Submitted:
31 July 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Spheroids
2.1. Limitations of 2D Cell Culture
2.2. Spheroids for Preclinical Drug Development
2.3. Spheroids for Drug Efficacy
2.4. Spheroids for Drug Toxicity
3. Organoids
Organoids for Preclinical Drug Development
4. Organ-On-A-Chip
4.1. Single-organ and Multi-Organ Chips
4.2. OoC Architecture
| Material | Fabrication method | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Polydimethylsiloxane (PDMS) | Soft Lithography | • Optically clear • Recapitulates high detail • Easy fabrication • Permeable to gasses • Hydrophilic/ hydrophobic capabilities • Biocompatible |
• Absorption, retention, and release of small molecules • Laborious for mass production |
[80,81] |
| Polymethylmethacrylate (PMMA) | Injection Molding | • Optically clear • Minimal absorption • Cost-effective for mass production |
• High stiffness • Low fidelity in complex microstructures • Low gas permeability • Difficult to seal |
[86,87] |
| Cyclic olefin copolymer (COC) | Injection Molding | • Optically clear • Minimal absorption • Cost-effective for mass production |
• High stiffness • Low fidelity in complex microstructures • Low gas permeability • Difficult to seal |
[85] |
| Silicon | Photolithography | • Compatible with electronic integration • Versatile surface treatments • Recapitulates high detail |
• Laborious and costly to produce • Requires cleanroom facilities • Poor optical transparency • Brittle |
[79] |
| Glass | Etching | • Optically clear • Inert • Chemically resistant • Biocompatible |
• Laborious and costly to produce • Brittle |
[82] |
| Resins | 3D Printing | • Low cost • Rapid prototyping • High throughput |
• Poor optical properties • Poor biocompatibility • Low permeability • Texturally rough • Low fidelity in complex microstructures |
[83,84] |
4.3. OoC for Preclinical Drug Development
4.4. Vascularization Capabilities of OoCs
5. D Bioprinting
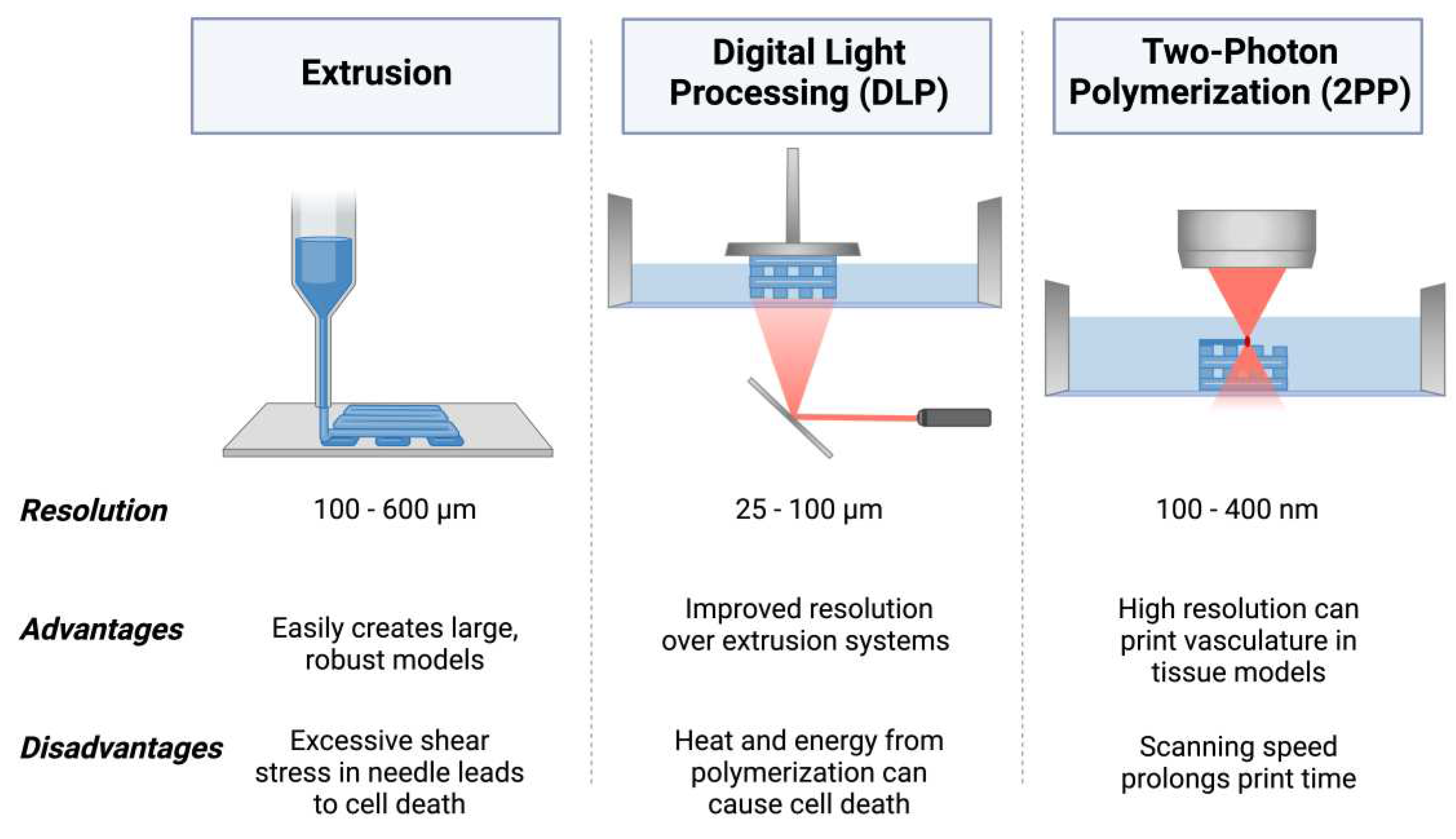
5.1. Extrusion 3D Bioprinting
5.2. Digital Light Processing (DLP) 3D Bioprinting
5.3. Two-Photon Polymerization (2PP) 3D Bioprinting
5.4. D Bioprinting Biomaterials
5.5. D Bioprinting for Preclinical Drug Development
5.6. D Bioprinting for Modeling Metastasis
5.7. Vascularization Capabilities of 3D Bioprinting
6. Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, A.M.; Mendicino, M.; Au, P. An FDA Perspective on Preclinical Development of Cell-Based Regenerative Medicine Products. Nat Biotechnol 2014, 32, 721–723. [CrossRef]
- Yang, W.; Meng, L.; Chen, K.; Tian, C.; Peng, B.; Zhong, L.; Zhang, C.; Yang, X.; Zou, J.; Yang, S.; et al. Preclinical Pharmacodynamic Evaluation of a New Src/FOSL 1 Inhibitor, LY-1816, in Pancreatic Ductal Adenocarcinoma. Cancer Sci 2019, 110, 1408–1419. [CrossRef]
- Lovitt, C.; Shelper, T.; Avery, V. Advanced Cell Culture Techniques for Cancer Drug Discovery. Biology (Basel) 2014, 3, 345–367. [CrossRef]
- Ikeda, K.; Iwasaki, Y. Edaravone, a Free Radical Scavenger, Delayed Symptomatic and Pathological Progression of Motor Neuron Disease in the Wobbler Mouse. PLoS One 2015, 10. [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm Sin B 2022, 12, 3049–3062. [CrossRef]
- Unger, C.; Kramer, N.; Walzl, A.; Scherzer, M.; Hengstschläger, M.; Dolznig, H. Modeling Human Carcinomas: Physiologically Relevant 3D Models to Improve Anti-Cancer Drug Development. Adv Drug Deliv Rev 2014, 79, 50–67.
