Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of myrrh resin extracts
2.3. Isolation and identification of airborne bacterial strains
2.4. Antibacterial activities evaluation of myrrh resin extracts
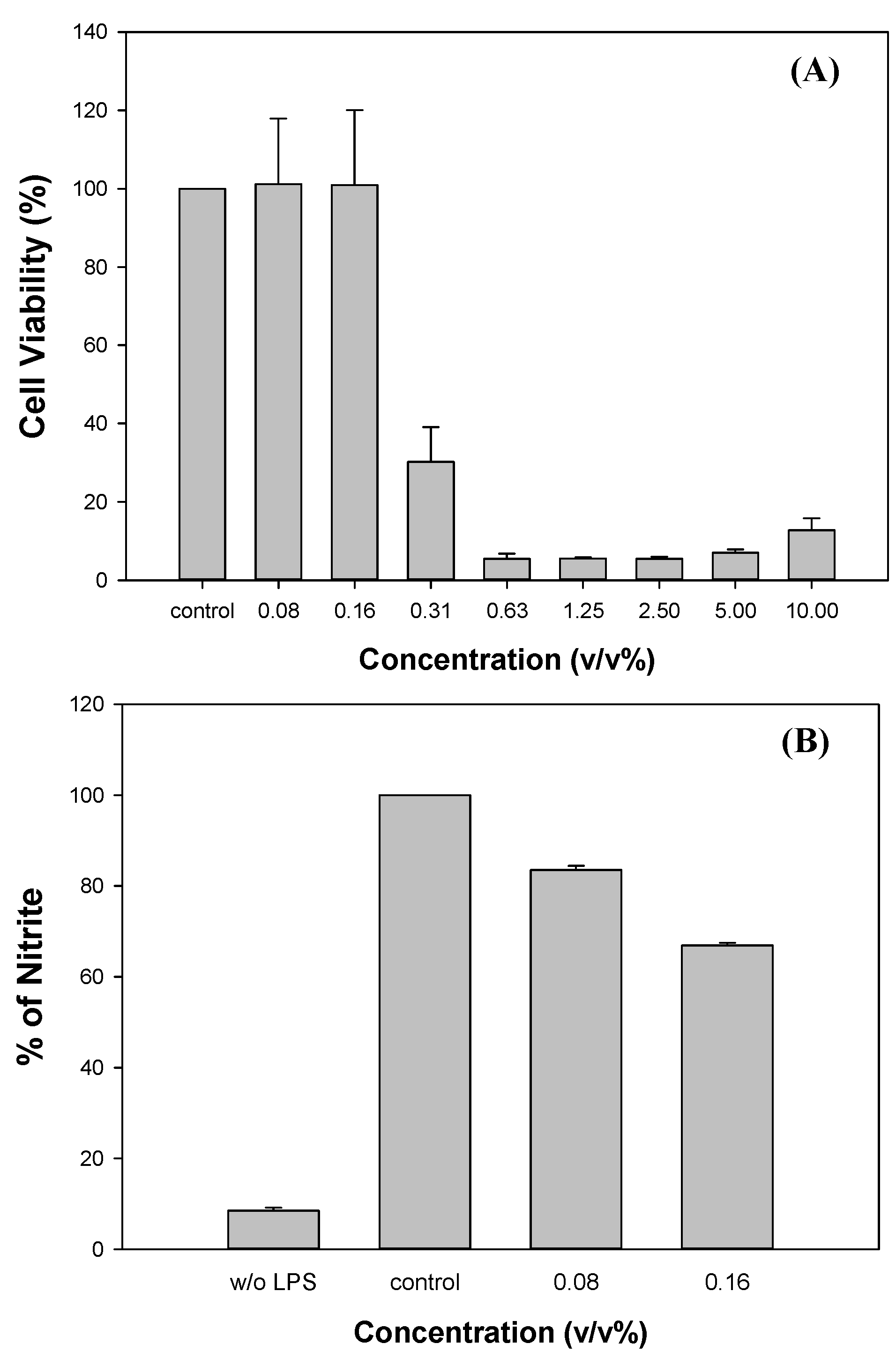
2.5. Evaluation of cytotoxicity and anti-inflammatory of myrrh resin extracts
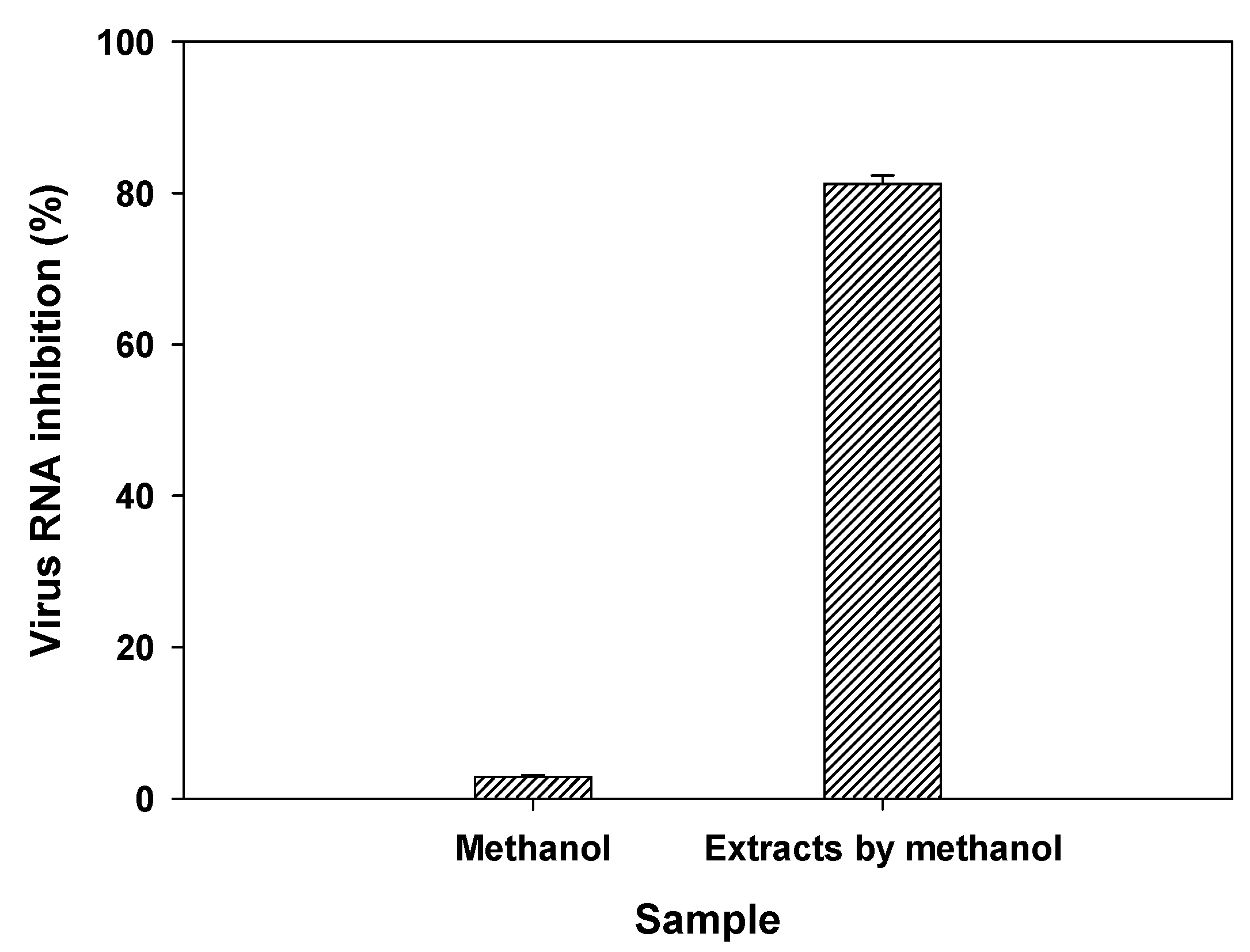
2.6. Antiviral activities by myrrh resin extracts and myrrh resin extracts coated biochar
2.7. Adsorption myrrh resin extracts onto biochar
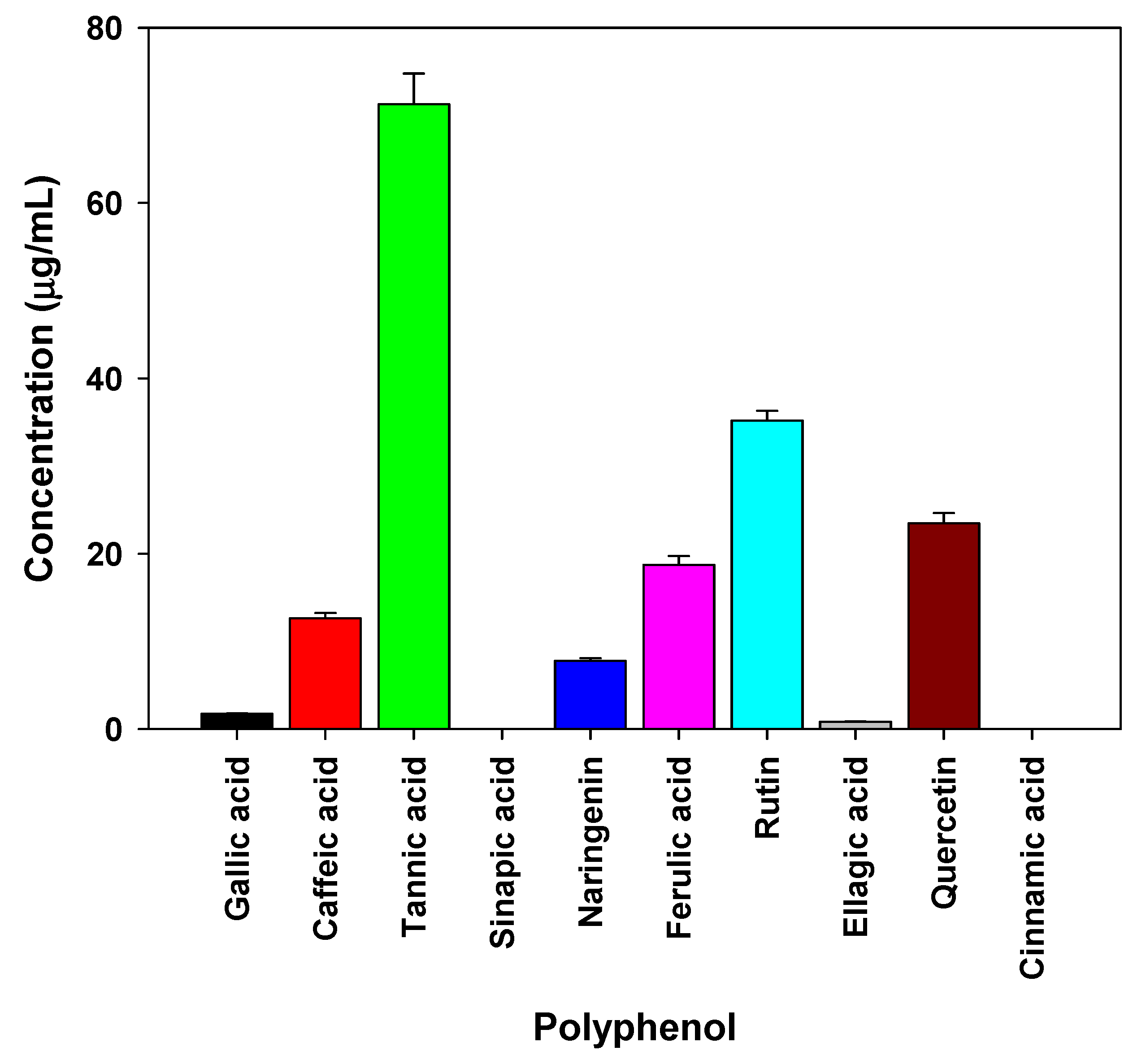
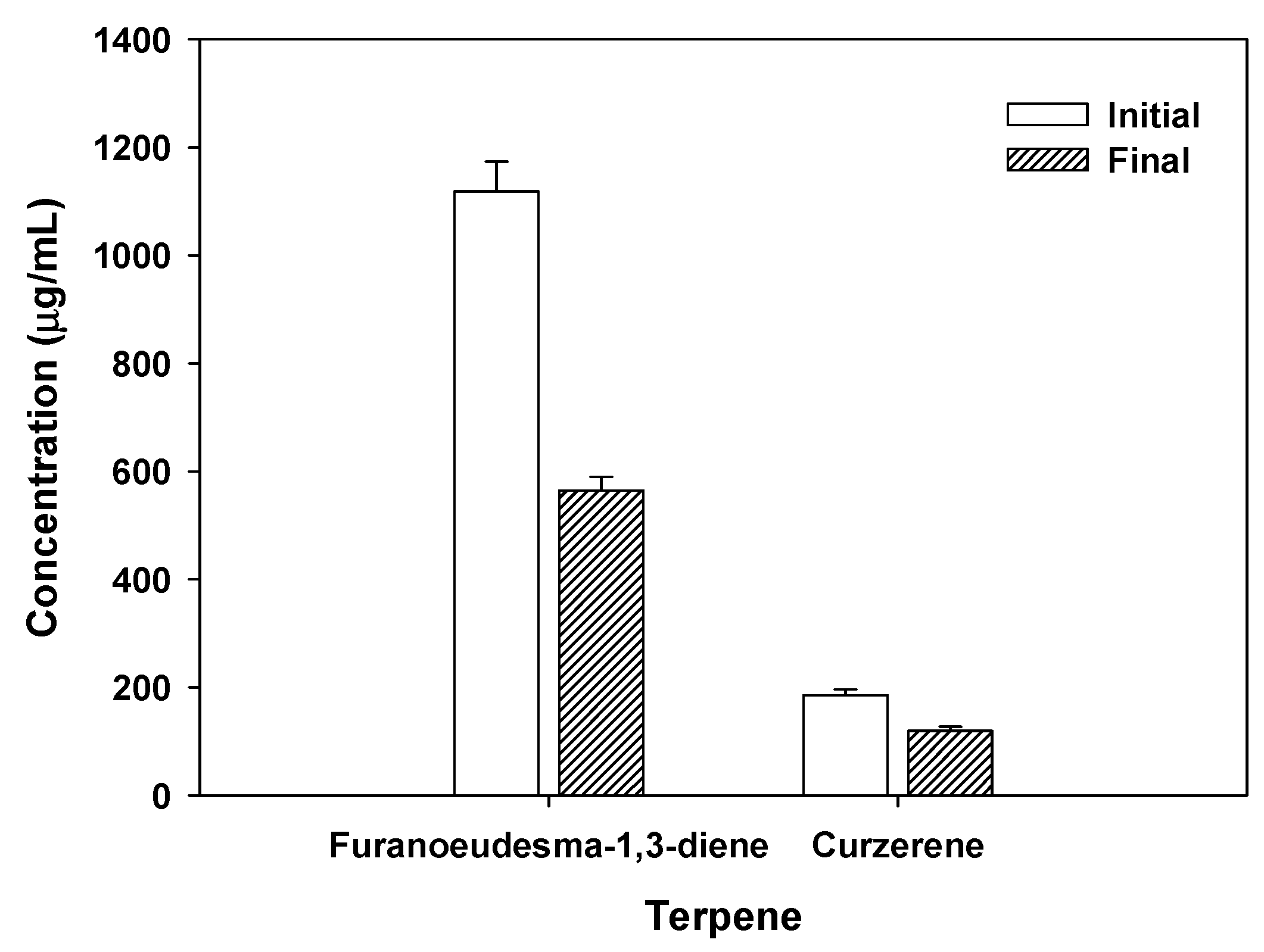
2.8. Natural compounds analysis of myrrh resin extracts
3. Results and Discussion
3.1. Identification of test microorganisms
3.2. Screening of optimal solvent for antibacterial activity of myrrh resin extracts on airborne bacterium
3.3. Evaluation of antibacterial activities of myrrh resin extracts
3.4. Evaluation of cytotoxicity and anti-inflammatory of myrrh resin extracts
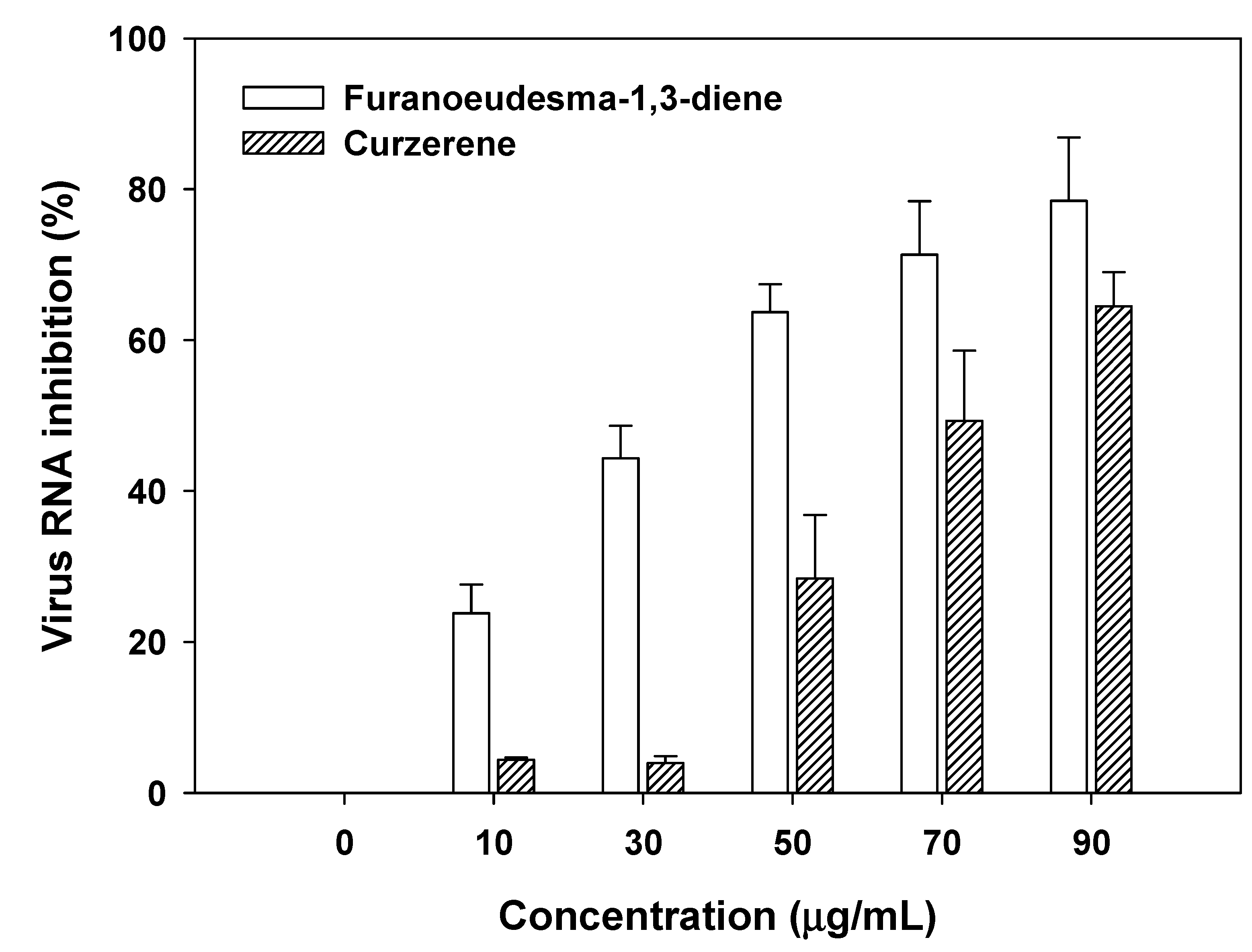
3.5. Evaluation of antiviral activities of myrrh resin extracts
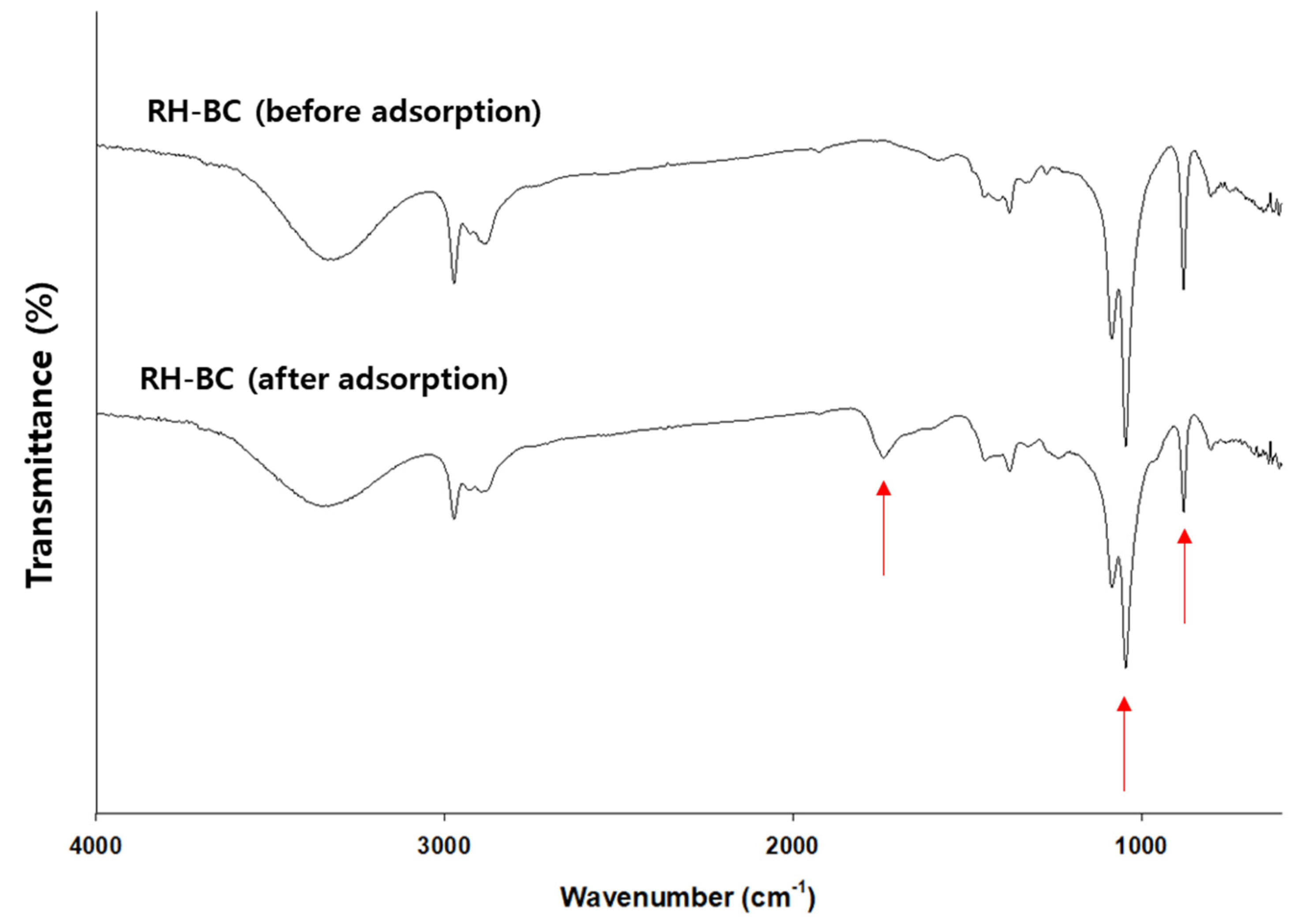
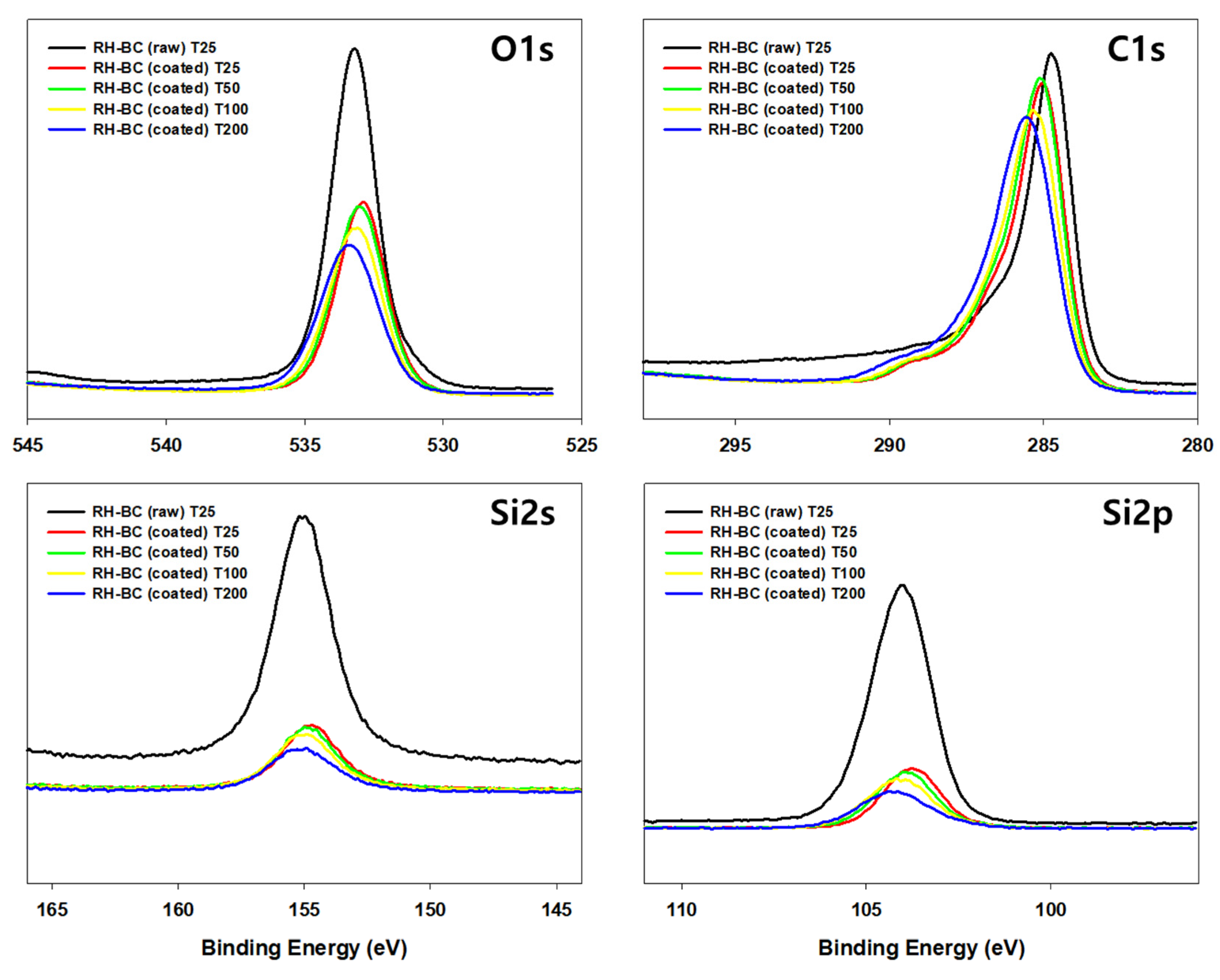
