1. Introduction
Orthognathic surgery, specifically, is a subset of oral and maxillofacial surgery that focuses on correcting conditions of the jaw and face related to structure, growth, sleep apnea, TMJ disorders, malocclusion problems owing to skeletal disharmonies, and other orthodontic problems that cannot be easily treated with orthodontics [
1]. These surgeries aim to improve chewing, speaking, and breathing functionality while enhancing the patient's appearance [
2]. On the other hand, oral maxillofacial surgery is a broader field that not only includes orthognathic surgery but also concerns the treatment of diseases and injuries of both the functional and aesthetic aspects of the hard and soft tissues of the oral and maxillofacial region [
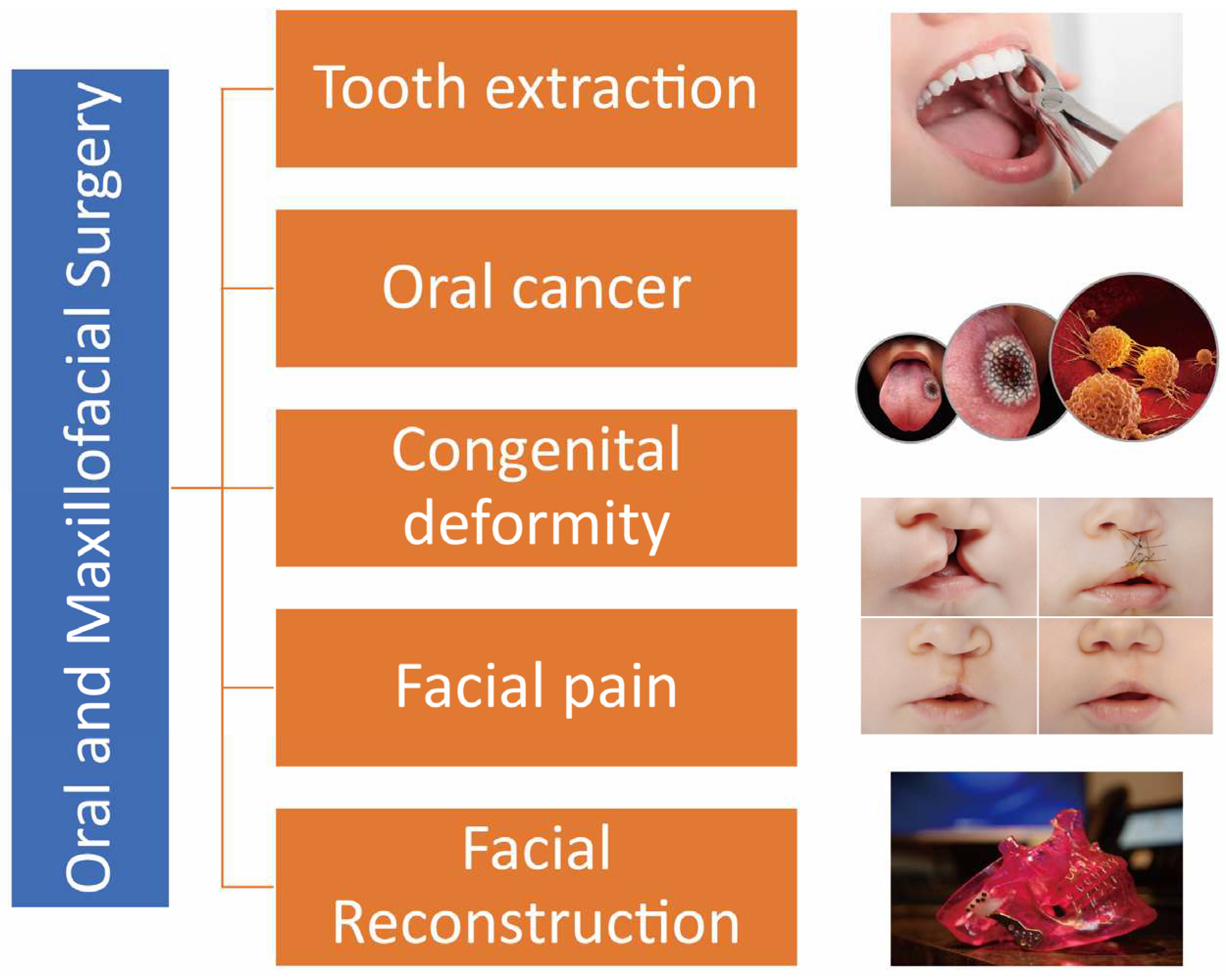
3]. This can range from removal of impacted teeth and administering of complex facial reconstructions to the treatment of oral cancer, cleft lip and palate, and chronic facial pain disorders (
Figure 1).
These fields require an in-depth understanding of the interplay between aesthetics and function, and the complex anatomical structures and their relationships in the cranio-maxillofacial region [
4]. Surgeons specializing in these areas combine their expertise in dentistry, surgery, and general medicine to provide comprehensive care for patients. Orthognathic and oral maxillofacial surgeries traditionally depend on detailed preoperative planning, including the creation of physical models and utilization of various imaging techniques [
2]. Dental casts, cephalometric analysis, and two-dimensional (2D) imaging such as panoramic radiography, cephalograms, and computed tomography (CT) scans have been the standard [
4]. However, these conventional methods present limitations. While 2D imaging has proven valuable, it does not provide comprehensive three-dimensional details of the patient's anatomy. Similarly, physical models, though helpful, cannot capture the dynamic nature of facial structures and movements [
5].
The traditional techniques employed in orthognathic and oral maxillofacial surgeries include a range of surgical osteotomies and bone grafting methods. The exact procedures depend on the specific patient case and could involve maxillary osteotomies, mandibular osteotomies, distraction osteogenesis, or even more complex craniofacial surgical approaches. Despite their proven efficacy, these techniques can be quite invasive, with significant post-operative morbidity in some cases [
6]. The predictability of outcomes can also be challenging due to variations in healing, relapse, or the lack of precision in executing the pre-surgical plan [
6].
Traditional methods come with inherent risks and complications such as infection, bleeding, nerve damage, issues with wound healing, unfavorable bone segment movement, and relapse [
5,
7]. In severe cases, these complications could lead to a second surgical intervention [
8] From a patient's perspective, traditional surgical methods can be intimidating due to the invasive nature of these procedures and the potential for long recovery times. Additionally, the traditional planning process may not allow patients to visualize the intended surgical outcome, leading to potential dissatisfaction with the postoperative results.
Traditional methods in orthognathic and oral maxillofacial surgery rely on the surgeon's skill and experience for precision, which can lead to variability in outcomes [
6]. Moreover, translating two-dimensional pre-surgical plans into three-dimensional surgical procedures can be challenging and may affect the accuracy of the operation [
9]. While experience and skill can help predict outcomes to some extent, the inherent unpredictability of human tissue responses post-surgery often leads to unexpected results [
2]. This lack of predictability can result in dissatisfaction from patients who had different expectations of surgical results [
9]. Every patient presents a unique anatomical framework and individual needs and expectations. Traditional methods, while customizable to an extent, do not provide the level of personalization and adaptability necessary to meet these varied needs [
10].
As medicine moves towards patient-centric care, the demand for personalized surgical methods increases [
11]. Surgeons need to tailor surgical plans to the individual patient's anatomy and desired outcomes. The need for greater surgical precision and predictable outcomes is paramount in improving patient satisfaction rates and reducing complications [
12]. Utilizing advanced technology can help achieve this by improving surgical planning, execution, and follow-up care. Incorporating 3D technology in surgical procedures can aid in better visualization of the surgical area, enhancing precision during surgery [
13]. Furthermore, the ability to simulate different surgical scenarios can lead to better preparedness and more predictable outcomes. By enabling patients to visualize their surgical outcomes beforehand through virtual surgical planning, we can manage their expectations better and potentially enhance satisfaction rates [
14]. Moreover, less invasive surgery due to precise planning can lead to quicker recovery times and less post-operative discomfort, further improving the patient experience [
15].
