Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Owners’ participation
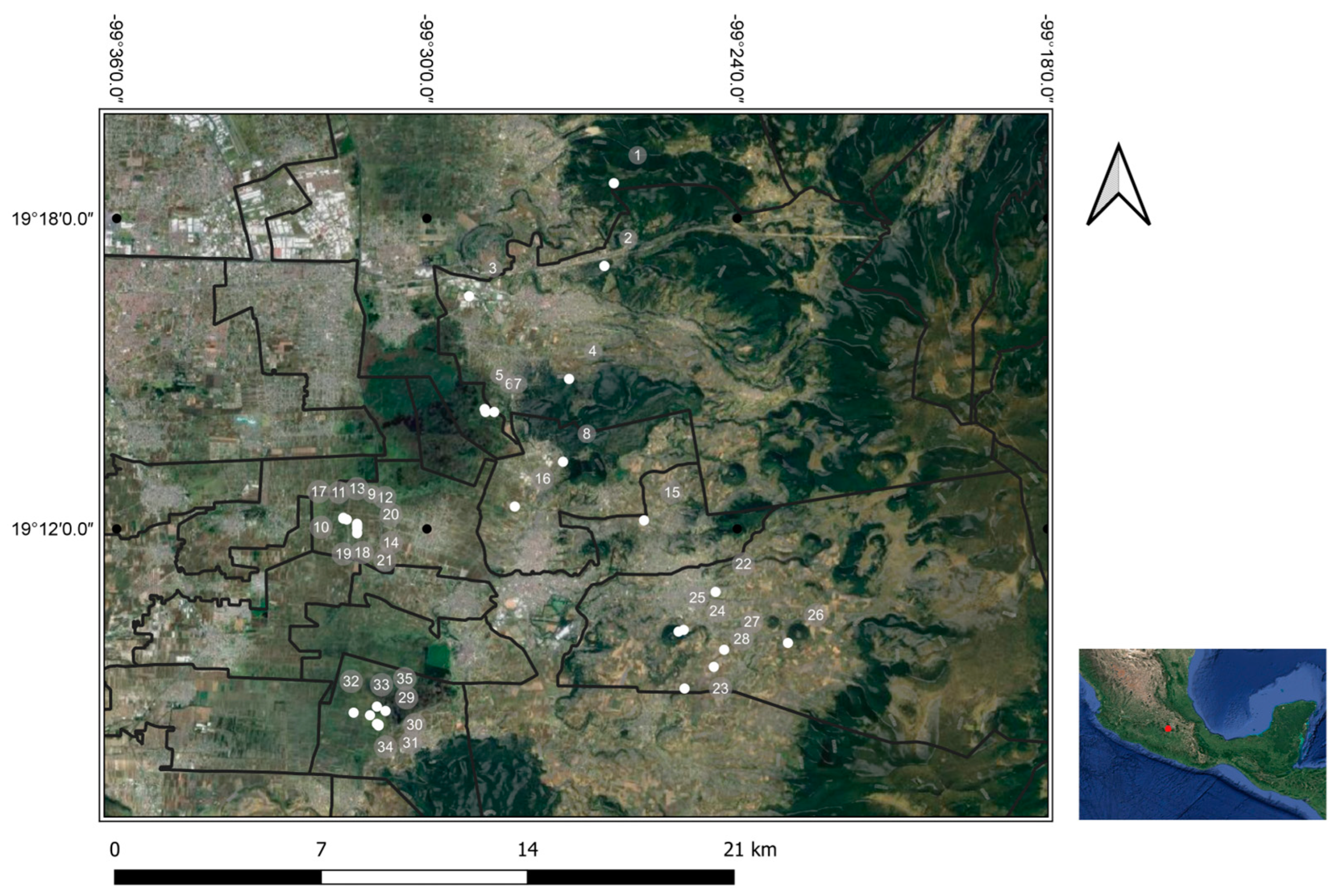
2.3. Geographic distribution of selected groups
2.4. Serological screening
2.5. Statistical analysis
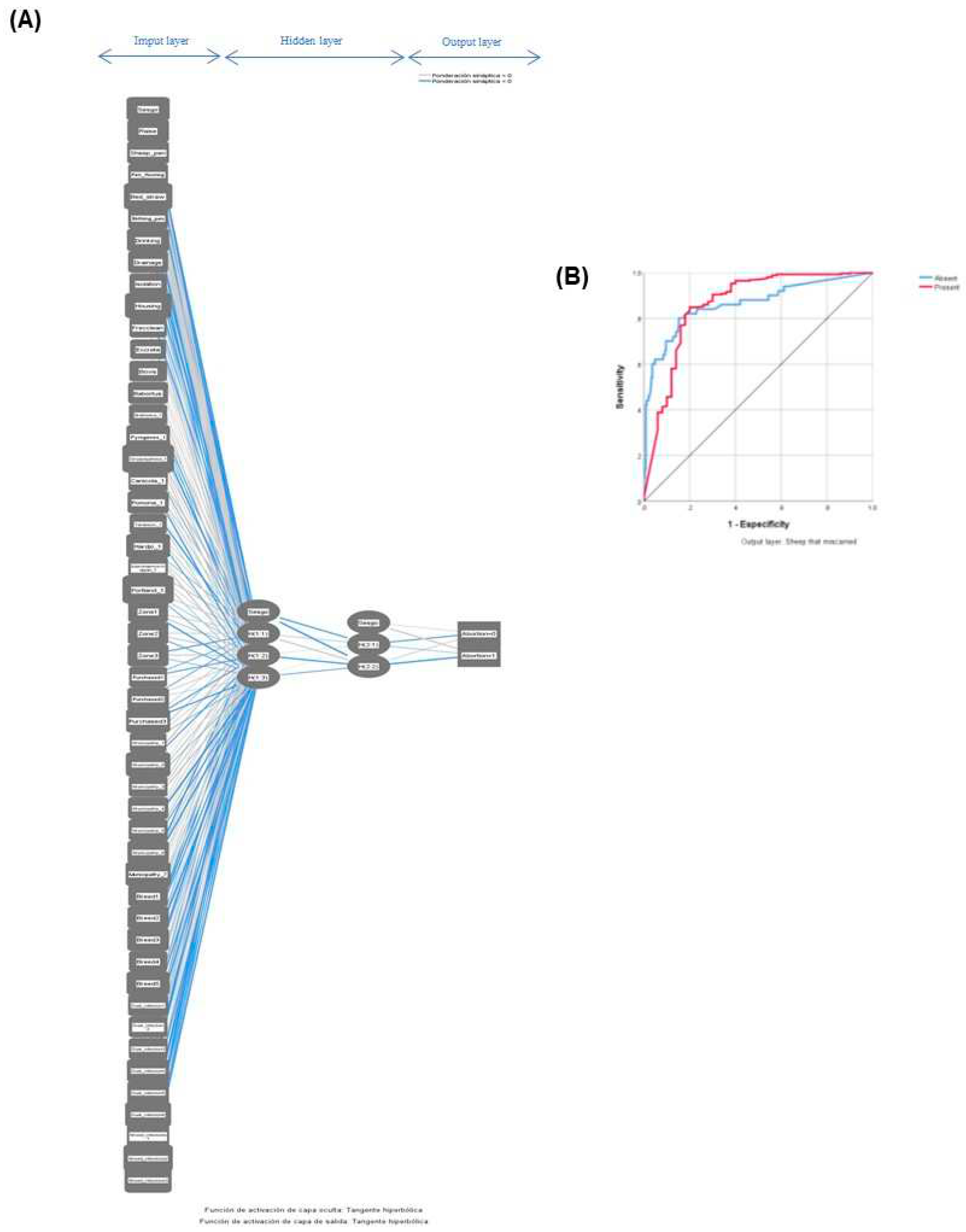
2.5.1. Artificial neural networks model (ANNm)
2.5.2. Generalized Linear Model (GLM)
3. Results
3.1. Places of sampling
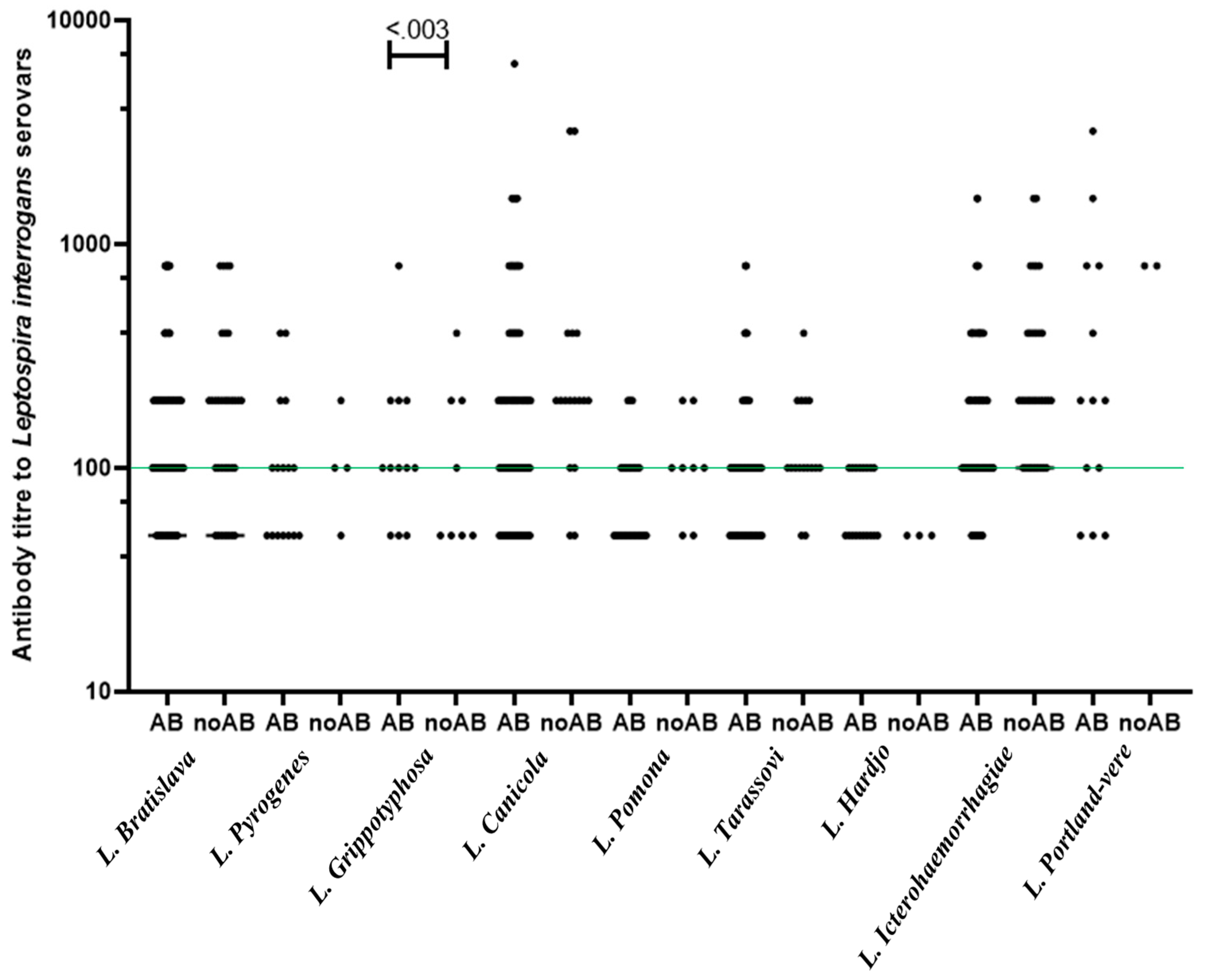
3.2. Antibody prevalence of multi-pathogens and history of abortion
3.3. Validation of the multilayer perceptron algorithm for abortion in ewes
