Submitted:
03 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Chemicals
2.3. Stock Solutions, Calibration Standards (STDs), Quality Controls (QCs), Plasma Patients Samples
2.4. Sample Preparation
2.5. Chromatographic System and Conditions
2.6. Statistical analysis
3. Results
3.1. Study Population
3.2. Imatinib Pharmacokinetics
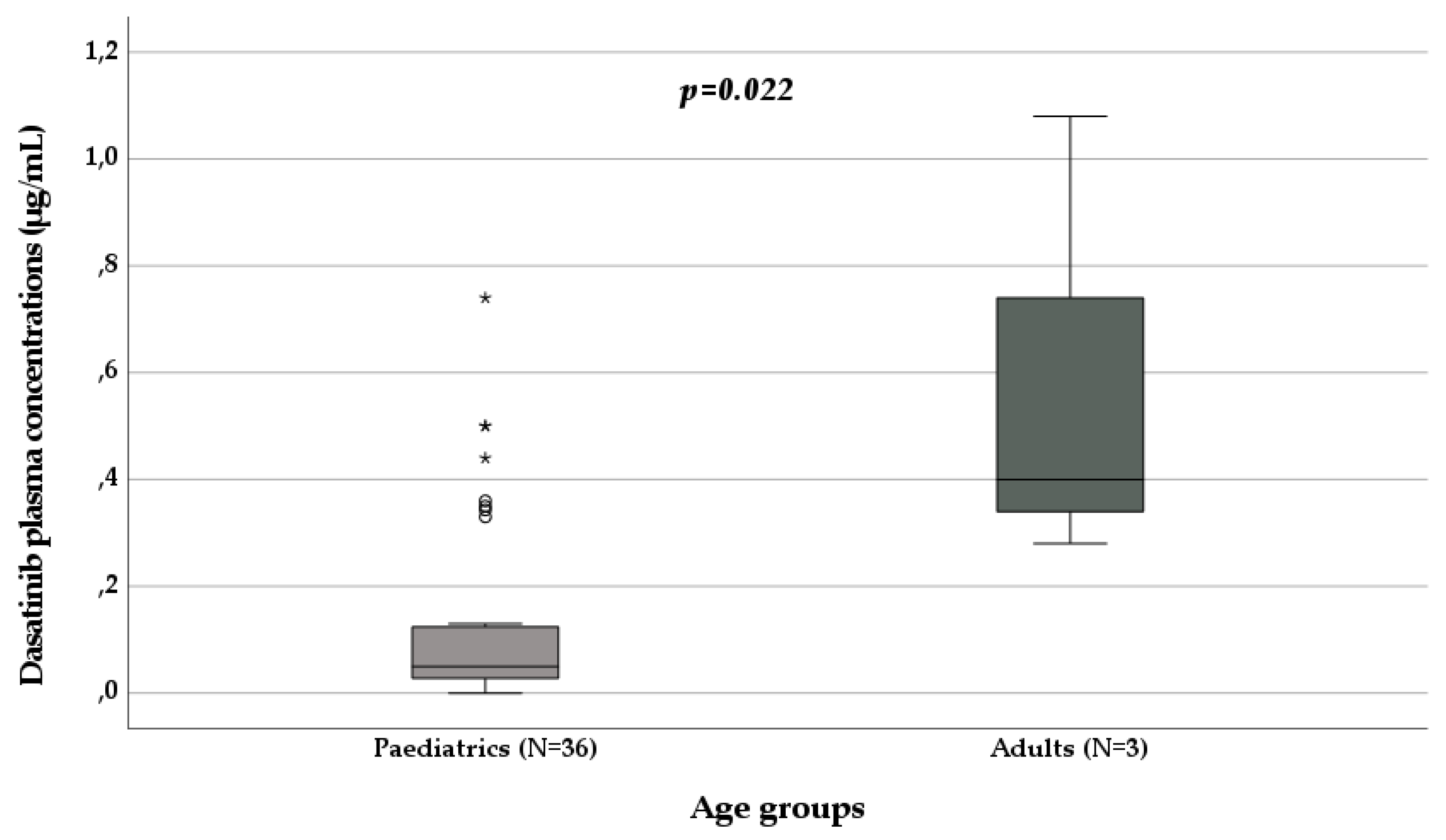
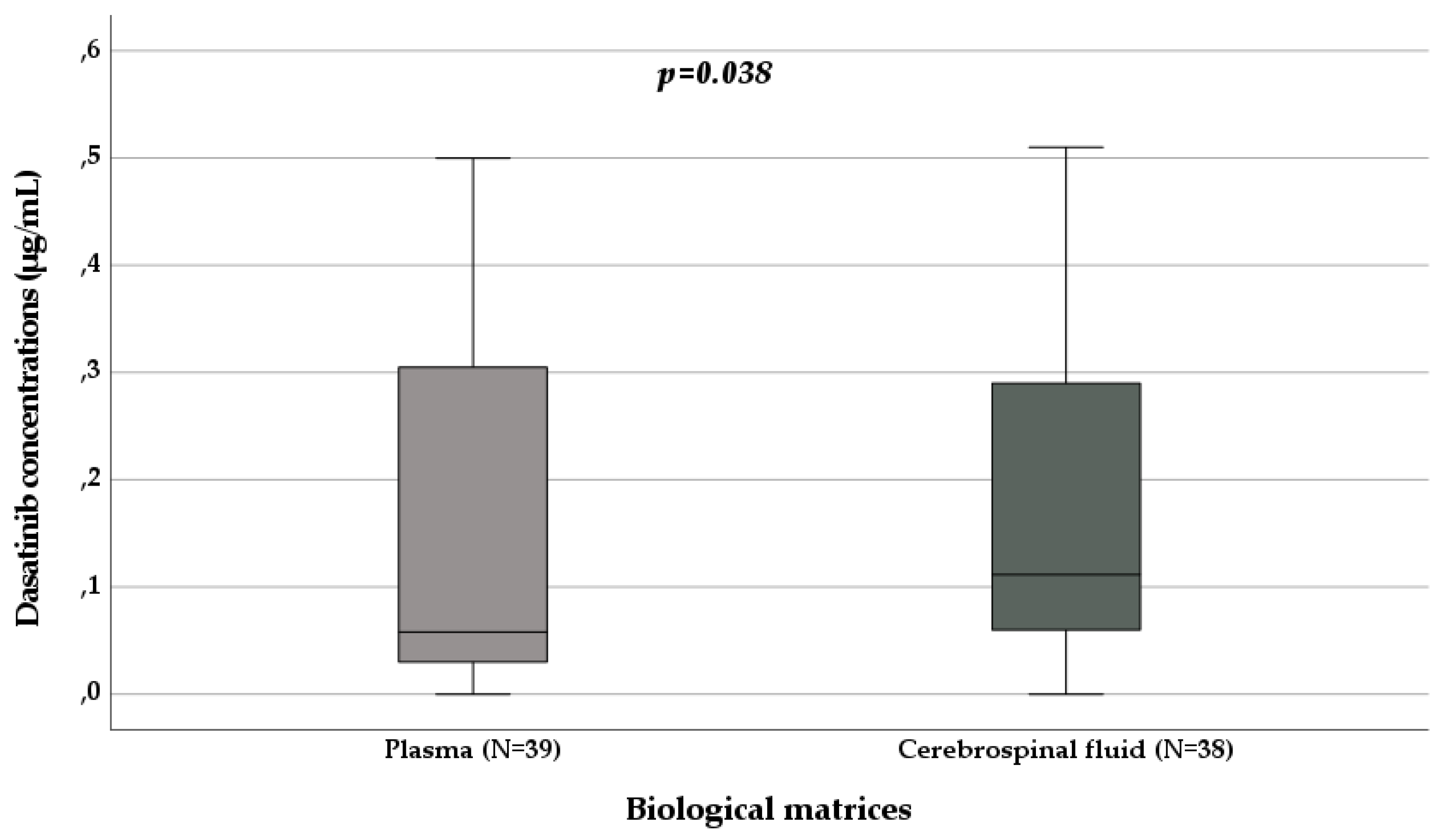
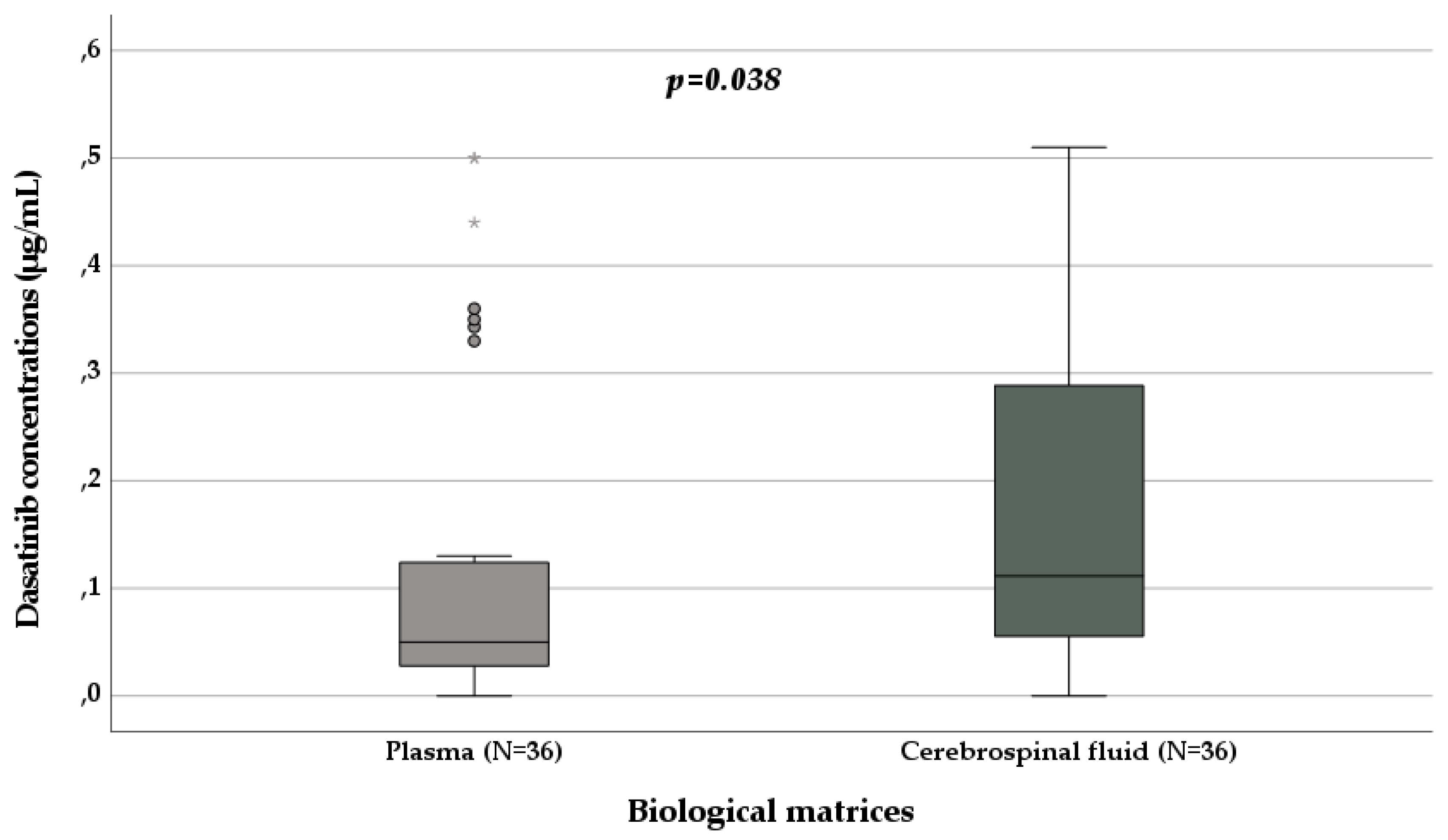
3.3. Dasatinib Pharmacokinetics
3.4. Nilotinib and Ponatinib Pharmacokinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gale, R.P. Radiation and leukaemia: Which leukaemias and what doses? Blood Rev 2023, 58, 101017. [CrossRef]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of chronic myeloid leukemia in 2023 - common ground and common sense. Blood Cancer J 2023, 13, 58. [CrossRef]
- Rosti, G.; Castagnetti, F.; Gugliotta, G.; Baccarani, M. Tyrosine kinase inhibitors in chronic myeloid leukaemia: Which, when, for whom? Nat Rev Clin Oncol 2017, 14, 141-154. [CrossRef]
- Groenland, S.L.; Mathijssen, R.H.J.; Beijnen, J.H.; Huitema, A.D.R.; Steeghs, N. Individualized dosing of oral targeted therapies in oncology is crucial in the era of precision medicine. Eur J Clin Pharmacol 2019, 75, 1309-1318. [CrossRef]
- Clarke, W.A.; Chatelut, E.; Fotoohi, A.K.; Larson, R.A.; Martin, J.H.; Mathijssen, R.H.J.; Salamone, S.J. Therapeutic drug monitoring in oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology consensus guidelines for imatinib therapy. Eur J Cancer 2021, 157, 428-440. [CrossRef]
