Submitted:
11 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
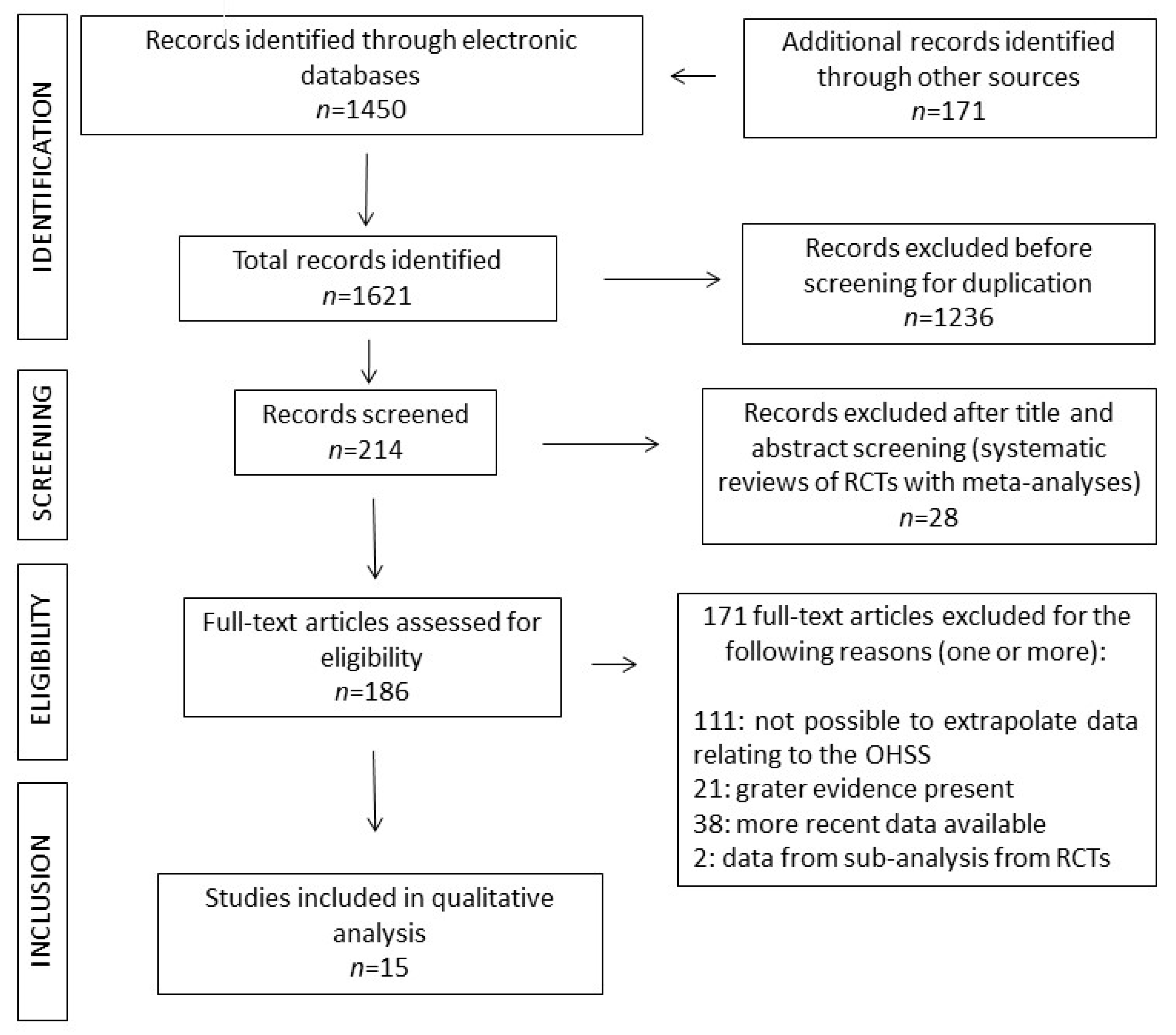
2.1. Literature Search
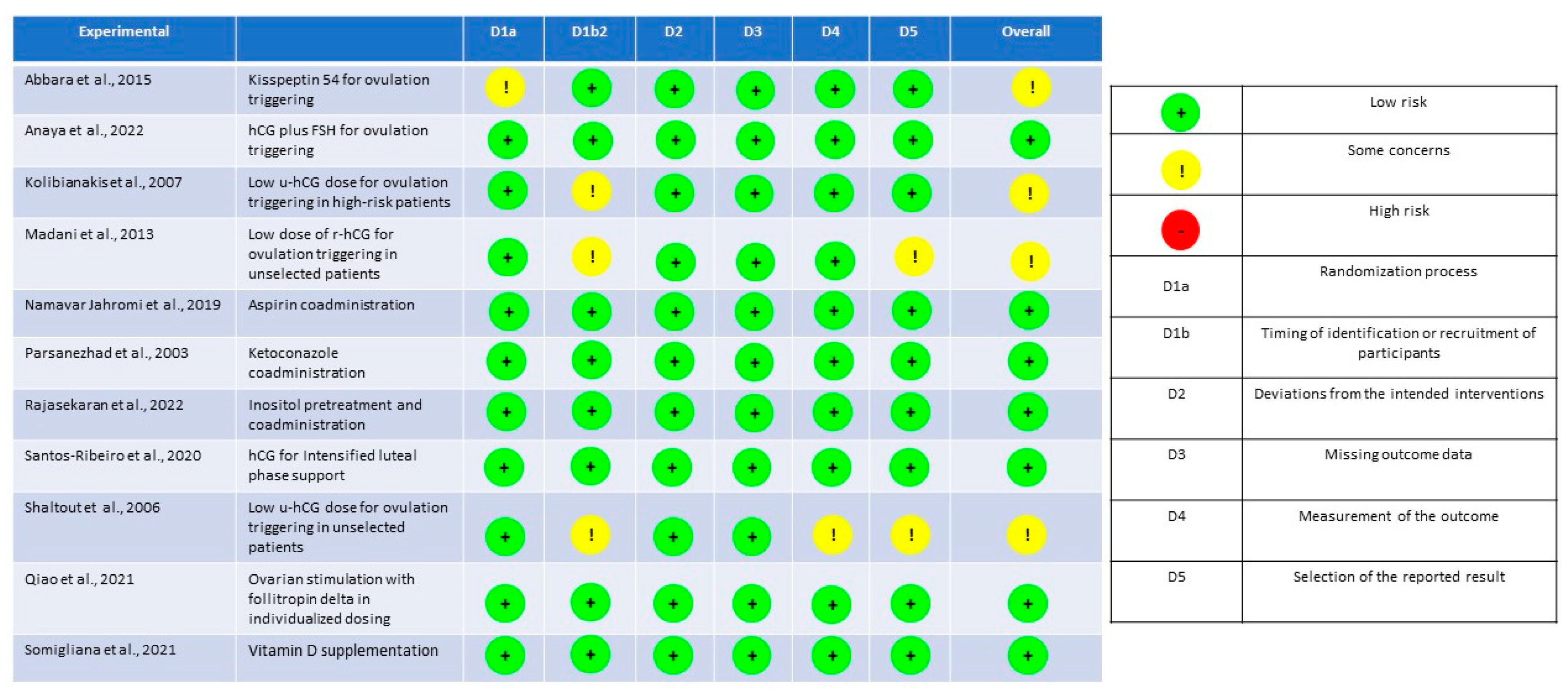
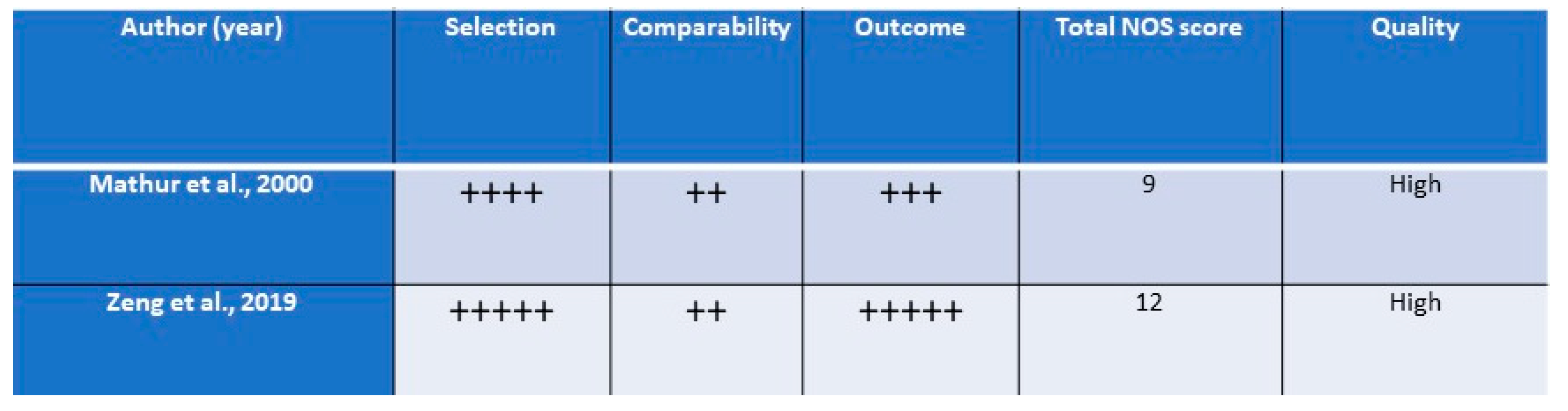
2.2. Quality Assessment and Data Analysis
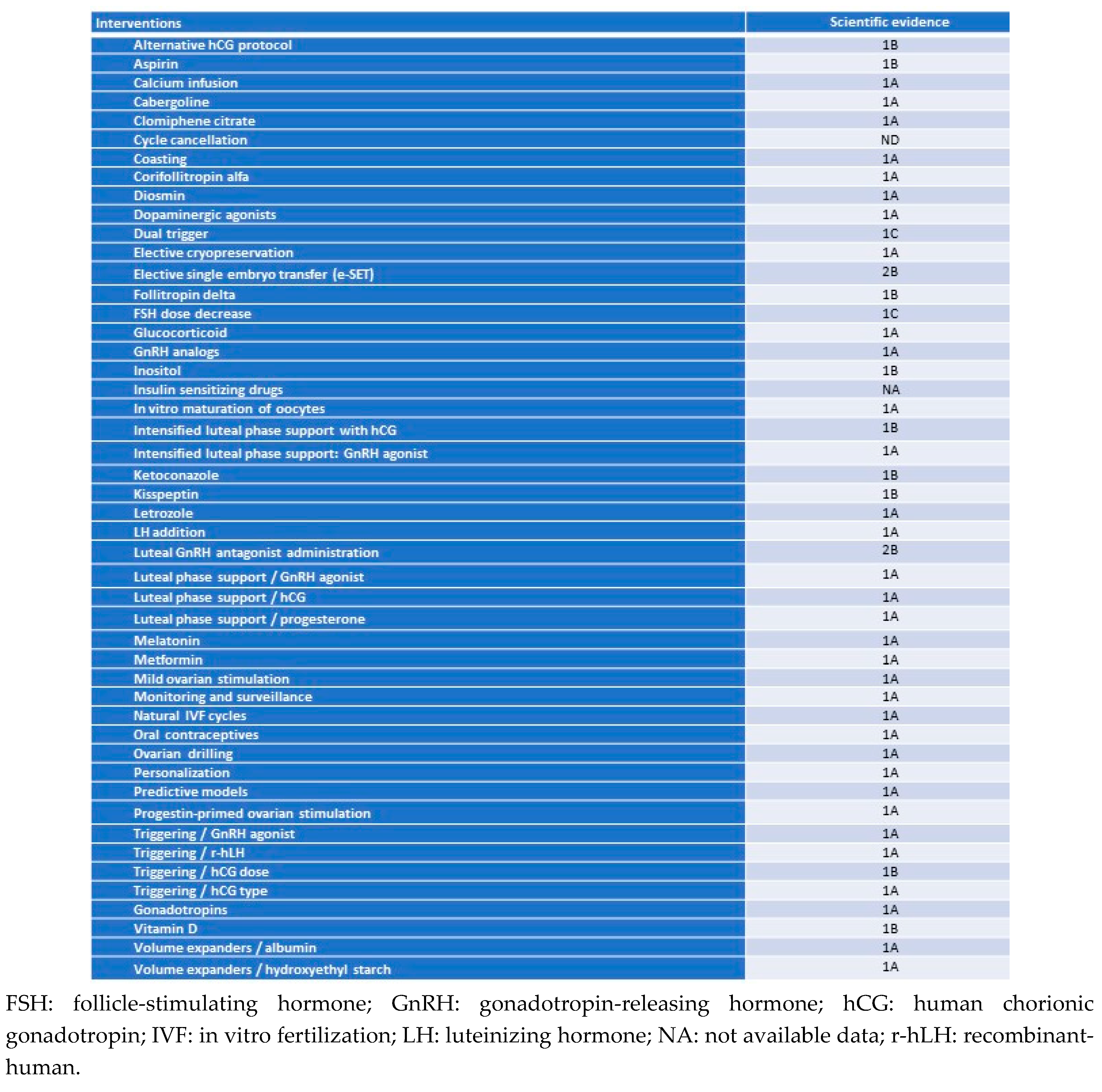
3. Results
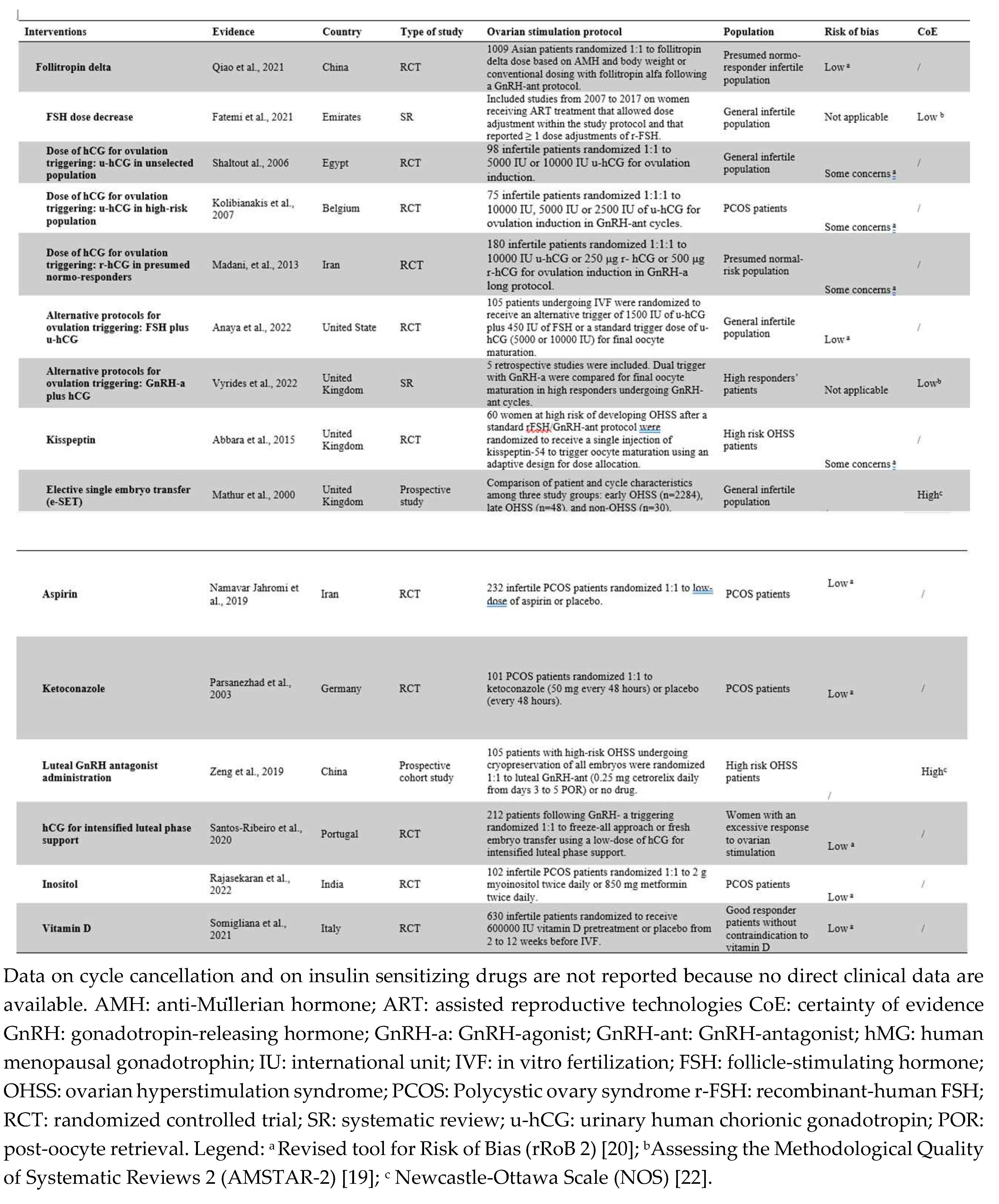
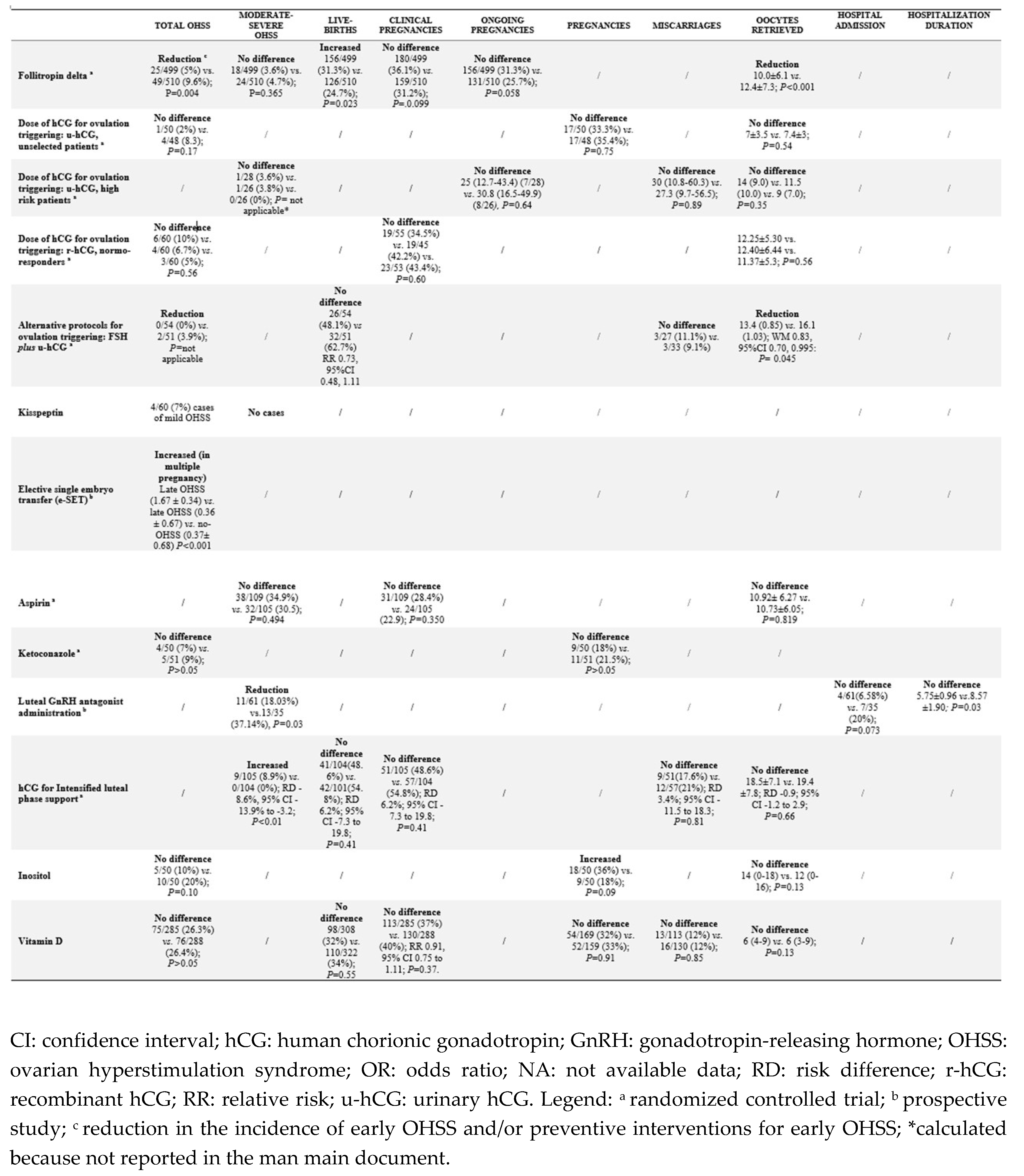
3.1. Use of Follitropin Delta for Ovarian Stimulation
3.2. FSH Dose Decrease for Ovarian Stimulation
3.3. Lower Doses of hCG for Ovulation Triggering
3.3.1. u-hCG
3.3.2. r-hCG
3.4. Alternative Protocols for Ovulation Triggering
3.4.1. u-hCG Plus FSH for Ovulation Triggering
3.4.2. GnRH-a Plus hCG vs. GnRH-a vs. hCG
3.4.3. Kisspeptin
3.5. Cycle Cancellation
3.6. Elective Single Embryo Transfer (e-SET)
3.7. Aspirin
3.8. Ketoconazole
3.9. Luteal GnRH-ant Administration
3.10. hCG Administration for Intensified Luteal Phase Support
3.11. Inositol
3.12. Insulin Sensitizing Drugs (ISDs)
3.13. Vitamin D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green-top guideline, No. 5: The management of ovarian hyperstimulation syndrome. Available online: https://www.rcog.org.uk/media/or1jqxbf/gtg_5_ohss.pdf (accessed on 11 January 2023).
- Humaidan, P.; Nelson, S.M.; Devroey, P.; Coddington, C.C.; Schwartz, L.B.; Gordon, K.; Frattarelli, J.L.; Tarlatzis, B.C.; Fatemi, H.M.; Lutjen, P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod 2016, 31, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.K.; Dubey, A.; Mittal, K.; Kale, S. Spontaneous ovarian hyperstimulation syndrome - understanding the dilemma. Gynecol Endocrinol 2015, 31, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Stimulation TEGGO.; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; Le Clef, N. et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open 2020, hoaa009. [CrossRef]
- Zaat, T.; Zagers, M.; Mol, F.; Goddijn, M.; van Wely, M, Mastenbroek, S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2021, 2, CD011184. [CrossRef]
- Roque, M.; Haahr, T.; Geber, S.; Esteves, S.C.; Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019, 25, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Shi, Y.; Sun, Y.; Zhang, B.; Liang, X.; Cao, Y.; Yang, J.; Liu, J.; Wei, D.; Weng, N.; Tian, L.; Hao, C.; Yang, D.; Zhou, F.; Shi, J.; Xu, Y.; Li, J.; Yan, J.; Qin, Y.; Zhao, H.; Zhang, H.; Legro, RS. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016, 375, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Wang, S.; Ye, X.; Zhang, D.; Hunt, S. GnRH agonist and hCG (dual trigger) versus hCG trigger for follicular maturation: a systematic review and meta-analysis of randomized trials. Reprod Biol Endocrinol 2021, 19, 78. [Google Scholar] [CrossRef]
- Ioannidou, P.G.; Bosdoum, J.K.; Lainas, G.T.; Lainas, T.G.; Grimbizis, G.F.; Kolibianakis, E.M. How frequent is severe ovarian hyperstimulation syndrome after GnRH agonist triggering in high-risk women? A systematic review and meta-analysis. Reprod Biomed Online 2021, 42, 635–650. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Kirsten, J.; D'Hooghe, T.; Sunkara, SK. Decision points for individualized hormonal stimulation with recombinant gonadotropins for treatment of women with infertility. Gynecol Endocrinol 2019, 35, 1027–1036. [Google Scholar] [CrossRef]