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Approval and Regulation of Pharmaceuticals, 1983-2018. JAMA 2020, 323, 164. [CrossRef]
- van Norman, G.A. Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs. JACC Basic Transl Sci 2016, 1, 170–179. [CrossRef]
- S.5002 - 117th Congress (2021-2022): FDA Modernization Act 2.0.
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [CrossRef]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of Multicellular Tumor Spheroids with Microwell-Based Agarose Scaffolds for Drug Testing. PLoS One 2015, 10, e0130348. [CrossRef]
- Tuveson, D.; Clevers, H. Cancer Modeling Meets Human Organoid Technology. Science (1979) 2019, 364, 952–955. [CrossRef]
- Drasdo, D.; Höhme, S. A Single-Cell-Based Model of Tumor Growth in Vitro : Monolayers and Spheroids. Phys Biol 2005, 2, 133–147. [CrossRef]
- Barisam, M.; Saidi, M.; Kashaninejad, N.; Nguyen, N.-T. Prediction of Necrotic Core and Hypoxic Zone of Multicellular Spheroids in a Microbioreactor with a U-Shaped Barrier. Micromachines (Basel) 2018, 9, 94. [CrossRef]
- Glicklis, R.; Merchuk, J.C.; Cohen, S. Modeling Mass Transfer in Hepatocyte Spheroids via Cell Viability, Spheroid Size, and Hepatocellular Functions. Biotechnol Bioeng 2004, 86, 672–680. [CrossRef]
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.-J.; Lee, K.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv Healthc Mater 2019, 1801363. [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol Sci 2021, 42, 119–133.
- Thompson, C.L.; Fu, S.; Heywood, H.K.; Knight, M.M.; Thorpe, S.D. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front Bioeng Biotechnol 2020, 8. [CrossRef]
- Kaarj; Yoon Methods of Delivering Mechanical Stimuli to Organ-on-a-Chip. Micromachines (Basel) 2019, 10, 700. [CrossRef]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A Multi-Throughput Multi-Organ-on-a-Chip System on a Plate Formatted Pneumatic Pressure-Driven Medium Circulation Platform. Lab Chip 2018, 18, 115–125. [CrossRef]
- Danku, A.E.; Dulf, E.-H.; Braicu, C.; Jurj, A.; Berindan-Neagoe, I. Organ-On-A-Chip: A Survey of Technical Results and Problems. Front Bioeng Biotechnol 2022, 10. [CrossRef]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D Bioprinting for Reconstituting the Cancer Microenvironment. NPJ Precis Oncol 2020, 4, 18. [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-Based 3D Bioprinting: A Comprehensive Review on Cell-Laden Hydrogels, Bioink Formulations, and Future Perspectives. Appl Mater Today 2020, 18, 100479. [CrossRef]
- Jia, L.; Zhang, Y.; Yao, L.; Zhang, P.; Ci, Z.; Zhang, W.; Miao, C.; Liang, X.; He, A.; Liu, Y.; et al. Regeneration of Human-Ear-Shaped Cartilage with Acellular Cartilage Matrix-Based Biomimetic Scaffolds. Appl Mater Today 2020, 20, 100639. [CrossRef]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and DECM/PCL Resins for 3D Printing of Acellular in Vitro Tissue Scaffolds by Stereolithography. Materials Science and Engineering: C 2020, 112, 110958. [CrossRef]
- Lewicki, J.; Bergman, J.; Kerins, C.; Hermanson, O. Optimization of 3D Bioprinting of Human Neuroblastoma Cells Using Sodium Alginate Hydrogel. Bioprinting 2019, 16, e00053. [CrossRef]
- Kingsley, D.M.; Roberge, C.L.; Rudkouskaya, A.; Faulkner, D.E.; Barroso, M.; Intes, X.; Corr, D.T. Laser-Based 3D Bioprinting for Spatial and Size Control of Tumor Spheroids and Embryoid Bodies. Acta Biomater 2019, 95, 357–370. [CrossRef]
- Hauptmann, N.; Lian, Q.; Ludolph, J.; Rothe, H.; Hildebrand, G.; Liefeith, K. Biomimetic Designer Scaffolds Made of D,L-Lactide-ɛ-Caprolactone Polymers by 2-Photon Polymerization. Tissue Eng Part B Rev 2019, 25, 167–186. [CrossRef]
- Potter, R.F.; Groom, A.C. Capillary Diameter and Geometry in Cardiac and Skeletal Muscle Studied by Means of Corrosion Casts. Microvasc Res 1983, 25, 68–84. [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem Rev 2020, 120, 11028–11055. [CrossRef]
- Hinton, T.J.; Lee, A.; Feinberg, A.W. 3D Bioprinting from the Micrometer to Millimeter Length Scales: Size Does Matter. Curr Opin Biomed Eng 2017, 1, 31–37. [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angewandte Chemie International Edition 2009, 48, 3244–3266. [CrossRef]
- Limberg, D.K.; Kang, J.-H.; Hayward, R.C. Triplet–Triplet Annihilation Photopolymerization for High-Resolution 3D Printing. J Am Chem Soc 2022, 144, 5226–5232. [CrossRef]
- Valente, F.; Hepburn, M.S.; Chen, J.; Aldana, A.A.; Allardyce, B.J.; Shafei, S.; Doyle, B.J.; Kennedy, B.F.; Dilley, R.J. Bioprinting Silk Fibroin Using Two-Photon Lithography Enables Control over the Physico-Chemical Material Properties and Cellular Response. Bioprinting 2022, 25, e00183. [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front Cell Dev Biol 2021, 9. [CrossRef]
- Saji Joseph, J.; Tebogo Malindisa, S.; Ntwasa, M. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery. In Cell Culture; IntechOpen, 2019.
- Anton, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int J Mol Sci 2015, 16, 5517–5527.
- Yue, B. Biology of the Extracellular Matrix: An Overview. J Glaucoma 2014, 23, S20–S23.