- Cheng, F.; Li, Q.; Cui, Z.; Hong, M.; Li, W.; Zhang, Y. Dose optimization strategy of the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib for chronic myeloid leukemia: From clinical trials to real-life settings. Front Oncol 2023, 13, 1146108. [CrossRef]
- He, S.; Bian, J.; Shao, Q.; Zhang, Y.; Hao, X.; Luo, X.; Feng, Y.; Huang, L. Therapeutic Drug Monitoring and Individualized Medicine of Dasatinib: Focus on Clinical Pharmacokinetics and Pharmacodynamics. Front Pharmacol 2021, 12, 797881. [CrossRef]
- Tian, X.; Zhang, H.; Heimbach, T.; He, H.; Buchbinder, A.; Aghoghovbia, M.; Hourcade-Potelleret, F. Clinical Pharmacokinetic and Pharmacodynamic Overview of Nilotinib, a Selective Tyrosine Kinase Inhibitor. J Clin Pharmacol 2018, 58, 1533-1540. [CrossRef]
- Poch Martell, M.; Sibai, H.; Deotare, U.; Lipton, J.H. Ponatinib in the therapy of chronic myeloid leukemia. Expert Rev Hematol 2016, 9, 923-932. [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct Target Ther 2021, 6, 201. [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966-984. [CrossRef]
- Hughes, T.P.; Saglio, G.; Kantarjian, H.M.; Guilhot, F.; Niederwieser, D.; Rosti, G.; Nakaseko, C.; De Souza, C.A.; Kalaycio, M.E.; Meier, S.; et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood 2014, 123, 1353-1360. [CrossRef]