3.6. Potential mechanisms for antibacterial and antiviral activities of myrrh resin extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials-A minireview. Materials 2020, 13, 3224. [CrossRef]
- Von Nussbaum, F.; Brands, M., Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry - Exodus or revival?. Angewandte Chemie - International Edition 2006, 45, 5072-5129. [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 2020, 11, 904. [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products. Critical Reviews in Food Science and Nutrition 2021, 61, 149–178. [CrossRef]
- Kim, T.; Song, B.; Cho, K. S.; Lee, I. S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. International Journal of Molecular Sciences 2020, 21, 2187. [CrossRef]
- Nasır, A.; Yabalak, E. Investigation of antioxidant, antibacterial, antiviral, chemical composition, and traditional medicinal properties of the extracts and essential oils of the Pimpinella species from a broad perspective: a review. Journal of Essential Oil Research 2021, 33, 411-426. [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K. M.; Latha, L. Y. Extraction, Isolation And Characterization Of Bioactive Compounds From Plants’ Extracts. Afr J Tradit Complement Altern Med 2011, 8, 1-10. [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Industrial Crops and Products 2019, 132, 413–429. [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from acacia dealbata and olea europaea. Antibiotics 2020, 9, 48. [CrossRef]
- Ahmadi, A.; Shahidi, S. A.; Safari, R.; Motamedzadegan, A.; Ghorbani-HasanSaraei, A. Evaluation of stability and antibacterial properties of extracted chlorophyll from alfalfa (Medicago sativa L.). Food and Chemical Toxicology 2022, 163, 112980. [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S. G.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. International Journal of Biological Macromolecules 2019, 124, . [CrossRef]
- Mukherjee, C., Suryawanshi, P. G., Kalita, M. C., Deka, D., Aranda, D. A. G., & Goud, V. V. (2022). Polarity-wise successive solvent extraction of Scenedesmus obliquus biomass and characterization of the crude extracts for broad-spectrum antibacterial activity. Biomass Convers Biorefin. [CrossRef]
- Thawabteh, A. M., Swaileh, Z., Ammar, M., Jaghama, W., Yousef, M., Karaman, R., A. Bufo, S., & Scrano, L. (2023). Antifungal and antibacterial activities of isolated marine compounds. Toxins, 15(2). [CrossRef]
