Submitted:
03 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Preparation of polyacrylate-polyacrylamide hydrogel grafted with 3-chloroaniline
2.3. Instrumental Techniques
2.3.1. Morphological studies
2.3.2. Transmission electron microscope (TEM)
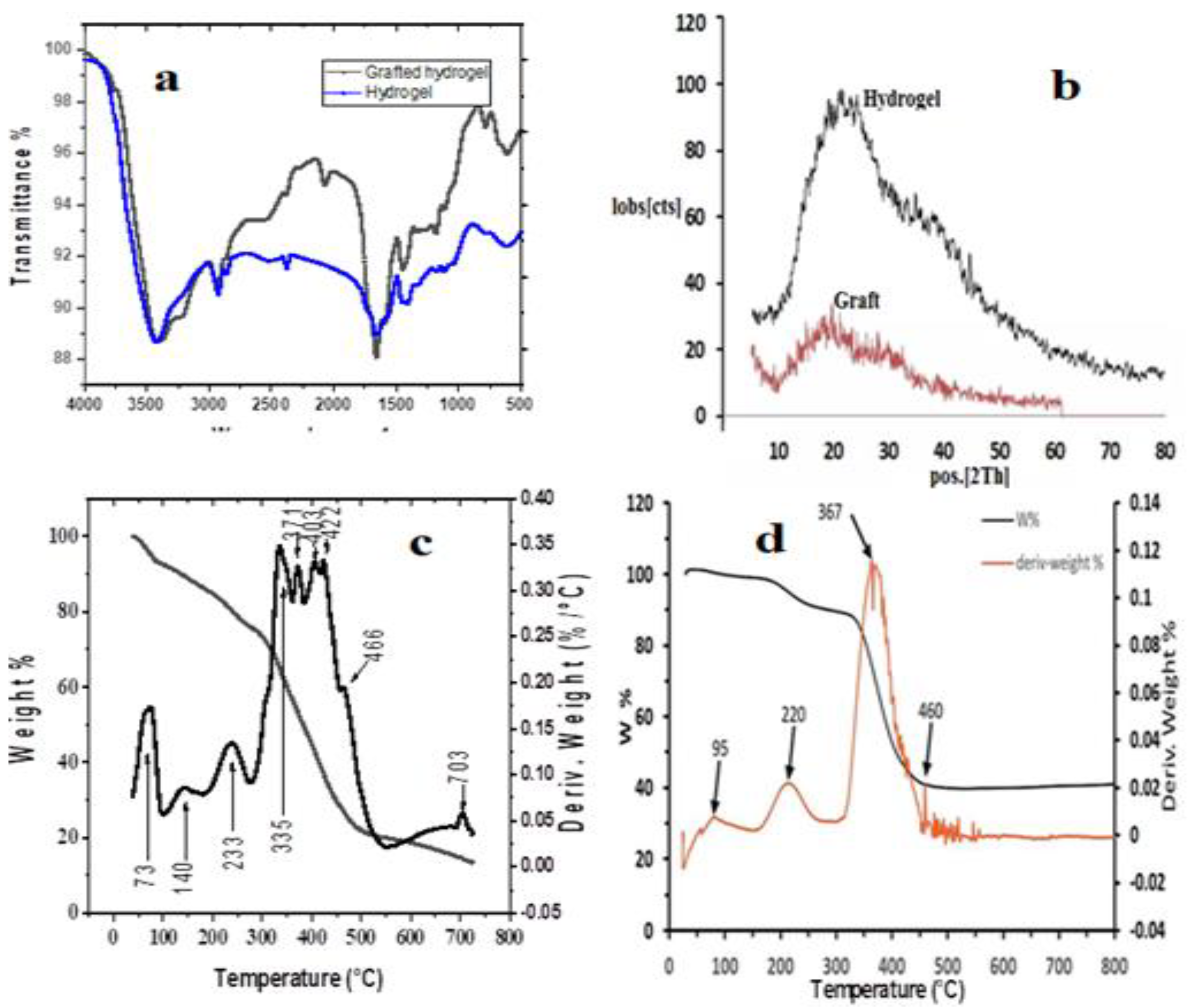
2.3.3. Thermogravimetric Analysis (TGA)
2.4. Sampling of ground water
2.4.1. Measurement of ammonia concentration
2.4.2. Measurement of iron concentration
2.4.3. Computational details
3. Results and Discussion
3.1. Characterization of Obtained Polymeric Samples
3.1.1. XRD and TGA analysis
3.1.2. SEM and TEM analysis
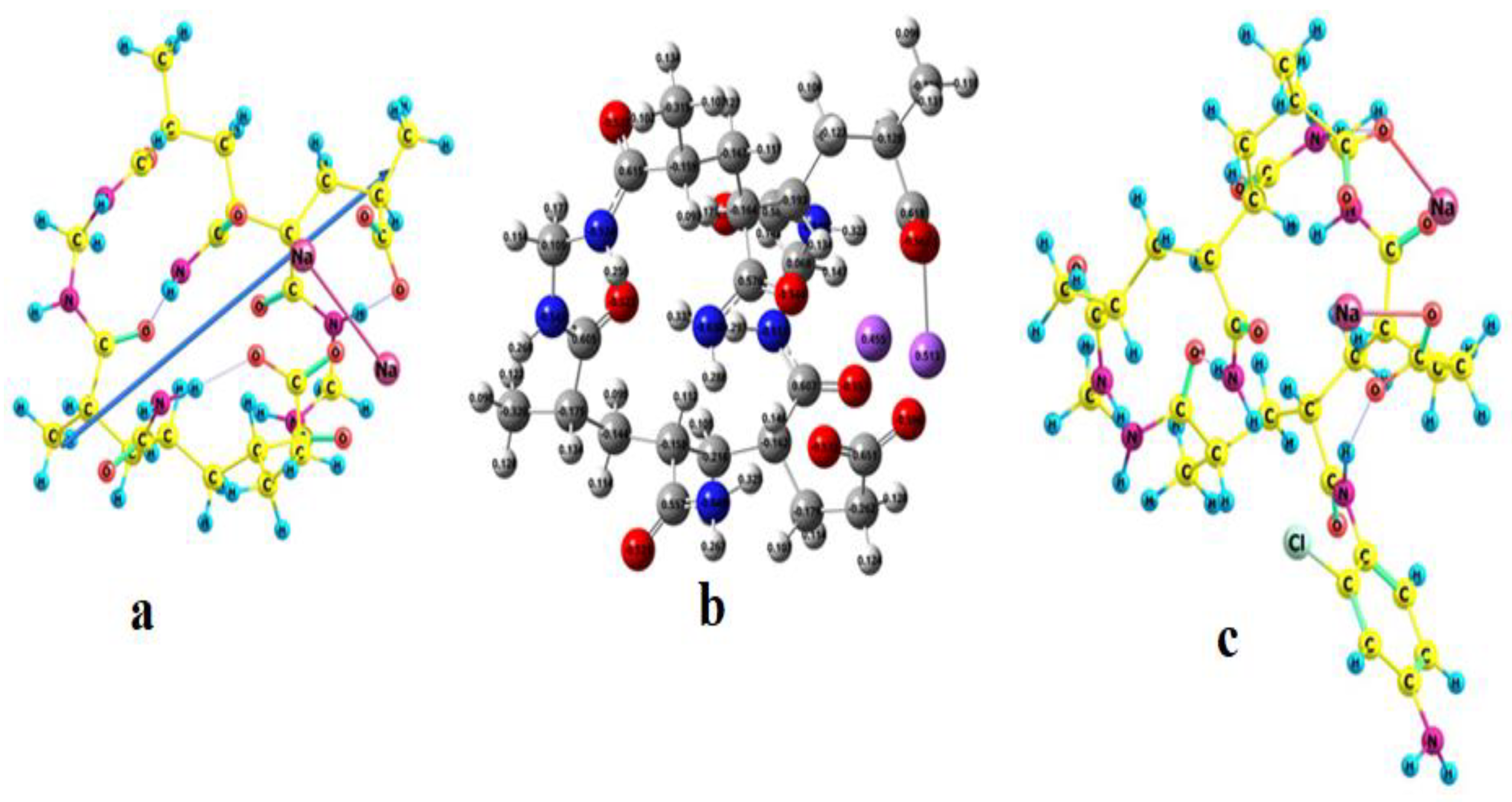
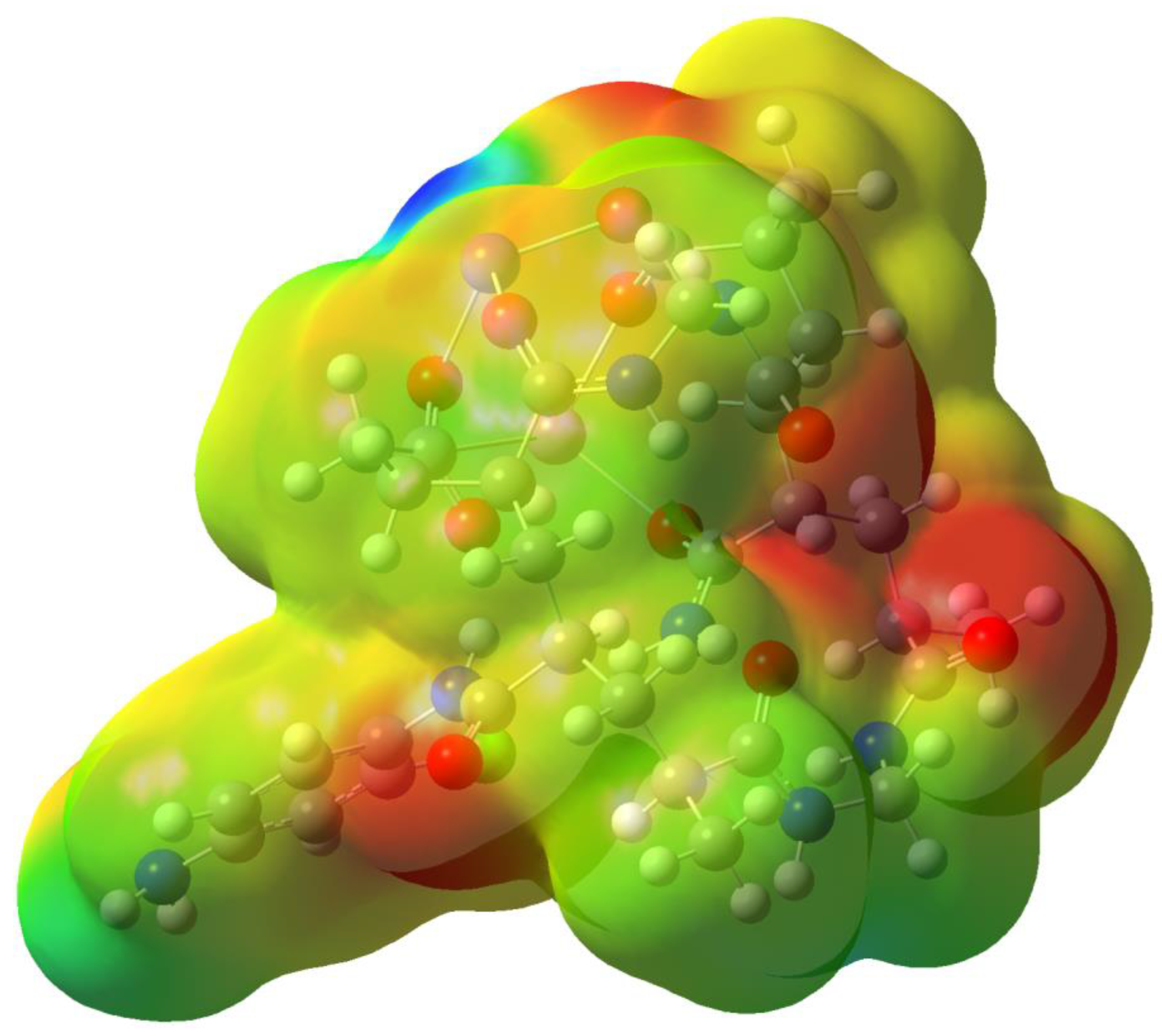
3.2. Optimized geometries