- Jabbour, E.; Kantarjian, H.M.; Saglio, G.; Steegmann, J.L.; Shah, N.P.; Boqué, C.; Chuah, C.; Pavlovsky, C.; Mayer, J.; Cortes, J.; et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014, 123, 494-500. [CrossRef]
- Cortes, J.E.; Gambacorti-Passerini, C.; Deininger, M.W.; Mauro, M.J.; Chuah, C.; Kim, D.W.; Dyagil, I.; Glushko, N.; Milojkovic, D.; le Coutre, P.; et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. J Clin Oncol 2018, 36, 231-237. [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044-1054. [CrossRef]
- Kantarjian, H.M.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Boquimpani, C.; Pasquini, R.; Clark, R.E.; et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia 2021, 35, 440-453. [CrossRef]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol 2016, 34, 2333-2340. [CrossRef]
- Kizaki, M.; Takahashi, N.; Iriyama, N.; Okamoto, S.; Ono, T.; Usui, N.; Inokuchi, K.; Nakaseko, C.; Kurokawa, M.; Sumi, M.; et al. Efficacy and safety of tyrosine kinase inhibitors for newly diagnosed chronic-phase chronic myeloid leukemia over a 5-year period: Results from the Japanese registry obtained by the New TARGET system. Int J Hematol 2019, 109, 426-439. [CrossRef]
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing chronic myeloid leukemia for treatment-free remission: A proposal from the GIMEMA CML WP. Blood Adv 2019, 3, 4280-4290. [CrossRef]