2. The Advent of 3D Printing in Medical Field
3D printing, also known as additive manufacturing, has emerged as a transformative technology in the last few decades [
16]. The process involves creating three-dimensional objects from a digital file, typically by adding material layer by layer [
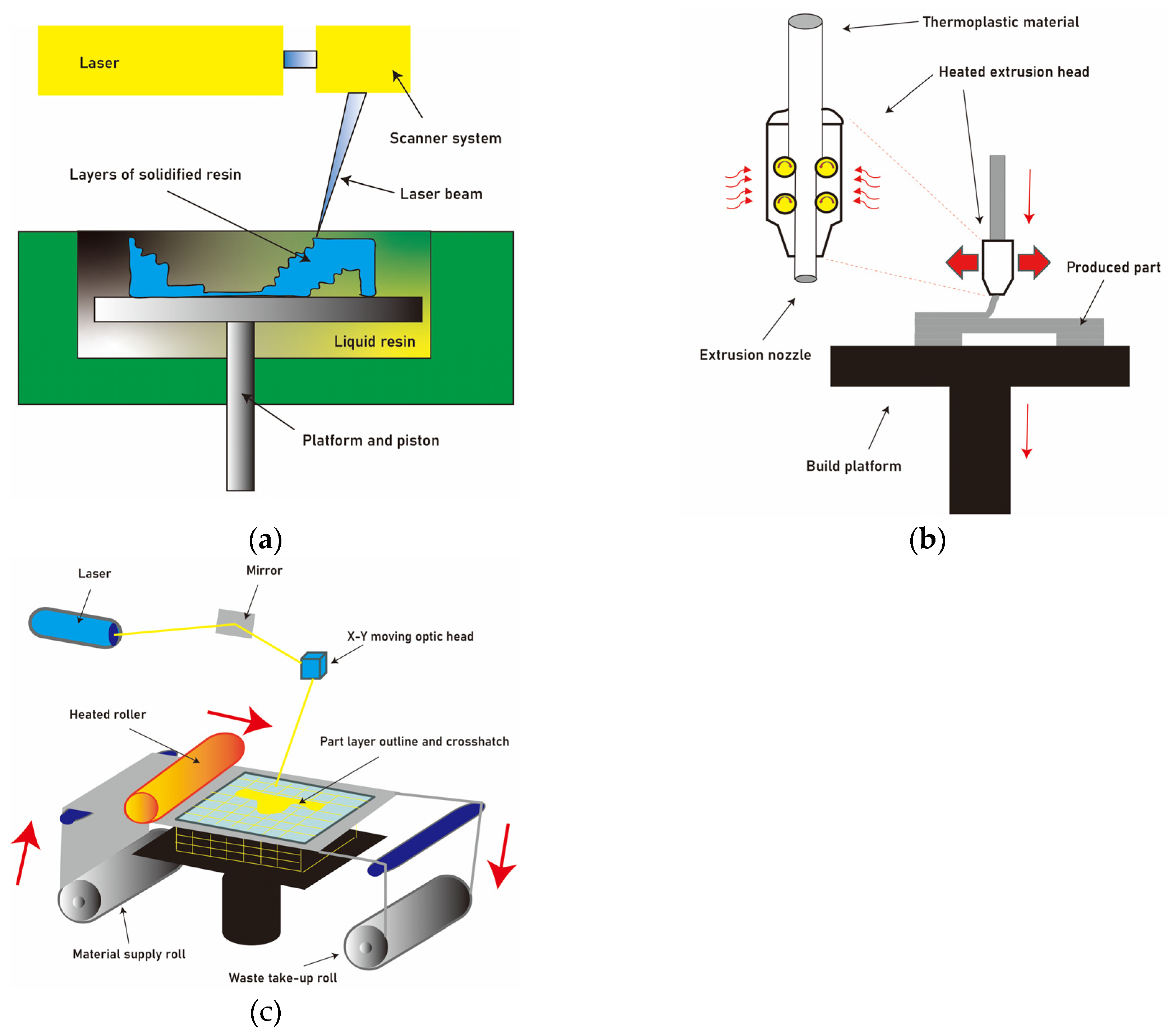
17]. This contrasts with traditional subtractive manufacturing methods, which rely on cutting away material. Various types of 3D printing exist, including stereolithography (SLA) [
18], fused deposition modeling (FDM) [
19], and selective laser sintering (SLS) [
20], each with its unique strengths and suitable applications (
Figure 2).
SLA stands out for its remarkable advantages, which include high resolution and clean surface finishes [
18]. The technique employs a liquid resin that is solidified layer by layer using a laser or a UV light source (
Figure 2a). This results in the production of intricate and detailed models. Furthermore, the SLA process enables the creation of smooth surfaces, enhancing the aesthetic appeal of the final product [
24]. However, it is important to acknowledge the drawbacks associated with SLA. One significant limitation is the relatively long processing time required for the completion of the printing process. Additionally, SLA is limited in terms of material choice, as it primarily relies on liquid resins. Moreover, the post-production step of removing supporting structures can be time-consuming and labor-intensive [
25].
On the other hand, FDM offers distinct benefits that make it a popular choice in many applications [
19]. Notably, FDM exhibits high production speed, making it suitable for rapid prototyping and small-scale manufacturing (
Figure 2b). Additionally, FDM is characterized by low startup and production costs, which makes it a cost-effective option for various industries [
24]. However, FDM has certain limitations that should be considered. One significant drawback is the poor mechanical characteristics of the printed objects, which often exhibit reduced strength and durability. Furthermore, FDM products may have a noticeable layered appearance, which can be visually unappealing. Additionally, the retained support structures in FDM prints require manual removal before the final product can be used [
26].
SLS is another prominent 3D printing technique that utilizes a powder-based approach [
21]. In SLS, a high-power laser selectively fuses powdered materials together, layer by layer, to create the desired object (
Figure 2c). SLS offers several advantages, particularly in the production of patient-specific implants, such as titanium mesh or cranial plates. These implants require high biocompatibility and mechanical strength, both of which are achieved through the SLS process. The technique also allows for the use of a wide range of materials, offering flexibility in terms of material selection. However, it is worth noting that SLS has its limitations. The process can be time-consuming, especially for complex structures, due to the need for multiple layers to be sintered. Additionally, the cost of SLS equipment and materials can be relatively high compared to other 3D printing techniques [
24].
Originally conceived for industrial design and manufacturing, the versatility and adaptability of 3D printing have seen its adoption across a multitude of sectors. The medical field has been one of the early adopters of this technology, recognizing its potential to revolutionize patient care and outcomes [
12,
15]. The use of 3D printing in the medical field has opened new possibilities for personalized medicine [
21]. By leveraging patient-specific data often obtained through imaging techniques such as CT or MRI scans, 3D printing can produce bespoke medical devices tailored to individual patient anatomy [
22]. These include custom prosthetics and orthotics, dental implants, hearing aids, and even patient-specific surgical implants [
27].
Moreover, 3D printing offers the unique advantage of producing exact replicas of patient-specific anatomical models, a feature that has profound implications for surgical planning and education [
23]. Surgeons can use these models to better understand complex pathologies, practice surgical procedures, and explain treatment strategies to patients, thereby improving patient understanding and satisfaction. Despite its benefits, 3D printing in medicine is not without challenges. Issues concerning regulatory approvals, quality control, biocompatibility of materials, and cost-effectiveness remain to be addressed. Nevertheless, the potential benefits that 3D printing brings to patient care make it an exciting area of ongoing development in medicine.
3.3. D Printing in Orthognathic and Oral Maxillofacial Surgery
The application of 3D printing technology in orthognathic and oral maxillofacial surgeries has revolutionized the field [
4]. This includes everything from preoperative planning, where patient-specific anatomical models can be constructed for visualization and surgical practice, to intraoperative guidance via personalized surgical guides [
2]. These advances have greatly reduced the ambiguity and unpredictability often associated with complex surgical procedures. Moreover, postoperative care has also been transformed with the introduction of 3D printed custom prosthetics and implants, leading to improved patient recovery and rehabilitation [
28].
The tangible benefits of 3D printed surgical models and guides are multifaceted. They offer unprecedented surgical precision and predictability by providing a tailored approach to each patient's unique anatomical structure [
29]. In addition, these patient-specific guides significantly shorten operating time and reduce surgical complications, leading to increased efficiency and cost-effectiveness [
24]. Furthermore, 3D models have proved to be an excellent tool for patient communication and education, helping patients to understand the surgical process and providing a more informed platform for consent [
30].
There are numerous case studies that illustrate the significant impact 3D printing technology has had on orthognathic and oral maxillofacial surgeries. For instance, one study found that the use of a 3D printed surgical guide resulted in a significant reduction in surgical time and improved the precision of the procedure, thereby reducing potential complications [
31]. Another study highlighted the success of a patient-specific 3D printed implant that enhanced postoperative recovery and resulted in improved aesthetic outcomes [
32]. Lastly, another study discussed the effectiveness of 3D models in facilitating patient understanding and consent, leading to enhanced patient satisfaction and confidence in their treatment [
33].
A customized 3D titanium implant has been utilized in various aspects of facial reconstruction, demonstrating its versatility and effectiveness [
34]. This implant not only provides the necessary mechanical strength to support the damaged area but also exhibits biocompatibility, reducing the risk of rejection or adverse reactions [
35]. Its application in orbital floor fractures is particularly noteworthy, as it can be tailored to the patient's specific needs based on the structure of the opposite orbit [
35]. This personalized design contributes significantly to the restoration of normal ocular movement, emphasizing the implant's ability to address functional aspects of facial reconstruction [
36].