- Zhang, H., Wang, X., He, D., Zou, D., Zhao, R., Wang, H., Li, S., Xu, Y., & Abudureheman, B. (2022). Optimization of flavonoid extraction from Xanthoceras sorbifolia bunge flowers, and the antioxidant and antibacterial capacity of the extract. Molecules, 27(1). [CrossRef]
- Abubakar, A. R., & Haque, M. (2020). Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci, 12(1). [CrossRef]
- Hardouin, K., Bedoux, G., Burlot, A. S., Donnay-Moreno, C., Bergé, J. P., Nyvall-Collén, P., & Bourgougnon, N. (2016). Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res, 16. [CrossRef]
- Madia, V. N., De Angelis, M., De Vita, D., Messore, A., De Leo, A., Ialongo, D., Tudino, V., Saccoliti, F., De Chiara, G., Garzoli, S., Scipione, L., Palamara, A. T., Di Santo, R., Nencioni, L., & Costi, R. (2021). Investigation of Commiphora myrrha (Nees) Engl. oil and its main components for antiviral activity. Pharm, 14(3). [CrossRef]
- Alara, O. R., Abdurahman, N. H., & Ukaegbu, C. I. (2021). Extraction of phenolic compounds: A review. Curr Res Food Sci, 4. [CrossRef]
- Asghar, N., Naqvi, S. A. R., Hussain, Z., Rasool, N., Khan, Z. A., Shahzad, S. A., Sherazi, T. A., Janjua, M. R. S. A., Nagra, S. A., Zia-Ul-Haq, M., & Jaafar, H. Z. (2016). Compositional difference in antioxidant and antibacterial activity of all parts of the Carica papaya using different solvents. Chem Cent J, 10(1). [CrossRef]
