Submitted:
02 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Cohorts
2.2. IHC Analysis and Scoring
2.3. Statistical Analysis
3. Results
3.1. Patient characteristics
3.2. Different Immunohistochemical Expression among Subtypes
3.3. Score and Cut-off Selection for Subtype Discrimination: Training Cohort
3.4. Validation Cohort
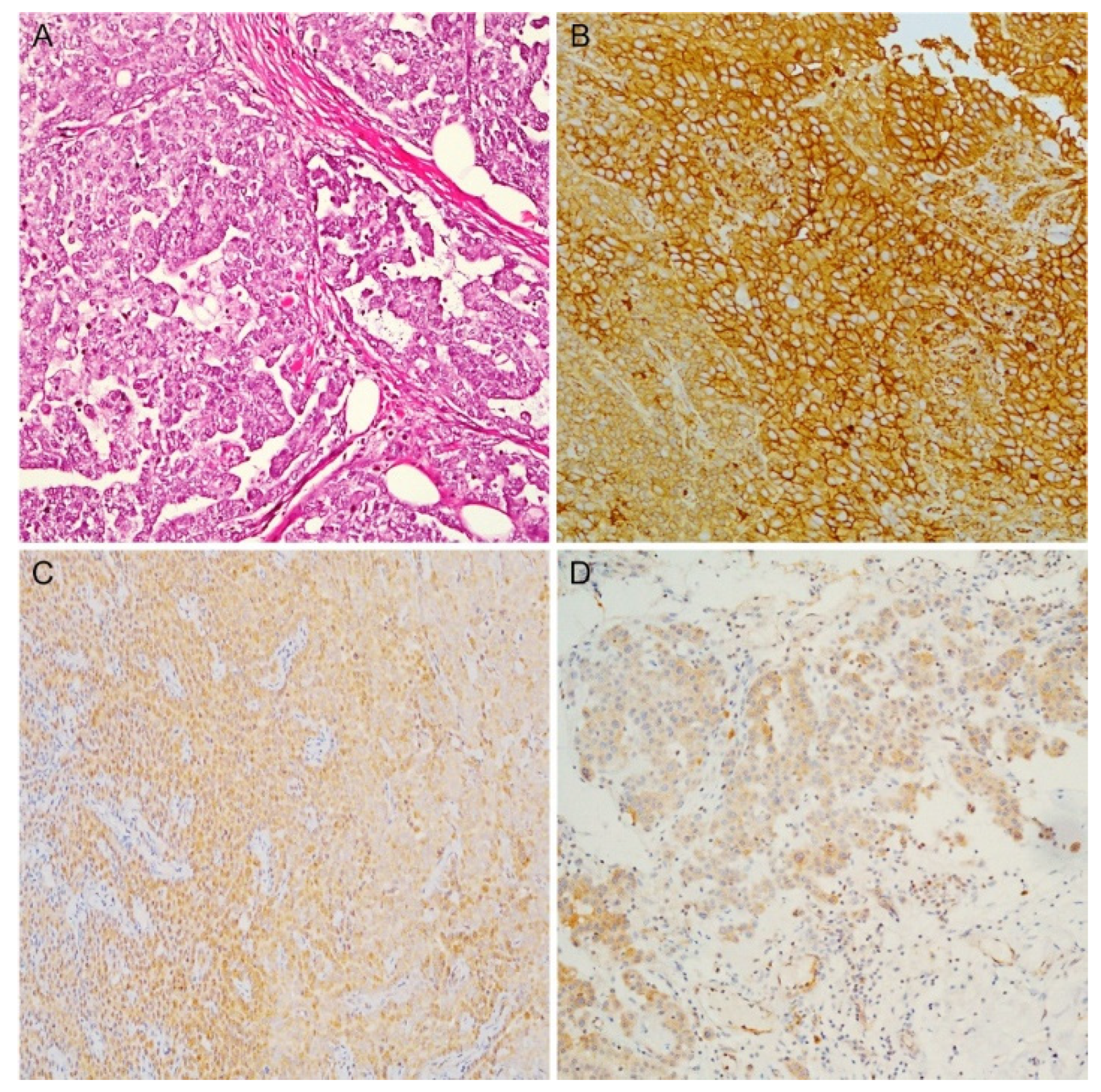
3.5. Epithelioid PMs: IHC and Histological Features
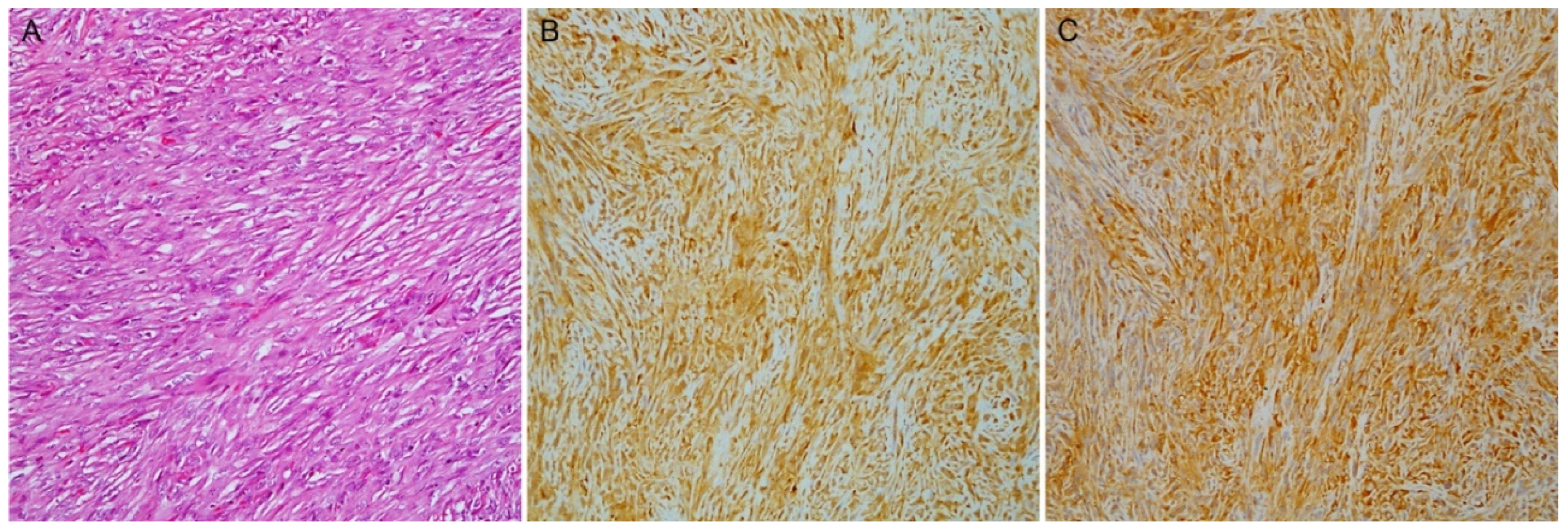
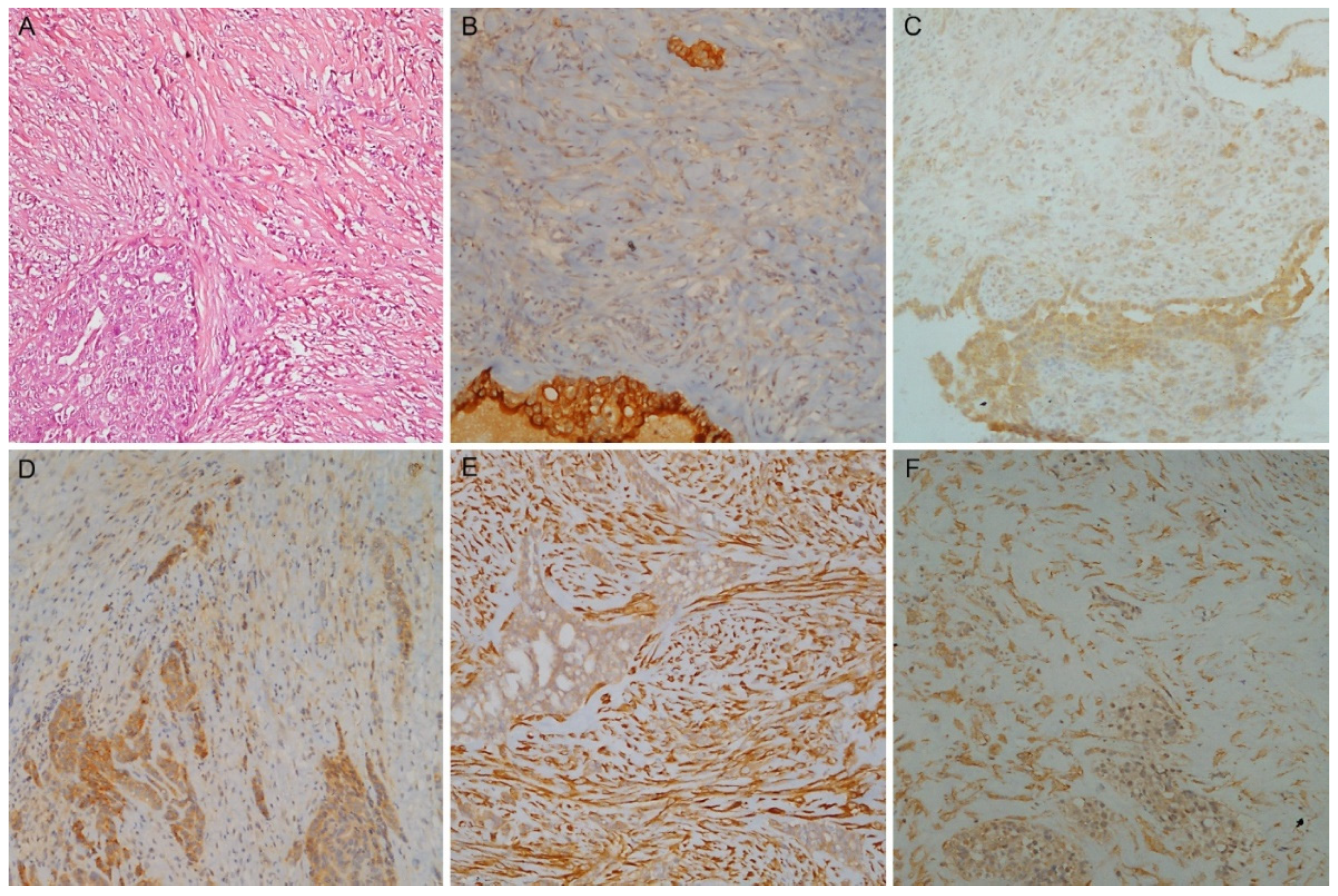
3.6. Biphasic PMs: IHC and Discrimination between Components
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sauter, J.B., R.; Dacic, S.; Gill, RR. . In Thoracic Tumours, 5th ed.; Board, T.W.C.o.T.E., Ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2021; Volume 5, pp. 204–219. [Google Scholar]
- Brims, F. Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ahern, C.A.; Berlind, C.G.; Lindsay, W.D.; Shabason, J.; Sharma, S.; Culligan, M.J.; Grover, S.; Friedberg, J.S.; Simone, C.B., 2nd. Survival by Histologic Subtype of Malignant Pleural Mesothelioma and the Impact of Surgical Resection on Overall Survival. Clin Lung Cancer 2018, 19, e901–e912. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Rice, D.C.; Niu, J.; Atay, S.; Vaporciyan, A.A.; Antonoff, M.; Hofstetter, W.L.; Walsh, G.L.; Swisher, S.G.; Roth, J.A.; et al. Long-Term Survival Outcomes of Cancer-Directed Surgery for Malignant Pleural Mesothelioma: Propensity Score Matching Analysis. J Clin Oncol 2017, 35, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Chirieac, L.R. Pathology of Malignant Pleural Mesothelioma. Thorac Surg Clin 2020, 30, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Chirieac, L.R.; Hung, Y.P.; Foo, W.C.; Hofer, M.D.; VanderLaan, P.A.; Richards, W.G.; Sugarbaker, D.J.; Bueno, R. Diagnostic value of biopsy sampling in predicting histology in patients with diffuse malignant pleural mesothelioma. Cancer 2019, 125, 4164–4171. [Google Scholar] [CrossRef]