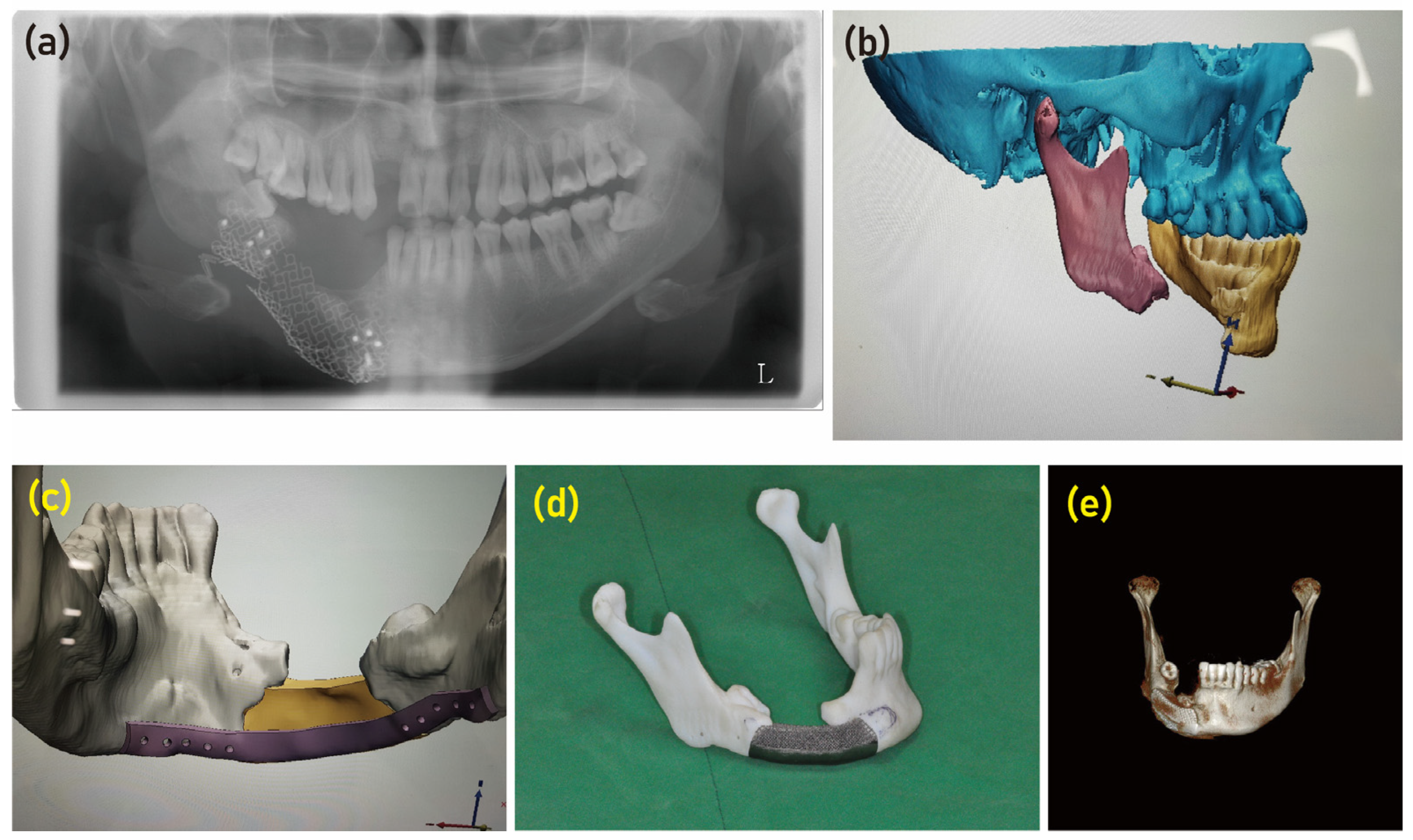
Moreover, the use of a customized titanium device has proven successful in the reconstruction of maxilla and mandible defects [
37]. Through the utilization of computer-aided design and manufacturing (CAD/CAM) and electron beam melting technology, a personalized mandibular reconstruction implant was manufactured according to the specific bone defect [
28]. This highlights the precision and accuracy of the manufacturing process, ensuring an optimal fit for the patient. The implant consisted of a lingual plate that was applied to the lingual surface of the mandible, addressing the specific needs of the individual case (
Figure 3). Furthermore, the upper surface of the implant was purposefully designed with a porous structure (
Figure 3). This design feature aimed to induce the migration of osteogenic cells from the residual mandible segment, facilitating the natural healing process and promoting bone regeneration [
28,
38]. This innovative approach demonstrates the implant's ability to not only provide structural support but also actively contribute to the biological aspects of tissue repair.
The adaptation of the reconstruction plate after tumor resection for the bridging of mandibular segments has involved risk of plate exposure and oro-cutaneous fistula [
39]. Postoperative radiotherapy and compromised soft tissue atrophy are major contributing factors [
40]. The patient having multiple previous surgeries frequently show severe wound shrinkage and fibrosis [
28]. The design for customized implant should be done considering the individual situation of patient. A specifically designed implant including the plate runs through to the lingual side of the mandible (
Figure 3). The plate for screw fixation extended from the implant body and adapted to the lingual surface of the mandible (
Figure 3). Using this lingual plate, it could be diminished the buccal volume of the implant and reduce the risk of plate exposure. The lingual application of a reconstruction implant plate has been successfully performed in previous study without complication [
41]. The customized implant did not need to fill the bone defect area. In previous mechanical tests, the EBM titanium implant showed enough strength to support the mandibular jaw movement and bite force [
42].
The porous structure of the EBM implant can lose the weight of device and provide the microenvironment for osteogenic cell migration and bone ingrowth [
38]. The upper, medial, and distal surfaces of the implant that contacted the residual bone segment had a porous structure. It was designed to permit alveolar bone ingrowth and osseointegration with surrounding bone (
Figure 3). Some of the bone tubercle that was previously grafted with iliac bone remained on both sides of the segment, and the porous structure is designed to surround that residual bone. The customized 3D implant has been successfully used for maxillofacial bone defects, and there have been attempts to repair the alveolar bone portion to further tooth rehabilitation [
43]. A customized titanium tray with an iliac bone graft was used to restore the bone defect and tooth implantation [
44]. The specific design of the implant composed of the mandibular condyle, ramus, body, and tooth prosthesis was used for the hemi-mandible reconstruction in a patient with hemifacial microsomia [
45].
4. The Emergence of Virtual Surgical Planning
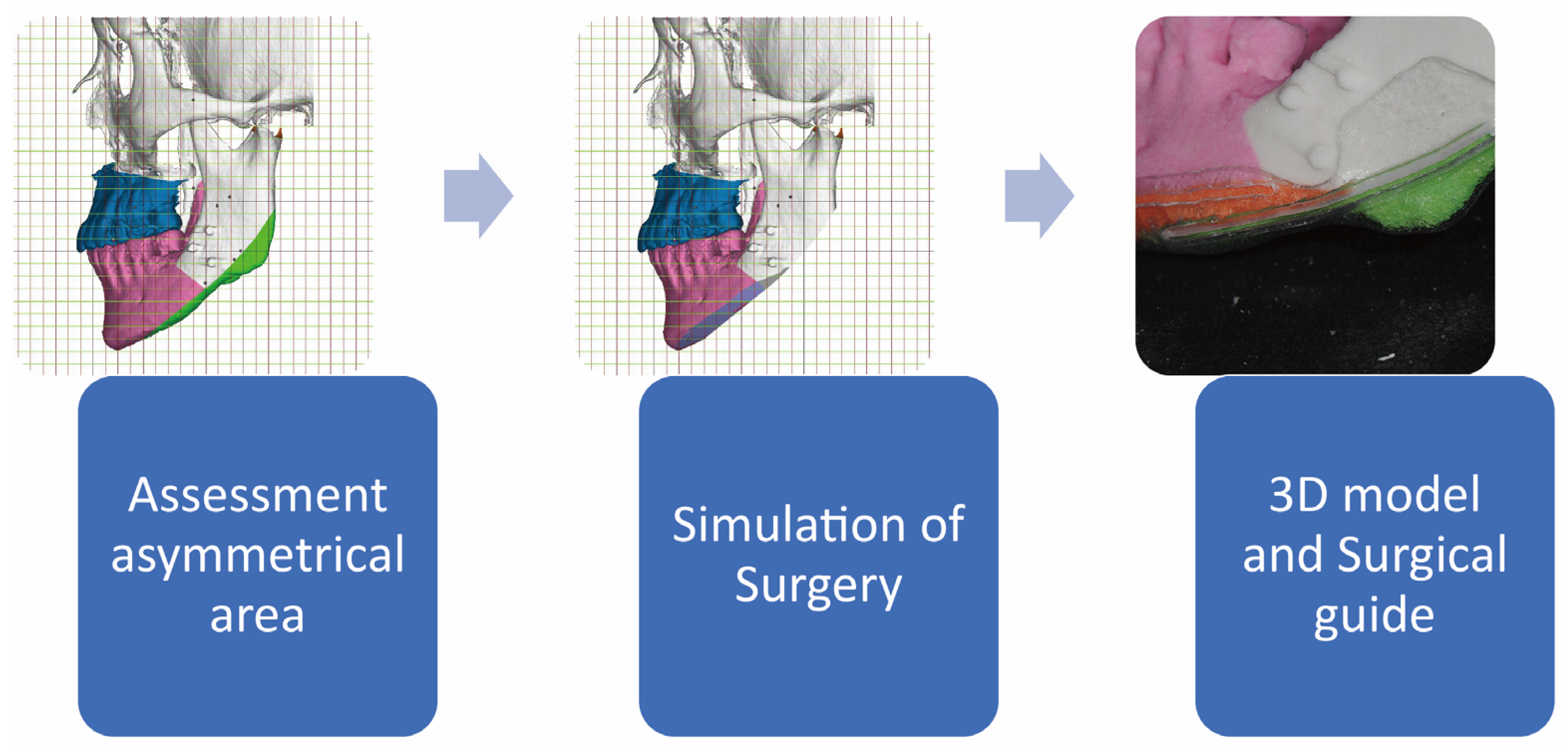
Virtual Surgical Planning (VSP) is a notable advancement in the field of orthognathic and oral maxillofacial surgery, signifying the ongoing evolution of these surgical practices. It is an outcome of the digital revolution in healthcare, employing computer technologies, advanced imaging, and simulation software to meticulously plan intricate surgical procedures [
46]. This innovative technique allows surgeons to visualize the operation in a virtual environment prior to its actual occurrence, thereby augmenting the predictability, precision, and customization of surgical procedures to meet the unique needs of individual patients (
Figure 4). By utilizing VSP, surgeons can gain a comprehensive understanding of the surgical procedure before operating on the patient. This ability to visualize and simulate the surgery in advance enhances the predictability of outcomes, as potential challenges and complications can be identified and addressed prior to the actual procedure. This can significantly reduce the risk of unforeseen complications and allow for better planning and preparation.
