Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Genetic analysis
2.3. Statistical analysis
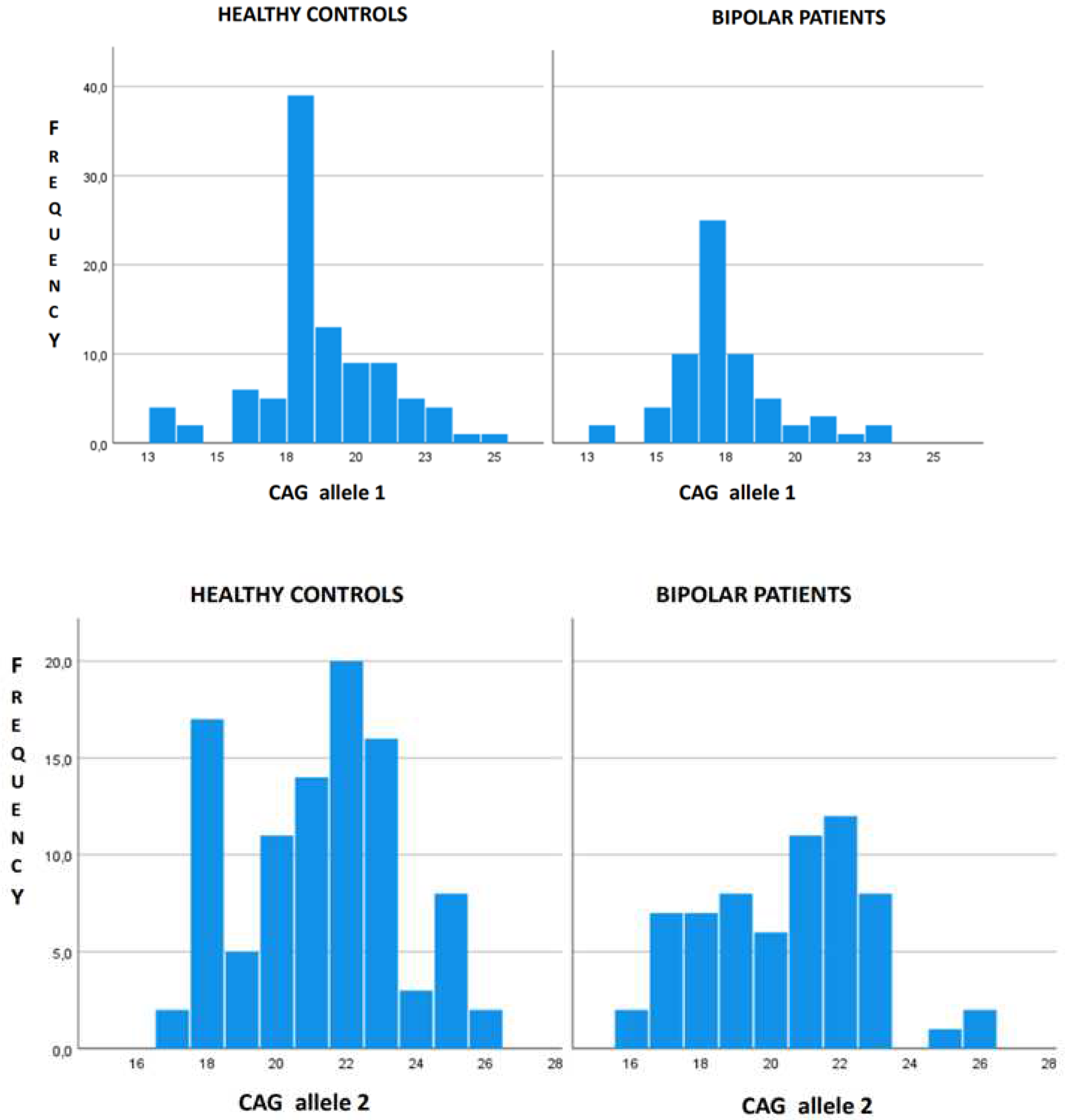
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur J Neurol 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Kay, C.; Hayden, M.R.; Leavitt, B.R. Epidemiology of Huntington disease. Handb Clin Neurol 2017, 144, 31–46. [Google Scholar] [PubMed]
- Ross, C.A.; Reilmann, R.; Cardoso, F.; McCusker, E.A.; Testa, C.M.; Stout, J.C.; et al. Movement Disorder Society Task Force Viewpoint: Huntington’s Disease Diagnostic Categories. Mov Disord Clin Pract 2019, 6, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.L.; Thompson, J.C.; Wild, S.; Yardumian, P.; Snowden, J.S.; Turner, G.; et al. Psychiatric disorders in preclinical Huntington’s disease. J Neurol Neurosurg Psychiatry 2007, 78, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.B.; Singleton, A.B. Common Premutations in the General Population. JAMA Neurol 2019, 76, 639–640. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, T.A.; Paiva, C.L.; Agostinho, L.A. REVIEW-ARTICLE Intermediate alleles of Huntington’s disease HTT gene in different populations worldwide: A systematic review. Genetics and molecular research 2017, 16. [Google Scholar] [CrossRef]
- Sundblom, J.; Niemelä, V.; Ghazarian, M.; Strand, A.S.; Bergdahl, I.A.; Jansson, J.H.; et al. High frequency of intermediary alleles in the HTT gene in Northern Sweden—The Swedish Huntingtin Alleles and Phenotype (SHAPE) study. Sci Rep 2020, 10, 9853. [Google Scholar] [CrossRef]
- Oosterloo, M.; Van Belzen, M.J.; Bijlsma, E.K.; Roos, R.A. Is There Convincing Evidence that Intermediate Repeats in the HTT Gene Cause Huntington’s Disease? J Huntingtons Dis 2015, 4, 141–148. [Google Scholar] [CrossRef]
- Killoran, A.; Biglan, K.M.; Jankovic, J.; Eberly, S.; Kayson, E.; Oakes, D.; et al. Characterization of the Huntington intermediate CAG repeat expansion phenotype in PHAROS. Neurology 2013, 80, 2022–2027. [Google Scholar] [CrossRef]
- Kenney, A.; Powell, S.; Jankovic, J. Autopsy-proven Huntington’s disease with 29 trinucleotide repeats. Mov Disord 2007, 22, 127–130. [Google Scholar] [CrossRef]