3.4. Removal of cations
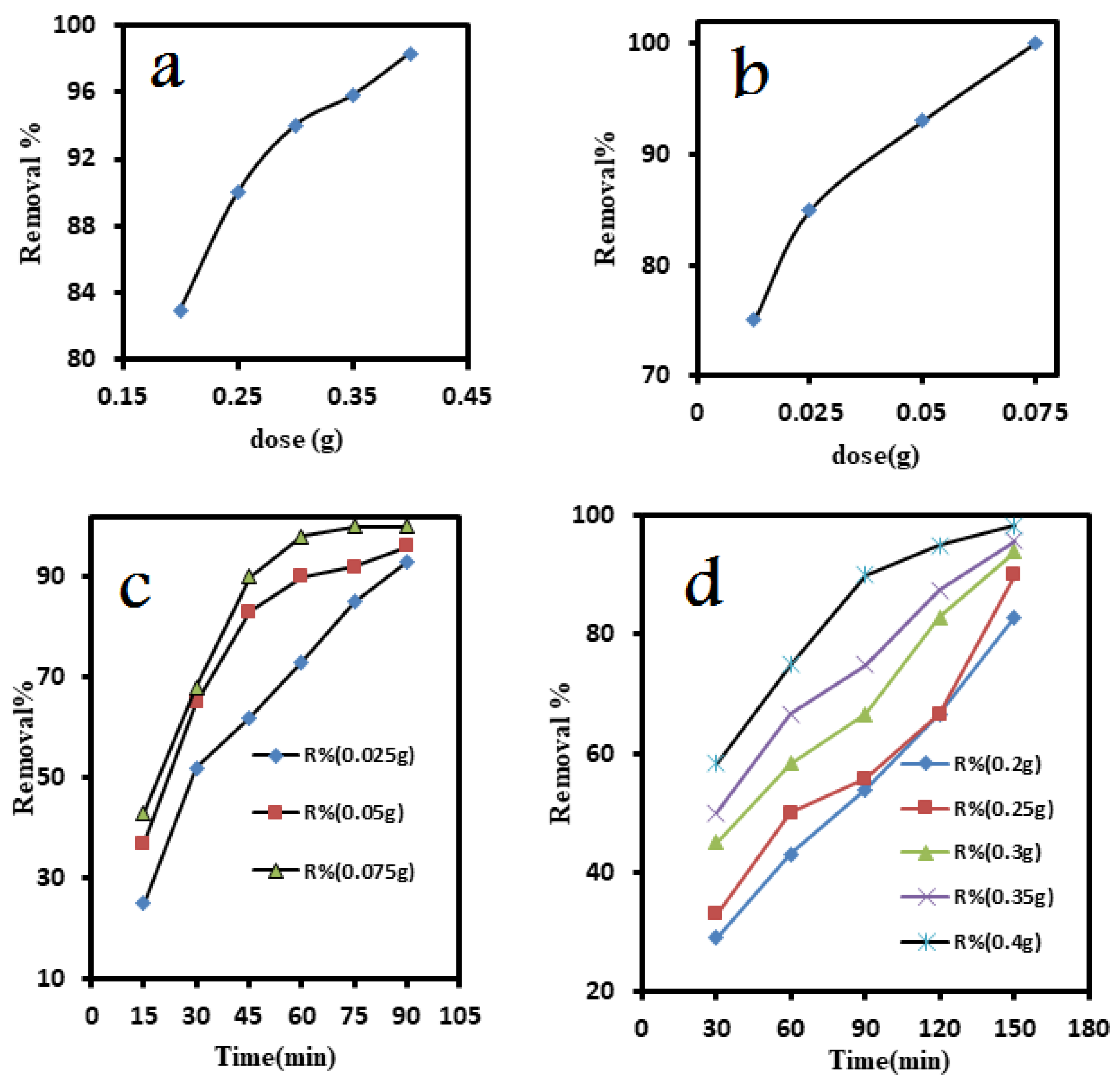
3.4.1. Removal of ammonia
3.4.2. Removal of iron
3.5. Effect of Temperature and Thermodynamics
3.5.1. Adsorption isotherms
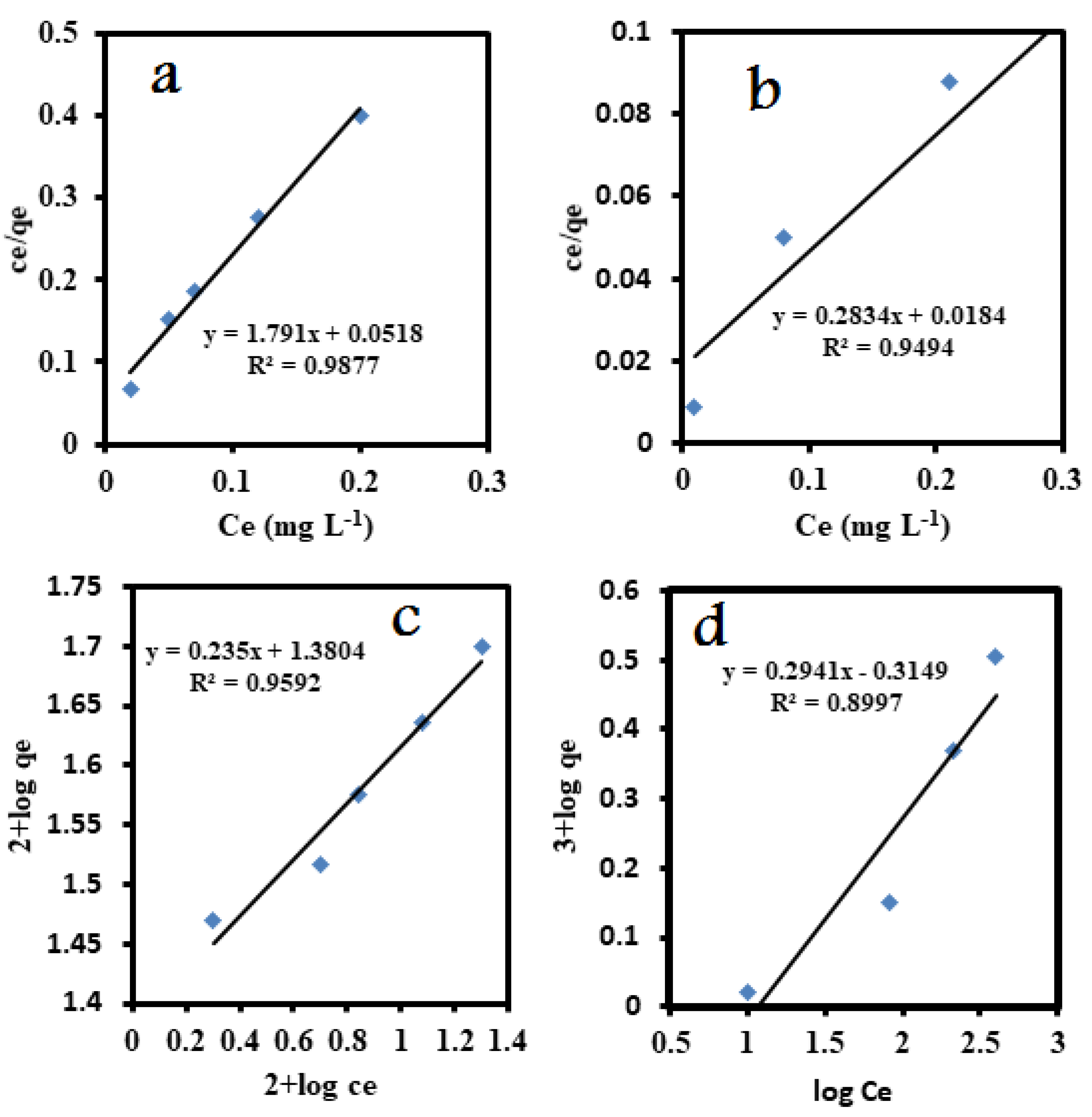
3.5.1.1. Langmuir isotherm
3.5.1.2. Freundlich isotherm
3.5.1.3. Temkin isotherms
3.5.1.4. Adsorption Kinetics
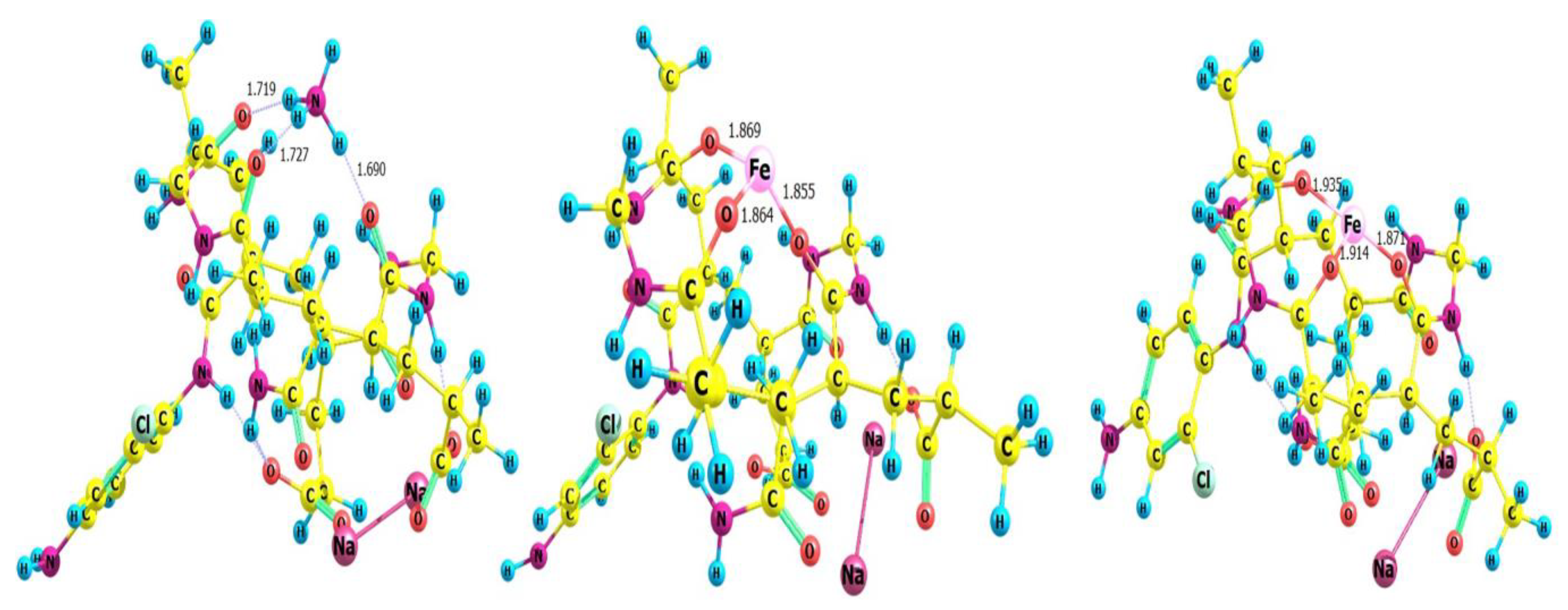
3.6. Binding (complexation) Energy of grafted hydrogel with cations
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gouzinis, A.; Kosmidis, N.; Vayenas, D.V.; Lyberatos, G. Removal of Mn and simultaneous removal of NH3, Fe and Mn from potable water using a trickling filter., Water Res 1998, 32 (8), 2442-2450. [CrossRef]