Furthermore, VSP enables surgeons to achieve a higher level of precision in their surgical interventions. By virtually manipulating the patient's anatomy and simulating different surgical approaches, surgeons can evaluate the potential impact of their interventions and make more informed decisions regarding the most appropriate course of action. This precision can lead to improved surgical outcomes and patient satisfaction. Another significant advantage of VSP is its ability to tailor surgical procedures to the specific needs of individual patients. Each patient's anatomy and condition are unique, and VSP allows surgeons to customize their approach based on these individual characteristics. This patient-specific tailoring can result in a more personalized and effective surgical intervention, ultimately leading to improved patient outcomes and a higher quality of care.
Traditional planning methods for orthognathic and oral maxillofacial surgeries predominantly relied on two-dimensional imaging techniques such as X-rays and hand-drawn sketches for preoperative planning [
4]. This approach, although functional, was associated with several limitations including lack of spatial context, variability in interpretation, and inability to customize the procedure based on individual patient anatomy [
47]. The advent of VSP has addressed these limitations, offering a three-dimensional, highly interactive, and patient-specific approach to surgical planning [
46]. With VSP, surgeons can manipulate 3D models of a patient's anatomy, plan the surgical approach, anticipate potential challenges, and even rehearse the procedure, all before the patient is on the operating table [
48]. This shift towards virtual techniques not only facilitates better surgical outcomes but also improves efficiency, reduces surgical risk, and enhances patient satisfaction by allowing for a clear preoperative dialogue [
15]. The use of these techniques represents a new era in orthognathic and oral maxillofacial surgery, one characterized by technological integration, precision, and individualized patient care.
5. Virtual Surgical Planning in Orthognathic and Oral Maxillofacial Surgery
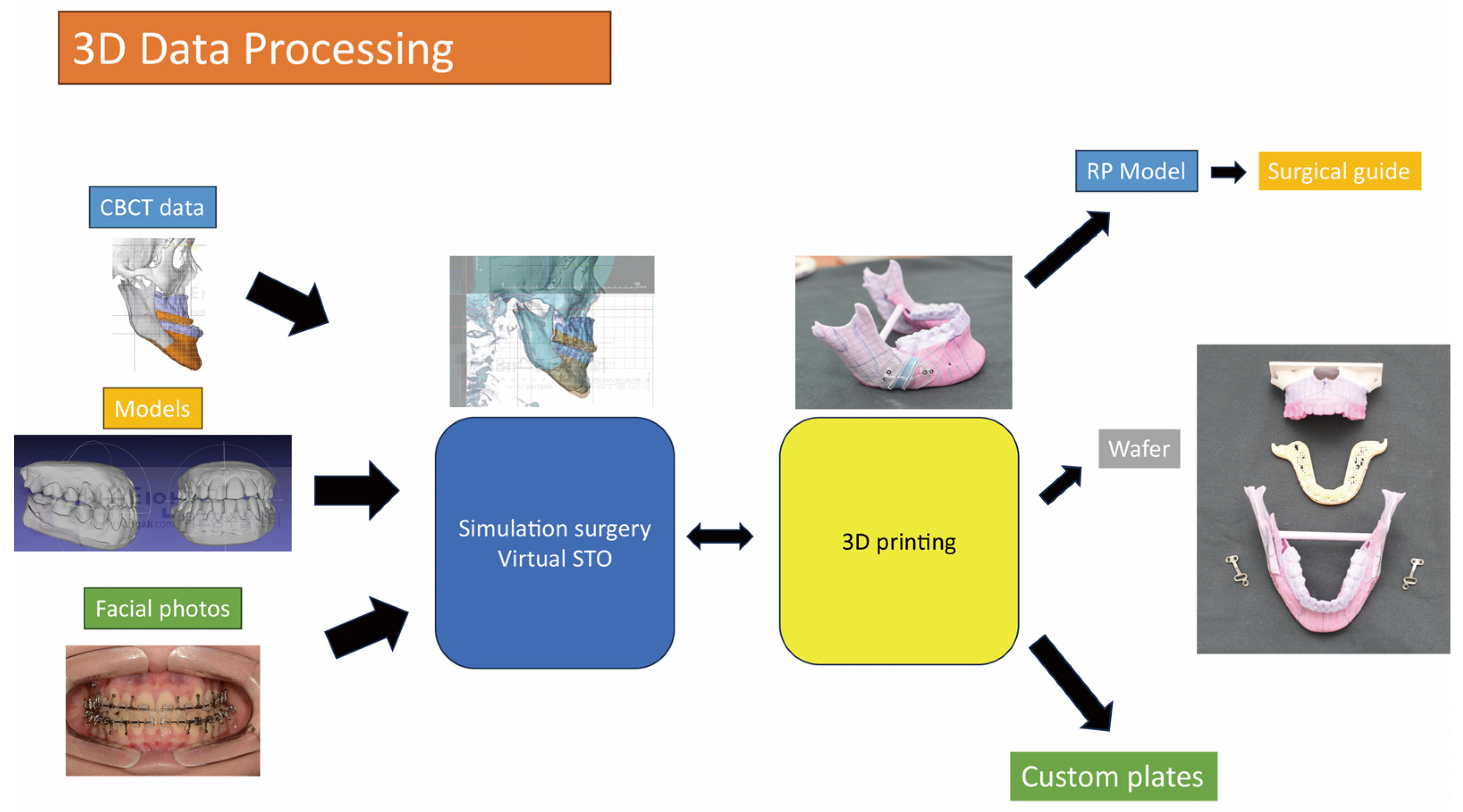
VSP has become an invaluable tool in the field of orthognathic and oral maxillofacial surgery. The application begins with the acquisition of patient-specific imaging data, typically through CT or CBCT scans, which are then converted into 3D digital models (
Figure 5). These models can be manipulated to simulate various surgical outcomes and to design patient-specific surgical guides [
6]. It allows the surgical team to visualize complex anatomical structures, understand the spatial relationships better, and perform virtual osteotomies and repositioning, thus fine-tuning the surgical plan [
24].
Several case studies have underscored the effectiveness of VSP. In a study conducted by Chen et al. [
49], the use of VSP in orthognathic surgery resulted in a significant reduction in operative time and an improved postoperative outcome in terms of facial symmetry and patient satisfaction. Another study by Valls-Ontañón et al. [
50] demonstrated improved accuracy in executing the surgical plan using VSP, resulting in better postoperative occlusal outcomes. These cases underscore the increasing role of VSP as an integral part of surgical planning in orthognathic and oral maxillofacial surgery.
In order to achieve precise mandibular ostectomy through an intraoral approach, the utilization of a 3D-printed surgical guide has proven effective [
51]. Despite limited visibility during the surgery, the surgeon can accurately identify the osteotomy site with the assistance of the surgical guide [
52]. This guide is prepared preoperatively by adapting it to a 3D digital model [
53]. Numerous variations of 3D-printed surgical guides or templates have been successfully employed in mandibular contouring surgeries, demonstrating their suitability for surgical purposes [
52]. When performing a mandibular ostectomy, adherence to the computer-assisted simulation planning (CASP) is crucial, ensuring proper transfer of the preoperative surgical template [
54].
