Submitted:
04 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
2.1. Characterization of (TPP)GaCl and (OEP)GaCl porphyrin materials
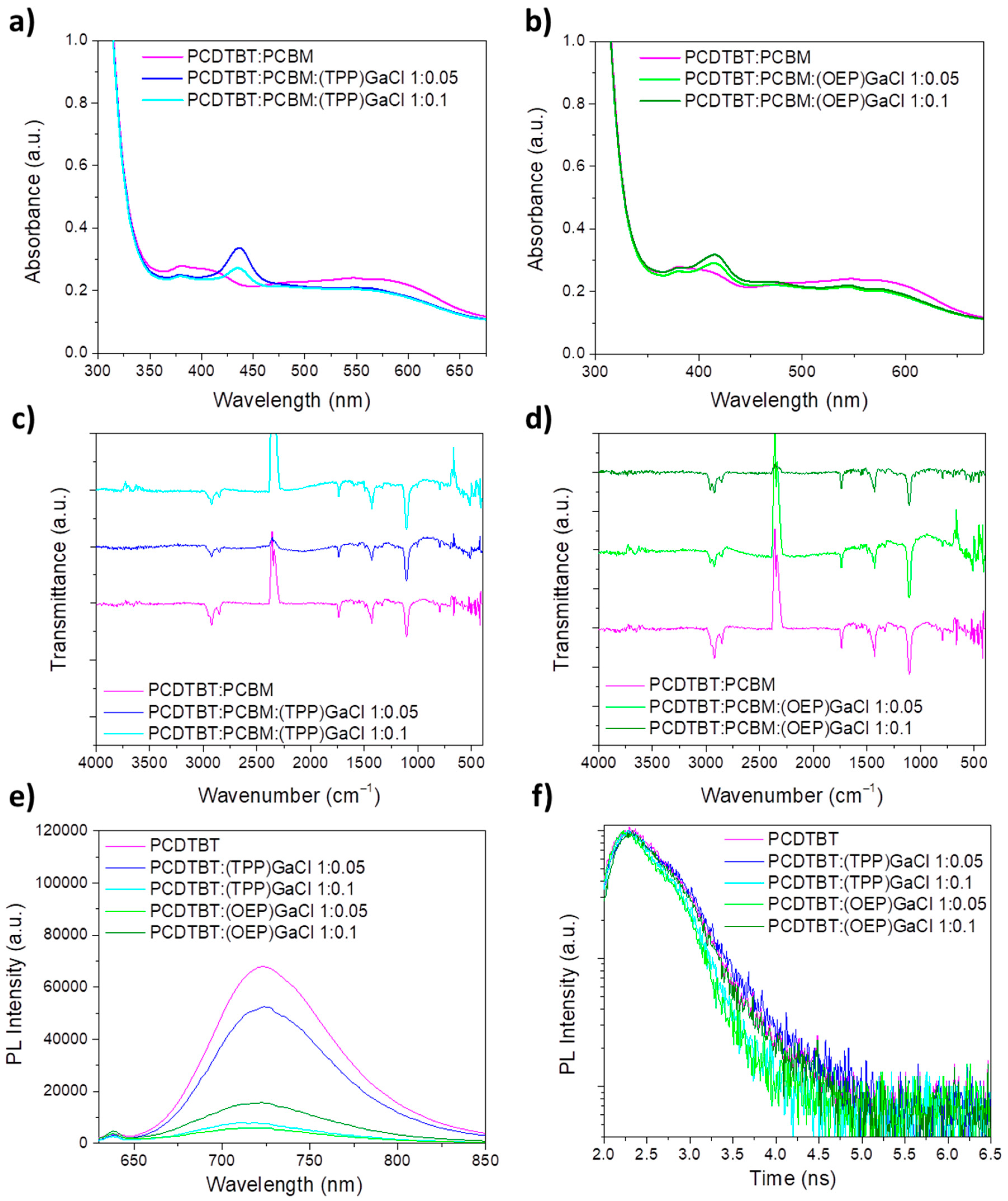
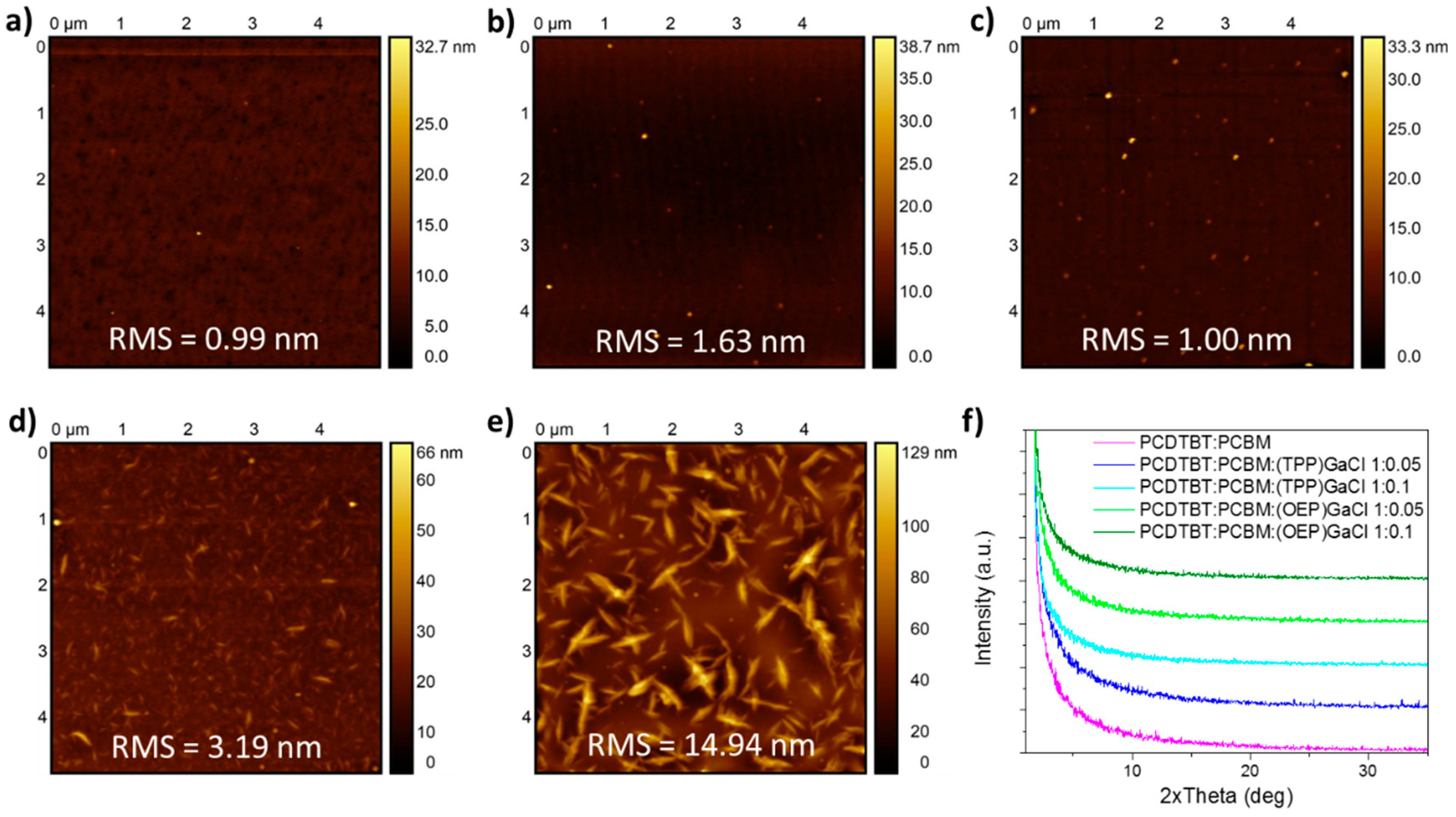
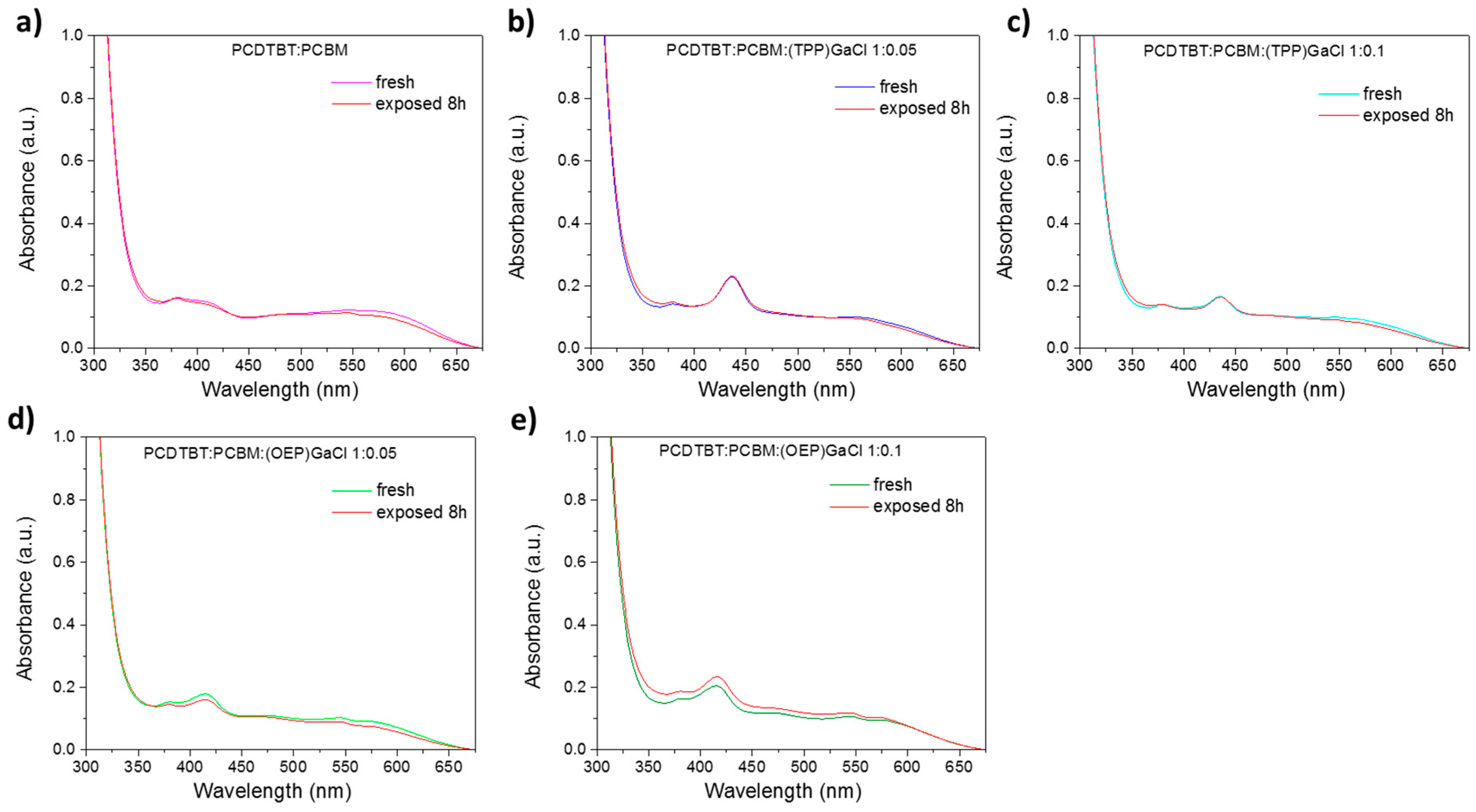
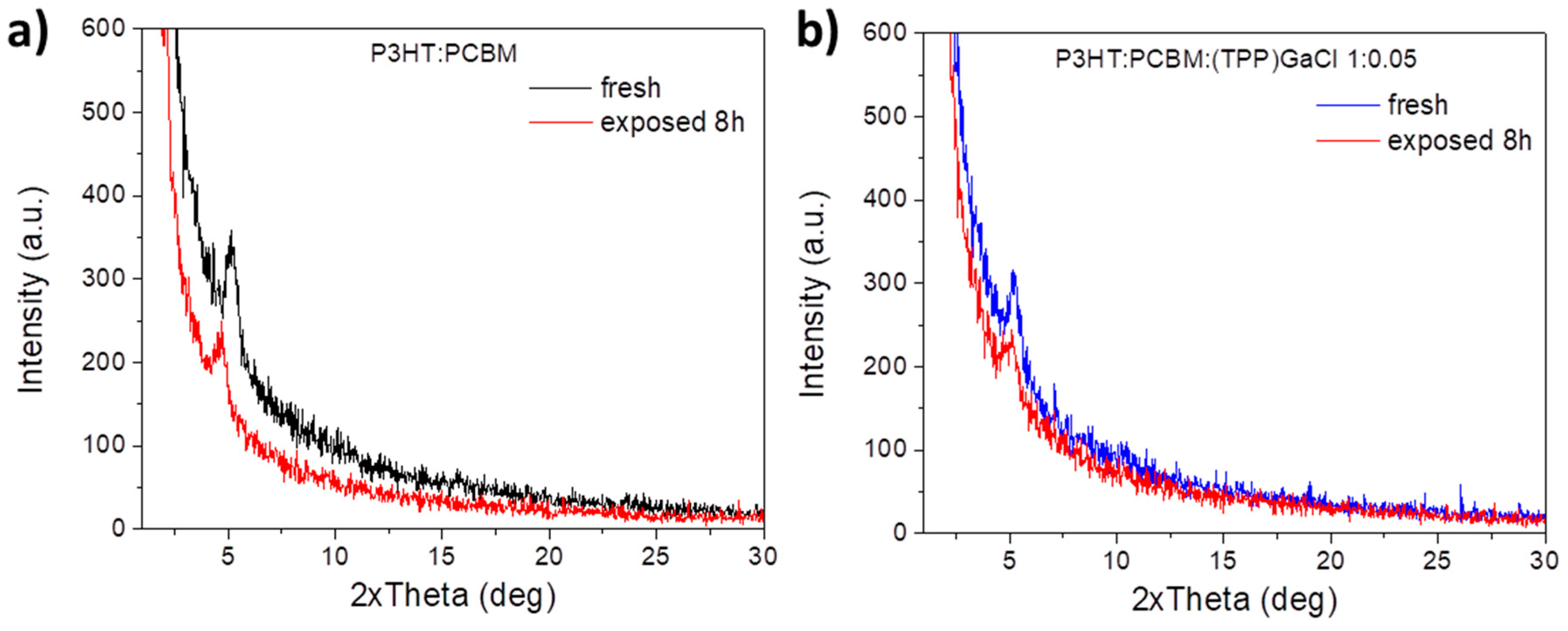
2.2. Characterization of ternary blend films
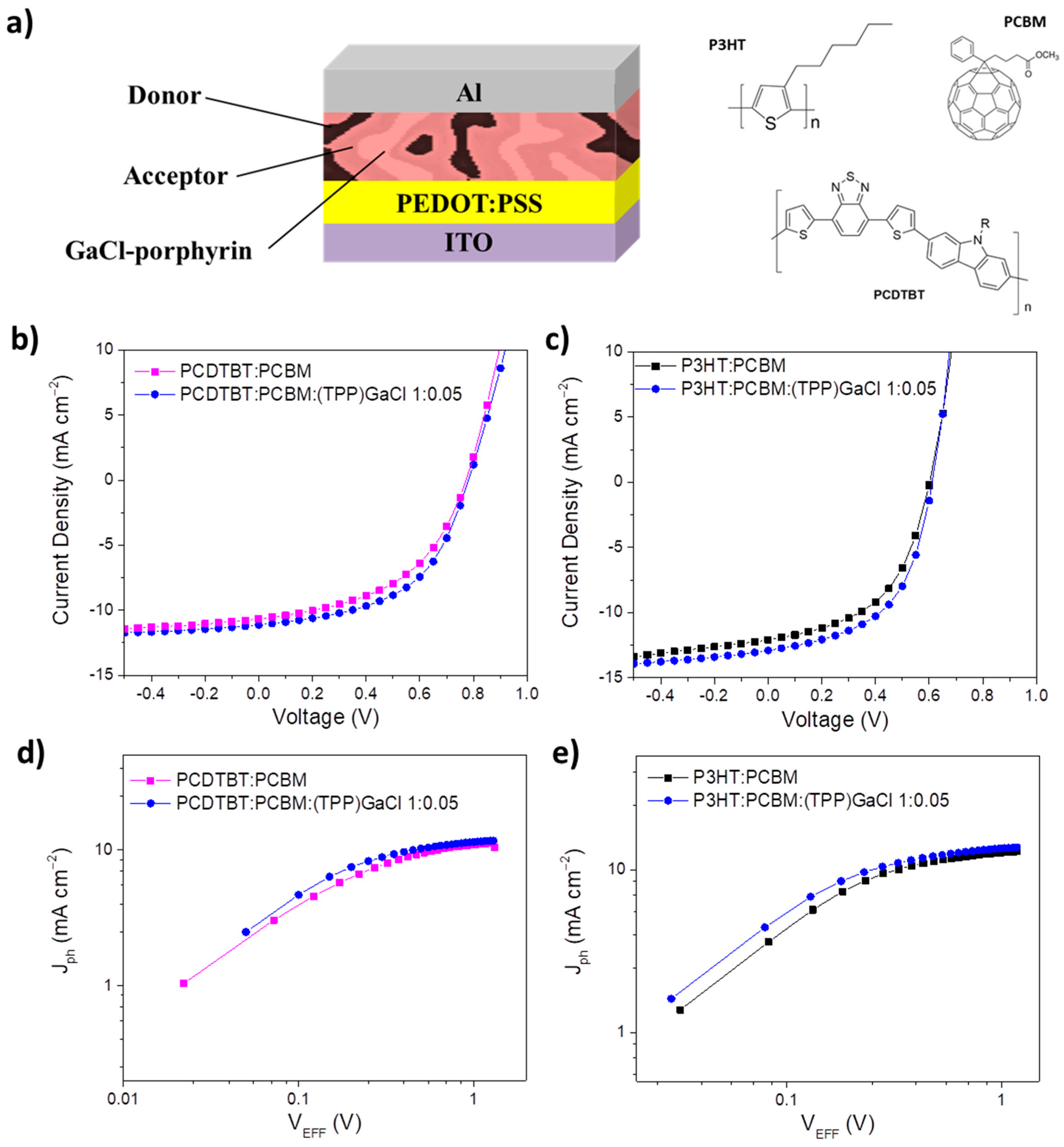
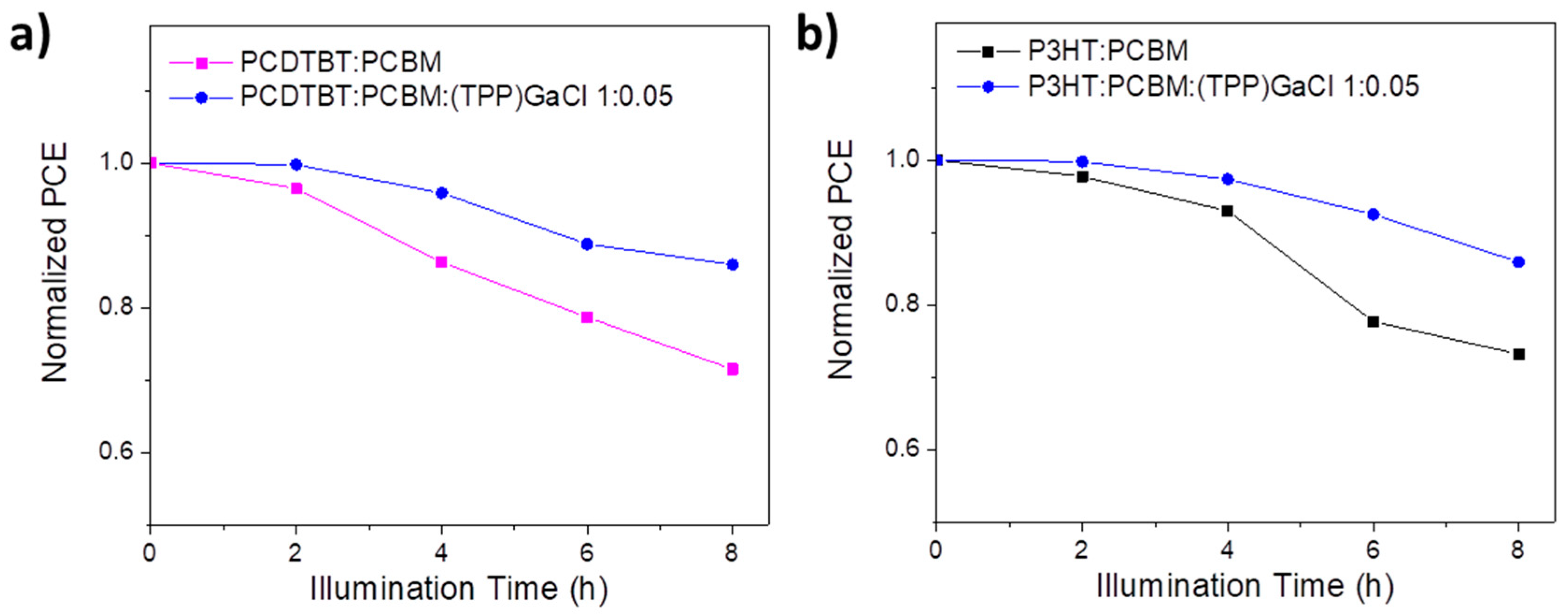
2.3. Fabrication of ternary-based organic solar cells
3. Conclusion
4. Experimental Section
Supplementary Materials
Author Contributions
Acknowledgments
References
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Bergqvist, J.; Österberg, T.; Melianas, A.; Aguirre, L.E.; Tang, Z.; Cai, W.; Ma, Z.; Kemerink, M.; Gedefaw, D.; Andersson, M.R.; Inganäs, O. Asymmetric photocurrent extraction in semitransparent laminated flexible organic solar cells. NPJ Flexible Electron. 2018, 2, 4. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596. [Google Scholar] [CrossRef]
- Duan, L.; Elumalai, N.K.; Zhang, Y.; Uddin, A. Progress in Stability of Organic Solar CellsSol. Energy Mater. Sol. Cells 2019, 193, 22. [Google Scholar] [CrossRef]
- Duan, L.; Yi, H.; Wang, Z.; Zhang, Y.; Haque, F.; Sang, B.; Deng, R.; Uddin, A. Semitransparent organic solar cells based on PffBT4T-2OD with a thick active layer and near neutral colour perception for window applications. Sustainable Energy Fuels 2019, 3, 2456. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Deshmukh, K.; Ahipa, T.N.; Muzaffar, A.; Ahamed, M.B.; Pasha, S.K.K.; Al-Maadeed, M.A.-A. Flexible, biodegradable and recyclable solar cells: a review. J. Mater. Sci.: Mater. Electron. 2019, 30, 951. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Li, Y.; Zhou, G.; Zhan, L.; Zhu, H.; Lu, X.; Chen, H.; Li, C.-Z. High-performance and eco-friendly semitransparent organic solar cells for greenhouse applications. Joule 2021, 5, 945. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.D.; Che, X.; Qu, Y.; Liu, F.; Liao, L.-S.; Forrest, S.R. High efficiency near-infrared and semitransparent non-fullerene acceptor organic photovoltaic cells. J. Am. Chem. Soc. 2017, 139, 17114. [Google Scholar] [CrossRef]
