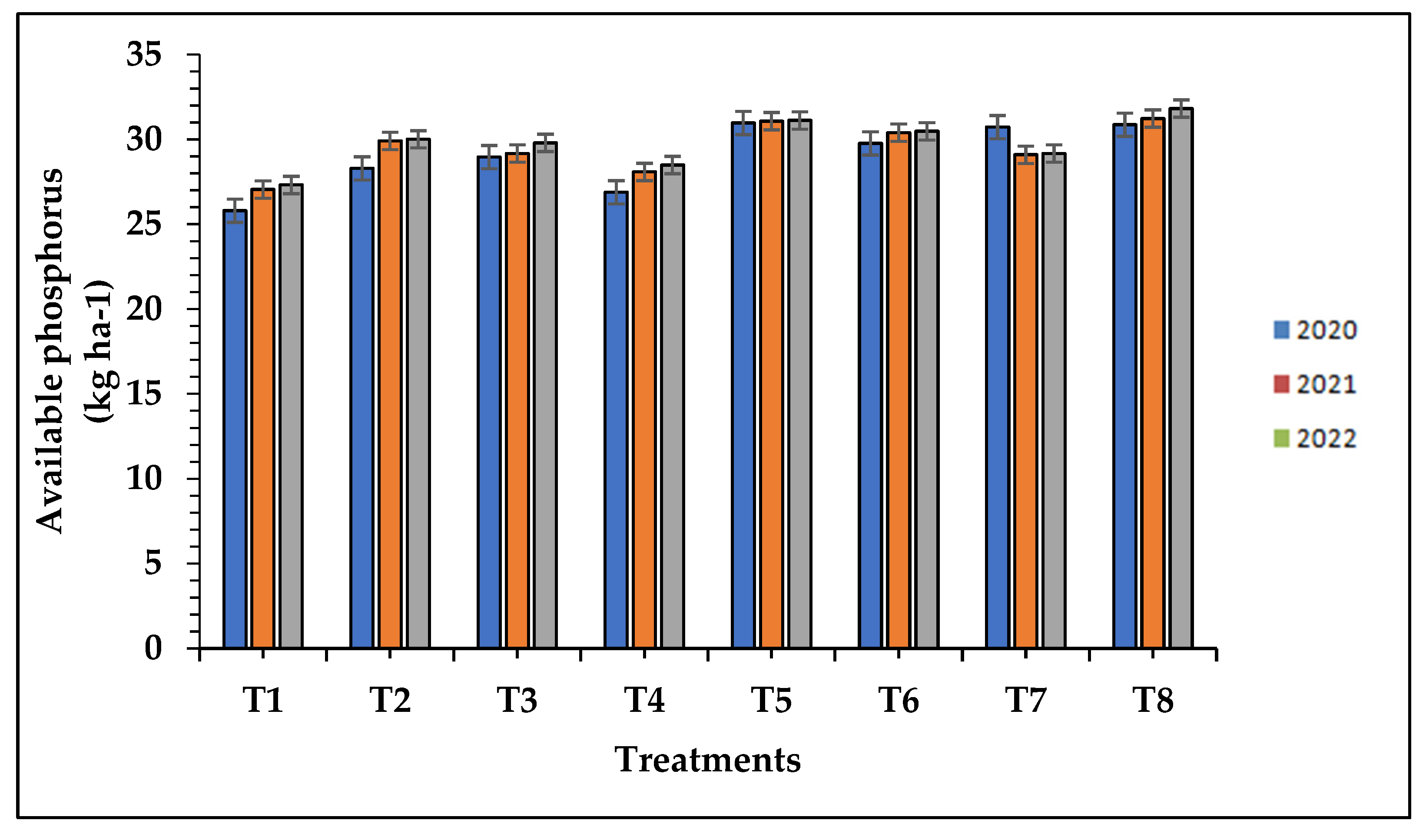
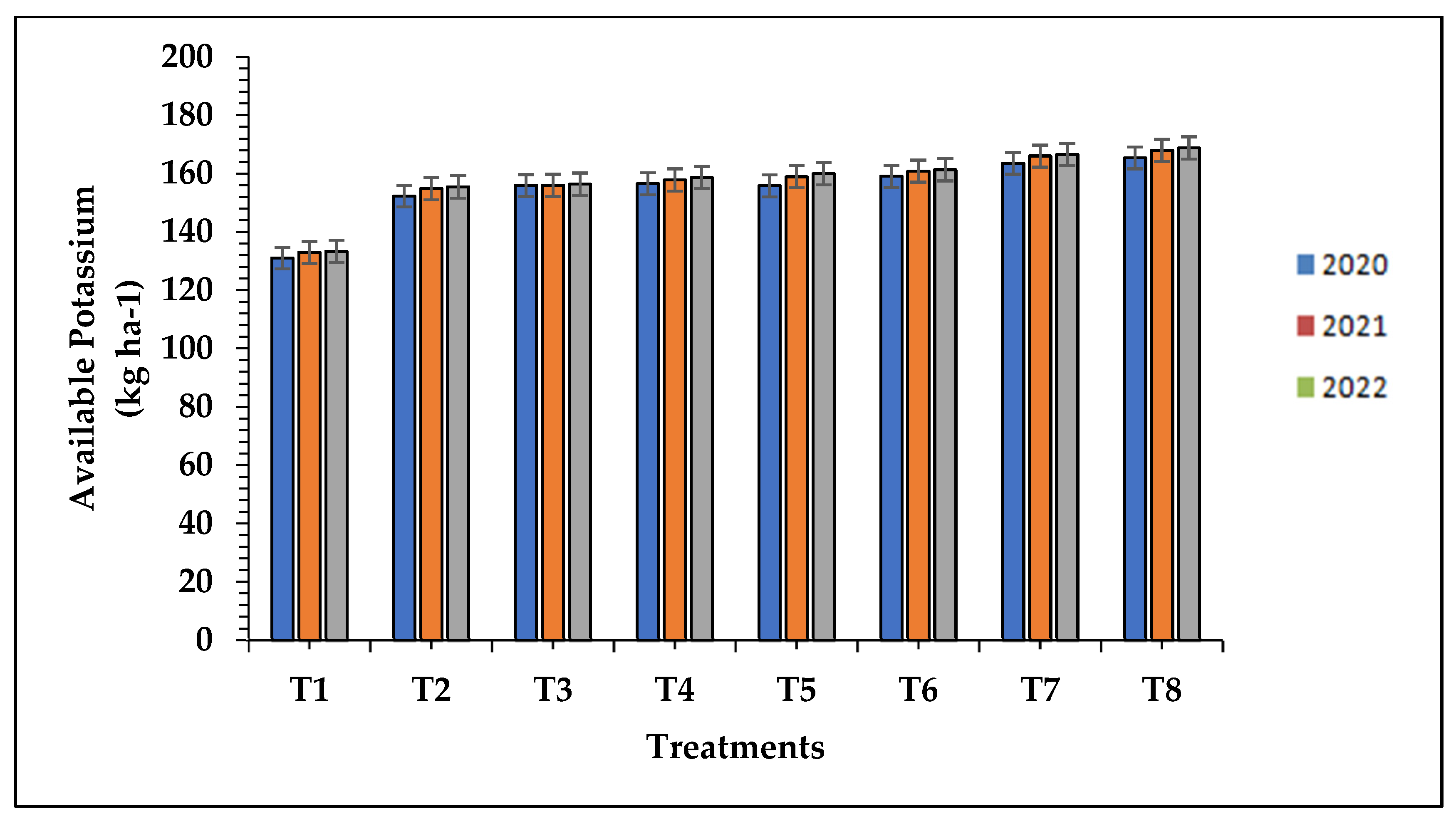
The data of the available nitrogen, phosphorous, and potassium differ significantly with respect to treatment applied with organic fertilizers along with biofertilizer as compared to chemical and control treatments during the study of the three successive seasons as presented in
Table 7. The treatment T8 (100% N from FYM + bio-fertilizer @ 10 kg ha-1) resulted in the significantly higher available N (349.25, 353.50 and 355.04 kg ha-1), P (30.87, 31.23 and 31.82kg ha-1) and K status (165.35, 167.91 and 168.74 kg ha-1) respectively, which was statistically at par with the 100% N from FYM + root soaking with bio-fertilizer (T7) and 100% N from FYM along with water soaking of roots (T6) while, the minimum available N (310.60, 312.40 and 313.63 kg ha-1), P (25.80, 27.05 and 27.32 kg ha-1) and K (131.06, 132.95 and 133.31kg ha-1) was recorded in control (T1) plots respectively, in the three successive seasons and shown in
Figure 1,
Figure 2 and
Figure 3. The increased uptake of micronutrients in the present study with the application of organic manures can be attributed to the higher bioavailability of nutrients in the soil. This enhanced availability is a result of the breakdown of organic matter, which releases nutrients through various mechanisms. Additionally, the addition of organic manures leads to a decrease in soil pH due to the release of various acids. This acidic environment promotes the improved bioavailability of micronutrients compared to the use of solely inorganic