Traditional mandibular contouring surgeries rely on 2D images for planning purposes [
55]. Unfortunately, the imprecise nature of these surgical plans has led to nerve-related complications and residual asymmetry in approximately 10% of patients [
55]. Evaluating the success of mandibuloplasty outcomes has typically involved a simple comparison of pre- and postoperative clinical photographs [
56]. The use of CASP and a 3D-printed surgical guide in mandibular contouring surgery offers advantages over traditional planning techniques relying on 2D images [
57]. By incorporating CASP and the surgical guide, operating time can be reduced and surgical accuracy increased [
57].
6. The Symbiosis between 3D Printing and Virtual Surgical Planning
A pivotal advancement in orthognathic and oral maxillofacial surgery is the amalgamation of 3D printing and VSP [
58]. This combination serves to revolutionize surgical planning and implementation by providing tactile 3D models for visualization and planning, and accurately designed surgical guides for execution (
Figure 6). The virtual environment allows for a meticulous preoperative plan, while 3D printing translates this plan into a tangible reality, serving as a road map during surgery.
The marriage of 3D printing and VSP creates a symbiotic relationship that enhances surgical precision, reduces operative time, and improves patient-specific outcomes [
59]. VSP provides the platform for simulation and evaluation of different surgical approaches in a risk-free virtual environment [
60]. On the other hand, 3D printing brings this virtual plan to life by creating patient-specific physical models and surgical guides, providing surgeons with a practical tool for intraoperative use [
61]. This convergence of digital planning and physical modeling facilitates a more predictable, personalized, and precise surgical process, paving the way for advanced patient care in orthognathic and oral maxillofacial surgery [
2,
5].
7. Challenges and Future Directions
Despite the immense potential of 3D printing and VSP in orthognathic and oral maxillofacial surgery, these technologies are not without their limitations. One of the major challenges in adopting these technologies is the need for extensive software training [
62]. Professionals need to acquire the necessary skills to operate the software used for designing and creating 3D printed models and surgical guides [
63]. This training can be time-consuming and may require additional financial resources. Moreover, the software itself may have a steep learning curve, especially for those who are not familiar with CAD programs [
64].
Financial constraints can also impede the widespread adoption of these technologies. The cost of acquiring and maintaining the necessary equipment and software can be significant [
65]. Additionally, the materials used for 3D printing, such as biocompatible resins, can be expensive [
66]. These financial considerations may limit the accessibility of these technologies to some healthcare institutions or individual practitioners. Another limitation is the reliance on high-quality digital imaging for accurate 3D printing and surgical planning [
67]. The accuracy of the printed models and surgical guides depends on the quality of the initial digital scans [
63]. Patient-related factors, such as movement during imaging or dental prosthetics, can affect the accuracy of the digital models [
68]. Technical factors, such as image resolution and artifact interference, can also impact the quality of the digital scans [
69]. Therefore, careful attention must be given to the imaging process to ensure accurate representation of the patient's anatomy.
Furthermore, the rapid advancement and adoption of 3D printing and VSP have outpaced the development of regulations and standards. As a result, there are concerns about patient safety and procedural accountability [
70]. It is crucial to establish guidelines and regulations to ensure the quality and reliability of these technologies. This includes standardized protocols for imaging, software validation, and quality control measures for 3D printing and surgical planning processes. According to the Regulation (EU) 2017/745 of the European Parliament and of the Council dated 5 April 2017, all 3D-printed products are classified as custom-made devices [
71]. As per this regulation, manufacturers of such custom-made devices are required to adhere to conformity assessment procedures in order to ensure compliance with safety and performance requirements [
72].
Looking ahead, continued technological evolution is likely to further refine the precision, affordability, and accessibility of 3D printing and VSP in orthognathic and oral maxillofacial surgery. Integration of artificial intelligence and machine learning could automate aspects of surgical planning, making these technologies more user-friendly and efficient. Moreover, advances in biomaterials may lead to the production of bioresorbable or tissue-engineered 3D printed implants, fostering innovation in patient-specific treatment. On the regulatory front, the establishment of clear guidelines and quality standards is necessary to ensure patient safety while supporting technological progress. As research progresses and the technology matures, these innovative tools are set to usher in a new era of precision and personalization in surgical care.
8. Conclusion
Traditional methods in orthognathic and oral maxillofacial surgery come with inherent risks and complications such as infection, bleeding, nerve damage, issues with wound healing, unfavorable bone segment movement, and relapse. These methods rely heavily on the surgeon's skill and experience for precision, which can lead to variability in outcomes. Translating two-dimensional pre-surgical plans into three-dimensional surgical procedures can be challenging and may affect the accuracy of the operation.
As medicine moves towards patient-centric care, the demand for personalized surgical methods increases. Surgeons need to tailor surgical plans to the individual patient's anatomy and desired outcomes. The need for greater surgical precision and predictable outcomes is paramount in improving patient satisfaction rates and reducing complications. Utilizing advanced technology can help achieve this by improving surgical planning, execution, and follow-up care. Incorporating 3D technology in surgical procedures can aid in better visualization of the surgical area, enhancing precision during surgery.
The amalgamation of 3D printing and VSP serves to revolutionize surgical planning and implementation by providing tactile 3D models for visualization and planning, and accurately designed surgical guides for execution. The virtual environment allows for a meticulous preoperative plan, while 3D printing translates this plan into a tangible reality, serving as a roadmap during surgery. This convergence of digital planning and physical modeling facilitates a more predictable, personalized, and precise surgical process, paving the way for advanced patient care in orthognathic and oral maxillofacial surgery.
Despite the immense potential of 3D printing and VSP in orthognathic and oral maxillofacial surgery, these technologies are not without their limitations. One of the major challenges in adopting these technologies is the need for extensive software training. Professionals need to acquire the necessary skills to operate the software used for designing and creating 3D printed models and surgical guides. This training can be time-consuming and may require additional financial resources. Moreover, the software itself may have a steep learning curve, especially for those who are not familiar with CAD programs.
Author Contributions
Conceptualization, K.J.Y. and K.S.G.; methodology, L.Y.C.; software, L.Y.C.; validation, K.S.G., K.J.Y. and G.U.; investigation, L.Y.C.; resources, G.U.; data curation, L.Y.C.; writing—original draft preparation, K.J.Y. and K.S.G.; writing—review and editing, L.Y.C. and G.U.; visualization, K.S.G. and L.Y.C.; supervision, G.U.; project administration, L.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
There is no data.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keser, E.; Naini, F. B. Accelerated orthodontic tooth movement: surgical techniques and the regional acceleratory phenomenon. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Sohn, H.B.; Park, Y.W.; Oh, J.H. Evaluation of postoperative changes in condylar positions after orthognathic surgery using balanced orthognathic surgery system. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 11. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Schmohl, J.; Cascant Ortolano, L.; Bayer, O.; Schweizer, S.; Welte-Jzyk, C.; Al-Nawas, B.; Daubländer, M. Therapy of neurophysiological changes after oral and maxillofacial surgery—A systematic review. Appl. Sci. 2022, 12, 1507. [Google Scholar] [CrossRef]