- Ravishankar, E.; Booth, R.E.; Saravitz, C.; Sederoff, H.; Ade, H.W.; O’Connor, B.T. Achieving net zero energy greenhouses by integrating semitransparent organic solar cells. Joule 2020, 4, 490. [Google Scholar] [CrossRef]
- Xie, L.; Song, W.; Ge, J.; Tang, B.; Zhang, X.; Wu, T.; Ge, Z. Recent progress of organic photovoltaics for indoor energy harvesting. Nano Energy 2021, 82, 105770. [Google Scholar] [CrossRef]
- Su, Y.-J.; Huang, S.-C.; Chen, t.-W.; Chueh, L.-C.; Cui, Y.; Hong, L.; Yao, H.; Hou, J.; Chen, J.-T.; Hsu, C.-S. Elucidating End-Group Modifications of Carbazole-Based Nonfullerene Acceptors in Indoor Applications for Achieving a PCE of over 20%. ACS Appl. Mater. Interfaces 2021, 13, 26247. [Google Scholar] [CrossRef]
- Cui, Y.; Hong, L.; Hou, J. Organic Photovoltaic Cells for Indoor Applications: Opportunities and Challenges. ACS Appl. Mater. Interfaces 2020, 12, 38815. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Park, S.Y.; Lee, T.H.; Kim, J.Y.; Woo, H.Y. Recent progress in indoor organic photovoltaics. Nanoscale 2020, 12, 5792. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Oh, J.; Yang, U.J.; Lee, S.M.; Lee, J.; Jeong, M.; Cho, Y.; Kim, S.; Baik, J.M.; Yang, C. 3D Cu ball-based hybrid triboelectric nanogenerator with non-fullerene organic photovoltaic cells for self-powering indoor electronics. Nano Energy 2020, 77, 105271. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, Z.; Chen, X.; Yao, J.; Yan, B.; Sheng, R.; Zhu, H.; Yang, Y.M. 19.34 cm2 large-area quaternary organic photovoltaic module with 12.36% certified efficiency. Photonics Res. 2021, 9, 324. [Google Scholar] [CrossRef]
- Qin, F.; Sun, L.; Chen, H.; Liu, Y.; Lu, X.; Wang, W.; Liu, T.; Dong, X.; Jiang, P.; Jiang, Y.; Wang, L.; Zhou, Y. 54 cm2 Large-Area Flexible Organic Solar Modules with Efficiency Above 13%. Adv. Mater. 2021, 2103017. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, Y.; Sun, L.; Qin, F.; Zhou, X.; Lu, X.; Wang, W.; Zhou, Y. Large-Area Organic Solar Modules with Efficiency Over 14%. Adv. Funct. Mater. 2021, 32, 2110209. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Y.; Li, Y.; Li, Y. Large-area flexible organic solar cells. npj Flexible Electronics 2021, 5, 30. [Google Scholar] [CrossRef]