- Oehler, V.G. First-generation vs second-generation tyrosine kinase inhibitors: Which is best at diagnosis of chronic phase chronic myeloid leukemia? Hematology Am Soc Hematol Educ Program 2020, 2020, 228-236. [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol 2020, 95, 691-709. [CrossRef]
- Höglund, M.; Sandin, F.; Hellström, K.; Björeman, M.; Björkholm, M.; Brune, M.; Dreimane, A.; Ekblom, M.; Lehmann, S.; Ljungman, P.; et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: Report from the population-based Swedish CML registry. Blood 2013, 122, 1284-1292. [CrossRef]
- Rohrbacher, M.; Hasford, J. Epidemiology of chronic myeloid leukaemia (CML). Best Pract Res Clin Haematol 2009, 22, 295-302. [CrossRef]
- Berger, U.; Maywald, O.; Pfirrmann, M.; Lahaye, T.; Hochhaus, A.; Reiter, A.; Hasford, J.; Heimpel, H.; Hossfeld, D.K.; Kolb, H.J.; et al. Gender aspects in chronic myeloid leukemia: Long-term results from randomized studies. Leukemia 2005, 19, 984-989. [CrossRef]
- Peng, B.; Lloyd, P.; Schran, H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet 2005, 44, 879-894. [CrossRef]
- Aziz, Z.; Iqbal, J.; Aaqib, M.; Akram, M.; Saeed, A. Assessment of quality of life with imatinib mesylate as first-line treatment in chronic phase-chronic myeloid leukemia. Leuk Lymphoma 2011, 52, 1017-1023. [CrossRef]
- Lee, J.P.; Birnstein, E.; Masiello, D.; Yang, D.; Yang, A.S. Gender and ethnic differences in chronic myelogenous leukemia prognosis and treatment response: A single-institution retrospective study. J Hematol Oncol 2009, 2, 30. [CrossRef]