- Cozzolino, M.; Vitagliano, A.; Cecchino, G.N.; Ambrosini, G.; Garcia-Velasco, J.A. Corifollitropin alfa for ovarian stimulation in in vitro fertilization: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2019, 111, 722–733. [Google Scholar] [CrossRef]
- Fernández Sánchez, M.; Višnová, H.; Larsson, P.; Yding Andersen, C.; Filicori, M.; Blockeel, C.; Pinborg, A.; Khalaf, Y.; Mannaerts, B; Rainbow Study Group. A randomized, controlled, first-in-patient trial of choriogonadotropin beta added to follitropin delta in women undergoing ovarian stimulation in a long GnRH agonist protocol. Hum Reprod 2022, 37, 1161–1174. [Google Scholar] [CrossRef]
- Gates, M.; Gates, A.; Pieper, D.; Fernandes, RM.; Tricco, AC.; Moher, D.; Brennan, S.E.; Li, T.; Pollock, M.; Lunny,C. ; et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ 2022, 9, e070849. [Google Scholar] [CrossRef]
- Palomba, S.; Costanzi, F.; Nelson, S.M.; Caserta, D.; Humaidan, P. Interventions to prevent or reduce the incidence and severity of ovarian hyperstimulation syndrome: a systematic umbrella review of the best clinical evidence. Reprod Biol Endocrinol 2023, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, T.F.; Bruun Nielsen, M.F.; Lindhardt, C.L.; Eriksen, M.B. Using the full PICO model as a search tool for systematic reviews resulted in lower recall for some PICO elements. J Clin Epidemiol 2020, 127, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Puhan, MA.; Vedula, S.S.; Singh, S.; Dickersin, K. Ad Hoc Network Meta-analysis Methods Meeting Working Group. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med 2011, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Christofilos, S.I.; Tsikopoulos, K.; Tsikopoulos, A.; Kitridis, D.; Sidiropoulos, K.; Stoikos, P.N.; Kavarthapu, V. Network meta-analyses: methodological prerequisites and clinical usefulness. World J Methodol 2022, 12, 92–98. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions; or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán. M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 12, 355:i4919. [Google Scholar] [CrossRef]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O'Connell, D.; Peterson, J.; Welch Losos, M.; Tugwell, P.; Ga, SW.; Zello, G.A.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Diet Suppl 2013, 10, 381–390. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Kirsten, J.; D'Hooghe, T.; Sunkara, SK. Decision points for individualized hormonal stimulation with recombinant gonadotropins for treatment of women with infertility. Gynecol Endocrinol 2019, 35, 1027–1036. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Liang, X.; Song, X.; Wei, Z.; Liu, J.; Yang, Y.; Tan, J.; Zhang, Q.; Sun, Y.; Wang, W.; Qian, W.; Jin, L.; Wang, S.; Xu, Y.; Yang, J.; Goethberg, M.; Mannaerts, B.; Wu, W:; Zheng, Z, Qiao, J. Comparative clinical outcome following individualized follitropin delta dosing in Chinese women undergoing ovarian stimulation for in vitro fertilization /intracytoplasmic sperm injection. Reprod Biol Endocrinol 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Nyboe Andersen, A.; Nelson, S.M.; Fauser, B.C.; García-Velasco, J.A.; Klein, B.M.; Arce, J. C; ESTHER-1 study group. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril 2017, 107, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, M.; Visnova, H.; Yuzpe, A.; Klein, BM.; Mannaerts, B.; Arce JC; ESTHER-1 and ESTHER-2 Study Group. Individualization of the starting dose of follitropin delta reduces the overall OHSS risk and/or the need for additional preventive interventions: cumulative data over three stimulation cycles. Reprod Biomed Online 2019, 38, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, Y.; Liang, X.; Ho, T.; Huang, H.Y.; Kim, S.H.; Goethberg, M.; Mannaerts, B.; Arce, J.C. A randomised controlled trial to clinically validate follitropin delta in its individualised dosing regimen for ovarian stimulation in Asian IVF/ICSI patients. Hum Reprod 2021, 36, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, O.; Arce, J. C; Japanese Follitropin Delta Phase 3 Trial (STORK) Group. Individualized follitropin delta dosing reduces OHSS risk in Japanese IVF/ICSI patients: a randomized controlled trial. Reprod Biomed Online 2021, 42, 909–918. [Google Scholar] [CrossRef]
- Ishihara, O.; Klein, B.M.; Arce, J. C; Japanese Follitropin Delta Phase 2 Trial Group. Randomized, assessor-blind, antimüllerian hormone-stratified.; dose-response trial in Japanese in vitro fertilization/intracytoplasmic sperm injection patients undergoing controlled ovarian stimulation with follitropin delta. Fertil Steril 2021, 115, 1478–1486. [Google Scholar] [CrossRef]
- Ishihara, O.; Nelson, S.M.; Arce, J.C. Comparison of ovarian response to follitropin delta in Japanese and White IVF/ICSI patients. Reprod Biomed Online 2022, 44, 177–184. [Google Scholar] [CrossRef]
- Fatemi, H.; Bilger, W.; Denis, D.; Griesinger, G.; La Marca, A.; Longobardi, S.; Mahony, M.; Yin, X.; D'Hooghe, T. Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: a systematic review covering 10 years (2007-2017). Reprod Biol Endocrinol 2021, 19, 68. [Google Scholar] [CrossRef]
- Shaltout, A.; Eid, M.; Shohayeb, A. Does triggering ovulation by 5000 IU of uhCG affect ICSI outcome? Middle East Fertility Society Journal 2006, 11, 99–103. [Google Scholar]
- Kolibianakis, E.M.; Papanikolaou, E.G.; Tournaye, H.; Camus, M.; Van Steirteghem, A.C.; Devroey, P. Triggering final oocyte maturation using different doses of human chorionic gonadotropin: a randomized pilot study in patients with polycystic ovary syndrome treated with gonadotropin-releasing hormone antagonists and recombinant follicle-stimulating hormone. Fertil Steril 2007, 88, 1382–1388. [Google Scholar] [CrossRef]
- Tiboni, G.M.; Colangelo, E.C.; Ponzano, A. Reducing the trigger dose of recombinant hCG in high-responder patients attending an assisted reproductive technology program: an observational study. Drug Des Devel Ther 2016, 10, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.; Mohammadi Yeganeh, L.; Ezabadi, Z.; Hasani, F.; Chehrazi, M. Comparing the efficacy of urinary and recombinant hCG on oocyte/follicle ratio to trigger ovulation in women undergoing intracytoplasmic sperm injection cycles: a randomized controlled trial. J Assist Reprod Genet 2013, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tapanainen, J.S.; Lapolt, P.S.; Perlas, E.; Hsueh, AJ. Induction of ovarian follicle luteinization by recombinant follicle-stimulating hormone. Endocrinology 1993, 133, 2875–2880. [Google Scholar] [CrossRef] [PubMed]