- Klebe, S.; Brownlee, N.A.; Mahar, A.; Burchette, J.L.; Sporn, T.A.; Vollmer, R.T.; Roggli, V.L. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010, 23, 470–479. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016, 48, 407–416. [Google Scholar] [CrossRef]
- Alcala, N.; Mangiante, L.; Le-Stang, N.; Gustafson, C.E.; Boyault, S.; Damiola, F.; Alcala, K.; Brevet, M.; Thivolet-Bejui, F.; Blanc-Fournier, C.; et al. Redefining malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions. EBioMedicine 2019, 48, 191–202. [Google Scholar] [CrossRef]
- Bruno, R.; Poma, A.M.; Ali, G.; Distefano, C.; Proietti, A.; Chella, A.; Lucchi, M.; Melfi, F.; Franco, R.; Fontanini, G. Gene Expression Analysis of Biphasic Pleural Mesothelioma: New Potential Diagnostic and Prognostic Markers. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Vizcaya, D.; Farahmand, B.; Walter, A.O.; Kneip, C.; Johrens, K.; Tukiainen, M.; Schmitz, A.A. Prognosis of patients with malignant mesothelioma by expression of programmed cell death 1 ligand 1 and mesothelin in a contemporary cohort in Finland. Cancer Treat Res Commun 2020, 25, 100260. [Google Scholar] [CrossRef]
- Inaguma, S.; Wang, Z.; Lasota, J.; Onda, M.; Czapiewski, P.; Langfort, R.; Rys, J.; Szpor, J.; Waloszczyk, P.; Okon, K.; et al. Comprehensive immunohistochemical study of mesothelin (MSLN) using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors with evaluation of its prognostic value in malignant pleural mesothelioma. Oncotarget 2017, 8, 26744–26754. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Higashi, T.; Ozeki, K.; Higashi, A.Y.; Sugimoto, K.; Mine, H.; Takagi, H.; Ozaki, Y.; Muto, S.; Okabe, N.; et al. CLDN15 is a novel diagnostic marker for malignant pleural mesothelioma. Sci Rep 2021, 11, 12554. [Google Scholar] [CrossRef] [PubMed]