fertilizers. The synergistic use of both organic and inorganic fertilizers can provide an optimal level of nutrition, contributing to chlorophyll biosynthesis. Other factors such as light, temperature, carbohydrates, and nitrogen availability significantly affect chlorophyll biosynthesis. In our study, the addition of farmyard manure and recommended doses of fertilizers promoted plant growth and production by enhancing root development, strengthening plant stems, and increasing photosynthetic rates. These factors ultimately led to higher nutrient amounts and uptake by the crop [
37]. Therefore, in the present study, slow mineralization of the nitrogen from manure and bio-fertilizer as compared to chemical fertilizer resulted in higher crop yield which resulted in higher herb and oil yield in bio-fertilizer and farmyard manure-treated soils [
38]. Similar results were observed in other crops also with regular application of farmyard manure improving the available N, P, and K status in soil [
39].
The soil pH, EC, and OC were influenced by recommended doses or treatments having organic manures and inorganic fertilizers along with biofertilizers during all three years of study. The significantly lower pH (7.44, 7.47, and 7.50) and EC values (0.14, 0.15, and 0.16 dSm-1) and higher values of organic carbon (0.56, 0.57, and 0.57%) were recorded under the treatment of 100% N from FYM + bio-fertilizer @ 10 kg ha-1(T8) during the three cropping seasons. The data (