- Zelinski-Wooten, M.B.; Hutchison, J.S.; Hess, D.L.; Wolf, D.P.; Stouffer, R.L. Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod 1995, 10, 1658–1666. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Leonardsen, L.; Ulloa-Aguirre, A.; Barrios-De-Tomasi, J.; Moore, L.; Byskov, A.G. FSH-induced resumption of meiosis in mouse oocytes: effect of different isoforms. Mol Hum Reprod 1999, 5, 726–731. [Google Scholar] [CrossRef]
- Andersen, C.Y. Effect of FSH and its different isoforms on maturation of oocytes from pre-ovulatory follicles. Reprod Biomed Online 2002, 5, 232–239. [Google Scholar] [CrossRef]
- Franciosi, F.; Manandhar, S.; Conti, M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence. Endocrinology 2016, 157, 872–882. [Google Scholar] [CrossRef]
- Rice, V.C.; Zusmanis, K.; Malter, H.; Mitchell-Leef, D. Pure FSH alone induces ovulation and subsequent pregnancy in the mouse resulting in fetal development. Life Sci 1993, 53, 31–39. [Google Scholar] [CrossRef]
- Wang, X.N.; Greenwald, G.S. Human chorionic gonadotropin or human recombinant follicle-stimulating hormone (FSH)-induced ovulation and subsequent fertilization and early embryo development in hypophysectomized FSH-primed mice. Endocrinology 1993, 132, 2009–2016. [Google Scholar] [CrossRef]
- Zelinski-Wooten, M.B.; Hutchison, J.S.; Hess, D.L.; WoIf, D.P.; Stouffer, R.L. A bolus of recombinant human follicle stimulating hormone at midcycle induces periovulatory events following multiple follicular development in macaques. Hum Reprod 1998, 13, 554–560. [Google Scholar] [CrossRef]
- Lin, M.H.; Wu, F.S.; Lee, R.K.; Li, S.H.; Lin, S.Y.; Hwu, Y.M. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 2013, 100, 1296–302. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Bassil, R.; Samara, N.; Zilberberg, E.; Mehta, C.; Orvieto, R.; Casper, R.F. GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod 2020, 35, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, X.; Wang, Y.; Xi, J.; Pan, H.; Wang, M.; Zhou, Y.; Xiao, Y. Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum Reprod 2022, 37, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Anaya, Y.; Cakmak, H.; Mata, D.A.; Letourneau, J.; Zhang, L.; Lenhart, N.; Juarez-Hernandez, F.; Jalalian, L.; Cedars, M.I.; Rosen, M. Triggering with 1500 IU of human chorionic gonadotropin plus follicle-stimulating hormone compared to a standard human chorionic gonadotropin trigger dose for oocyte competence in in vitro fertilization cycles: a randomized, double-blinded, controlled noninferiority trial. Fertil Steril 2022, 118, 266–278. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Hudson, C. Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril 2011, 95, 2715–2717. [Google Scholar] [CrossRef]
- González, V.G.; Triana, A.M.; García, I.S.; Nieto, S.O.; Urrutia, M.C.; García, I.C.; Gastañaga-Holguera, T. Dual trigger vs. conventional trigger outcomes in in vitro fertilization. Systematic review and meta-analysis. JBRA Assist Reprod 2023, 27, 112–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Guo, L.; Chang, H.M.; Shu, J.; Leung, P.C.K. Outcomes comparison of IVF/ICSI among different trigger methods for final oocyte maturation: A systematic review and meta-analysis. FASEB J, 2021, 35, e21696. [Google Scholar] [CrossRef]
- Mourad, S.; Brown, J.; Farquhar, C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev 2017, 1, CD012103. [Google Scholar] [CrossRef]