- Tekerlekopoulou, A.G.; Pavlou, S.; Vayenas, D.V. Removal of ammonium, iron and manganese from potable water in biofiltration units: a review. J. Chem.Technol. Biotechnol 2013, 88 (5), 751-773. [CrossRef]
- Piper, R.G.; Smith, C.E. Use of clinoptilolite for ammonia removal in fish culture systems, In Pond, W.G. & Mumpton, F. A. (eds.) Zeo-agriculture, use of natural zeolites in agriculture and aquaculture, Western Press 1984, 224–234.
- Lindenbaum, J. Identification of sources of ammonium in groundwater using stable nitrogen and boron isotopes in Nam Du, Hanoi, Master’s thesis ohanLindenbaum 2012, 45.
- Shaban, M.; Abukhadra, M. R.; Shahien, M. G.; Khan, A. A. P. Upgraded modified forms of bituminous coal for the removal of safranin-T dye from aqueous solution. Environmental Science and Pollution Research 2017a. [CrossRef]
- WHO. Guidelines for drinking-water quality, Recommendations 2008, 3rd ed. Vol.1.
- Chen, Q. Competitive mechanisms of ammonia, iron and manganese for dissolved oxygen using pilot-scale bio filter at different dissolved oxygen concentrations. Water Sci, Technol, Water Supply 2016, 16 (3), 766-774.
- Alshameri, A.; Ibrahim, A.; Assabri, A. M.; Lei, X.; Wang, H.; Yan. C. The investigation into the ammonium removal performance of Yemeni natural zeolite: modification, ion exchange mechanism, and thermodynamics, Powder Technology 2014, 258, 20–31. [CrossRef]