- Sidi, R.; Pasello, G.; Opitz, I.; Soltermann, A.; Tutic, M.; Rehrauer, H.; Weder, W.; Stahel, R.A.; Felley-Bosco, E. Induction of senescence markers after neo-adjuvant chemotherapy of malignant pleural mesothelioma and association with clinical outcome: an exploratory analysis. Eur J Cancer 2011, 47, 326–332. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Akerley, W.; Bazhenova, L.A.; Borghaei, H.; Camidge, D.R.; Cheney, R.T.; Chirieac, L.R.; D'Amico, T.A.; Dilling, T.; et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016, 14, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Capkova, L.; Koubkova, L.; Kodet, R. Expression of carbonic anhydrase IX (CAIX) in malignant mesothelioma. An immunohistochemical and immunocytochemical study. Neoplasma 2014, 61, 161–169. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Hocking, A.; Kim, C.; Hussey, M.; Klebe, S. The potential utility of GATA binding protein 3 for diagnosis of malignant pleural mesotheliomas. Hum Pathol 2020, 105, 1–8. [Google Scholar] [CrossRef]
- Kadota, K.; Villena-Vargas, J.; Nitadori, J.; Sima, C.S.; Jones, D.R.; Travis, W.D.; Adusumilli, P.S. Tumoral CD10 expression correlates with aggressive histology and prognosis in patients with malignant pleural mesothelioma. Ann Surg Oncol 2015, 22, 3136–3143. [Google Scholar] [CrossRef]
- Pasello, G.; Urso, L.; Mencoboni, M.; Grosso, F.; Ceresoli, G.L.; Lunardi, F.; Vuljan, S.E.; Bertorelle, R.; Sacchetto, V.; Ciminale, V.; et al. MDM2 and HIF1alpha expression levels in different histologic subtypes of malignant pleural mesothelioma: correlation with pathological and clinical data. Oncotarget 2015, 6, 42053–42066. [Google Scholar] [CrossRef]
- Chu, G.J.; Linton, A.; Kao, S.; Klebe, S.; Adelstein, S.; Yeo, D.; Rasko, J.E.J.; Cooper, W.A. High mesothelin expression by immunohistochemistry predicts improved survival in pleural mesothelioma. Histopathology 2023, 83, 202–210. [Google Scholar] [CrossRef]