- Kini, G.P.; Jeon, S.J; Moon, D.K. Latest progress on photoabsorbent materials for multifunctional semitransparent organic solar cells. Adv. Funct. Mater. 2021, 31, 2007931. [Google Scholar] [CrossRef]
- Brus, V.V.; Lee, J.; Luginbuhl, B.R.; Ko, S.J.; Bazan, G.C.; Nguyen, T.Q. Solution-processed semitransparent organic photovoltaics: from molecular design to device performance. Adv. Mater. 2019, 31, 1900904. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Xu, G.; Xue, R.; Li, Y.; Zhou, Y.; Hou, J.; Li, Y. A semitransparent inorganic perovskite film for overcoming ultraviolet light instability of organic solar cells and achieving 14.03% efficiency. Adv. Mater. 2018, 30, 1800855. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394. [Google Scholar] [CrossRef]
- Xue, P.; Cheng, P.; Han, R.P.S.; Zhan, X. Printing fabrication of large-area non-fullerene organic solar cells. Mater. Horiz. 2022, 9, 194. [Google Scholar] [CrossRef]
- Wang, G.; Adil, M.A; Zhang, J.; We, Z. Large-Area Organic Solar Cells: Material Requirements, Modular Designs, and Printing Methods. Adv. Mater. 2019, 31, 1805089. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhandari, S.; Sundaram, S.; Mallick, T.K. Carbon counter electrode mesoscopic ambient processed & characterised perovskite for adaptive BIPV fenestration. Renew. Energy 2020, 145, 2151. [Google Scholar] [CrossRef]
- Cannavale, A.; Ierardi, L.; Hörantner, M.; Eperon, G.E.; Snaith, H.J.; Ayr, U.; Martellotta, F. Improving energy and visual performance in offices using building integrated perovskite-based solar cells: A case study in Southern Italy. Appl. Energy 2017, 205, 834. [Google Scholar] [CrossRef]
- Cannavale, A.; Hörantner, M.; Eperon, G.E.; Snaith, H.J.; Fiorito, F.; Ayr, U.; Martellotta, F. Building integration of semitransparent perovskite-based solar cells: Energy performance and visual comfort assessment. Appl. Energy 2017, 194, 94–107. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Dai, L. Efficiently photo-charging lithium-ion battery by perovskite solar cell. Nat. Commun. 2015, 6, 8103. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef]