- Haminiuk, C. W. I., Plata-Oviedo, M. S. V., de Mattos, G., Carpes, S. T., & Branco, I. G. (2014). Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J Food Sci Technol, 51(10). [CrossRef]
- Lim, S., Choi, A. H., Kwon, M., Joung, E. J., Shin, T., Lee, S. G., Kim, N. G., & Kim, H. R. (2019). Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem, 278, 178–184. [CrossRef]
- Nguyen, L. T. T., Nguyen, T. T., Nguyen, H. N., & Bui, Q. T. P. (2020). Simultaneous determination of active compounds in Piper betle Linn. leaf extract and effect of extracting solvents on bioactivity. Eng Rep, 2(10). [CrossRef]
- Rahmani, A. H., Anwar, S., Raut, R., Almatroudi, A., Babiker, A. Y., Khan, A. A., Alsahli, M. A., & Almatroodi, S. A. (2022). Therapeutic potential of myrrh, a natural resin, in health management through modulation of oxidative stress, inflammation, and advanced glycation end products formation using in Vitro and in Silico analysis. Appl Sci, 12(18). [CrossRef]
- Rezaei, M., & Ghasemi Pirbalouti, A. (2019). Phytochemical, antioxidant and antibacterial properties of extracts from two spice herbs under different extraction solvents. J Food Meas Charact, 13(3), 2470–2480. [CrossRef]