- Savitt, D.; Jankovic, J. Clinical phenotype in carriers of intermediate alleles in the huntingtin gene. J Neurol Sci 2019, 402, 57–61. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical manual of mental disorders, 4th ed.; 2000. [Google Scholar]
- Ingannato, A.; Bagnoli, S.; Bessi, V.; Ferrari, C.; Mazzeo, S.; Sorbi, S.; Nacmias, B. Intermediate alleles of HTT: A new pathway in longevity. J. Neurol. Sci. 2022, 438, 120274. [Google Scholar] [CrossRef] [PubMed]
- Jama, M.; Millson, A.; Miller, C.E.; Lyon, E. Triplet repeat primed PCR simplifies testing for Huntington disease. J. Mol. Diagn. 2013, 15, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Smoller, J.W.; Mysore, J.; Sun, M.; Gillis, T.; Purcell, S.; et al. Prevalence of incompletely penetrant Huntington’s disease alleles among individuals with major depressive disorder. Am J Psychiatry 2010, 167, 574–579. [Google Scholar] [CrossRef]
- Gardiner, S.L.; van Belzen, M.J.; Boogaard, M.W.; van Roon-Mom, W.M.C.; Rozing, M.P.; van Hemert, A.M.; et al. Huntingtin gene repeat size variations affect risk of lifetime depression. Transl Psychiatry 2017, 7, 1277. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.M.; Gillis, T.; Mysore, J.S.; Lee, J.M.; Alonso, I.; Gusella, J.F.; et al. Prevalence of Huntington’s disease gene CAG trinucleotide repeat alleles in patients with bipolar disorder. Bipolar Disord 2015, 17, 403–408. [Google Scholar] [CrossRef]
- Raskin, S.; Allan, N.; Teive, H.A.; Cardoso, F.; Haddad, M.S.; Levi, G.; et al. Huntington disease: DNA analysis in Brazilian population. Arq Neuropsiquiatr 2000, 58, 977–985. [Google Scholar] [CrossRef]
- Lee, J.K.; Conrad, A.; Epping, E.; Mathews, K.; Magnotta, V.; Dawson, J.D.; et al. Effect of Trinucleotide Repeats in the Huntington’s Gene on Intelligence. EBioMedicine 2018, 3, 47–53. [Google Scholar] [CrossRef]
- Hannan, A.J. Tandem Repeat Polymorphisms: Genetic Plasticity, Neural Diversity and Disease; Landes Bioscience and Springer Science: New York, 2012. [Google Scholar]
- Mühlau, M.; Winkelmann, J.; Rujescu, D.; Giegling, I.; Koutsouleris, N.; Gaser, C.; et al. Variation within the Huntington’s disease gene influences normal brain structure. PLoS ONE 2012, 7, e29809. [Google Scholar] [CrossRef]