- Sandeck, H.P.; Roe, O.D.; Kjaerheim, K.; Willen, H.; Larsson, E. Re-evaluation of histological diagnoses of malignant mesothelioma by immunohistochemistry. Diagn Pathol 2010, 5, 47. [Google Scholar] [CrossRef]
- Kindler, H.L.; Novello, S.; Bearz, A.; Ceresoli, G.L.; Aerts, J.; Spicer, J.; Taylor, P.; Nackaerts, K.; Greystoke, A.; Jennens, R.; et al. Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): a randomised, open-label phase 2 trial. Lancet Oncol 2022, 23, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Mesothelioma with signet-ring cell features: report of 23 cases. Mod Pathol 2013, 26, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.; Borchert, S.; Wessolly, M.; Mathilakathu, A.; Mairinger, E.; Kollmeier, J.; Mairinger, T.; Hegedus, B.; Greimelmaier, K.; Wohlschlaeger, J.; et al. One Third of Malignant Pleural Mesothelioma Shows High Immunohistochemical Expression of MSLN or CXCR4 Which Indicates Potent Candidates for Endo-Radiotherapy. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Weidemann, S.; Gagelmann, P.; Gorbokon, N.; Lennartz, M.; Menz, A.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Blessin, N.C.; Fraune, C.; et al. Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Kajino, K.; Momose, S.; Masaoka, A.; Sasahara, K.; Shiomi, K.; Izumi, H.; Abe, M.; Ohtsuji, N.; Wang, T.; et al. Mesothelin (MSLN) promoter is hypomethylated in malignant mesothelioma, but its expression is not associated with methylation status of the promoter. Hum Pathol 2010, 41, 1330–1338. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, B.; Zhao, X.; Sun, H.; An, J.; Lin, X.; Zhu, D.; Zhao, X.; Wang, X.; Liu, F.; et al. Twist1 accelerates tumour vasculogenic mimicry by inhibiting Claudin15 expression in triple-negative breast cancer. J Cell Mol Med 2020, 24, 7163–7174. [Google Scholar] [CrossRef]