- Kim, B.-S.; Nikolovski, J.; Bonadio, J.; Smiley, E.; Mooney, D.J. Engineered Smooth Muscle Tissues: Regulating Cell Phenotype with the Scaffold. Exp Cell Res 1999, 251, 318–328. [CrossRef]
- 4Yin, Z.; Dong, C.; Jiang, K.; Xu, Z.; Li, R.; Guo, K.; Shao, S.; Wang, L. Heterogeneity of Cancer-Associated Fibroblasts and Roles in the Progression, Prognosis, and Therapy of Hepatocellular Carcinoma. J Hematol Oncol 2019, 12.
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 2019, 24, 41–53.
- Jong, B.K. Three-Dimensional Tissue Culture Models in Cancer Biology. Semin Cancer Biol 2005, 15, 365–377.
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int J Mol Sci 2020, 21, 1–17.
- Tancioni, I.; Miller, N.L.G.; Uryu, S.; Lawson, C.; Jean, C.; Chen, X.L.; Kleinschmidt, E.G.; Schlaepfer, D.D. FAK Activity Protects Nucleostemin in Facilitating Breast Cancer Spheroid and Tumor Growth. Breast Cancer Research 2015, 17. [CrossRef]
- Del Duca, D.; Werbowetski, T.; Del Maestro, R.F. Spheroid Preparation from Hanging Drops: Characterization of a Model of Brain Tumor Invasion. J Neurooncol 2004, 67, 295–303. [CrossRef]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. Journal of Visualized Experiments 2011. [CrossRef]
- Mirab, F.; Kang, Y.J.; Majd, S. Preparation and Characterization of Size-Controlled Glioma Spheroids Using Agarose Hydrogel Microwells. PLoS One 2019, 14, e0211078. [CrossRef]
- Tang, Y.; Liu, J.; Chen, Y. Agarose Multi-Wells for Tumour Spheroid Formation and Anti-Cancer Drug Test. Microelectron Eng 2016, 158, 41–45. [CrossRef]
- Kang, S.; Kim, D.; Lee, J.; Takayama, S.; Park, J.Y. Engineered Microsystems for Spheroid and Organoid Studies. Adv Healthc Mater 2021, 10, 2001284. [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent Advances in Three-Dimensional Multicellular Spheroid Culture for Biomedical Research. Biotechnol J 2008, 3, 1172–1184. [CrossRef]
- Kim, S.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv Healthc Mater 2020, 9, 2000608. [CrossRef]
- Ryu, J.H.; Kim, M.S.; Lee, G.M.; Choi, C.Y.; Kim, B.-S. The Enhancement of Recombinant Protein Production by Polymer Nanospheres in Cell Suspension Culture. Biomaterials 2005, 26, 2173–2181. [CrossRef]
- Shin, J.-Y.; Park, J.; Jang, H.-K.; Lee, T.-J.; La, W.-G.; Bhang, S.H.; Kwon, I.K.; Kwon, O.H.; Kim, B.-S. Efficient Formation of Cell Spheroids Using Polymer Nanofibers. Biotechnol Lett 2012, 34, 795–803. [CrossRef]
- Anil-Inevi, M.; Yaman, S.; Yildiz, A.A.; Mese, G.; Yalcin-Ozuysal, O.; Tekin, H.C.; Ozcivici, E. Biofabrication of in Situ Self Assembled 3D Cell Cultures in a Weightlessness Environment Generated Using Magnetic Levitation. Sci Rep 2018, 8, 7239. [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-Dimensional Tissue Culture Based on Magnetic Cell Levitation. Nat Nanotechnol 2010, 5, 291–296. [CrossRef]
- Türker, E.; Demirçak, N.; Arslan-Yildiz, A. Scaffold-Free Three-Dimensional Cell Culturing Using Magnetic Levitation. Biomater Sci 2018, 6, 1745–1753. [CrossRef]
- Baek, N.; Seo, O.W.; Lee, J.; Hulme, J.; An, S.S.A. Real-Time Monitoring of Cisplatin Cytotoxicity on Three-Dimensional Spheroid Tumor Cells. Drug Des Devel Ther 2016, 10, 2155–2165. [CrossRef]
- Wong, C.W.; Han, H.W.; Tien, Y.W.; Hsu, S. hui Biomaterial Substrate-Derived Compact Cellular Spheroids Mimicking the Behavior of Pancreatic Cancer and Microenvironment. Biomaterials 2019, 213. [CrossRef]
- Antunes, J.; Gaspar, V.M.; Ferreira, L.; Monteiro, M.; Henrique, R.; Jerónimo, C.; Mano, J.F. In-Air Production of 3D Co-Culture Tumor Spheroid Hydrogels for Expedited Drug Screening. Acta Biomater 2019, 94, 392–409. [CrossRef]
- Culp, M.B.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020, 77, 38–52.
- Weydert, Z.; Lal-Nag, M.; Mathews-Greiner, L.; Thiel, C.; Cordes, H.; Küpfer, L.; Guye, P.; Kelm, J.M.; Ferrer, M. A 3D Heterotypic Multicellular Tumor Spheroid Assay Platform to Discriminate Drug Effects on Stroma versus Cancer Cells. SLAS Discovery 2020, 25, 265–276. [CrossRef]
- Courau, T.; Bonnereau, J.; Chicoteau, J.; Bottois, H.; Remark, R.; Assante Miranda, L.; Toubert, A.; Blery, M.; Aparicio, T.; Allez, M.; et al. Cocultures of Human Colorectal Tumor Spheroids with Immune Cells Reveal the Therapeutic Potential of MICA/B and NKG2A Targeting for Cancer Treatment. J Immunother Cancer 2019, 7. [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat Rev Cancer 2018, 18, 407–418. [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science (1979) 2014, 345. [CrossRef]
- Quadrato, G.; Brown, J.; Arlotta, P. The Promises and Challenges of Human Brain Organoids as Models of Neuropsychiatric Disease. Nat Med 2016, 22, 1220–1228. [CrossRef]
- Jiang, S.; Zhao, H.; Zhang, W.; Wang, J.; Liu, Y.; Cao, Y.; Zheng, H.; Hu, Z.; Wang, S.; Zhu, Y.; et al. An Automated Organoid Platform with Inter-Organoid Homogeneity and Inter-Patient Heterogeneity. Cell Rep Med 2020, 1. [CrossRef]
- Maenhoudt, N.; Defraye, C.; Boretto, M.; Jan, Z.; Heremans, R.; Boeckx, B.; Hermans, F.; Arijs, I.; Cox, B.; Van Nieuwenhuysen, E.; et al. Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models. Stem Cell Reports 2020, 14, 717–729. [CrossRef]
- Steele, N.G.; Chakrabarti, J.; Wang, J.; Biesiada, J.; Holokai, L.; Chang, J.; Nowacki, L.M.; Hawkins, J.; Mahe, M.; Sundaram, N.; et al. An Organoid-Based Preclinical Model of Human Gastric Cancer. Cell Mol Gastroenterol Hepatol 2019, 7, 161–184. [CrossRef]
- Schmitz, C.H.J.; Rowat, A.C.; Köster, S.; Weitz, D.A. Dropspots: A Picoliter Array in a Microfluidic Device. Lab Chip 2009, 9, 44–49. [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [CrossRef]
- Ronaldson-Bouchard, K.; Teles, D.; Yeager, K.; Tavakol, D.N.; Zhao, Y.; Chramiec, A.; Tagore, S.; Summers, M.; Stylianos, S.; Tamargo, M.; et al. A Multi-Organ Chip with Matured Tissue Niches Linked by Vascular Flow. Nat Biomed Eng 2022, 6, 351–371. [CrossRef]
- Ma, L.-D.; Wang, Y.-T.; Wang, J.-R.; Wu, J.-L.; Meng, X.-S.; Hu, P.; Mu, X.; Liang, Q.-L.; Luo, G.-A. Design and Fabrication of a Liver-on-a-Chip Platform for Convenient, Highly Efficient, and Safe in Situ Perfusion Culture of 3D Hepatic Spheroids. Lab Chip 2018, 18, 2547–2562. [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nature Reviews Methods Primers 2022, 2.