- Aziz, N.A.; Jurgens, C.K.; Landwehrmeyer, G.B.; EHDN Registry Study Group; van Roon-Mom, W.M.; van Ommen, G.J.; et al. Normal and mutant HTT interact to affect clinical severity and progression in Huntington disease. Neurology 2009, 73, 1280–1285, Erratum in Neurology 2009, 73, 1608. Erratum in Neurology 2011, 76, 202. A. Ciarmielo [corrected to Ciarmiello, Andrea]. [Google Scholar] [CrossRef]
- Djoussé, L.; Knowlton, B.; Hayden, M.; Almqvist, E.W.; Brinkman, R.; Ross, C.; et al. Interaction of normal and expanded CAG repeat sizes influences age at onset of Huntington disease. Am J Med Genet. A 2003, 119A, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Terenzi, F.; Bagnoli, S.; Latorraca, S.; Nacmis, B.; Sorbi, S.; et al. Late-onset Huntington disease: An Italian cohort. J Clin Neurosci 2021, 86, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Jia, Y.; Zhong, S.; Sun, Y.; Zhou, Z.; Zhang, Z.; Huang, L. Microstructural Abnormalities of Basal Ganglia and Thalamus in Bipolar and Unipolar Disorders: A Diffusion Kurtosis and Perfusion Imaging Study. Psychiatry Investig. 2017, 14, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Aylward, E.H.; Roberts-Twillie, J.V.; Barta, P.E.; Kumar, A.J.; Harris, G.J.; Geer, M.; Peyser, C.E.; Pearlson, G.D. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am. J. Psychiatry 1994, 151, 687–693. [Google Scholar] [CrossRef] [PubMed]
| Patients (n = 69) | |
|---|---|
| Age (SD) | 53.91 (10.2) |
| Age at disease onset (SD) | 34.81 (13.4) |
|
Sex Male (%) Female (%) |
27 (39.1) 42 (60.9) |
| Education in years (SD) | 10.25 (3.1) |
|
Symptoms at onset Maniacal (%) Depression (%) Mix (%) |
11 (15.9) 34 (49.2) 24 (34.7) |
| Bipolar disorder Type 1 (%) | 44 (63.7) |
| Family history for psychiatric disorders (%) | 30 (43.5) |
HTT gene *
|
17.7 (2.1) 21.03 (3.3) - 0 7 (10.2) |
| No IA-carriers (n = 62) | IA-carriers (n = 7) | P | |
|---|---|---|---|
| Age at disease onset (SD) | 33.7 (13.5) | 43.3 (9.9) | 0.048 |
|
Sex Male (%) Female (%) |
23 (37.1%) 39 (62.9%) |
4 (57.3%) 3 (42.8) |
0.38 |
|
Symptoms at onset Maniacal (%) Depression (%) Mix (%) |
9 (14.5) 30 (48.4) 23 (37.1) |
2 (28.5) 4 (57.1) 1 (14.2) |
0.89 |
| Family history for psychiatric disorder (%) | 39 (45.1) | 2 (28.5) | 0.09 |
| CAG 1 (SD) | 17.37 (1.8) | 20.6 (2.3) | 0.09 |
| CAG 2 (SD) | 20.2 (2.08) | 28.6 (1.4) | < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