- Tabaries, S.; Annis, M.G.; Hsu, B.E.; Tam, C.E.; Savage, P.; Park, M.; Siegel, P.M. Lyn modulates Claudin-2 expression and is a therapeutic target for breast cancer liver metastasis. Oncotarget 2015, 6, 9476–9487. [Google Scholar] [CrossRef]
- He, C.; Li, Y.; Zhang, R.; Chen, J.; Feng, X.; Duan, Y. Low CFB expression is independently associated with poor overall and disease-free survival in patients with lung adenocarcinoma. Oncol Lett 2021, 21, 478. [Google Scholar] [CrossRef]
- Shimazaki, R.; Takano, S.; Satoh, M.; Takada, M.; Miyahara, Y.; Sasaki, K.; Yoshitomi, H.; Kagawa, S.; Furukawa, K.; Takayashiki, T.; et al. Complement factor B regulates cellular senescence and is associated with poor prognosis in pancreatic cancer. Cell Oncol (Dordr) 2021, 44, 937–950. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Dabritz, J.H.M.; Zhao, Z.; Yu, Y.; Dorr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Dou, Z.; Berger, S.L. Senescence Elicits Stemness: A Surprising Mechanism for Cancer Relapse. Cell Metab 2018, 27, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Li, Z.; Wang, X.; Qu, X.; Liu, Y. Activated Pak4 expression correlates with poor prognosis in human gastric cancer patients. Tumour Biol 2015, 36, 9431–9436. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Ye, Z.; Wang, X.; Pan, Y.; Weng, Y.; Lao, S.; Wei, H.; Li, L. Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion. J Exp Clin Cancer Res 2015, 34, 48. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.; Imoto, I.; Kozaki, K.; Tsuda, H.; Suzuki, E.; Amagasa, T.; Inazawa, J. Identification of PAK4 as a putative target gene for amplification within 19q13.12-q13.2 in oral squamous-cell carcinoma. Cancer Sci 2009, 100, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Y.; Liu, Y.; Liu, H.; Zhang, W.; Xu, L.; Zhu, Y.; Xu, J. p21-Activated kinase 4 predicts early recurrence and poor survival in patients with nonmetastatic clear cell renal cell carcinoma. Urol Oncol 2015, 33, 205 e213-221. [Google Scholar] [CrossRef]