- Güler, E.N.; Distler, A.; Basu, R.; Brabec, C.J.; Egelhaaf, E.-J. Fully solution-processed, light-weight, and ultraflexible organic solar cells. Flex. Print. Electron. 2022, 7, 025003. [Google Scholar] [CrossRef]
- Chen, X.; Xu, G.; Zeng, G.; Gu, H.; Chen, H.; Xu, H.; Yao, H.; Li, Y.; Hou, J.; Li, Y. Realizing ultrahigh mechanical flexibility and >15% efficiency of flexible organic solar cells via a “welding” flexible transparent electrode. Adv. Mater. 2020, 32, 1908478. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.; Wan, X.; Chen, Y. Recent progress in flexible organic solar cells. eScience 2022. [Google Scholar] [CrossRef]
- Li, X.; Xia, R.; Yan, K.; Ren, J.; Yip, H.-L.; Li, C.-Z.; Chen, H. Semitransparent organic solar cells with vivid colors. ACS Energy Lett. 2020, 5, 3115. [Google Scholar] [CrossRef]
- Jeong, E.G.; Jeon, Y.; Cho, S.H.; Choi, K.C. Textile-based washable polymer solar cells for optoelectronic modules: toward self-powered smart clothing. Energy Environ. Sci. 2019, 12, 1878. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Ramakrishna, S.; Aberle, A.G. Recent progress in flexible–wearable solar cells for self-powered electronic devices. Energy Environ. Sci. 2020, 13, 685. [Google Scholar] [CrossRef]
- Lv, D.; Jiang, Q.; Shang, Y. Highly efficient fiber-shaped organic solar cells toward wearable flexible electronics. npj Flex Electron 2022, 6, 38. [Google Scholar] [CrossRef]
- Nicolaidis, N.C.; Hollott, P.V.; Stanwell, B.; Gill, I.A.; Bull, J.E.; Bentsen, S.; Iredale, J.; Pappenfus, T.M.; Dastoor, P.C.; Feron, K.; Griffith, M.J.; Holmes, N.P. Developing a Portable Organic Solar Cell Kit Suitable for Students to Fabricate and Test Solar Cells in the Laboratory. J. Chem. Educ. 2020, 97, 3751. [Google Scholar] [CrossRef]
- Jahandar, M.; Kim, S.; Lim, D.C. Indoor organic photovoltaics for self-sustaining IoT devices: progress, challenges and practicalization. ChemSusChem 2021, 14, 1–27. [Google Scholar] [CrossRef]