- Bartella, A.K.; Kamal, M.; Scholl, I.; Schiffer, S.; Steegmann, J.; Ketelsen, D.; Hölzle, F.; Lethaus, B. Virtual reality in preoperative imaging in maxillofacial surgery: implementation of “the next level”? Br. J. Oral Maxillofac. Surg. 2019, 57, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Koyachi, M.; Odaka, K.; Matsunaga, S.; Katakura, A. A safe, stable, and convenient three-dimensional device for high Le Fort I osteotomy. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 32. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, D.S.; Song, J.M.; Kim, U.K. Comparison of time and cost between conventional surgical planning and virtual surgical planning in orthognathic surgery in Korea. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 18. [Google Scholar] [CrossRef]
- O'Connor, M.K.; Emanuelli, E.; Garg, R.K. Le Fort I maxillary osteotomy in a Jehovah's Witness patient: strategies for minimizing blood loss and maximizing safety. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 10. [Google Scholar] [CrossRef] [PubMed]
- Aydil, B.A.; Akbaş, M.; Ayhan, M.; Atalı, O.; Can, S.; Çömlekçioğlu, Y. Retrospective examination of complications observed in orthognathic surgical surgery in 85 patients. Turk. J. Trauma Emerg. Surg. 2022, 28, 698. [Google Scholar]
- Alkhayer, A.; Piffkó, J.; Lippold, C.; Segatto, E. Accuracy of virtual planning in orthognathic surgery: a systematic review. Head Face Med. 2020, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Kämmerer, P.W.; Hennig, M.; Schön, G.; Thiem, D.G.; Bschorer, R. Customized virtual surgical planning in bimaxillary orthognathic surgery: a prospective randomized trial. Clin. Oral Investig. 2019, 23, 3115–3122. [Google Scholar] [CrossRef]
- Büyükçoban, S.; Öner, Ö.; Hanci, V. A bibliometric analysis of the most cited articles in geriatric anesthesia. Turk. J. Geriatr. 2020, 23, 410–418. [Google Scholar] [CrossRef]
- Pimkhaokham, A.; Jiaranuchart, S.; Kaboosaya, B.; Arunjaroensuk, S.; Subbalekha, K.; Mattheos, N. Can computer-assisted implant surgery improve clinical outcomes and reduce the frequency and intensity of complications in implant dentistry? A critical review. Periodontol. 2000 2022, 90, 197–223. [Google Scholar] [PubMed]
- Wang, Y.; Cao, D.; Chen, S.L.; Li, Y.M.; Zheng, Y.W.; Ohkohchi, N. Current trends in three-dimensional visualization and real-time navigation as well as robot-assisted technologies in hepatobiliary surgery. World J. Gastrointest. Surg. 2021, 13, 904. [Google Scholar] [PubMed]
- Youn, S.; Geismar, H.N.; Pinedo, M. Planning and scheduling in healthcare for better care coordination: Current understanding, trending topics, and future opportunities. Prod. Oper. Manag. 2022, 31, 4407–4423. [Google Scholar]
- Sadeghi, A.H.; El Mathari, S.; Abjigitova, D.; Maat, A.P.; Taverne, Y.J.; Bogers, A.J.; Mahtab, E.A. Current and future applications of virtual, augmented, and mixed reality in cardiothoracic surgery. Ann. Thorac. Surg. 2022, 113, 681–691. [Google Scholar]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit. Manuf. 2022, 55, 102867. [Google Scholar]
- Praveena, B.A.; Lokesh, N.; Buradi, A.; Santhosh, N.; Praveena, B.L.; Vignesh, R. A comprehensive review of emerging additive manufacturing (3D printing technology): Methods, materials, applications, challenges, trends and future potential. Mater. Today: Proc. 2022, 52, 1309–1313. [Google Scholar]
- Ge, Q.; Li, Z.; Wang, Z.; Kowsari, K.; Zhang, W.; He, X.; Zhou, J.; Fang, N.X. Projection micro stereolithography based 3D printing and its applications. Int. J. Extreme Manuf. 2020, 2, 022004. [Google Scholar]
- Wasti, S.; Adhikari, S. Use of biomaterials for 3D printing by fused deposition modeling technique: a review. Front. Chem. 2020, 8, 315. [Google Scholar]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [PubMed]
- dos Santos, J.; de Oliveira, R.S.; de Oliveira, T.V.; Velho, M.C.; Konrad, M.V.; da Silva, G.S.; Deon, M.; Beck, R.C. 3D printing and nanotechnology: a multiscale alliance in personalized medicine. Adv. Funct. Mater. 2021, 31, 2009691. [Google Scholar] [CrossRef]
- Paxton, N.C.; Nightingale, R.C.; Woodruff, M.A. Capturing patient anatomy for designing and manufacturing personalized prostheses. Curr. Opin. Biotechnol. 2022, 73, 282–289. [Google Scholar]
- Jaksa, L.; Pahr, D.; Kronreif, G.; Lorenz, A. Development of a multi-material 3D printer for functional anatomic models. Int. J. Bioprinting 2021, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Dod, G.; Jibhakate, R.; Walke, P. A review on 3D printing maxillofacial surgery: Present work and future prospects. Mater. Today: Proc 2023. [Google Scholar] [CrossRef]
- Prakash, K.S.; Nancharaih, T.; Rao, V.V.S. Additive manufacturing techniques in manufacturing-an overview. Mater. Today: Proc. 2018, 5, 3873–3882. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Kim, S.G.; Kim, M.K.; Shin, S.H.; Ahn, J.; Seok, H. Mandibular reconstruction using a customized three-dimensional titanium implant applied on the lingual surface of the mandible. J. Craniofac. Surg. 2018, 29, 415–419. [Google Scholar] [CrossRef]
- Carneiro, N.C.; Oliveira, D.V.; Real, F.H.; da Silva Tabosa, A.K.; Júnior, J.T. A new model of customized maxillary guide for orthognathic surgery: precision analysis. J. Craniomaxillofac. Surg. 2020, 48, 1119–1125. [Google Scholar] [CrossRef]
- Seok, J.; Yoon, S.; Ryu, C.H.; Kim, S.K.; Ryu, J.; Jung, Y.S. A personalized 3D-printed model for obtaining informed consent process for thyroid surgery: A randomized clinical study using a deep learning approach with mesh-type 3D modeling. J. Pers. Med. 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- May, M.M.; Howe, B.M.; O'Byrne, T.J.; Alexander, A.E.; Morris, J.M.; Moore, E.J.; Kasperbauer, J.L.; Janus, J.R.; Van Abel, K.M.; Dickens, H.J.; et al. Short and long-term outcomes of three-dimensional printed surgical guides and virtual surgical planning versus conventional methods for fibula free flap reconstruction of the mandible: Decreased nonunion and complication rates. Head Neck 2021, 43, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Khanal, N.; Chaulagain, R.; Sharma, N.; Thieringer, F.M. Is the pre-shaping of an orbital implant on a patient-specific 3D-printed model advantageous compared to conventional free-hand shaping? A systematic review and meta-analysis. J. Clin. Med. 2023, 12, 3426. [Google Scholar] [CrossRef] [PubMed]
- Hertanto, M.; Ayoub, A.F.; Benington, P.C.; Naudi, K.B.; McKenzie, P.S. Orthognathic patient perception of 3D facial soft tissue prediction planning. J. Craniomaxillofac. Surg. 2021, 49, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, D.M. Three-dimensional analysis and surgical planning in craniomaxillofacial surgery. J. Oral Maxillofac. Surg. 2015, 73, S40–S56. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jo, E.; Cho, H.; Kim, H.J. Temporomandibular joint reconstruction with alloplastic prosthesis: the outcomes of four cases. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 6. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Ellis, E. 3rd.; Aniceto, G.S.; Schramm, A.; Wagner, M.E.; Grant, M.P.; Cornelius, C.P.; Strong, E.B.; Rana, M.; Chye, L.T.; Calle, A.R.; Wilde, F.; et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J. Craniomaxillofac. Surg. 2016, 44, 1485–1497. [Google Scholar]
- Shan, X.F.; Chen, H.M.; Liang, J.; Huang, J.W.; Cai, Z.G. Surgical reconstruction of maxillary and mandibular defects using a printed titanium mesh. J. Oral Maxillofac. Surg. 2015, 73, 1437–e1. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, N.; Da Assuncao, R.; Hancock, N.J.; Lau, A.; Walsh, W.R. Influence of electron beam melting manufactured implants on ingrowth and shear strength in an ovine model. J. Arthroplasty 2012, 27, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Al Deek, N.F.; Wei, F.C.; Tsao, C.K. Fistulae after successful free tissue transfer to head and neck: Its prevention and treatment. Clin. Plast. Surg. 2016, 43, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Hwang, J.H.; Ahn, K.M. Fibular flap for mandible reconstruction in osteoradionecrosis of the jaw: selection criteria of fibula flap. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 46. [Google Scholar] [CrossRef]
- Probst, F.A.; Metzger, M.; Ehrenfeld, M.; Cornelius, CP. Computer-assisted designed and manufactured procedures facilitate the lingual application of mandible reconstruction plates. J. Oral Maxillofac. Surg. 2016, 74, 1879–1895. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.V.; Duan, Y.; Neidigh, J.; Koike, M.; Chahine, G.; Kovacevic, R.; Okabe, T.; Griggs, J.A. Fatigue testing of electron beam-melted Ti-6Al-4V ELI alloy for dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Nakaoka, K.; Sonoyama, T.; Kumagai, K.; Ikawa, T.; Shigeta, Y.; Harada, N.; Kawamura, N.; Ogawa, T.; Hamada, Y. Clinical usefulness of mandibular reconstruction using custom-made titanium mesh tray and autogenous particulate cancellous bone and marrow harvested from tibia and/or ilia. J. Craniofac. Surg. 2016, 27, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.B.; Shang, H.T.; He, L.S.; Bo, B.; Liu, G.C.; Liu, Y.P.; Zhao, J.L. Accurate reconstruction of discontinuous mandible using a reverse engineering/computer-aided design/rapid prototyping technique: a preliminary clinical study. J. Oral Maxillofac. Surg. 2010, 68, 2115–2121. [Google Scholar] [CrossRef]