Table 7) showed that the decrease in the soil pH and slight increment in the OC with the application of farmyard manure alone or along with biofertilizer might be due to the release of organic acids on decomposition which created favourable environment for the formation of humic acid which encouraged the activity of soil microorganisms resulting in an increase in the organic carbon content of soil [
40]. The EC value did not significantly influence by different treatments, however, a slightly higher EC value was recorded in the T2 (RDF) treatment, which might be attributed to the accumulation of soluble salts due to the addition of inorganic ions as compared to other treatments. No change in electrical conductivity with chemical and organic treatments was also reported in other crops [
41].
4. Conclusions
The results of the present investigation which was conducted during three successive seasons (2020 to 2022), revealed that the treatment of 100% N from FYM + bio-fertilizer @ 10 kg ha-1 proved to be superior over RDF + bio-fertilizer @ 10 kg ha-1 and other inorganic and organic treatments in term of fresh, dry herb yield, oil content, oil yield and physicochemical properties of soil. This method can help in reducing costs and the environmental impact caused by excessive chemical application of fertilizers. It can also help farmers achieve higher yields and profits. This approach will help in improving soil fertility, and environmental safety and will reduce the indiscriminate use of chemical application fertilizers in mentha crops. It is believed that the use of organic manures and bio-fertilizers can lead to sustainable mentha production.