- Björkholm, M.; Ohm, L.; Eloranta, S.; Derolf, A.; Hultcrantz, M.; Sjöberg, J.; Andersson, T.; Höglund, M.; Richter, J.; Landgren, O.; et al. Success story of targeted therapy in chronic myeloid leukemia: A population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol 2011, 29, 2514-2520. [CrossRef]
- Yamaguchi, H.; Takezako, N.; Ohashi, K.; Oba, K.; Kumagai, T.; Kozai, Y.; Wakita, H.; Yamamoto, K.; Fujita, A.; Igarashi, T.; et al. Treatment-free remission after first-line dasatinib treatment in patients with chronic myeloid leukemia in the chronic phase: The D-NewS Study of the Kanto CML Study Group. Int J Hematol 2020, 111, 401-408. [CrossRef]
- Kimura, S.; Imagawa, J.; Murai, K.; Hino, M.; Kitawaki, T.; Okada, M.; Tanaka, H.; Shindo, M.; Kumagai, T.; Ikezoe, T.; et al. Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): A single-arm, multicentre, phase 2 trial. Lancet Haematol 2020, 7, e218-e225. [CrossRef]
- Shah, N.P.; García-Gutiérrez, V.; Jiménez-Velasco, A.; Larson, S.; Saussele, S.; Rea, D.; Mahon, F.X.; Levy, M.Y.; Gómez-Casares, M.T.; Pane, F.; et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: The DASFREE study. Leuk Lymphoma 2020, 61, 650-659. [CrossRef]
- Lyseng-Williamson, K.; Jarvis, B. Imatinib. Drugs 2001, 61, 1765-1774; discussion 1775-1766. [CrossRef]
- Dagher, R.; Cohen, M.; Williams, G.; Rothmann, M.; Gobburu, J.; Robbie, G.; Rahman, A.; Chen, G.; Staten, A.; Griebel, D.; et al. Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res 2002, 8, 3034-3038.