- Hwang, S.; Yasuda, T. Indoor photovoltaic energy harvesting based on semiconducting π-conjugated polymers and oligomeric materials toward future IoT applications, Polym. J. 2022. [Google Scholar] [CrossRef]
- Arai, R.; Furukawa, S.; Hidaka, Y.; Komiyama, H.; Yasuda, T. High-performance organic energy-harvesting devices and modules for self-sustainable power generation under ambient indoor lighting environments. ACS Appl Mater Interfaces 2019, 11, 9259. [Google Scholar] [CrossRef]
- Luke, J.; Corrêa, L.; Rodrigues, J.; Martins, J.; Daboczi, M.; Bagnis, D.; Kim, J.-S. A Commercial Benchmark: Light-Soaking Free, Fully Scalable, Large-Area Organic Solar Cells for Low-Light Applications. Adv. Energy Mater. 2021, 11, 2003405. [Google Scholar] [CrossRef]
- Chen, B.; Baek, S.-W.; Hou, Y.; Aydin, E.; De Bastiani, M.; Scheffel, B.; Proppe, A.; Huang, Z.; Wei, M.; Wang, Y.-K.; Jung, E.-H. , Allen, T.G.; Van Kerschaver, E.; de Arquer, F.P.G.; Saidaminov, M.I.; Hoogland, S.; De Wolf, S.; Sargent, E.H. Enhanced optical path and electron diffusion length enable high-efficiency perovskite tandems. Nat. Commun. 2020, 11, 1257. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.H.; Wu, J.; Barbé, J.; Jain, S.M.; Wood, S.; Speller, E.M.; Li, Z.; Castro, F.A.; Durrant, J.R.; Tsoi, W.C. Organic photovoltaic cells-promising indoor light harvesters for self-sustainable electronics. J. Mater. Chem. A 2018, 6, 5618. [Google Scholar] [CrossRef]
- Burlingame, Q.; Song, B.; Ciammaruchi, L.; Zanotti, G.; Hankett, J.; Chen, Z.; Katz, E.A.; Forrest, S.R. Reliability of Small Molecule Organic Photovoltaics with Electron-Filtering Compound Buffer Layers. Adv. Energy Mater. 2016, 6, 1601094. [Google Scholar] [CrossRef]
- Peters, C.H.; Sachs-Quintana, I.-T.; Kastrop, J.P.; Beaupré, S.; Leclerc, M.; McGehee, M.D. High efficiency polymer solar cells with long operating lifetimes. Adv. Energy Mater. 2011, 1, 491. [Google Scholar] [CrossRef]
- Du, X.; Heumueller, T.; Gruber, W.; Classen, A.; Unruh, T.; Li, N.; Brabec, C.J. Efficient Polymer Solar Cells Based on Non-fullerene Acceptors with Potential Device Lifetime Approaching 10 Years. Joule 2019, 3, 215. [Google Scholar] [CrossRef]