- Taneva, N. Removal of ammonium and phosphates from aqueous solutions by activated and modified Bulgarian clinoptilolite, Journal of Chemical Engineering and Materials Science 2012, 3(5), 79–85.
- Xiong, W. H.; Peng, J. Development and characterization of ferrihydrite-modified diatomite as a phosphorus adsorbent, Water Research 2008, 42, 4869–4877. [CrossRef]
- Cai, Y.A.; Li, D.; Liang, Y.; Luo, Y.; Zeng, H.; Zhang. J. Effective start-up bio filtration method for Fe, Mn, and ammonia removal and bacterial community analysis, Bioresour. Technol 2015, 176, 149-155. [CrossRef]
- WHO. Guidelines for Drinking Water Quality. Health Criteria and Other Supporting Information, 2nd edition 1993, (2).
- Obiri-Nyarko Franklin. Geochemical modelling for predicting the long-term performance of zeolite-PRB to trea lead contaminated groundwater, J Contam Hydrol 2015, 177–178: 76–84.
- Dvorak, BI.; Skipton, SO. (2007). Drinking water: iron and manganese. Neb Guide published by University of Nebraska-Lincoln Extension 2007, Institute of Agriculture and Natural Resources.
- Casey. TJ. Iron and manganese in water: Occurrence, drinking water standards, treatment options. Aquavarra Research Publications Water Engineering Papers Aquavarra Research Limited, 22a brook field avenue, Blackrock, County Dublin, Ireland 2009.
- Keyser SL. Iron and manganese in drinking water, UCD EXTOXNET FAQ Team. Accessed June. 1997.