- Termentzi, A., Fokialakis, N., & Leandros Skaltsounis, A. (2012). Natural resins and bioactive natural products thereof as potential anitimicrobial agents. Curr Pharm Des, 17(13). [CrossRef]
- Adam, M. E., & Selim, S. A. (2013). Antimicrobial activity of essential oil and methanol extract from Commiphora molmol (Engl.) resin. Int J Curr Microbiol, App Sci, 2(12). http://www.ijcmas.com.
- Bhattacharjee, M. K., & Alenezi, T. (2020). Antibiotic in myrrh from Commiphora molmol preferentially kills nongrowing bacteria. Future Sci, 6(4). [CrossRef]
- Hana, D. B., Kadhim, H. M., Jasim, G. A., & Latif, Q. N. (2016). Antibacterial activity of Commiphora molmol extracts on some bacterial species in Iraq. Sch Acad J Pharm, 5(12), 406–412. [CrossRef]
- Mahboubi, M., & Kazempour, N. (2016). The antimicrobial and antioxidant activities of Commiphora molmol extracts. Biharean Biol, 10(2).
- Mohamed, A., Shahid, S. M. A., Alkhamaiseh, S. I., Ahmed, M. Q., Albalwi, F. O., Al-Gholaigah, H., Alqhtani, M., & Alshammari, G. (2017). Antibacterial activity of Commiphora molmol in wound infections. J Diagn Pathol Oncol, 2(2).