- Pindolia, V.K.; Zarowitz, B.J. Imatinib mesylate, the first molecularly targeted gene suppressor. Pharmacotherapy 2002, 22, 1249-1265. [CrossRef]
- Champagne, M.A.; Capdeville, R.; Krailo, M.; Qu, W.; Peng, B.; Rosamilia, M.; Therrien, M.; Zoellner, U.; Blaney, S.M.; Bernstein, M.; et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: Results from a Children’s Oncology Group phase 1 study. Blood 2004, 104, 2655-2660. [CrossRef]
- Larson, R.A.; Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Riviere, G.J.; Krahnke, T.; Gathmann, I.; Wang, Y.; Group, I.I.R.I.v.S.S. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: A subanalysis of the IRIS study. Blood 2008, 111, 4022-4028. [CrossRef]
- Wilkinson, G.R. Cytochrome P4503A (CYP3A) metabolism: Prediction of in vivo activity in humans. J Pharmacokinet Biopharm 1996, 24, 475-490. [CrossRef]
- Porkka, K.; Koskenvesa, P.; Lundán, T.; Rimpiläinen, J.; Mustjoki, S.; Smykla, R.; Wild, R.; Luo, R.; Arnan, M.; Brethon, B.; et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 2008, 112, 1005-1012. [CrossRef]
- Frigeri, F.; Arcamone, M.; Luciano, L.; Di Francia, R.; Pane, F.; Pinto, A. Systemic dasatinib fails to prevent development of central nervous system progression in a patient with BCR-ABL unmutated Philadelphia chromosome-positive leukemia. Blood 2009, 113, 5028-5029. [CrossRef]
- Papageorgiou, S.G.; Pappa, V.; Economopoulou, C.; Tsirigotis, P.; Konsioti, F.; Ionnidou, E.D.; Chondropoulos, S.; Vasilatou, D.; Papageorgiou, E.; Economopoulos, T.; et al. Dasatinib induces long-term remission in imatinib-resistant Philadelphia chromosome-positive acute megakaryoblastic leukemia but fails to prevent development of central nervous system progression. Leuk Res 2010, 34, e254-256. [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017, 7, 42717. [CrossRef]
- Zhou, H.S.; Dai, M.; Wei, Y.; Wang, Q.; Xu, N.; Yin, C.; Meng, F. Isolated central nervous system relapse in patient with blast-crisis chronic myeloid leukemia in durable complete cytogenetic remission on dasatinib treatment: Pharmacokinetics and ABL mutation analysis in cerebrospinal fluid. Leuk Lymphoma 2013, 54, 1557-1559. [CrossRef]
- Kondo, T.; Tasaka, T.; Matsumoto, K.; Matsumoto, R.; Koresawa, L.; Sano, F.; Tokunaga, H.; Matsuhashi, Y.; Nakanishi, H.; Morita, K.; et al. Philadelphia chromosome-positive acute lymphoblastic leukemia with extramedullary and meningeal relapse after allogeneic hematopoietic stem cell transplantation that was successfully treated with dasatinib. Springerplus 2014, 3, 177. [CrossRef]
- Levêque, D.; Becker, G.; Bilger, K.; Natarajan-Amé, S. Clinical Pharmacokinetics and Pharmacodynamics of Dasatinib. Clin Pharmacokinet 2020, 59, 849-856. [CrossRef]
- Gong, X.; Li, L.; Wei, H.; Liu, B.; Zhou, C.; Zhang, G.; Liu, K.; Lin, D.; Gong, B.; Wei, S.; et al. A Higher Dose of Dasatinib May Increase the Possibility of Crossing the Blood-brain Barrier in the Treatment of Patients With Philadelphia Chromosome-positive Acute Lymphoblastic Leukemia. Clin Ther 2021, 43, 1265-1271.e1261. [CrossRef]

| All | Imatinib | Dasatinib | Nilotinib | Ponatinib | |
|---|---|---|---|---|---|
| N | 153 | 60 | 77 | 2 | 14 |
| Males (N, %) | 126, 82.4 | 33, 55 | 77, 100 | 2 | 14 |
| Females (N, %) | 27, 17.6 | 27, 45 | 0 | 0 | 0 |
| Median age (years; IQR) | 14 (12-17) | 15 (25-56.75) | 13 (12-17) | 52 (50-54) | 14 (13-16.5) |
| Paediatrics (N, %) | 116, 75.8 | 31, 51.7 | 72, 93.5 | 0 | 13, 92.9 |
| Median paediatric age (years; IQR) | 13 (12-14) | 13 (9-14) | 13 (12-14) | / | 14 (13-14) |
| Adults (N, %) | 37, 24.2 | 29, 48.3 | 5, 6.5 | 2, 100 | 1, 7.1 |
| Median adult age (years; IQR) | 54 (42.5-58) | 57 (50.5-59) | 18 (18-48) | 52 (50-54) | / |
| Plasma sampling (N, %) | 111, 72.5 | 60, 100 | 39, 50.6 | 2, 100 | 10, 71.4 |
| Plasmatic concentrations (µg/mL) | / | 1.82 (0.88-2.93) | 0.06 (0.03-0.33) | 0.19 (0.16-0.22) | 0.19 (0.15-0.51) |
| Cerebrospinal fluid sampling (N, %) | 42, 27.5 | 0 | 38, 49.4 | 0 | 4, 28.6 |
| Cerebrospinal fluid concentrations (µg/mL) | / | / | 0.11 (0.06-0.32) | / | 0.65 (0.18-1.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