- Song, B.; Wang, W.; Zheng, Y.; Yang, J.; Xu, Z. P21-activated kinase 1 and 4 were associated with colorectal cancer metastasis and infiltration. J Surg Res 2015, 196, 130–135. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.W.; Kim, H.; Kim, J.W.; Kim, Y.J.; Lee, K.W.; Kim, J.H.; Kim, J.H.; Hwang, J.H.; Choi, Y.R.; et al. Prognostic value of p21-activated kinase 4 in resected pancreatic cancer. APMIS 2017, 125, 699–707. [Google Scholar] [CrossRef]
| Characteristics | Training Cohort (n=73) | Validation Cohort (n=30) |
| Age, years, median (range) | 71 (40-85) | 75 (54-87) |
| Sex, male, n (%) | 58 (79.5) | 25 (83.3) |
| Mesothelioma Subtype | ||
| Epithelioid, n (%) | 31 (42.5) | 11 (36.7) |
| Biphasic, n (%) | 25 (34.2) | 11 (36.7) |
| Sarcomatoid, n (%) | 17 (23.3) | 8 (26.6) |
| Epithelioid subtype (n=42) | Training Cohort (n=31) | Validation Cohort (n=11) |
| High grade, n (%) | 12 (38.7) | 3 (27.3) |
| Mitosis number score | ||
| 1 (≤ 1 mitosis/2 mm2) | 11 (35.5) | 5 (45.4) |
| 2 (2–4 mitoses/2 mm2) | 15 (48.4) | 3 (27.3) |
| 3 (≥ 5 mitoses/2 mm2) | 5 (16.1) | 3 (27.3) |
| Nuclear atypia score | ||
| 1 | 8 (25.8) | 3 (27.3) |
| 2 | 17 (54.8) | 5 (45.4) |
| 3 | 6 (19.4) | 3 (27.3) |
| Necrosis presence | 13 (41.9) | 4 (36.4) |
| Scores |
CFB Median (IQR) |
Mesothelin Median (IQR) |
Claudin-15 Median (IQR) |
PAI1 Median (IQR) |
PAK4 Median (IQR) |
|
| ES | TPS H-score |
70 (55-90) 120 (55-180) |
92.5 (81.25-95) 270 (190-285) |
85 (70-95) 190 (130-210) |
60 (50-80) 120 (82.5-170) |
70 (52.5-82.5) 120 (85-160) |
| BS | TPS H-score |
60 (30-70) 80 (40-120) |
50 (30-70) 117.5 (60-187.5) |
70 (60-75) 150 (130-195) |
85 (80-90) 210 (160-270) |
80 (70-90) 210 (180-240) |
| SS | TPS H-score |
10 (5-20) 10 (5-20) |
0 (0-10) 0 (0-15) |