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Functionally and Genetically Different From Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 754–765. [CrossRef]
- Diederichs, S.; Tuan, R.S. Functional Comparison of Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Cells and Bone Marrow-Derived Mesenchymal Stromal Cells from the Same Donor. Stem Cells Dev 2014, 23, 1594–1610. [CrossRef]
- van den Berg, A.; Mummery, C.L.; Passier, R.; van der Meer, A.D. Personalised Organs-on-Chips: Functional Testing for Precision Medicine. Lab Chip 2019, 19, 198–205. [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [CrossRef]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R.; et al. A Linked Organ-on-Chip Model of the Human Neurovascular Unit Reveals the Metabolic Coupling of Endothelial and Neuronal Cells. Nat Biotechnol 2018, 36, 865–874. [CrossRef]
- da Ponte, R.M.; Gaio, N.; van Zeijl, H.; Vollebregt, S.; Dijkstra, P.; Dekker, R.; Serdijn, W.A.; Giagka, V. Monolithic Integration of a Smart Temperature Sensor on a Modular Silicon-Based Organ-on-a-Chip Device. Sens Actuators A Phys 2021, 317, 112439. [CrossRef]
- Quirós-Solano, W.F.; Gaio, N.; Stassen, O.M.J.A.; Arik, Y.B.; Silvestri, C.; Van Engeland, N.C.A.; Van der Meer, A.; Passier, R.; Sahlgren, C.M.; Bouten, C.V.C.; et al. Microfabricated Tuneable and Transferable Porous PDMS Membranes for Organs-on-Chips. Sci Rep 2018, 8, 13524. [CrossRef]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater Sci Eng 2021, 7, 2880–2899. [CrossRef]
- Hirama, H.; Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Kanamori, T.; Inoue, T. Glass-Based Organ-on-a-Chip Device for Restricting Small Molecular Absorption. J Biosci Bioeng 2019, 127, 641–646. [CrossRef]
- Fritschen, A.; Bell, A.K.; Königstein, I.; Stühn, L.; Stark, R.W.; Blaeser, A. Investigation and Comparison of Resin Materials in Transparent DLP-Printing for Application in Cell Culture and Organs-on-a-Chip. Biomater Sci 2022, 10, 1981–1994. [CrossRef]
- Warr, C.; Valdoz, J.C.; Bickham, B.P.; Knight, C.J.; Franks, N.A.; Chartrand, N.; Van Ry, P.M.; Christensen, K.A.; Nordin, G.P.; Cook, A.D. Biocompatible PEGDA Resin for 3D Printing. ACS Appl Bio Mater 2020, 3, 2239–2244. [CrossRef]
- Alsharhan, A.T.; Acevedo, R.; Warren, R.; Sochol, R.D. 3D Microfluidics via Cyclic Olefin Polymer-Based in Situ Direct Laser Writing. Lab Chip 2019, 19, 2799–2810. [CrossRef]
- Busek, M.; Nøvik, S.; Aizenshtadt, A.; Amirola-Martinez, M.; Combriat, T.; Grünzner, S.; Krauss, S. Thermoplastic Elastomer (TPE)–Poly(Methyl Methacrylate) (PMMA) Hybrid Devices for Active Pumping PDMS-Free Organ-on-a-Chip Systems. Biosensors (Basel) 2021, 11, 162. [CrossRef]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.-E.; Ahn, S.; Kang, J.H. Robust Chemical Bonding of PMMA Microfluidic Devices to Porous PETE Membranes for Reliable Cytotoxicity Testing of Drugs. Lab Chip 2019, 19, 3706–3713. [CrossRef]
- Lee, H.-M.; Yu, M.-S.; Kazmi, S.R.; Oh, S.Y.; Rhee, K.-H.; Bae, M.-A.; Lee, B.H.; Shin, D.-S.; Oh, K.-S.; Ceong, H.; et al. Computational Determination of HERG-Related Cardiotoxicity of Drug Candidates. BMC Bioinformatics 2019, 20, 250. [CrossRef]
- Chramiec, A.; Teles, D.; Yeager, K.; Marturano-Kruik, A.; Pak, J.; Chen, T.; Hao, L.; Wang, M.; Lock, R.; Tavakol, D.N.; et al. Integrated Human Organ-on-a-Chip Model for Predictive Studies of Anti-Tumor Drug Efficacy and Cardiac Safety. Lab Chip 2020, 20, 4357–4372. [CrossRef]
- Liu, Y.; Sakolish, C.; Chen, Z.; Phan, D.T.T.; Bender, R.H.F.; Hughes, C.C.W.; Rusyn, I. Human in Vitro Vascularized Micro-Organ and Micro-Tumor Models Are Reproducible Organ-on-a-Chip Platforms for Studies of Anticancer Drugs. Toxicology 2020, 445. [CrossRef]
- Hachey, S.J.; Movsesyan, S.; Nguyen, Q.H.; Burton-Sojo, G.; Tankazyan, A.; Wu, J.; Hoang, T.; Zhao, D.; Wang, S.; Hatch, M.M.; et al. An: In Vitro Vascularized Micro-Tumor Model of Human Colorectal Cancer Recapitulates in Vivo Responses to Standard-of-Care Therapy. Lab Chip 2021, 21, 1333–1351. [CrossRef]
- Yu, J.; Lee, S.; Song, J.; Lee, S.R.; Kim, S.; Choi, H.; Kang, H.; Hwang, Y.; Hong, Y.K.; Jeon, N.L. Perfusable Micro-Vascularized 3D Tissue Array for High-Throughput Vascular Phenotypic Screening. Nano Converg 2022, 9. [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf 2019, 35, 1286–1296. [CrossRef]
- Hwang, S.; Reyes, E.I.; Moon, K.; Rumpf, R.C.; Kim, N.S. Thermo-Mechanical Characterization of Metal/Polymer Composite Filaments and Printing Parameter Study for Fused Deposition Modeling in the 3D Printing Process. J Electron Mater 2015, 44, 771–777. [CrossRef]