- Burlingame, Q.; Huang, X.; Liu, X.; Forrest, S.R. Intrinsically stable organic solar cells under high-intensity illumination. Nature 2019, 573, 394. [Google Scholar] [CrossRef]
- Kawano, K.; Pacios, R.; Poplavskyy, D.; Nelson, J.; Bradley, D.D.C.; Durrant, J.R. Degradation of organic solar cells due to air exposure. Sol. Energy Mater. Sol. Cells 2006, 20, 3520. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.X.; Xu, G.; Chen, Z.-K.; Zhu, F. Degradation mechanisms in organic solar cells: Localized moisture encroachment and cathode reaction. Sol Energy Sol. Cells 2012, 104, 1–4. [Google Scholar] [CrossRef]
- Ye, L.; Hu, H.; Ghasemi, M.; Wang, T.; Collins, B.A.; Kim, J.-H.; Jiang, K.; Carpenter, J.H.; Li, H.; Li, Z.; McAfee, T.; Zhao, J.; Chen, X.; Lai, J.L.Y.; Ma, T.; Bredas, J.-L.; Yan, H.; Ade, H. Quantitative relations between interaction parameter, miscibility and function in organic solar cells. Nat. Mater. 2018, 17, 253–260. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mat. Sol. Cells 2008, 92, 686. [Google Scholar] [CrossRef]
- Pacios, R.; Chatten, A.J.; Kawano, K.; Durrant, J.R.; Bradley, D.D.C.; Nelson, J. Effects of Photo-oxidation on the Performance of Poly[2-methoxy-5-(3′,7′-dimethyloctyloxy)-1,4-phenylene vinylene]:[6,6]-Phenyl C61-Butyric Acid Methyl Ester Solar Cells. Adv. Funct. Mater. 2006, 16, 2117. [Google Scholar] [CrossRef]
- Howard, I.A.; Mauer, R.; Meister, M.; Laquai, F. Effect of Morphology on Ultrafast Free Carrier Generation in Polythiophene:Fullerene Organic Solar Cells. J. Am. Chem. Soc. 2010, 132, 14866. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yuan, Y.; Yang, B.; VanDerslice, J.; Chen, J.; Dyck, O.; Duscher, G.; Huang, J. Universal formation of compositionally graded bulk heterojunction for efficiency enhancement in organic photovoltaics. Adv. Mater. 2014, 26, 3068. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Wang, Z.; Ma, W.; Xie, Z.; Liu, J.; Wang, L. A Cross-Linkable Donor Polymer as the Underlying Layer to Tune the Active Layer Morphology of Polymer Solar Cells. Adv. Funct. Mater. 2016, 26, 226. [Google Scholar] [CrossRef]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photon. 2015, 9, 174. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photon. 2012, 6, 591. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Tsao, C.-S.; Lin, H.-K.; Cha, H.-C.; Chung, T.-Y.; Sung, Y.-M.; Huang, Y.-C. Encapsulation improvement and stability of ambient roll-to-roll slot-die-coated organic photovoltaic modules. Sol. Energy 2021, 213, 136. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Hong, K.; Messman, J.M.; Pickel, D.L.; Xiao, K.; Dadmun, M.D.; Mays, J.W.; Rondinone, A.J.; Sumpterand, B.G.; Kilbey II, S.M. , Ternary Behavior and Systematic Nanoscale Manipulation of Domain Structures in P3HT/PCBM/P3HT-b-PEO Films. J. Mater. Chem. 2012, 22, 13013. [Google Scholar] [CrossRef]
- Chen, H.P.; Chen, J.H.; Yin, W.; Yu, X.; Shao, M.; Xiao, K.; Hong, K.L.; Pickel, D.L.; Kochemba, W.M.; Kilbey II, S.M.; Dadmun, M. Correlation of Polymeric Compatibilizer Structure to its Impact on the Morphology and Function of P3HT:PCBM Bulk Heterojunctions. J. Mater. Chem. A 2013, 1, 5309. [Google Scholar] [CrossRef]
- Cheng, P.; Zhan, X. Versatile Third Components for Efficient and Stable Organic Solar Cells. Mater. Horiz. 2015, 2, 462. [Google Scholar] [CrossRef]
- An, Q.; Zhang, F.; Zhang, J.; Tang, W.; Deng, Z.; Hu, B. Versatile Ternary Organic Solar Cells: A Critical Review. Energy Environ. Sci. 2016, 9, 281. [Google Scholar] [CrossRef]
- Balis, N.; Verykios, A.; Soultati, A.; Constantoudis, V.; Papadakis, M.; Kournoutas, F.; Drivas, C.; Skoulikidou, M.-C.; Gardelis, S.; Fakis, M.; Kennou, S.; Kontos, A.G.; Coutsolelos, A.G.; Falaras, P.; Vasilopoulou, M. Triazine-substituted zinc porphyrin as an electron transport interfacial material for efficiency enhancement and degradation retardation in planar perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 3216. [Google Scholar] [CrossRef]
- Tountas, M.; Verykios, A.; Polydorou, E.; Kaltzoglou, A.; Soultati, A.; Balis, N.; Angaridis, P.A.; Papadakis, M.; Nikolaou, V.; Auras, F.; Palilis, L.C.; Tsikritzis, D.; Evangelou, E.K.; Gardelis, S.; Koutsoureli, M.; Papaioannou, G.; Petsalakis, I.D.; Kennou, S.; Davazoglou, D.; Argitis, P.; Falaras, P.; Coutsolelos, A.G.; Vasilopoulou, M. Engineering of porphyrin molecules for use as effective cathode interfacial modifiers in organic solar cells of enhanced efficiency and stability, ACS Appl. Mater. Interfaces. 2018, 10, 20728. [Google Scholar] [CrossRef]