- Das, R., & Panda, S. N. (2022). Preparation and applications of biochar based nanocomposite: A review. J Anal Appl Pyrolysis, 167. [CrossRef]
- Nguyen, D. T. C., Tran, T. Van, Nguyen, T. T. T., Nguyen, D. H., Alhassan, M., & Lee, T. (2023). New frontiers of invasive plants for biosynthesis of nanoparticles towards biomedical applications: A review. Sci Total Environ, 857. [CrossRef]
- Pirtarighat, S., Ghannadnia, M., & Baghshahi, S. (2019). Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. Journal of Nanostructure in Chemistry, 9, 1-9. [CrossRef]
- Strasakova, M., Pummerova, M., Filatova, K., Sedlarik, V., & Fernández-García, M. (2021). Immobilization of caraway essential oil in a polypropylene matrix for antimicrobial modification of a polymeric surface. Polymers, 13(6), 906. [CrossRef]
- Choi, Y. K., Choi, T. R., Gurav, R., Bhatia, S. K., Park, Y. L., Kim, H. J., Kan, E., & Yang, Y. H. (2020). Adsorption behavior of tetracycline onto Spirulina sp. (microalgae)-derived biochars produced at different temperatures. Sci Total Environ, 710. [CrossRef]
- Kim, J. E., Bhatia, S. K., Song, H. J., Yoo, E., Jeon, H. J., Yoon, J. Y., Yang, Y., Gurav, R., Yang, Y. H., Kim, H. J., & Choi, Y. K. (2020). Adsorptive removal of tetracycline from aqueous solution by maple leaf-derived biochar. Bioresour Technol, 306. [CrossRef]
- Adhikari, S., Timms, W., & Mahmud, M. A. P. (2022). Optimising water holding capacity and hydrophobicity of biochar for soil amendment – A review. Sci Total Environ, 851. [CrossRef]
- Choi, Y. K., & Kan, E. (2019). Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere, 218. [CrossRef]
- Das, R., & Panda, S. N. (2022). Preparation and applications of biochar based nanocomposite: A review. J Anal Appl Pyrolysis, 167. [CrossRef]
- Liu, M., Ke, X., Liu, X., Fan, X., Xu, Y., Li, L., Solaiman, Z. M., & Pan, G. (2022). The effects of biochar soil amendment on rice growth may vary greatly with rice genotypes. Sci Total Environ, 810. [CrossRef]
- Liu, Z., Zhu, M., Wang, J., Liu, X., Guo, W., Zheng, J., Bian, R., Wang, G., Zhang, X., Cheng, K., Liu, X., Li, L., & Pan, G. (2019). The responses of soil organic carbon mineralization and microbial communities to fresh and aged biochar soil amendments. Glob Change Biol Bioenergy, 11(12). [CrossRef]
- Song, H. J., Gurav, R., Bhatia, S. K., Lee, E. Bin, Kim, H. J., Yang, Y. H., Kan, E., Kim, H. H., Lee, S. H., & Choi, Y. K. (2021). Treatment of microcystin-LR cyanotoxin contaminated water using Kentucky bluegrass-derived biochar. J Water Process Eng, 41. [CrossRef]
- Kang, Y., Choi, Y. K., Kim, H. J., Song, Y., & Kim, H. (2015). Preparation of anti-bacterial cellulose fiber via electrospinning and crosslinking with β-cyclodextrin. Fash Text, 2(1). [CrossRef]
- Chuang, S., Yang, H., Wang, X., Xue, C., Jiang, J., & Hong, Q. (2021). Potential effects of Rhodococcus qingshengii strain djl-6 on the bioremediation of carbendazim-contaminated soil and the assembly of its microbiome. J Hazard Mater, 414. [CrossRef]
- Fang, Y., Wu, L., Chen, G., & Feng, G. (2016). Complete genome sequence of Pseudomonas azotoformans S4, a potential biocontrol bacterium. J Biotechnol, 227. [CrossRef]
- Langlois, B. E., Harmon, R. J., & Akers, K. (1983). Identification of Staphylococcus species of bovine origin with the API staph-ident system. J Clin Microbiol, 18(5). [CrossRef]
- Osman, S., Satomi, M., & Venkateswaran, K. (2006). Paenibacillus pasadenensis sp. nov. and Paenibacillus barengoltzii sp. nov., isolated from a spacecraft assembly facility. Int J Syst Evol Microbiol, 56(7). [CrossRef]
- White, O., Eisen, J. A., Heidelberg, J. F., Hickey, E. K., Peterson, J. D., Dodson, R. J., Haft, D. H., Gwinn, M. L., Nelson, W. C., Richardson, D. L., Moffat, K. S., Qin, H., Jiang, L., Pamphile, W., Crosby, M., Shen, M., Vamathevan, J. J., Lam, P., McDonald, L., Fraser, C. M. (1999). Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Sci, 286(5444). [CrossRef]
- Zhao, G. Z., Li, J., Qin, S., Zhang, Y. Q., Zhu, W. Y., Jiang, C. L., Xu, L. H., & Li, W. J. (2009). Micrococcus yunnanensis sp. nov., a novel actinobacterium isolated from surface-sterilized Polyspora axillaris roots. Int J Syst Evol Microbiol, 59(10). [CrossRef]
- Kapoor, G., Saigal, S., & Elongavan, A. (2017). Action and resistance mechanisms of antibiotics : A guide for clinicians basic anatomy of bacterial cell. J Anaesthesiol Clin Pharmacol, 33(3). [CrossRef]
- Markom, M., Hasan, M., Daud, W. R. W., Singh, H., & Jahim, J. M. (2007). Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep Purif Technol, 52(3). [CrossRef]
- Ng, Z. X., Samsuri, S. N., & Yong, P. H. (2020). The antioxidant index and chemometric analysis of tannin, flavonoid, and total phenolic extracted from medicinal plant foods with the solvents of different polarities. J Food Process Preserv, 44(9). [CrossRef]
- Ouyang, J., Ding, J., Lefebvre, J., Li, Z., Guo, C., Kell, A. J., & Malenfant, P. R. L. (2018). Sorting of semiconducting single-walled carbon nanotubes in polar solvents with an amphiphilic conjugated polymer provides general guidelines for enrichment. ACS Nano, 12(2). [CrossRef]
- Al-Madi, E. M., Almohaimede, A. A., Al-Obaida, M. I., & Awaad, A. S. (2019). Comparison of the antibacterial efficacy of Commiphora molmol and sodium hypochlorite as root canal irrigants against Enterococcus faecalis and Fusobacterium nucleatum. Evid Based Complement Alternat Med, 2019. [CrossRef]
- Abdallah, E., & Khalid, A. (2012). A preliminary evaluation of the antibacterial effects of Commiphora molmol and Boswellia papyrifera oleo-gum resins vapor. Int J Chem Biochem Res, 1.
- Abdallah, E. M., Khalid, A. S., & Ibrahim, N. (2009). Antibacterial activity of oleo-gum resins of Commiphora molmol and Boswellia papyrifera against methicillin resistant Staphylococcus aureus (MRSA). Sci Res Essays, 4(4).