- Valino, A.D.; Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D Printing of Thermoplastic Polymer Composites and Nanocomposites. Prog Polym Sci 2019, 98, 101162. [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater Res 2018, 22, 11. [CrossRef]
- Zhu, Y.; Joralmon, D.; Shan, W.; Chen, Y.; Rong, J.; Zhao, H.; Xiao, S.; Li, X. 3D Printing Biomimetic Materials and Structures for Biomedical Applications. Biodes Manuf 2021, 4, 405–428. [CrossRef]
- Zhang, B.; Pei, X.; Zhou, C.; Fan, Y.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.; Sun, Y.; et al. The Biomimetic Design and 3D Printing of Customized Mechanical Properties Porous Ti6Al4V Scaffold for Load-Bearing Bone Reconstruction. Mater Des 2018, 152, 30–39. [CrossRef]
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3D Bioprinting and Photocrosslinking: Emerging Strategies & Future Perspectives. Biomaterials Advances 2022, 134, 112576. [CrossRef]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Advanced Materials 2017, 29, 1604983. [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl Mater Interfaces 2017, 9, 43449–43458. [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Advanced Materials 2015, 27, 5075–5079. [CrossRef]
- Kuzucu, M.; Vera, G.; Beaumont, M.; Fischer, S.; Wei, P.; Shastri, V.P.; Forget, A. Extrusion-Based 3D Bioprinting of Gradients of Stiffness, Cell Density, and Immobilized Peptide Using Thermogelling Hydrogels. ACS Biomater Sci Eng 2021, 7, 2192–2197. [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography Apparatus and Digital Light Processing-Based 3D Bioprinting for Tissue Fabrication. iScience 2023, 26, 106039. [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and Continuous 3D Bioprinting of Human Tissues with Decellularized Extracellular Matrix. Biomaterials 2019, 194, 1–13. [CrossRef]
- Godar, D.E.; Gurunathan, C.; Ilev, I. 3D Bioprinting with UVA1 Radiation and Photoinitiator Irgacure 2959: Can the ASTM Standard L929 Cells Predict Human Stem Cell Cytotoxicity? Photochem Photobiol 2019, 95, 581–586. [CrossRef]
- Gittard, S.D. Two-Photon Polymerization Microstructuring in Regenerative Medicine. Frontiers in Bioscience 2013, E5, E642. [CrossRef]
- Tromayer, M.; Gruber, P.; Markovic, M.; Rosspeintner, A.; Vauthey, E.; Redl, H.; Ovsianikov, A.; Liska, R. A Biocompatible Macromolecular Two-Photon Initiator Based on Hyaluronan. Polym Chem 2017, 8, 451–460. [CrossRef]
- Li, Z.; Torgersen, J.; Ajami, A.; Mühleder, S.; Qin, X.; Husinsky, W.; Holnthoner, W.; Ovsianikov, A.; Stampfl, J.; Liska, R. Initiation Efficiency and Cytotoxicity of Novel Water-Soluble Two-Photon Photoinitiators for Direct 3D Microfabrication of Hydrogels. RSC Adv 2013, 3, 15939–15946. [CrossRef]
- Masuma, R.; Kashima, S.; Kurasaki, M.; Okuno, T. Effects of UV Wavelength on Cell Damages Caused by UV Irradiation in PC12 Cells. J Photochem Photobiol B 2013, 125, 202–208. [CrossRef]
- Moon, S.H.; Choi, H.N.; Yang, Y.J. Natural/Synthetic Polymer Materials for Bioink Development. Biotechnology and Bioprocess Engineering 2022, 27, 482–493. [CrossRef]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.H.; Sriram, G.; Cao, T. 3D Bioprinting and Microscale Organization of Vascularized Tissue Constructs Using Collagen-based Bioink. Biotechnol Bioeng 2021, 118, 3150–3163. [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu Rev Biochem 2009, 78, 929–958. [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a Glance. J Cell Sci 2007, 120, 1955–1958. [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers (Basel) 2018, 10, 1290. [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers (Basel) 2017, 9, 401. [CrossRef]
- Foox, M.; Zilberman, M. Drug Delivery from Gelatin-Based Systems. Expert Opin Drug Deliv 2015, 12, 1547–1563. [CrossRef]
- Lee, B.H.; Shirahama, H.; Cho, N.-J.; Tan, L.P. Efficient and Controllable Synthesis of Highly Substituted Gelatin Methacrylamide for Mechanically Stiff Hydrogels. RSC Adv 2015, 5, 106094–106097. [CrossRef]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic Optimization of Visible Light-Induced Crosslinking Conditions of Gelatin Methacryloyl (GelMA). Sci Rep 2021, 11, 23276. [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci Rep 2019, 9, 6863. [CrossRef]
- Kneser, U.; Voogd, A.; Ohnolz, J.; Buettner, O.; Stangenberg, L.; Zhang, Y.H.; Stark, G.B.; Schaefer, D.J. Fibrin Gel-Immobilized Primary Osteoblasts in Calcium Phosphate Bone Cement: In Vivo Evaluation with Regard to Application as Injectable Biological Bone Substitute. Cells Tissues Organs 2005, 179, 158–169. [CrossRef]