- Barloková, D.; Ilavský, J. Removal of Iron and Manganese from Water Using Filtration by Natural Materials, Polish J. of Environ. Stud 2010, 19 (6), 1117-1122.
- Okoniewska, E.; Lach, J.; Kacprzak, M.; Neczaj, E. The Removal of Manganese, Iron and Ammonium Nitrogen on Impregnated Activated Carbon, Desalination 2007, 206, 251-258. [CrossRef]
- Kontari, N. Groundwater, Iron and Manganese: An Unwelcome Trio, Water Engineering and Management 1988, 135, 25-26.
- Gage, B.; O’Dowd; D.; Williams, P. Biological Iron and Manganese Removal, Pilot Plant and Full Scale Application, Proceedings of the Ontario Water Works Association Conference, Ontario 2001, 3 May.
- WHO. Guidelines for Drinking Water Quality. Health Criteria and Other Supporting Information, 2nd edition (2). 1996, Geneva.
- Anonymous, Water: A millennial priority. The Acme Agrovat and Beverage Ltd., Dhaka, Bangladesh 2004.
- Kneeper, W. A. Iron, In: Kirk-Othmer encyclopedia of chemical technology, New York, USA, Wiley Interscience 1987, 13, 735-753.
- Huo, H.; Lin, H.; Dong, Y.; Cheng, H.; Wang, H.; Cao, L. (2012). Ammonia- nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite, Journal of Hazardous Materials 2012, 229, 292–297. [CrossRef]
- Shaban, M.; AbuKhadra M. R.; Nasief F. M.; Abd El-Salam, H. M. (2017). Removal of Ammonia from Aqueous Solutions, Ground Water, and Waste water Using Mechanically Activated Clinoptilolite and Synthetic Zeolite-A: Kinetic and Equilibrium Studies. Water Air Soil Pollut 2017, 228:450.
- Abd El-Salam, H. M.; Zaki T. Removal of hazardous cationic organic dyes from water using nickel- based metal- organic fromeworks. Inorganic chimica 2018, 471, 203-210.
- El Shafey, A. M.; Abdel-Latif, M. K.; Abd El-Salam, H.M. The facile synthesis of poly (Acrylate/Acrylamide) Titanium Dioxide Nano composite for Ground water Ammonia Removal, Desalination and Water Treatment 2020, 212, 61-70.
- Ilavský, J.; BarlokovÁ, D.; Biskupič, F. Chémia vody a hydrobiológia, STU Bratislava 2008, ISBN 978-80-227- 2930-7, pp. 303.
- Barud, H. G. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration, Carbohydrate Polymer 2015, 128, 41–51. [CrossRef]
- Abd El-Mageed, H. R.; Abd El-Salam, H. M.; Eissa, M. F. Spectroscopic study on poly (Acrylic Acid-co- Acrylamide)-graft-poly aniline As A Radiation Dosimeter for Alpha particles, Radiation Protection Dosimetry 2017, 1-8.
- Abd El-Salam, H. M.; Mohamed, R. A.; Shokry, A. Preparation and characterization of novel and selective polyacrylamide-graft-poly (2 methoxyaniline) adsorbent for lead removal. Verlag GmbH Germany 2017. [CrossRef]
- Kamal, E. H. M.; Abd El-salam, H.M.; Sayyah, S.M. Enhancing both the mechanical and chemical properties of paper sheet by graft Co- polymerization with acrylonitrile/methyl methacrylate. Journal of Basic and Applied Science 2014. [CrossRef]
- Abd El-Salam, H. M.; Kamal, E. H. M.; Ibrahim, M. S. Synthesis and Characterization of Chitosan-Grafted-Poly(2-Hydroxyaniline) Microstructures for Water Decontamination, J Polym Environ 2017, 25, 973–982. [CrossRef]
- https://gaussian.com/glossary/g03/, Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford CT, 2004.
- Becke, A. D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing, J Chem Phys 1996, 104(3):1040–1046. [CrossRef]
- Becke, A. D. Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals, J Chem Phys 1997, 107(20):8554–8560.
- Raghavachari, K.; Trucks, GW.; Pople, J. A.; Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theories, Chem Phys Lett 1989, 157(6):479–483. [CrossRef]
- Becke, A. D. Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 1993, 98(7):5648–5652.
- Dunning, T. H.; Hay, P. J. In Modern Theoretical Chemistry, Ed. H. F. Schaefer III, 1977, Vol. 3 (Plenum, New York,) 1-28.