- Vyrides, A.A.; Mahdi, E.E.; Lamnisos, D.; Giannakou, K. Dual trigger with gonadotropin releasing hormone agonist and human chorionic gonadotropin of fresh autologous cycles in high responders: a systematic review. J Reprod Infertil 2022, 23, 3–17. [Google Scholar] [CrossRef]
- Abbara, A.; Eng, P.C.; Phylactou, M.; Clarke, S.A.; Richardson, R.; Sykes, C.M.; Phumsatitpong, C.; Mills, E.; Modi, M.; et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest 2020, 130, 6739–6753. [Google Scholar] [CrossRef]
- Abbara, A.; Jayasena, C.N.; Christopoulos, G.; Narayanaswamy, S.; Izzi-Engbeaya, C.; Nijher, G.M.; Comninos, A.N.; Peters, D.; Buckley, A. ; Ratnasabapathy, R et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab 2015, 100, 3322–3331. [Google Scholar] [CrossRef]
- Mathur, R.S.; Akande, A.V.; Keay, S.D.; Hunt, L.P.; Jenkins, JM. Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril 2000, 73, 901–907. [Google Scholar] [CrossRef]
- Vane, JR.; Botting, RM. The mechanism of action of aspirin. Thromb Res 2003, 110, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Moini, A.; Zafarani, F.; Haddadian, S.; Ahmadi, J.; Honar, H.; Riazi, K. Effect of low-dose aspirin therapy on implantation rate in women undergoing in-vitro fertilization cycles. Saudi Med J 2007, 28, 732–736. [Google Scholar] [PubMed]
- Várnagy, A.; Bódis, J.; Mánfai, Z.; Wilhelm, F.; Busznyák, C.; Koppán, M. Low-dose aspirin therapy to prevent ovarian hyperstimulation syndrome. Fertil Steril 2010, 93, 2281–2284. [Google Scholar] [CrossRef] [PubMed]
- Namavar Jahromi, B.; Zolghadri, J.; Rahmani, E.; Alipour, S.; Anvar, Z.; Zarei, A.; Keramati, P. Effect of low-dose aspirin on the development of ovarian hyperstimulation syndrome and outcomes of assisted reproductive techniques in the women with PCOS, a randomized double-blinded clinical trial. Taiwan J Obstet Gynecol 2019, 58, 255–260. [Google Scholar] [CrossRef]
- Owj, M.; Tehrani-Nejad, E.S.; Amirchaghmaghi, E.; Baghestani, A.R.; Ahmadi, J. The role of ketoconazole in the prevention of ovarian hyperstimulation syndrome in patients with polycystic ovary syndrome during assisted reproductive technology cycles. Saudi Med J 2005, 26, 1584–1587. [Google Scholar]
- Parsanezhad, M.E.; Alborzi, S.; Pakniat, M.; Schmidt, E.H. A double-blind, randomized, placebo-controlled study to assess the efficacy of ketoconazole for reducing the risk of ovarian hyperstimulation syndrome. Fertil Steril 2003, 80, 1151–1155. [Google Scholar] [CrossRef]
- Lainas, G.T.; Kolibianakis, E.M.; Sfontouris, I.A.; Zorzovilis, I.Z.; Petsas, G.K.; Tarlatzi, T.B.; Tarlatzis, B.C.; Lainas, T.G. Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study. Reprod Biol Endocrinol 2012, 10, 69. [Google Scholar] [CrossRef]
- Lainas, G.T.; Kolibianakis, E.M.; Sfontouris, I.A.; Zorzovilis, I.Z.; Petsas, G.K.; Lainas, T.G.; Tarlatzis, B.C. Serum vascular endothelial growth factor levels following luteal gonadotrophin-releasing hormone antagonist administration in women with severe early ovarian hyperstimulation syndrome. BJOG 2014, 121, 848–855. [Google Scholar] [CrossRef]