- Abelseth, E.; Abelseth, L.; De la Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater Sci Eng 2019, 5, 234–243. [CrossRef]
- Piard, C.; Baker, H.; Kamalitdinov, T.; Fisher, J. Bioprinted Osteon-like Scaffolds Enhance in Vivo Neovascularization. Biofabrication 2019, 11, 025013. [CrossRef]
- Ding, Y.-W.; Zhang, X.-W.; Mi, C.-H.; Qi, X.-Y.; Zhou, J.; Wei, D.-X. Recent Advances in Hyaluronic Acid-Based Hydrogels for 3D Bioprinting in Tissue Engineering Applications. Smart Mater Med 2023, 4, 59–68. [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue Engineering—A Review. Carbohydr Polym 2013, 92, 1262–1279. [CrossRef]
- Perng, C.-K.; Wang, Y.-J.; Tsi, C.-H.; Ma, H. In Vivo Angiogenesis Effect of Porous Collagen Scaffold with Hyaluronic Acid Oligosaccharides. Journal of Surgical Research 2011, 168, 9–15. [CrossRef]
- Wang, X.; He, J.; Wang, Y.; Cui, F.-Z. Hyaluronic Acid-Based Scaffold for Central Neural Tissue Engineering. Interface Focus 2012, 2, 278–291. [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of Collagen Type I-Hyaluronan Hybrid Bioink for 3D Bioprinted Liver Microenvironments. Biofabrication 2018, 11, 015003. [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J Tissue Eng 2017, 8, 204173141772646. [CrossRef]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater Sci Eng 2018, 4, 2643–2652. [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-Based Inks for 3D Printing and Bioprinting. Green Chemistry 2022, 24, 62–101. [CrossRef]
- He, Y.; Derakhshanfar, S.; Zhong, W.; Li, B.; Lu, F.; Xing, M.; Li, X. Characterization and Application of Carboxymethyl Chitosan-Based Bioink in Cartilage Tissue Engineering. J Nanomater 2020, 2020, 1–11. [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J Pharm Sci 2021, 16, 280–306. [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling Alginate Oxidation Conditions for Making Alginate-Gelatin Hydrogels. Carbohydr Polym 2018, 198, 509–517. [CrossRef]
- Zhang, X.; Wang, K.; Hu, J.; Zhang, Y.; Dai, Y.; Xia, F. Role of a High Calcium Ion Content in Extending the Properties of Alginate Dual-Crosslinked Hydrogels. J Mater Chem A Mater 2020, 8, 25390–25401. [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog Polym Sci 2012, 37, 106–126. [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84, 25–34. [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat Rev Mater 2018, 3, 159–173. [CrossRef]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-Based Bioinks for Engineering Tissue- and Organ-Specific Microenvironments. Chem Rev 2020, 120, 10608–10661. [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking Strategies for Preparation of Extracellular Matrix-Derived Cardiovascular Scaffolds. Regen Biomater 2014, 1, 81–89. [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers (Basel) 2022, 14, 1153. [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive Manufacturing of PLA-Based Scaffolds Intended for Bone Regeneration and Strategies to Improve Their Biological Properties. e-Polymers 2020, 20, 571–599. [CrossRef]
- Muthe, L.P.; Pickering, K.; Gauss, C. A Review of 3D/4D Printing of Poly-Lactic Acid Composites with Bio-Derived Reinforcements. Composites Part C: Open Access 2022, 8, 100271. [CrossRef]
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid Microscaffold-Based 3D Bioprinting of Multi-Cellular Constructs with High Compressive Strength: A New Biofabrication Strategy. Sci Rep 2016, 6, 39140. [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-Dimensional Bioprinting of Mesenchymal Stem Cells Using an Osteoinductive Bioink Containing Alginate and BMP-2-Loaded PLGA Nanoparticles for Bone Tissue Engineering. Biomaterials Advances 2022, 136, 212789. [CrossRef]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.-S.; Kim, B.S.; Choi, Y.-J.; Jang, W.B.; Hong, Y.J.; Kwon, S.-M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv Funct Mater 2017, 27, 1700798. [CrossRef]
- Naseri, E.; Butler, H.; MacNevin, W.; Ahmed, M.; Ahmadi, A. Low-Temperature Solvent-Based 3D Printing of PLGA: A Parametric Printability Study. Drug Dev Ind Pharm 2020, 46, 173–178. [CrossRef]
- Arcaute, K.; Mann, B.; Wicker, R. Stereolithography of Spatially Controlled Multi-Material Bioactive Poly(Ethylene Glycol) Scaffolds. Acta Biomater 2010, 6, 1047–1054. [CrossRef]