Author Contributions
Conceptualization, S.S.W.; methodology, V.K.; data collection, V.K., V.P.K.; analysis of data, V.K., S.A., A.T.A., L.A.H.; writing—original draft preparation, R.K.G., V.K., S.S.W., V.P.K., T.K.; writing—review and editing, S.S.W., S.A., A.T.A., L.A.H., F.U.H.; visualization S.S.W.; supervision S.S.W.; project administration, S.S.W.; funding acquisition, S.A., A.T.A., L.A.H. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
This research was funded by the Researchers Supporting Project number (RSP2023R194), King Saud University, Riyadh, Saudi Arabia. This study was conducted at the Student’s Research Farm, School of Organic Farming, Punjab Agricultural University, Ludhiana, India.
Conflicts of Interest
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- FiBL; IFOAM – Organics International. The World of Organic Agriculture: Statistics and Emerging Trends 2022. Helga Willer, Jan Travnicek, Claudia Meier and Bernhard Schlatter, Research Institute of Organic Agriculture (FiBL), Frick, Switzerland. 2022.
- Pandey, A.K.; Rai, M.K.; Acharya, D. Chemical composition and antimycotic activity of the essential oils of corn mint (Mentha arvensis) and lemon grass (Cymbopogon flexuosus) against human pathogenic fungi. Pharm. Biol. 2003, 41, 421–425. [Google Scholar] [CrossRef]
- Mahantesh, P.S.; Sampath, P.M.; Pooja, M.R.; Nishchitha, M.; Bhat, D.S.; Harishkumar, K. Effect of row spacing and nitrogen levels on growth, physiological factors and oil yield of Japanese mint (Mentha arvensis L.). Int. J. Agric. Sci. 2017; 9, 4304–4307. [Google Scholar]
- Anonymous. Package of Practices for Kharif crops. Punjab Agricultural University, Ludhiana, Punjab. 2023. pp. 109–112.
- Salim, E.A.; Hassan, El.G.M.; Hassan, El.S.K. Effect of spacing and seasonal variation on growth parameters, yield and oil content of mint plants. J. For. Prod. Indust. 2014, 3, 71–74. [Google Scholar]
- Duhan, S.P.S; Bhattacharya, A.K.; Hussain, A. Effect of nitrogen and its method of application on the herb and oil quality of Japanese mint (Mentha arvensis L.). Indian Perf. 1977; 21, 47–50. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Shabi, M.; Ngassoum. M.B. Comparative investigation of essential oil and volatiles of spearmint. Perfum. Flav. 2002, 27, 16–22. [Google Scholar]
- Drinkwater, L.E.; Letourneau, D.K.; Workneh, F.; van Bruggen, A.H.C.; Shennan, C. Fundamental differences between conventional and organic tomato agroecosystems in California. Ecol. Appl. 1995, 5, 1098–1112. [Google Scholar] [CrossRef]
- Stamatiadis, S.; Werner, M.; Buchanan, M. Field assessment of soil quality as affected by compost and fertilizer application in a broccoli field (San Benito County, California). Appl. Soil Ecol. 1999, 12, 217–225. [Google Scholar] [CrossRef]
- Walkley, W.; Blacks, C.A. An examination of the digtjareff method for determination of soil organic matter and a prosed modification of the chromic acid titration method. Soil Sci. 1934, 37, 173–179. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 1956, 25, 259–268. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorous by extraction with sodium bicarbonate. USDA Cir No. 1954, pp. 19, 939–1019. [Google Scholar]
- Piper, C.S. Soil and plant analysis. Hans Publishers, Bombay. 1966.
- Muhur, G.R.; Dutta, N.P.; Sankara, S. Soil testing in India. USAID, New Delhi, India. 1965.