35 (30-60) 60 (35-80) |
90 (80-95) 210 (190-255) |
70 (65-90) 160 (110-195) |
|
ES vs BS p-value |
TPS H-score |
0.04 0.05 |
<0.0001 <0.0001 |
0.0006 0.17 |
0.001 <0.0001 |
0.05 <0.0001 |
|
ES vs SS p-value |
TPS H-score |
<0.0001 <0.0001 |
<0.0001 <0.0001 |
<0.0001 <0.0001 |
<0.0001 <0.0001 |
0.23 0.04 |
|
BS vs SS p-value |
TPS H-score |
0.0003 0.0001 |
0.0001 <0.0001 |
0.004 <0.0001 |
0.09 0.23 |
0.25 0.03 |
| Training cohort | |||||
| Mesothelin | Claudin-15 | CFB | PAI1 | PAK4 | |
| Cut-off | 67.5 % | 77.5 % | 65 % | 72.5 % | 62.5 % |
| AUC | 0.97 (0.93-0.99) | 0.85 (0.75-0.93) | 0.76 (0.64-0.87) | 0.79 (0.67-0.89) | 0.60 (0.47-0.73) |
| Sensitivity | 0.88 (0.76-0.98) | 0.88 (0.57-1) | 0.86 (0.55-1) | 0.88 (0.62-0.98) | 0.86 (0.43-0.98) |
| Specificity | 1 (0.94-1) | 0.71 (0.51-0.94) | 0.61 (0.32-0.90) | 0.65 (0.42-0.84) | 0.42 (0.23-0.81) |
| Accuracy | 0.93 (0.86-0.97) | 0.81 (0.70-0.89) | 0.75 (0.66-0.82) | 0.77 (0.67-0.85) | 0.67 (0.58-0.77) |
| NPV | 0.86 (0.76-0.97) | 0.83 (0.61-1) | 0.76 (0.57-1) | 0.79 (0.60-0.95) | 0.70 (0.50-0.89) |
| PPV | 1 (0.95-1) | 0.80 (0.72-0.94) | 0.76 (0.67-0.90) | 0.76 (0.68-0.87) | 0.67 (0.61-0.77) |
| Validation cohort | |||||
| Mesothelin | Claudin-15 | CFB | PAI1 | PAK4 | |
| AUC* | 0.98 (0.92-1) | 0.84 (0.66-0.97) | 0.80 (0.59-0.97) | NA | 0.75 (0.57-0.90) |
| Sensitivity* | 0.79 (0.58-0.95) | 0.84 (0.68-1) | 0.89 (0.74-1) | NA | 0.84 (0.68-1) |
| Specificity* | 0.91 (0.73-1) | 0.73 (0.45-1) | 0.64 (0.36-0.91) | NA | 0.36 (0.09-0.64) |
| Accuracy* | 0.83 (0.70-0.93) | 0.80 (0.63-0.93) | 0.80 (0.67-0.93) | NA | 0.67 (0.53-0.80) |
| NPV* | 0.71 (0.56-0.92) | 0.73 (0.50-1) | 0.78 (0.55-1) | NA | 0.57 (0.25-1) |
| PPV* | 0.94 (0.81-1) | 0.84 (0.71-1) | 0.81 (0.70-0.95) | NA | 0.70 (0.60-0.81) |
| Best cut-off on Validation cohort | |||||
| 87.5% | 75% | 47.5% | 65% | 77.5% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