- Tu, X.; Wang, L.; Wei, J.; Wang, B.; Tang, Y.; Shi, J.; Zhang, Z.; Chen, Y. 3D Printed PEGDA Microstructures for Gelatin Scaffold Integration and Neuron Differentiation. Microelectron Eng 2016, 158, 30–34. [CrossRef]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D Bioprinting of Photo-crosslinkable Silk Methacrylate (SilMA)-polyethylene Glycol Diacrylate (PEGDA) Bioink for Cartilage Tissue Engineering. J Biomed Mater Res A 2022, 110, 884–898. [CrossRef]
- Fathi, A.; Kermani, F.; Behnamghader, A.; Banijamali, S.; Mozafari, M.; Baino, F.; Kargozar, S. Three-Dimensionally Printed Polycaprolactone/Multicomponent Bioactive Glass Scaffolds for Potential Application in Bone Tissue Engineering. Biomedical Glasses 2020, 6, 57–69. [CrossRef]
- Elomaa, L.; Kokkari, A.; Närhi, T.; Seppälä, J. V. Porous 3D Modeled Scaffolds of Bioactive Glass and Photocrosslinkable Poly(ε-Caprolactone) by Stereolithography. Compos Sci Technol 2013, 74, 99–106. [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers (Basel) 2021, 13, 2754. [CrossRef]
- Cai, Z.; Wan, Y.; Becker, M.L.; Long, Y.-Z.; Dean, D. Poly(Propylene Fumarate)-Based Materials: Synthesis, Functionalization, Properties, Device Fabrication and Biomedical Applications. Biomaterials 2019, 208, 45–71. [CrossRef]
- Walker, J.M.; Bodamer, E.; Krebs, O.; Luo, Y.; Kleinfehn, A.; Becker, M.L.; Dean, D. Effect of Chemical and Physical Properties on the In Vitro Degradation of 3D Printed High Resolution Poly(Propylene Fumarate) Scaffolds. Biomacromolecules 2017, 18, 1419–1425. [CrossRef]
- Wang, S.; Lu, L.; Yaszemski, M.J. Bone-Tissue-Engineering Material Poly(Propylene Fumarate): Correlation between Molecular Weight, Chain Dimensions, and Physical Properties. Biomacromolecules 2006, 7, 1976–1982. [CrossRef]
- Bordonaro, M.; Lazarova, D.L.; Augenlicht, L.H.; Sartorelli, A.C. Estimates of the World-Wide Prevalence of Cancer for 25 Sites in the Adult Population. Int J Cancer 2002, 97, 72–81. [CrossRef]
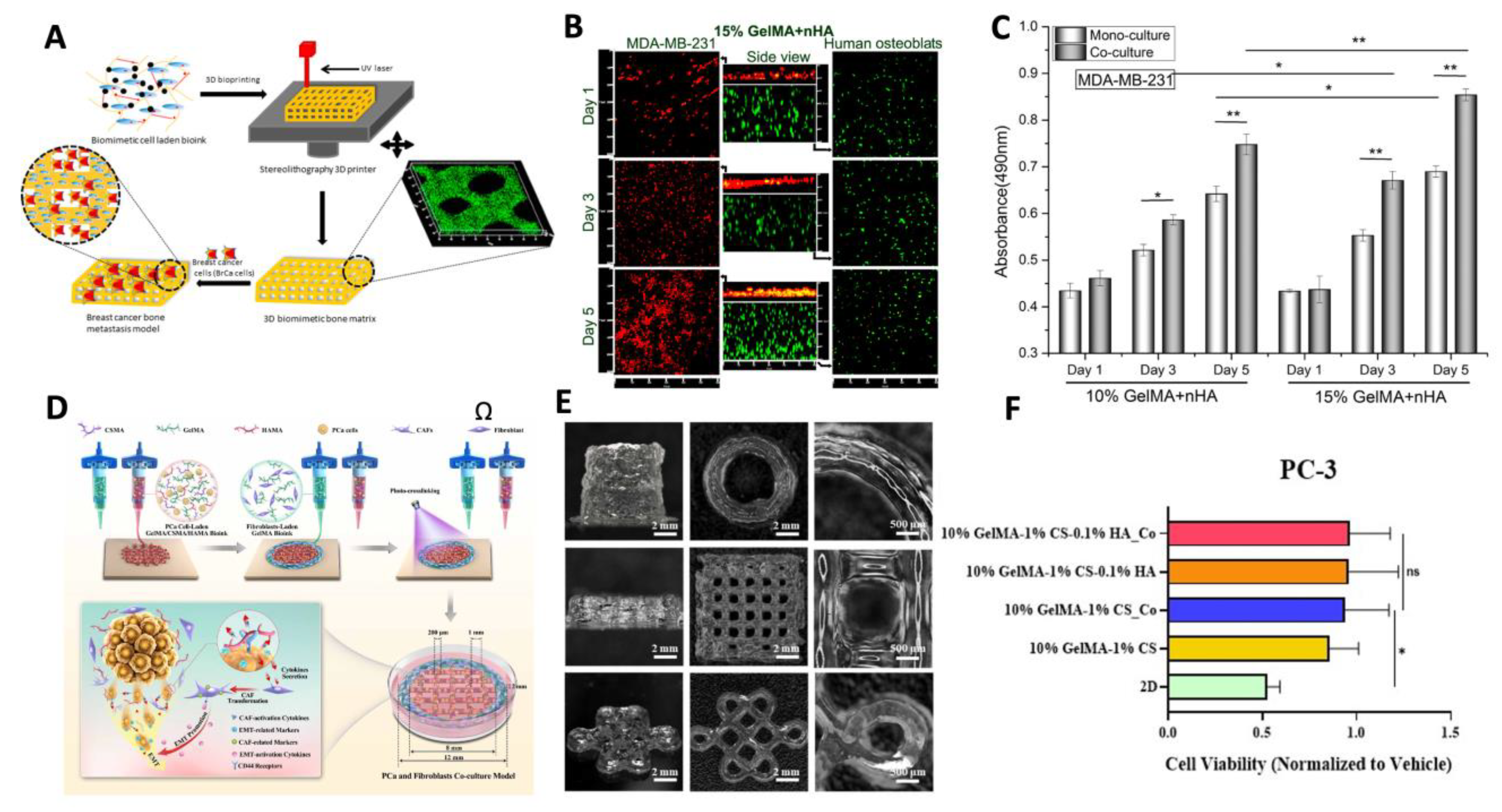
- Sztankovics, D.; Moldvai, D.; Petővári, G.; Gelencsér, R.; Krencz, I.; Raffay, R.; Dankó, T.; Sebestyén, A. 3D Bioprinting and the Revolution in Experimental Cancer Model Systems—A Review of Developing New Models and Experiences with in Vitro 3D Bioprinted Breast Cancer Tissue-Mimetic Structures. Pathology and Oncology Research 2023, 29. [CrossRef]
- Zhou, X.; Zhu, W.; Nowicki, M.; Miao, S.; Cui, H.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl Mater Interfaces 2016, 8, 30017–30026. [CrossRef]
- Xu, K.; Huang, Y.; Wu, M.; Yin, J.; Wei, P. 3D Bioprinting of Multi-Cellular Tumor Microenvironment for Prostate Cancer Metastasis. Biofabrication 2023, 15, 035020. [CrossRef]
- Pulido, C.; Vendrell, I.; Ferreira, A.R.; Casimiro, S.; Mansinho, A.; Alho, I.; Costa, L. Bone Metastasis Risk Factors in Breast Cancer. Ecancermedicalscience 2017, 11.
- Huang, T.Q.; Qu, X.; Liu, J.; Chen, S. 3D Printing of Biomimetic Microstructures for Cancer Cell Migration. Biomed Microdevices 2014, 16, 127–132. [CrossRef]
- Ning, L.; Shim, J.; Tomov, M.L.; Liu, R.; Mehta, R.; Mingee, A.; Hwang, B.; Jin, L.; Mantalaris, A.; Xu, C.; et al. A 3D Bioprinted in Vitro Model of Neuroblastoma Recapitulates Dynamic Tumor-Endothelial Cell Interactions Contributing to Solid Tumor Aggressive Behavior. Advanced Science 2022, 9. [CrossRef]
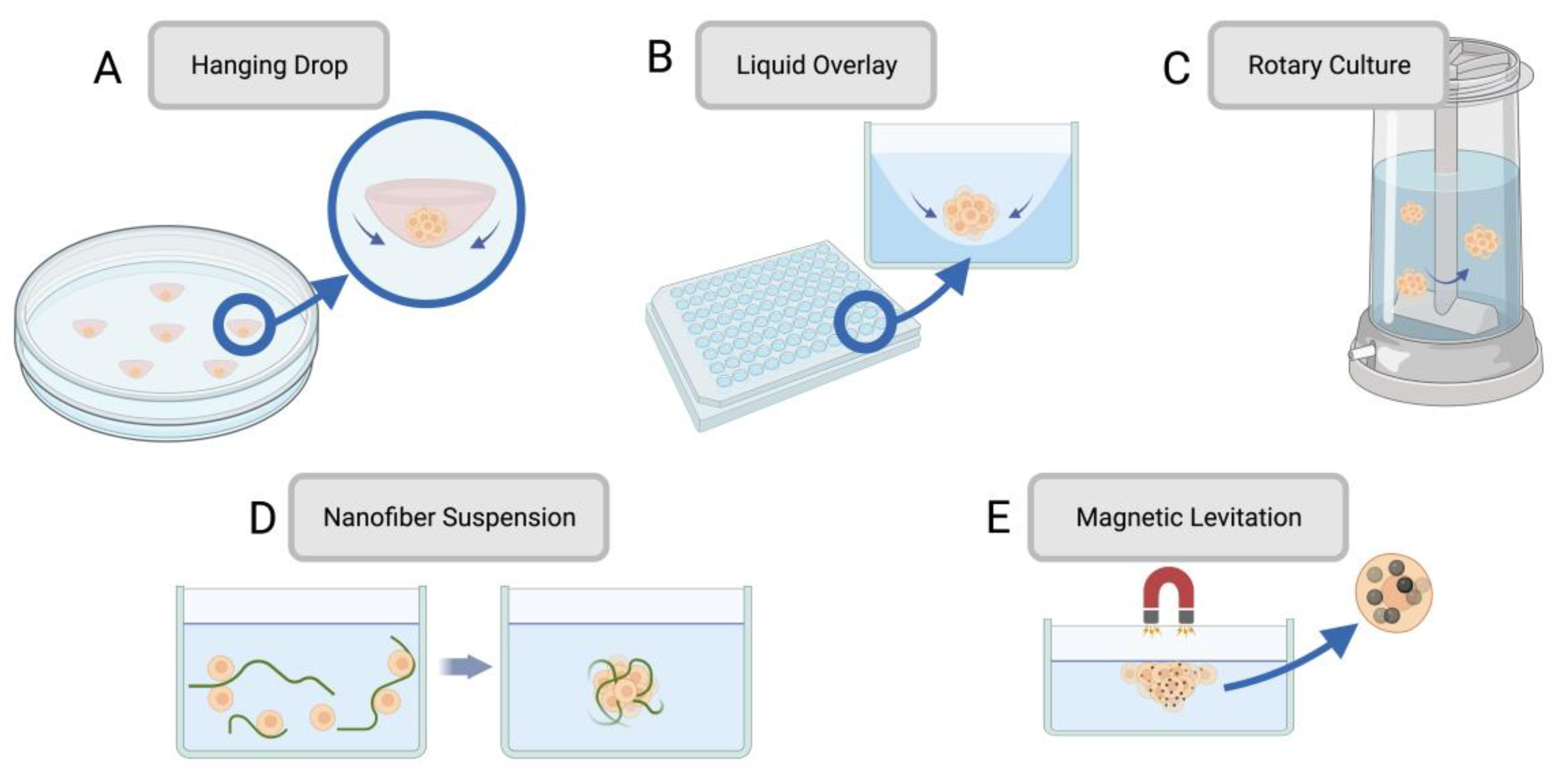
| Spheroid Fabrication Method | Overview | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Hanging drop | A drop of cell suspension is placed onto the inside of a cell culture plate lid, which is then inverted without disturbing the droplets held by surface tension. Over time, cells concentrate and cluster into a spheroid at the bottom of the hanging droplet. | • Simple • Requires no specialized equipment • Can be used with small cell suspension volumes |
• Laborious • Low throughput • High shear force • Limited cell lines form spheroids through this method |
[45,46] |
| Liquid Overlay | Cell suspension is seeded onto a nonadherent surface with recesses that promote cell aggregation. | • High throughput • Control over spheroid size |
• Some cell lines may need added ECM proteins to promote spheroid formation | [47,48] |
| Rotary cell culture | Cells are cultured in a container with an agitator that disrupts the cells’ ability to adhere to the substrate, forcing them to self-assemble into spheroids. | • Simple • High throughput • Large scale • |
• Spheroid size variation • Viability challenges due to mechanical damage |
[43,49,50] |