- Piradi, V.; Xu, X.; Wang, Z.; Ali, J.; Peng, Q.; Liu, F.; Zhu, X. Panchromatic ternary organic solar cells with porphyrin dimer and absorption-complementary benzodithiophene-based small molecules. ACS Appl. Mater. Interfaces 2019, 11, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Kadish, K.M.; Boisselier-Cocolios, B.; Coutsolelos, A.; Mitaine, P.; Guilard, R. Electrochemistry and Spectroelectrochemistry of Gallium(III) Porphyrins. Redox properties of Five-Coordinate Ionic and Sigma-Bonded Complexes. Inorg. Chem 1985, 24, 4521–4528. [Google Scholar] [CrossRef]
- Coutsolelos, A.; Guilard, R. Synthese et caracteristiques physicochimiques de gallioporphyrines a laison σ metal carbone. J. Organometallic Chem. 1983, 253, 273–282. [Google Scholar] [CrossRef]
- Coutsoleleos, A.; Guilard, R.; Bayeul, D.; Lecomte, C. Gallium (III) porphyrins: synthesis and physicochemical characteristics of halogeno gallium (III) porphyrins-X-ray crystal structure of chloro-(5,10,15,20-tetraphenylporphyrinato) gallium (III). Polyhedron 1986, 5, 1157–1164. [Google Scholar] [CrossRef]
- Bieniasz, L.K. Cyclic Voltammetric Current Functions Determined with a Prescribed Accuracy by the Adaptive Huber Method for Abel Integral Equations. Anal. Chem. 2008, 80, 9659–9665. [Google Scholar] [CrossRef]
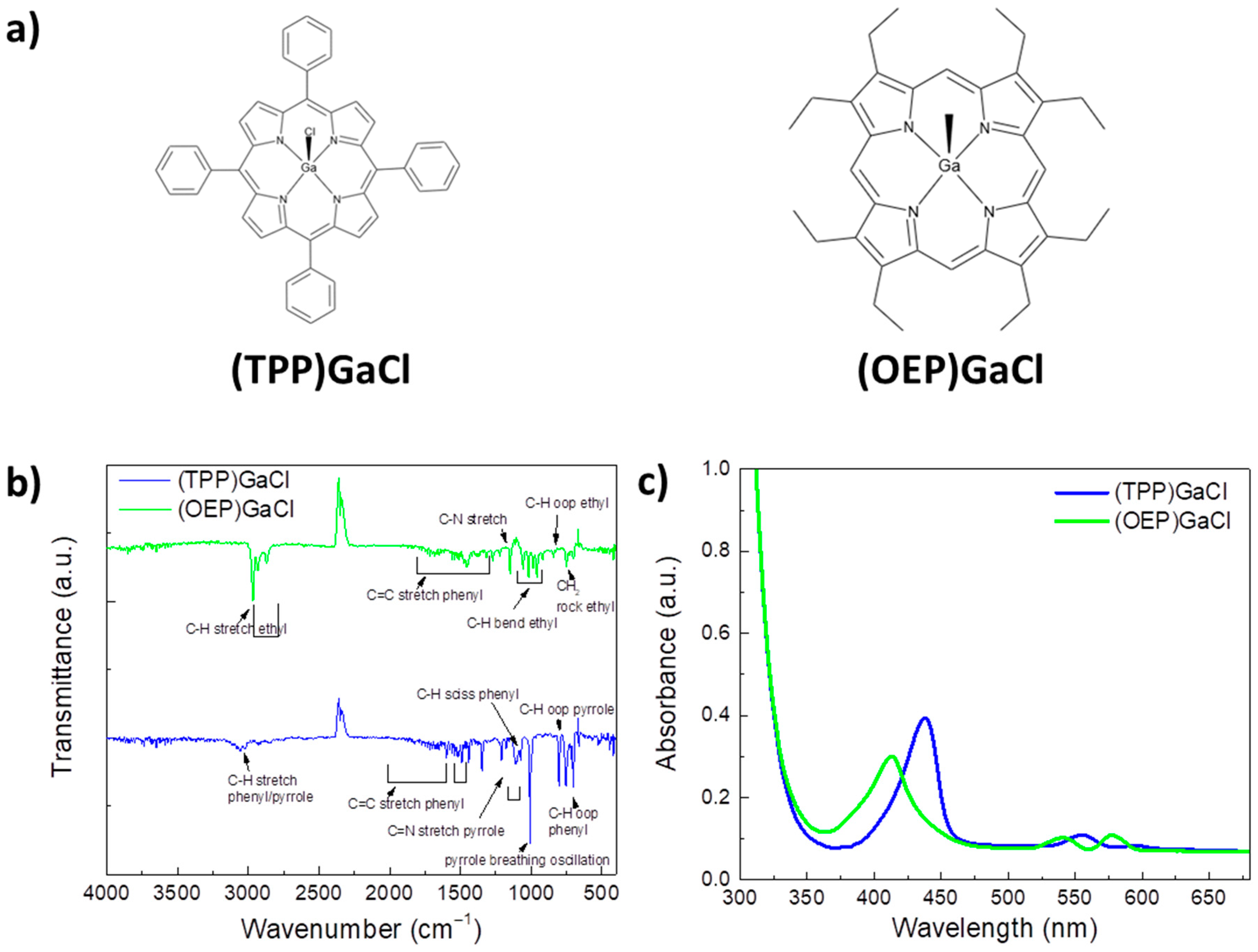
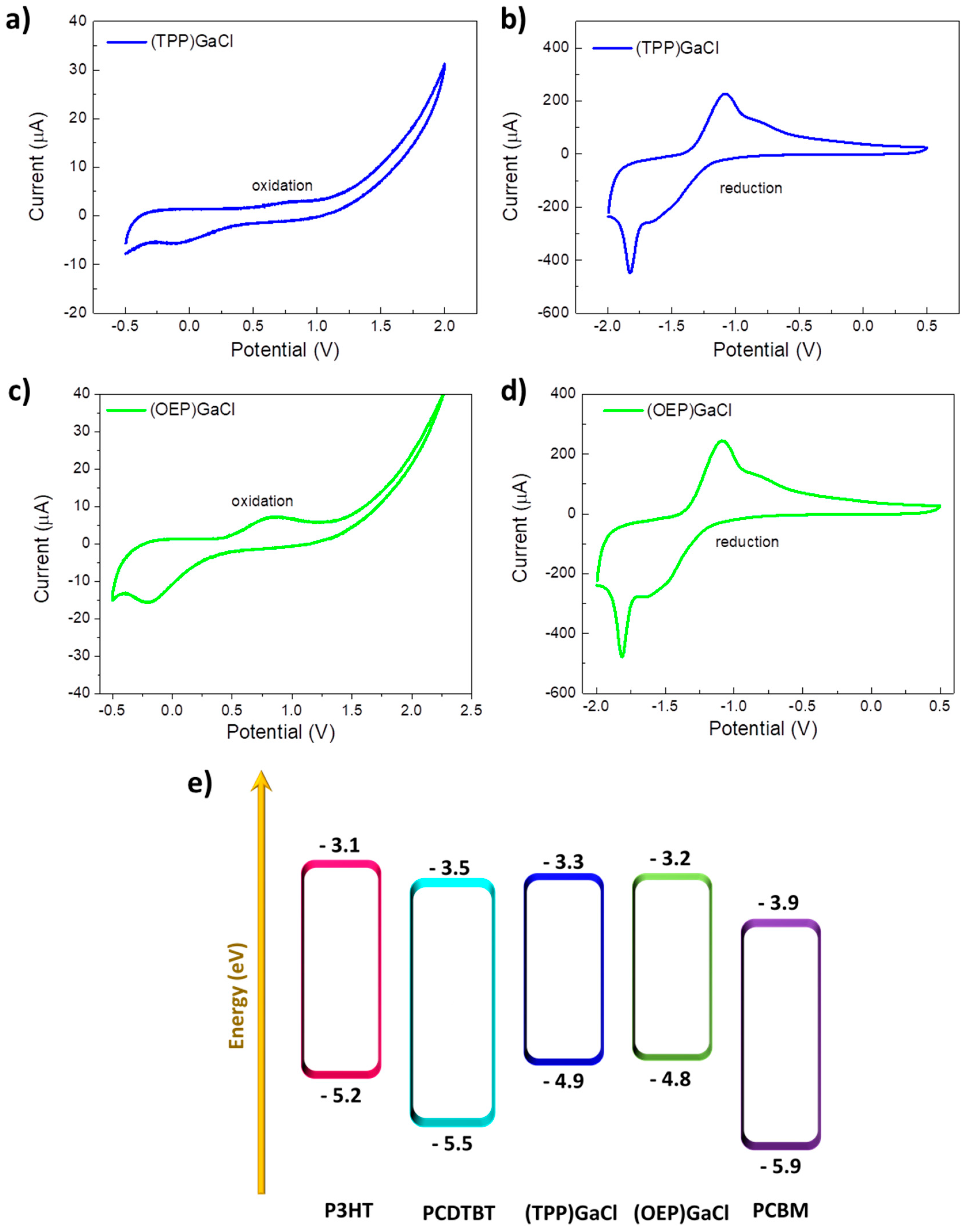
| Porphyrin | Eox (eV) | Ered (eV) | EHOMO | ELUMO |
|---|---|---|---|---|
| (TTP)GaCl | 0.5 | -1.1 | -4.9 | -3.3 |
| (OEP)GaCl | 0.4 | -1.2 | -4.8 | -3.2 |
| Active layer | JSC(mA cm-2) | VOC (V) | FF | PCE (%) | RS (Ω cm2) |
RSH (Ω cm2) |
|---|---|---|---|---|---|---|
| PCDTBT:PCBM | -10.66 | 0.77 | 0.48 | 3.94 | 11.5 | 401 |
| PCDTBT:PCBM:(TTP)GaCl 1:0.05 | -11.14 | 0.78 | 0.53 | 4.61 | 8.2 | 489 |
| P3HT:PCBM | -12.09 | 0.60 | 0.50 | 3.63 | 5.3 | 289 |
| P3HT:PCBM:(TTP)GaCl 1:0.05 | -12.90 | 0.61 | 0.54 | 4.25 | 3.7 | 318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