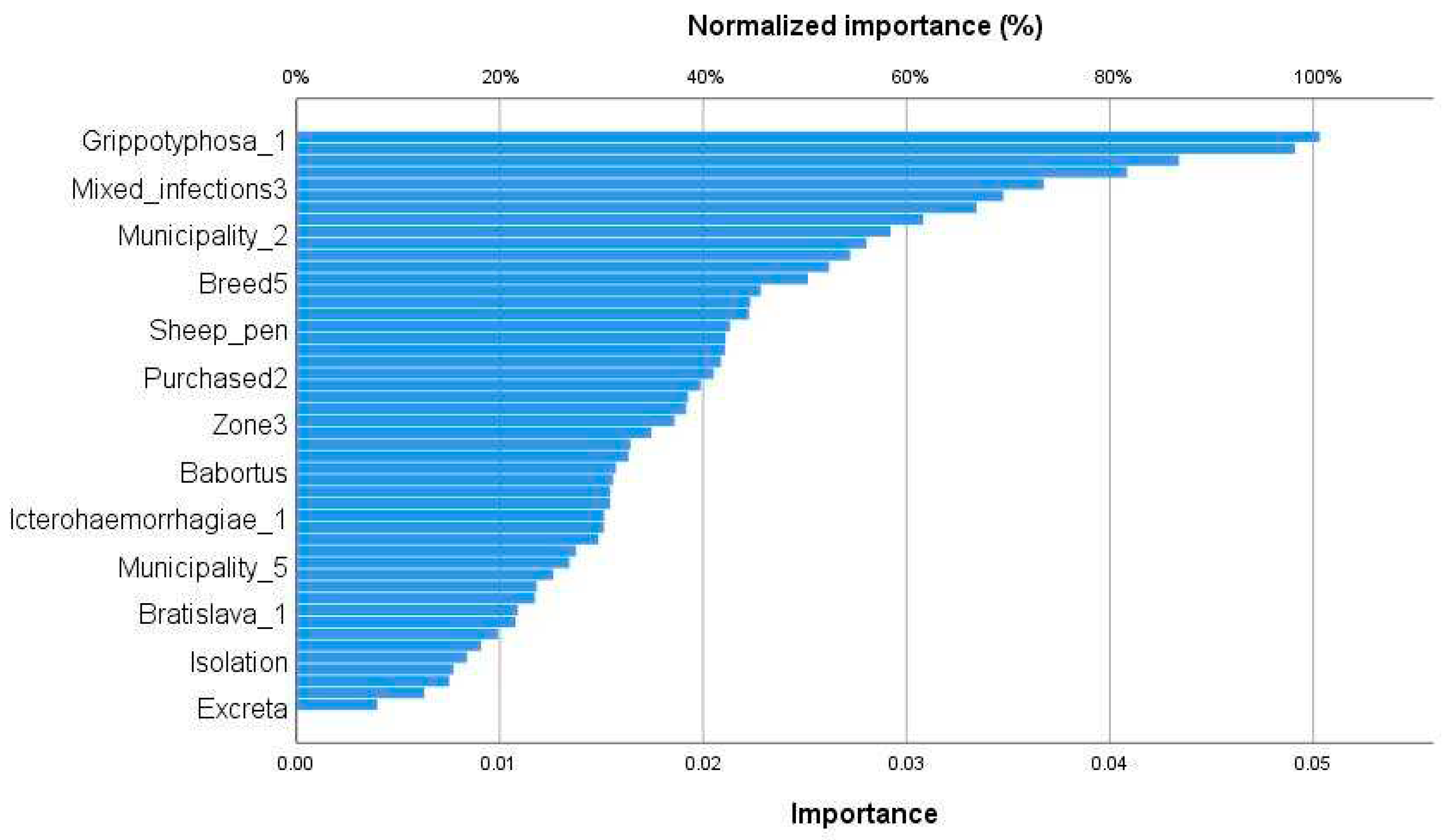
3.4. Determinants of infectious abortion in ewes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
1. SHEEP FLOCK DATA:
2. SHEEP YARD DESIGN AND CONSTRUCTION:
3. HEALTH AND MANAGEMENT:
4. CLINICAL FEATURES
| No. | Abortion/Stillbirth, jaundiced/Haemorrhagic fetus | Haemoglobinuria | Jaundice | Haemorrhages | Anaemia | Conjunctivitis | Mastitis (Black udder) | Panic | Depression or sleepy sickness |
| Diseases/syndromes | Serious problem | Moderate problem | Present but no problem | Non-existent |
| Infertility | ||||
| Bearing troubles (Prolapse of vagina) | ||||
| Lambing difficulties | ||||
| Mastitis (Black udder) | ||||
| Diarrhea in lambs | ||||
| Arthritis in lambs | ||||
| Lamb deaths during first two weeks of life | ||||
| Facial eczema | ||||
| Pneumonia | ||||
| Salmonellosis (Diarrhea) | ||||
| Pinkeye | ||||
| Pregnancy toxaemia |
References
- Guernier, V.; Allan, K. J.; Goarant, C. Advances and challenges in barcoding pathogenic and environmental Leptospira. Parasitology 2018, 145, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Hagan, J. E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira M., S.; et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO. Report of the second meeting of the leptospirosis burden epidemiology reference group. (2011).
- Levett, P. N. Leptospirosis: a forgotten zoonosis? Clin. and Appl. Immunol. Rev. 2004, 4, 435–448. [Google Scholar] [CrossRef]
- Comia, I.; Madureira, A.C.; Schooley, R.T.; Vieira, M.L.; Noormahomed, E.V. Molecular Detection of Leptospira spp. in Rodents Trapped in the Mozambique Island City, Nampula Province, Mozambique. E.C. Microbiol. 2018, 14, 813-821.