- Zeng, C.; Shang, J.; Jin, AM.; Wu, PL.; Li, X.; Xue, Q. The effect of luteal GnRH antagonist on moderate and severe early ovarian hyperstimulation syndrome during in vitro fertilization treatment: a prospective cohort study. Arch Gynecol Obstet 2019, 300, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Humaidan, P.; Bredkjaer, H.E.; Bungum, L.; Bungum, M.; Grøndahl, M.L.; Westergaard, L.; Andersen, C.Y. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod 2005, 20, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Humaidan, P.; Polyzos, N.P.; Alsbjerg, B.; Erb, K.; Mikkelsen, A.L.; Elbaek, H.O.; Papanikolaou, E.G.; Andersen, C.Y. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod 2013, 28, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.Y.; Elbaek, H.O.; Alsbjerg, B.; Laursen, R.J.; Povlsen, B.B.; Thomsen, L.; Humaidan, P. Daily low-dose hCG stimulation during the luteal phase combined with GnRHa triggered IVF cycles without exogenous progesterone: a proof of concept trial. Hum Reprod 2015, 30, 2387–2395. [Google Scholar] [CrossRef]
- Svenstrup, L.; Möller, S.; Fedder, J.; Pedersen, D.E.; Erb, K.; Andersen, C.Y.; Humaidan, P. Does the HCG trigger dose used for IVF impact luteal progesterone concentrations? a randomized controlled trial. Reprod Biomed Online 2022, 45, 793–804. [Google Scholar] [CrossRef]
- Santos-Ribeiro, S.; Mackens, S.; Popovic-Todorovic, B.; Racca, A.; Polyzos, N.P.; Van Landuyt, L.; Drakopoulos, P.; de Vos, M.; Tournaye, H.; Blockeel, C. The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Hum Reprod 2020, 35, 2808–2818. [Google Scholar] [CrossRef]
- Berridge, M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Greff, D.; Juhász, A.E.; Váncsa, S.; Váradi, A.; Sipos, Z.; Szinte, J.; Park, S; Hegyi, P. ; Nyirády, P.; Ács, N.; Várbíró, S.; Horváth, E.M. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol 2023, 21, 10. [Google Scholar] [CrossRef]
- Mendoza, N.; Diaz-Ropero, MP.; Aragon, M.; Maldonado, V.; Llaneza, P.; Lorente, J.; Mendoza-Tesarik, R.; Maldonado-Lobon, J.; Olivares, M.; Fonolla, J. Comparison of the effect of two combinations of myo-inositol and D-chiro-inositol in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecol Endocrinol 2019, 35, 695–700. [Google Scholar] [CrossRef]
- Agrawal, A.; Mahey, R.; Kachhawa, G.; Khadgawat, R.; Vanamail, P.; Kriplani, A. Comparison of metformin plus myoinositol vs metformin alone in PCOS women undergoing ovulation induction cycles: randomized controlled trial. Gynecol Endocrinol 2019, 35, 511–514. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Malhotra, N.; Mahey, R.; Khadgawat, R.; Kalaivani, M. Myoinositol versus metformin pretreatment in GnRH-antagonist cycle for women with PCOS undergoing IVF: a double-blinded randomized controlled study. Gynecol Endocrinol 2022, 38, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.; Seidler, K.; Neil, J. Vitamin D deficiency and female infertility: a mechanism review examining the role of vitamin D in ovulatory dysfunction as a symptom of polycystic ovary syndrome. J Reprod Immunol 2022, 151, 103633. [Google Scholar] [CrossRef] [PubMed]
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The association between vitamin D and anti-Müllerian hormone: a systematic review and meta-analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Turan, G.A.; Eskicioglu, F.; Sivrikoz, O.N.; Cengiz, H.; Gur, E.B.; Tatar, S.; Sahin, N.; Yilmaz, O. Prophylactic vitamin D supplementation in ovarian hyperstimulation syndrome: an animal study. Arch Gynecol Obstet 2015, 292, 421–427. [Google Scholar] [CrossRef]