- Lee, U.L.; Kwon, J.S.; Woo, S.H.; Choi, Y.J. Simultaneous bimaxillary surgery and mandibular reconstruction with a 3-dimensional printed titanium implant fabricated by electron beam melting: A preliminary mechanical testing of the printed mandible. J. Oral Maxillofac. Surg. 2016, 74. 1501.e1–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Klasen, J.R.; Thatcher, G.P.; Bleedorn, J.A.; Soukup, J.W. Virtual surgical planning and 3D printing: Methodology and applications in veterinary oromaxillofacial surgery. Front. Vet. Sci. 2022, 9, 971318. [Google Scholar] [CrossRef]
- Unberath, M.; Gao, C.; Hu, Y.; Judish, M.; Taylor, R.H.; Armand, M.; Grupp, R. The impact of machine learning on 2d/3d registration for image-guided interventions: A systematic review and perspective. Front. Robot. AI. 2021, 8, 716007. [Google Scholar] [CrossRef]
- Vyas, K.; Gibreel, W.; Mardini, S. Virtual surgical planning (VSP) in craniomaxillofacial reconstruction. Facial Plast. Surg. Clin. North Am. 2022, 30, 239–253. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, S.; Fan, X.; You, Y.; Ye, G.; Zhou, N. A meta-analysis and systematic review comparing the effectiveness of traditional and virtual surgical planning for orthognathic surgery: based on randomized clinical trials. J. Oral Maxillofac. Surg. 2021, 79, 471–e1. [Google Scholar] [CrossRef]
- Valls-Ontañón, A.; Ascencio-Padilla. R.D.; Vela-Lasagabaster, A.; Sada-Malumbres, A.; Haas-Junior, O.L.; Masià-Gridilla, J.; Hernández-Alfaro, F. Relevance of 3D virtual planning in predicting bony interferences between distal and proximal fragments after sagittal split osteotomy. Int. J. Oral Maxillofac. Surg. 2020, 49, 1020–1028. [Google Scholar] [CrossRef]
- Zhang, C.; Teng, L.; Chan, F.C.; Xu, J.J.; Lu, J.J.; Xie, F.; Zhao, J.Y.; Xu, M.B.; Jin, X.L. Single stage surgery for contouring the prominent mandibular angle with a broad chin deformity: en-bloc Mandibular Angle-Body-Chin Curved Ostectomy (MABCCO) and Outer Cortex Grinding (OCG). J. Craniomaxillofac. Surg. 2014, 42, 1225–1233. [Google Scholar] [CrossRef]
- Ye, N.; Long, H.; Zhu, S.; Yang, Y.; Lai, W.; Hu, J. The accuracy of computer image-guided template for mandibular angle ostectomy. Aesthetic Plast. Surg. 2015, 39, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Habu, M.; Tsurushima, H.; Takahashi, O.; Yoshioka, I. CAD/CAM splint based on soft tissue 3D simulation for treatment of facial asymmetry. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, M.K.; Kang, S.H. Genioplasty using a simple CAD/CAM (computer-aided design and computer-aided manufacturing) surgical guide. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 44. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Shan, L.; Wang, J. Intraoral curved ostectomy for prominent mandibular angle by grinding, contiguous drilling, and chiseling. J. Craniofac. Surg. 2011, 22, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.A.; Ribeiro, R.D.; Azevedo, F.; Freitas, P.H. Reduction mandibuloplasty for facial aesthetic enhancement in western women-a case report. Biosci. J. 2016, 32, 781–786. [Google Scholar] [CrossRef]
- Seok, H.; Kim, S.G.; Park, Y.W.; Lee, Y.C. Postoperative three-dimensional evaluation of mandibular contouring surgery using computer-assisted simulation planning and a three-dimensional-printed surgical guide. J. Craniofac. Surg. 2017, 28, 768–770. [Google Scholar] [CrossRef]
- Pandian, S.M.; Gandedkar, N.H.; kumar Palani, S.; Kim, Y.J.; Adel, S.M. An integrated 3D-driven protocol for surgery first orthognathic approach (SFOA) using virtual surgical planning (VSP). In Seminars in Orthodontics. 2022, 28, 320–333, WB Saunders. [Google Scholar] [CrossRef]
- Hurley, C.M.; Walsh, R.M.; Shine, N.P.; O'Neill, J.P.; Martin, F.; O'Sullivan, J.B. Current trends in craniofacial reconstruction. Surgeon. 2023, 21, e118–e125. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Y.; Lu, L.; Chen, Y.; Long, H.; Wang, J. Virtual simulation in undergraduate medical education: a scoping review of recent practice. Front. Med. 2022, 9, 855403. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.H.; Darwood, A.R.; Shaunak, S.; Kulatilake, P.; Abdulrahman, A.; Mulki, O.; Baskaradas, A. Three-dimensional printing in surgery: a review of current surgical applications. J. Surg. Res. 2015, 199, 512–522. [Google Scholar] [CrossRef]
- Cho, K.H.; Papay, F.A.; Yanof, J.; West, K.; Bassiri Gharb, B.; Rampazzo, A.; Gastman, B.; Schwarz, G.S. Mixed reality and 3D printed models for planning and execution of face transplantation. Ann. Surg. 2021, 274, e1238–e1246. [Google Scholar] [CrossRef]
- Sears, V.A.; Morris, J.M. Establishing a point-of-care virtual planning and 3D printing program. In Seminars in Plastic Surgery. 2022, 36, 133–148, 333 Seventh Avenue, 18th Floor, New York, NY 10001, USA: Thieme Medical Publishers, Inc.. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Qiao, J.; Girod, S.; Niu, F.; feng Liu, J.; Lee, G.K.; Gui, L. Standardized protocol for virtual surgical plan and 3-dimensional surgical template–assisted single-stage mandible contour surgery. Ann. Plast. Surg. 2017, 79, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ostaș, D.; Almășan, O.; Ileșan, R.R.; Andrei, V.; Thieringer, F.M.; Hedeșiu, M.; Rotar, H. Point-of-care virtual surgical planning and 3D printing in oral and cranio-maxillofacial surgery: a narrative review. J. Clin. Med. 2022, 11, 6625. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhal, K.; Gupta, R.; Tappa, K.; Rybicki, F.J.; Ravi, P. Medical 3D printing using desktop inverted vat photopolymerization: Background, clinical applications, and challenges. Bioeng. 2023, 10, 782. [Google Scholar] [CrossRef]
- Zoabi, A.; Redenski, I.; Oren, D.; Kasem, A.; Zigron, A.; Daoud, S.; Moskovich, L.; Kablan, F.; Srouji, S. 3D printing and virtual surgical planning in oral and maxillofacial surgery. J. Clin. Med. 2022, 11, 2385. [Google Scholar] [CrossRef]
- Beek, D.M.; Baan, F.; Liebregts, J.; Bergé, S.; Maal, T.; Xi, T. Surgical accuracy in 3D planned bimaxillary osteotomies: intraoral scans and plaster casts as digital dentition models. Int. J. Oral Maxillofac. Surg. 2022, 51, 922–928. [Google Scholar] [CrossRef]
- Francisco, I.; Ribeiro, M.P.; Marques, F.; Travassos, R.; Nunes, C.; Pereira, F.; Caramelo, F.; Paula, A.B.; Vale, F. Application of three-dimensional digital technology in orthodontics: the state of the art. Biomim. 2022, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Kuruoglu, D.; Yan, M.; Bustos, S.S.; Morris, J.M.; Alexander, A.E.; Sharaf, B. Point of care virtual surgical planning and 3D printing in facial gender confirmation surgery: a narrative review. Ann. Transl. Med. 2021, 9, 614. [Google Scholar] [CrossRef]
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and regulation (EC) No 1223/2009 and repealing Council Directive 90/985/EEC and 93/42/EEC.
- Article 20, Comma 1 of the Regulation (EU) 2017/745.
Figure 1.
The field of oral maxillofacial surgery encompasses a wide range of treatments and procedures. It not only involves orthognathic surgery but also encompasses the management of various diseases and injuries affecting the functional and aesthetic aspects of the oral and maxillofacial region. These treatments can include the removal of impacted teeth, complex facial reconstructions, as well as the management of conditions such as oral cancer, cleft lip and palate, and chronic facial pain disorders.
Figure 1.
The field of oral maxillofacial surgery encompasses a wide range of treatments and procedures. It not only involves orthognathic surgery but also encompasses the management of various diseases and injuries affecting the functional and aesthetic aspects of the oral and maxillofacial region. These treatments can include the removal of impacted teeth, complex facial reconstructions, as well as the management of conditions such as oral cancer, cleft lip and palate, and chronic facial pain disorders.
Figure 2.
There are several forms of 3D printing, such as (a) stereolithography, (b) fused deposition modeling (FDM), and (c) selective laser sintering (SLS), each possessing distinct advantages and appropriate areas of application.
Figure 2.
There are several forms of 3D printing, such as (a) stereolithography, (b) fused deposition modeling (FDM), and (c) selective laser sintering (SLS), each possessing distinct advantages and appropriate areas of application.
Figure 3.