- Joshi, E.B.; Nepalia, V.; Verma, A.K.; Singh, D.K. Effect of integrated nutrient management on growth, productivity and economics of maize (Zea mays). Indian J. Agron. 2013, 58, 434–436. [Google Scholar]
- Habib, M.D.; Asif, M.; Aziz, M.; Ali, A.; Ashraf, M.; Javaid, M.M. Growth performance of spring maize and soil fertility status as influenced by nutrient sources. 2012, 4, 35–41.
- Kumar, A.; Shukla, R.; Singh, P.; Singh, A.K.; Dubey, N.K. Use of essential oil from Mentha arvensis L. to control storage moulds and insects in stored chickpea. J. Sci. Food Agric. 2009, 89, 2643–2649. [Google Scholar] [CrossRef]
- Al-Fraihat, A.H.; Al-dalain, S.Y.A.; Al-Rawashdeh, Z.B.; Abu-Darwish, M.S.; Al-Tabbal, J.A. Effect of organic and biofertilizers on growth, herb yield and volatile oil of marjoram plant grown in Ajloun region, Jordan. J. Med. Plants Res. 2011, 5, 2822–2833. [Google Scholar]
- Biswas, N.; Chattopadhyay, N.; Jamir, A.R.; Bandyopadhyay, A.; Ghosh, D.K. Organic management of mint (Mentha arvensis L.) towards improving productivity and quality. J. Crop. Weed, 2022; 18, 51–55. [Google Scholar]
- Bajeli, J.; Tripathi, S.; Kumar, A.; Tripathi, A.; Upadhyay, R.K. Organic manures a convincing source for quality production of Japanese mint (Mentha arvensis L.). Ind. Crops Prod. 2016, 83, 603–606. [Google Scholar] [CrossRef]
- Al-Amri, S.M. Response of growth, essential oil composition, endogenous hormones and microbial activity of Mentha piperita to some organic and biofertilizers agents. Saudi J. Biol. Sci. 2021, 28, 5435–5441. [Google Scholar] [CrossRef]
- Randhawa, S.S. Effect of row spacing and nitrogen levels on herb and oil yields of spearmint (Mentha spicata L.). M Sc Thesis, Punjab Agricultural University, Ludhiana, India. 1995.
- Patra, A.; Sharma, V.K.; Purakayastha, T.J.; Barman, M.; Kumar, S.; Chakraborty, D.; Chobhe, K.A.; Nath, D.J. ; Long-term effect of integrated nutrient management on yield and nutrients uptake by rice (Oryza sativa) in acid soil. Indian J. Agric. Sci. 2018, 88, 579–583. [Google Scholar] [CrossRef]
- Moharana, P.C.; Sharma, B.M.; Biswas, D.R.; Dwivedi, B.S.; Singh, R.V. Long-term effect of nutrient management on soil fertility and soil organic carbon pools under a 6-year-old pearl millet-wheat cropping system in an Inceptisol of sub-tropical India. Field Crop. Res. 2012, 136, 32–41. [Google Scholar] [CrossRef]
- Trivedi, P.; Singh, K.; Pankaj, U.; Verma, S.K.; Verma, R.K.; Patra, D.D. Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecol. Eng. 2017, 102, 127–136. [Google Scholar] [CrossRef]
- Dauda, S.N.; Ajayi, F.A.; Ndor, E. Growth and yield of watermelon (Citrullus lanatus) as affected by poultry manure application. Electron. J. Environ. Agric. Food Chem. 2009, 8, 305–311. [Google Scholar]
- Dhull, S.; Goyal, S.; Kapoor, K.; Mundra, M. Microbial Biomass carbon and microbial activities of soils receiving chemical fertilizers and organic amendments. Int. J. Phytoremediation 2004, 21, 641–647. [Google Scholar] [CrossRef]
- Arafa, M.S.; Sarhan, A.M.Z.; Mohamed, M.A.; Rabbu, H.S.A. Effect of organic manures with biofertilizers on growth and volatile oil production of Mentha longifolia plant. Middle East J. Agric. Res. 2017, 6, 748–756. [Google Scholar]