- Irani, M.; Minkoff, H.; Seifer, D.B.; Merhi, Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J Clin Endocrinol Metab 2014, 99, E886–90. [Google Scholar] [CrossRef]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Julka, N.; Bhatt, D.; Kalgi; Irani, S. ; Tal, O.; Lambert-Messerlian, G.; Tal, R. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab 2015, 100, 4307–4314. [Google Scholar] [CrossRef]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Irani, S.; Rosenwaks, Z.; Tal, R. Vitamin D decreases serum VEGF correlating with clinical improvement in vitamin D-deficient women with PCOS: a randomized placebo-controlled trial. Nutrients 2017, 9, 334. [Google Scholar] [CrossRef]
- Lv, S.S.; Wang, J.Y.; Wang, X.Q.; Wang, Y.; Xu, Y. Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 2016, 293, 1339–1345. [Google Scholar] [CrossRef]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod 2018, 33, 65–80. [Google Scholar] [CrossRef]
- Iliuta, F.; Pijoan, J.I.; Lainz, L.; Exposito, A.; Matorras, R. Women's vitamin D levels and IVF results: a systematic review of the literature and meta-analysis, considering three categories of vitamin status (replete, insufficient and deficient). Hum Fertil (Camb) 2022, 25, 228–246. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Xu, B.; Yan, Y.; Zhang, Q.; Li, Y. Whether vitamin D was associated with clinical outcome after IVF/ICSI: a systematic review and meta-analysis. Reprod Biol Endocrinol 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Busnelli, A.; Pellegrini, L.; Riviello, E.; Vitagliano, A. How vitamin D level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril 2020, 114, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, X.; Luo, X.; Shao, J.; Guo, D.; Deng, B.; Wu, Z. Effect of vitamin D supplementation on in vitro fertilization outcomes: a trial sequential meta-analysis of 5 randomized controlled trials. Front Endocrinol (Lausanne) 2022, 13, 852428. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, J.; Wan, Q.; Huang, J.; Han, T.; Qu, T.; Yu, LL. Influence of vitamin D supplementation on reproductive outcomes of infertile patients: a systematic review and meta-analysis. Reprod Biol Endocrinol 2023, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Eller, A.B.P.; Ejzenberg, D.; Monteleone, P.A.A.; Soares, J.M. Jr; Baracat, E.C. Vitamin D and in vitro fertilization: a systematic review. J Assist Reprod Genet 2023, 40, 735–743. [Google Scholar] [CrossRef]
- Aflatoonian, A.; Arabjahvani, F.; Eftekhar, M.; Sayadi, M. Effect of vitamin D insufficiency treatment on fertility outcomes in frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J Reprod Med 2014, 12, 595–600. [Google Scholar]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S; Kamali, K. ; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; Giahi, L. Role of vitamin E and D3 supplementation in intra-cytoplasmic sperm injection outcomes of women with polycystic ovarian syndrome: a double blinded randomized placebo-controlled trial. Clin Nutr ESPEN 2017, 18, 23–30. [Google Scholar] [CrossRef]
- Abedi, S.; Taebi, M.; Nasr Esfahani, M.H. Effect of vitamin D supplementation on intracytoplasmic sperm injection outcomes: a randomized double-blind placebo-controlled trial. Int J Fertil Steril 2019, 13, 18–23. [Google Scholar] [CrossRef]