- Hay, P. J.; Wadt, W. R. Ab initio effective core potentials for molecular calculations – potentials for the transition-metal atoms Sc to Hg, J. Chem. Phys. 1985, 82: 270-83. [CrossRef]
- Abd El-Mageed, H. R.; Abd El-Salam, H. M.; Abdel- Latif, M. K.; Mustafa, F. M. Preparation and spectroscopic properties, density functional theory calculation and nonlinear optical properties of poly (acrylic acid-co- acrylamide)-graft-polyaniline. Journal of molecular structure 2018, 1173, 268-279.
- Monteiro & Neves, S. Characterization of Banana Fibers Functional Groups by Infrared Spectroscopy, Materials Science Forum 2014, 775–776, 250–54.
- Paiva, M. C. Alfa Fibres: Mechanical, Morphological and Interfacial Characterization, Composites Science and Technology 2007, 67(6), 1132–38. [CrossRef]
- Silverstein, R. M.; Bassler, C. G.; Morill, T. C. Spectroscopic identification of organic compounds, Wiley, New York 1974.
- Reddy, K. R.; Lee, K. P. Novel electrically conductive and ferromagnetic composites of poly (aniline-co amino naphthalene sulfonic acid) with iron oxide nanoparticles: synthesis and characterization, Appl Polym Sci, 2007, 106, 1181–1191.
- Asabe, D.; Bashar, Mechanical, Spectroscopic and Micro-Structural Characterization of Banana Particulate Reinforced PVC Composite as Piping Material, Tribology in Industry 2016, 38(2), 255–67.
- Rojas; Carlos. Thermal Insulation Materials Based on gricultural Residual Wheat Straw and Corn Husk Biomass, for Application in Sustainable Buildings, Sustainable Materials and Technologies 2019, 20, e00102.
- Alshameri, A.; Ibrahim, A.; Assabri, A. M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: modification, ion exchange mechanism, and thermodynamics, Powder Technology 2014, 258, 20-31. [CrossRef]
- Paudel, S. R.; Kansakar, B. R. Dissolved Ammonia Adsorption in water using over Burnt Brick, Energy Research Journal 2010, 1, 1-5.
- Namasivayam, C.; Yamuna, R.T. Adsorption of direct red 12 B by biogas residual slurry: equilibrium and rate processes, Environ. Pollut 1995, 89, 1–7. [CrossRef]
- Freundlich, I.; Helle, W.J., J. Am. Chem. Soc. 1939, 61, 2–28.
- Temkin, M. J.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalysts, Acta Physiochimica, URSS, 1940, 12, 217–222.
- Lagergren, S. Zur theorie der sogenannten adsorption gel oster stoffe. Kungliga SvenskaVetenskapsakademiens. Handlingar 1898, 25, 1–39.
- Ho, Y. S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem 1999, 34, 451–465. [CrossRef]
- Hui, K. S.; Chao, C. Y.; Kot, S. C. (2005). Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash, Journal of Hazardous Materials , 2005, 127, 89–101. [CrossRef]
- Boys, S. F.; Bernardi, F. D. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors, Mol Phys 1970, 19(4), 553–566. [CrossRef]

| Parameter | Before treatment | After treatment |
|---|---|---|
| Turbidity | 1.3 NTU | 1.2 NTU |
| Chlorides | 140 mg/L | 130 mg/L |
| Alkalinity | 320 mg/L | 320 mg/L |
| TDS (Total dissolved salts) |
710 mg/L | 650 mg/L |
| Total hardness | 280 mg/L | 250 mg/L |
| Ca hardness | 130 mg/L | 120 mg/L |
| Mg hardness | 150 mg/L | 130 mg/L |
| Ammonia | 0.8 mg/L | 0.0 mg/L |
| Iron | 1.2 mg/L | 0.02 mg/L |
| Model | Parameter | Parameter value | |
|---|---|---|---|
| Ammonia | Iron | ||
| Langmuir | Qm(mg g-1) | 3.52 | 0.55 |
| b | 15.439 | 35.1 | |
| R2 | 0.9494 | 0.9877 | |
| Freundlich | n | 3.4 | 4.25 |
| Kf | 2.06 | 24 | |
| R2 | 0.8997 | 0.9592 | |
| Temkin | BT(J/mol) | 0.5739 | 0.0904 |
| KT(L/g) | 2.76 | 85.39 | |
| R2 | 0.8502 | 0.9354 | |
| Ion | Complexation Energy kcal/mol | |
|---|---|---|
| Method | (raw) | (corrected) BSSE |
| Iron(II) | -410.49 | -401.65 |
| Iron(III) | -990.86 | -920.7 |
| Ammonioum | -74.6 | -71.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