- Amooaghaie, R.; Golmohammadi, S. Effect of vermicompost on growth, essential oil, and health of Thymus vulgaris. Compost Sci. Util. 2017, 25, 166–177. [Google Scholar] [CrossRef]
- Edris, A.E.; Shalaby, A.S.; Fadel, H.M. Effect of organic agriculture practices on the volatile flavor components of some essential oil plants growing in Egypt: Iii. Thymus vulgaris L. Essential Oil. J. Essent. Oil-Bearing Plants 2009, 12, 319–326. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, R.D.; Kim, Y.W.; Shim, J.H.; Chae, D.H.; Rim, Y.S.; Sohn, B.K.; Kim, T. H.; Kim, K.Y. Effect of food waste compost on microbial population, soil enzyme activity, and lettuce growth. Bioresour. Technol. 2004, 93, 21–28. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Pokluda, R.; Abdelwahab, M. Influence of compost, microorganisms and NPK fertilizer upon growth, chemical composition and essential oil production of Rosmarinus officinalis L. Not. Bot. Horti Agrobot. Cluj-Napoca 2007, 35, 86–90. [Google Scholar]
- Gharib, F.A.; Moussa, L.A.; Massoud, O.N. Effect of compost and bio-fertilizers on growth, yield and essential oil of sweet marjoram (Majorana hortensis) plant. Int. J. Agric. Biol. 2008, 10, 381–387. [Google Scholar]
- Mahboobeh, Z.; Morteza, A.S.; Mryam, T.; Reza, S.A. Effects of organic and chemical fertilizers on quantitative and qualitative characteristics of peppermint (Mentha piperita L.). Int. J. Agric. Crop Sci. 2014, 7, 237–244. [Google Scholar]
- Hajagha, R.I.; Kirici, S.; Tabrizi, L.; Asgharzadeh, A.; Hamidi, A. Evaluation of growth and yield of purple coneflower (Echinacea purpurea L.) in response to biological and chemical fertilizers. J. Agric. Sci. 2017, 9, 160. [Google Scholar] [CrossRef]
- Kaur, S. Agronomic management for higher herb and oil productivity in Japanese mint (Mentha arvensis L.). Ph. D Dissertation submitted to Punjab Agricultural University, Ludhiana. 2012.
- Dhaliwal, S. S.; Sharma, S.; Sharma, V.; Shukla, A. K.; Walia, S. S. Long-term integrated nutrient management in the maize-wheat cropping system in alluvial soils of north-western India : Influence on soil organic carbon, microbial activity and nutrient status. Agronomy 2021, 11, 2258. [Google Scholar] [CrossRef]
- Anwar, M.; Patra, D.D.; Chand, S.; Alpesh, K.; Naqvi, A.A.; Khanuja, S.P.S. Effect of organic manures and inorganic fertilizer on growth, herb and oil yield, nutrient accumulation, and oil quality of french basil. Commun. Soil Sci. Plant Anal. 2005, 36, 1737–1746. [Google Scholar] [CrossRef]
- Jamwal, J.S. Effect of integrated nutrient management in maize (Zea mays) on succeeding winter crops under rainfed conditions. Indian J. Agron. 2006, 51, 14–16. [Google Scholar] [CrossRef]
- Bajpai, R.K.; Chitale, S.; Upadhyay, S.K.; Urkurkar, J.S. Long-term studies on soil physicochemical properties and productivity of rice-wheat system as influenced by integrated nutrient management in inceptisol of Chhattisgarh. J. Indian Soc. Soil Sci. 2006, 54, 24–29. [Google Scholar]
- Kumar, V.H.S.; Joshi, S.C.; Rana, D.K. Response of vermicompost on growth and yield of pea (Pisum sativum L.) cv. Arkel. Nature and Science, 1995; 106, 87–92. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).