- Kermack, A.J.; Lowen, P.; Wellstead, S.J.; Fisk, H.L.; Montag, M.; Cheong, Y.; Osmond, C.; Houghton, F.D.; Calder, P.C.; Macklon, N.S. Effect of a 6-week "Mediterranean" dietary intervention on in vitro human embryo development: the Preconception Dietary Supplements in Assisted Reproduction double-blinded randomized controlled trial. Fertil Steril 2020, 113, 260–269. [Google Scholar] [CrossRef]
- Wdowiak, A.; Filip, M. The effect of myo-inositol, vitamin D3 and melatonin on the oocyte quality and pregnancy in in vitro fertilization: a randomized prospective controlled trial. Eur Rev Med Pharmacol Sci 2020, 24, 8529–8536. [Google Scholar] [CrossRef]
- Doryanizadeh, L.; Morshed-Behbahani, B.; Parsanezhad, M.E.; Dabbaghmanesh, M.H.; Jokar, A. Calcitriol effect on outcomes of in vitro fertilization in infertile women with Vitamin D deficiency: a double-blind randomized clinical trial. Z Geburtshilfe Neonatol 2021, 225, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Espinola, M.S.; Bilotta, G.; Aragona, C. Positive effect of a new supplementation of vitamin D3 with myo-inositol, folic acid and melatonin on IVF outcomes: a prospective randomized and controlled pilot study. Gynecol Endocrinol 2021, 37, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Sarais, V.; Reschini, M.; Ferrari, S.; Makieva, S.; Cermisoni, G.C.; Paffoni, A.; Papaleo, E.; Vigano, P. Single oral dose of vitamin D3 supplementation prior to in vitro fertilization and embryo transfer in normal weight women: the SUNDRO randomized controlled trial. Am J Obstet Gynecol 2021, 225, 283–e1. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.F.; Larsson, P.; Serrano, M.F.; Bosch, E.; Velasco, J.A.G.; López, E.S.; Mannaerts, B. Live birth rates following individualized dosing algorithm of follitropin delta in a long GnRH agonist protocol. Reprod Biol Endocrinol 2023, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- 77. Broekmans, F.J. Individualization of FSH doses in assisted reproduction: facts and fiction. Front Endocrinol (Lausanne) 2019, 10, 181. [Google Scholar] [CrossRef]
- 78. Nakhuda, G.S.; Douglas, N.C.; Thornton, M.H.; Guarnaccia, M.M.; Lobo, R.; Sauer, M.V. Anti-Mullerian hormone testing is useful for individualization of stimulation protocols in oocyte donors. Reprod Biomed Online 2010, 20, 42–47. [Google Scholar] [CrossRef]
- 79.Yovich, J.; Stanger, J.; Hinchliffe, P. Targeted gonadotrophin stimulation using the PIVET algorithm markedly reduces the risk of OHSS. Reprod Biomed Online 2012, 24, 81–92. [CrossRef]
- 80. Wu, D.; Shi, H.; Yu, Y.; Yu, T.; Zhai, J. Comparison of the effectiveness of various medicines in the prevention of ovarian hyperstimulation syndrome: a network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2022, 13, 808517. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Seifer, D.B. Women with PCOS who undergo IVF: a comprehensive review of therapeutic strategies for successful outcomes. Reprod Biol Endocrinol 2023, 21, 70. [Google Scholar] [CrossRef]
- Hu, K.L.; Gan, K.; Wang, R.; Li, W.; Wu, Q.; Zheng, B.; Zou, L.; Zhang, S.; Liu, Y.; Wu, Y.; Chen, R.; Cao, W.; Yang, S.; Liu, F.T.; Tian, L.; Zeng, H.; Xu, H.; Qiu, S.; Yang, L.; Chen, X.; Pan, X.; Wu, X.; Mol, B.W.; Li, R.; Zhang, D. Vitamin D supplementation prior to in vitro fertilisation in women with polycystic ovary syndrome: a protocol of a multicentre randomised, double-blind, placebo-controlled clinical trial. BMJ Open 2020, 10, e041409. [Google Scholar] [CrossRef]
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