| Nanofiber cell suspension | Adding polymer nanofibers to the cell suspension increases spheroid production by cells interacting with the nanofibers. | • Reduced cell death due to non-adherence • Suitable for anchorage-dependent cells • More time-efficient than other adaptations for anchorage-dependent cells |
• Polymer nanofibers may have unintended impacts on cell behaviour | [51,52,53] |
| Magnetic levitation | Magnetic particles are combined with cells, and a magnetic force is introduced. Negative magnetophoresis induces a weightless environment where cell aggregation is promoted | • Low-cost • Allows for real-time imaging • Minimizes additional forces on cells |
• Can lead to apoptosis | [54,55,56] |
| Material | Overview | Properties for Bioinks | Crosslinking Mechanics | References |
|---|---|---|---|---|
| Collagen | Triple helical protein for tissue scaffolding and tensile strength in tendon, cartilage, bone, and skin | • Biodegradable • Biocompatible • Contributes to printability • Bioactive properties |
Covalent bonding of fibrils | [112,113,114] |
| Gelatin | Hydrogel from the hydrolysis of collagen, solid when cooled and can be used to synthesize gelatin methacryloyl (GelMA) | • Temperature-based gelation • Printable • Tunable mechanical properties |
Gelation under cold temperatures | [115,116,117,118] |
| Gelatin Methacryloyl (GelMA) | Gelatin derivative with methacrylated functional groups, mechanically stable after photocrosslinking | • Selective crosslinking • Mimics the ECM • Cell-binding sites • Biocompatible • Tunable |
Photocrosslinked under UV light exposure | [119,120] |
| Fibrin | High-viscosity, insoluble biopolymer that allows for paracrine signalling due to non-linear elasticity | • Biocompatible • Biodegradable • Regenerative • Nanofibrous structural properties • Imitates both hard and soft tissues |
Cleaved by thrombin which induces polymerization | [121,122,123] |
| Hyaluronic Acid | Bioresorbable material found in mammalian ECM, maintains a hydrated environment | • High porosity allows for compound diffusion • Must be combined with other biomaterials for bioink synthesis as it lacks mechanical stability and cell adhesion alone |
Enzyme-crosslinking, Schiff-base reaction, thiol-modified HA crosslinking, Diels-Alder click crosslinking, ionic crosslinking, photo-crosslinking | [124,125,126,127,128,129] |
| Chitosan | Polysaccharide derived from chitin deacetylation with solubility at low pH levels | • Nontoxic • Bio-adhesive • Suitable for soft tissues due to low mechanical strength |
Chemical crosslinking with glutaraldehyde (amine groups), or citric acid (covalent) | [130,131,132] |
| Alginate | Polymer derived from brown algae, can form hydrogels that mimic the ECM and be crosslinked through its aldehyde groups | • Biocompatible • Low cost • Low bioactivity • Can degrade easily due to hydrolytic degradation |
Ionically crosslinked with divalent cations | [133,134,135,136] |
| Decellularized ECM | Produced by removing cellular components from tissues by chemical or physical processes | • Can retain tissue-specific behaviours post-decellularization • May not require additional crosslinking |
Glutaraldehyde, thermal gelation | [137,138,139,140] |
| Material | Overview | Properties for Bioinks | References |
|---|---|---|---|
| Polylactic acid (PLA) | Semi-crystalline structure with high molecular weight, used in extrusion-based bioprinting | • Useful for dental models • Accurate surface properties • Can be brittle |
[141,142,143] |
| Poly(lactic-co-glycolic acid) (PLGA) | Synthesized through co-polymerization of both glycolic acid and lactic acid | • Cell-compatible • Can perform controlled drug release • Properties can be tuned through glycolic to lactic ratio |
[144,145,146,147] |
| Poly(ethylene glycol) diacrylate (PEGDA) | Long-chain photo-crosslinkable monomer that forms hydrogels | • Photo-crosslinkability allows for use in light-based printing • Hydrophilic for cell maintenance and encapsulation • Modular though tunable functional groups |
[148,149,150] |
| Poly e-caprolactone (PCL) | Semi-crystalline thermoplastic with high thermal stability, long degradation rate | • Rubber-like flexibility in physiological conditions • High permeability • Useful for bone models due to degradation rate |
[151,152,153] |
| Poly(propylene fumarate) (PPF) | Linear unsaturated polyester with fumaric acid backbone chains | • High viscosity • Light-responsive • Useful in degradable materials as its ester bonds can be hydrolyzed, allowing for excretable products |
[154,155,156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