- Mahboubi, M., & Kazempour, N. (2016). The antimicrobial and antioxidant activities of Commiphora molmol extracts. Biharean Biol, 10(2).
- Mandal, S. M., Dias, R. O., & Franco, O. L. (2017). Phenolic compounds in antimicrobial therapy. J Med Food, 20(10). [CrossRef]
- Belhaoues, S., Amri, S., & Bensouilah, M. (2020). Major phenolic compounds, antioxidant and antibacterial activities of Anthemis praecox Link aerial parts. S Afr J Bot, 131. [CrossRef]
- Pandey, A., & Negi, P. S. (2018). Phytochemical composition, in vitro antioxidant activity and antibacterial mechanisms of Neolamarckia cadamba fruits extracts. Nat Prod Res, 32(10). [CrossRef]
- Suzilla, W. Y., Izzati, A., Isha, I., Zalina, A., & Rajaletchumy, V. K. (2020). Formulation and evaluation of antimicrobial herbosomal gel from Quercus infectoria extract. IOP Conference Series: Mater Sci Eng, 736(2). [CrossRef]
- Vance, S. H., Tucci, M., & Benghuzzi, H. (2011). Evaluation of the antimicrobial efficacy of green tea extract (EGCG) against Streptococcus pyogenes in vitro. Biomed Sci Instrum, 47.
- Roberts, I. S. (1996). The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol, 50. [CrossRef]
- Cheng, Y. W., Cheah, K. P., Lin, C. W., Li, J. S., Yu, W. Y., Chang, M. L., Yeh, G. C., Chen, S. H., Choy, C. S., & Hu, C. M. (2011). Myrrh mediates haem oxygenase-1 expression to suppress the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages. J Pharm Pharmacol, 63(9). [CrossRef]
- Wang, W., Cao, J., Yang, J., Niu, X., Liu, X., Zhai, Y., Qiang, C., Niu, Y., Li, Z., Dong, N., Wen, B., Ouyang, Z., Zhang, Y., Li, J., Zhao, M., & Zhao, J. (2023). Antimicrobial activity of tannic acid in Vitro and its protective effect on mice against Clostridioides difficile. Microbiol Spectr, 11(1). [CrossRef]
- Gullón, B., Lú-Chau, T. A., Moreira, M. T., Lema, J. M., & Eibes, G. (2017). Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol, 67. [CrossRef]
- Qi, W., Qi, W., Xiong, D., & Long, M. (2022). Quercetin: its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules, 27(19). [CrossRef]
- Dinku, W., Isaksson, J., Rylandsholm, F. G., Bouř, P., Brichtová, E., Choi, S. U., Lee, S. H., Jung, Y. S., No, Z. S., Svendsen, J. S. M., Aasen, A. J., & Dekebo, A. (2020). Anti-proliferative activity of a novel tricyclic triterpenoid acid from Commiphora africana resin against four human cancer cell lines. Appl Biol Chem, 63(1). [CrossRef]
- Al-Nami, S. Y., & Fouda, A. E. A. S. (2020). Corrosion inhibition effect and adsorption activities of methanolic myrrh extract for cu in 2 M HNO3. International Journal of Electrochemical Science, 15(2), 1187–1205. [CrossRef]
| Name of strains | Homology (%) | Morphology | Gram staining | Remark | Ref. |
|---|---|---|---|---|---|
| Paenibacillus pasadenensis NBRC 161214 | 98 | Rods | Positive | Soil, Water, Sewage, etc. | [47] |
| Micrococcus yunnanensis YIM 65004 | 99 | Cocci | Positive | Pollutants-degrading | [49] |
| Pseudomonas azotoformans NBRC 12693 | 99 | Rods | Negative | Infecting cereal grains | [45] |
| Rhodococcus qingshengii dj1-6-2 | 99 | Ovoid | Positive | Carbendazim-degrading | [44] |
| Staphylococcus capitis JCM 2420 | 99 | Cocci | Positive | Human skin | [46] |
| Staphylococcus epidermidis NBRC 100911 | 98 | Cocci | Positive | Human skin | [46] |
| Deinococcus radiodurans R1 | 99 | Cocci | Positive | Radiation-resistant | [48] |
| No. | Name of strains | Antibacterial activity | ||||
|---|---|---|---|---|---|---|
| Hot water | DMSO | Hexane | Ethanol | Methanol | ||
| 1 | Paenibacillus pasadenensis NBRC 161214 | - | - | - | + | + |
| 2 | Micrococcus yunnanensis YIM 65004 | - | + | - | + | + |
| 3 | Pseudomonas azotoformans NBRC 12693 | - | - | - | - | - |
| 4 | Rhodococcus qingshengii dj1-6-2 | - | - | - | - | + |
| 5 | Staphylococcus capitis JCM 2420 | - | - | - | - | - |
| 6 | Staphylococcus epidermidis NBRC 100911 | - | - | - | - | - |
| 7 | Deinococcus radiodurans R1 | - | - | - | + | + |
| No. | Name of strains | Inhibition zone diameter (mm) |
|---|---|---|
| 1 | Paenibacillus pasadenensis NBRC 161214 | 14 ± 2.8 |
| 2 | Micrococcus yunnanensis YIM 65004 | 10 ± 0.0 |
| 3 | Pseudomonas azotoformans NBRC 12693 | ND |
| 4 | Rhodococcus qingshengii dj1-6-2 | 10 ± 1.4 |
| 5 | Staphylococcus capitis JCM 2420 | ND |
| 6 | Staphylococcus epidermidis NBRC 100911 | ND |
| 7 | Deinococcus radiodurans R1 | 12 ± 4.2 |
| Sample | Ct value | Remained virus (%) |
|---|---|---|
| Water (without virus) | 0 | - |
| Virus (Initial) | 24.809 | 100.00 |
| WD-BC (Final) | 25.121 | 96.69 |
| Extracts coated WD-BC (Final) | 27.105 | 75.66 |
| RH-BC (Final) | 25.137 | 96.52 |
| Extracts coated RH-BC (Final) | 26.941 | 77.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