The research investigates the utilization of 3D printing and virtual surgical planning in addressing mandibular defects. (a) The initial examination revealed a broken mesh and defect in the right mandibular body region. (b) To gain better insights, a 3D simulated image was produced to visualize the extent of the defect. (c) Subsequently, a prosthesis was designed using computer-aided design/computer-aided manufacturing (CAD/CAM) technology, with a lingual plate being incorporated and applied to the lingual surface of the mandible. (d) To create the actual implant, a 3D printed titanium version was applied to a 3D model of the patient. Notably, the upper surface of the implant was intentionally designed with a porous structure to improve integration. (e) Following the surgical procedure, the 3D printed implant was seen to integrate successfully into the bony defect.
Figure 3.
The research investigates the utilization of 3D printing and virtual surgical planning in addressing mandibular defects. (a) The initial examination revealed a broken mesh and defect in the right mandibular body region. (b) To gain better insights, a 3D simulated image was produced to visualize the extent of the defect. (c) Subsequently, a prosthesis was designed using computer-aided design/computer-aided manufacturing (CAD/CAM) technology, with a lingual plate being incorporated and applied to the lingual surface of the mandible. (d) To create the actual implant, a 3D printed titanium version was applied to a 3D model of the patient. Notably, the upper surface of the implant was intentionally designed with a porous structure to improve integration. (e) Following the surgical procedure, the 3D printed implant was seen to integrate successfully into the bony defect.
Figure 4.
A virtual surgical plan designed specifically for facial contouring surgery. By employing the mirroring technique, areas of asymmetry can be identified and visualized using different colors. Furthermore, during the simulation surgery, an accurate visualization of the post-operative mandible can be achieved. This virtual surgical plan includes the use of a surgical guide, which greatly aids in the precise removal of bony excess. Additionally, the surgical guide can be produced on a 3D model, enhancing the overall accuracy and efficiency of the surgical procedure.
Figure 4.
A virtual surgical plan designed specifically for facial contouring surgery. By employing the mirroring technique, areas of asymmetry can be identified and visualized using different colors. Furthermore, during the simulation surgery, an accurate visualization of the post-operative mandible can be achieved. This virtual surgical plan includes the use of a surgical guide, which greatly aids in the precise removal of bony excess. Additionally, the surgical guide can be produced on a 3D model, enhancing the overall accuracy and efficiency of the surgical procedure.
Figure 5.
The initial steps involved in the application process of acquiring patient-specific imaging data and subsequently creating a 3D printed prosthesis. The process typically commences with the acquisition of imaging data, such as CT or CBCT scans, which provide detailed information about the patient's anatomy. These scans serve as the foundation for creating accurate 3D digital models. Once the imaging data is obtained, it is then converted into digital models through a series of advanced software algorithms. These algorithms meticulously reconstruct the scanned data into a three-dimensional representation that can be manipulated and visualized. The next step in this process involves transferring the digital models to a 3D printer. This transfer can occur through various means, such as direct file transfer or by utilizing specialized software that interfaces with the specific 3D printing technology being employed. The 3D printer then utilizes this digital model to create a physical replica of the intended prosthesis through additive manufacturing techniques, layer by layer. Finally, the printed prosthesis undergoes a critical evaluation process to ensure its compatibility and functionality for the intended application. This evaluation often involves fitting the prosthesis to the patient, assessing its biomechanical properties, and verifying that it meets quality standards.
Figure 5.
The initial steps involved in the application process of acquiring patient-specific imaging data and subsequently creating a 3D printed prosthesis. The process typically commences with the acquisition of imaging data, such as CT or CBCT scans, which provide detailed information about the patient's anatomy. These scans serve as the foundation for creating accurate 3D digital models. Once the imaging data is obtained, it is then converted into digital models through a series of advanced software algorithms. These algorithms meticulously reconstruct the scanned data into a three-dimensional representation that can be manipulated and visualized. The next step in this process involves transferring the digital models to a 3D printer. This transfer can occur through various means, such as direct file transfer or by utilizing specialized software that interfaces with the specific 3D printing technology being employed. The 3D printer then utilizes this digital model to create a physical replica of the intended prosthesis through additive manufacturing techniques, layer by layer. Finally, the printed prosthesis undergoes a critical evaluation process to ensure its compatibility and functionality for the intended application. This evaluation often involves fitting the prosthesis to the patient, assessing its biomechanical properties, and verifying that it meets quality standards.
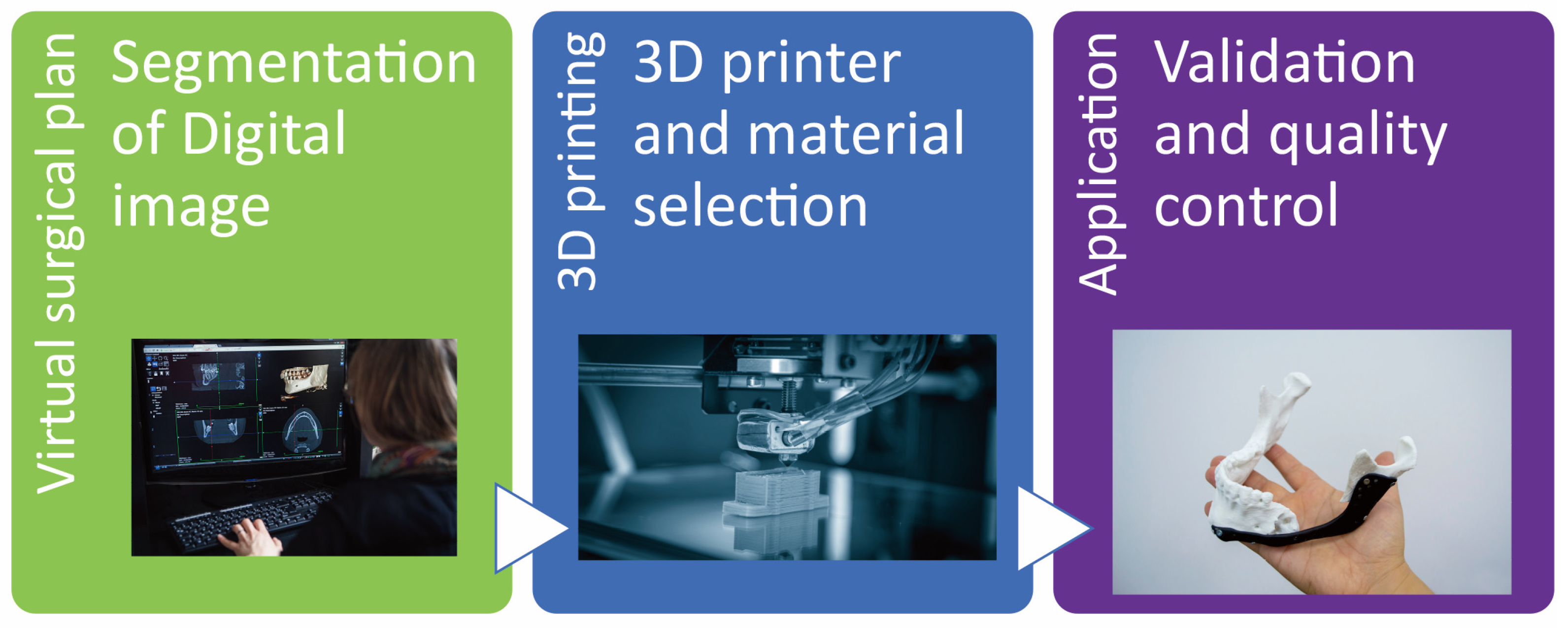
Figure 6.
The utilization of surgical guides in orthognathic surgery has significantly transformed the field of surgical treatment objective (STO) and execution. This innovative combination incorporates tactile rapid prototype (RP) models that offer enhanced visualization and planning capabilities, as well as accurately designed surgical guides that facilitate precise execution of the surgical procedure. Surgical guides play a pivotal role in orthognathic surgery by providing an unparalleled means of visualizing and planning the surgical intervention. By creating tactile 3D models, surgeons can gain a comprehensive understanding of the anatomical structures involved and can assess the potential outcomes of the proposed procedure. This visual representation assists in accurate preoperative planning, allowing for increased precision and reduced surgery time. Furthermore, the ability to manipulate and examine the model from various angles ensures a more thorough analysis of the proposed treatment, ultimately resulting in improved surgical outcomes.
Figure 6.
The utilization of surgical guides in orthognathic surgery has significantly transformed the field of surgical treatment objective (STO) and execution. This innovative combination incorporates tactile rapid prototype (RP) models that offer enhanced visualization and planning capabilities, as well as accurately designed surgical guides that facilitate precise execution of the surgical procedure. Surgical guides play a pivotal role in orthognathic surgery by providing an unparalleled means of visualizing and planning the surgical intervention. By creating tactile 3D models, surgeons can gain a comprehensive understanding of the anatomical structures involved and can assess the potential outcomes of the proposed procedure. This visual representation assists in accurate preoperative planning, allowing for increased precision and reduced surgery time. Furthermore, the ability to manipulate and examine the model from various angles ensures a more thorough analysis of the proposed treatment, ultimately resulting in improved surgical outcomes.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).