- Gamage, C.D.; Sato, Y.; Kimura, R.; Yamashiro, T.; Toma, C. Understanding leptospirosis eco-epidemiology by environmental DNA metabarcoding of irrigation water from two agro-ecological regions of Sri Lanka. PLoS Negl. Trop. Dis. 2020, 14, e0008437. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P. R.; Hagan, J. E.; Costa, F.; Calcagno, J.; Kane, M.; Martinez-Silveira, M. S. , et al. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl. Trop. Dis. 2015; 9, e0004122. [Google Scholar]
- Dorsch, M.A.; Francia, M.E.; Tana, L.R.; González, F.C.; Cabrera, A.; Calleros, L.; Sanguinetti, M.; Barcellos, M.; Zarantonelli, L.; Ciuffo, C.; Maya, L.; Castells, M.; Mirazo, S.; da Silva Silveira, C.; Rabaza, A.; Caffarena, R.D.; Doncel Díaz, B.; Aráoz, V.; Matto, C.; Armendano, J.I.; Salada, S.; Fraga, M.; Fierro, S. and Giannitti, F. Diagnostic Investigation of 100 Cases of Abortion in Sheep in Uruguay: 2015–2021. Front. Vet. Sci. 2022,9,904786. [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife-Livestock-Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Abdul Mutalip, M.H.; Mahmud, M.A.F.; Lodz, N.A.; Yoep, N.; Muhammad, E.N.; Ahmad, A.; Hashim, M.H.; Muhamad, N.A. Environmental risk factors of leptospirosis in urban settings: a systematic review protocol. BMJ. Open. 2019, 9, e023359. [Google Scholar] [CrossRef]
- Suwannarong, K.; Soonthornworasiri, N.; Maneekan, P.; Yimsamran, S.; Balthip, K.; Maneewatchararangsri, S.; Saisongkorh, W.; Saengkul, C.; Sangmukdanun, S.; Phunta, N.; Singhasivanon, P. Rodent-Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand. Vet. Sci. 2022, 9, 85. [Google Scholar] [CrossRef]
- Mendoza, M.V.; Rivera, W.L. Identification of Leptospira spp. from environmental sources in areas with high human leptospirosis incidence in the Philippines. Pathog. Glob. Health 2019, 113, 109–116. [Google Scholar] [CrossRef]
- Sun, J. Dirty environment for adult life: The bad, the good, the unknown. Genes & Diseases 2016,3,167-168. [CrossRef]
- Li, Y.; Baldridge, M.T. Modelling human immune responses using microbial exposures in rodents. Nat. Microbiol. 2023, 8, 363–366. [Google Scholar] [CrossRef]
- Aikowe, J.O.; Mazancová, J. Barriers to Water Access in Rural Communities: Examining the Factors Influencing Water Source Choice. Water 2021, 13, 2755. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Trop. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef]
- Arteaga-Troncoso, G.; Jiménez-Estrada, J.; Montes de Oca, R.; López-Hurtado, M.; Luna-Alvarez, M. , et al. Seroprevalence and risk factors associated with within-flock transmission of Leptospira interrogans in transhumant farming systems in Mexico. Epidemiology & Infection 2015,143,2894-2902. [CrossRef]
- Jiménez-Estrada, J. M.; Escobedo-Guerra, M. R.; Arteaga-Troncoso, G.; López-Hurtado, M.; De Haro-Cruz, M. D. J.; Jiménez, R. M. D. O. , & Guerra-Infante, F. M. Detection of Chlamydophila abortus in sheep (Ovis aries) in Mexico. American Journal of Animal and Veterinary Sciences 2008,3, 91-95. [CrossRef]
- A Guide to Designing a Sheep Handling Unit. Teagasc – Agriculture and Food Development Authority of Ireland 2020. Available online: https://www.teagasc.ie/media/website/publications/2020/A-Guide-to-Designing-a-Sheep-Handling-Unit.pdf (accessed on 18, March 2023).
- INEGI, 2017. Carta de Uso del Suelo y Vegetación, Serie VI, escala 1: 250 000. INEGI, México, 2014.
- INEGI, 2005. Carta de Uso del Suelo y Vegetación, Serie III, escala 1: 250 000 (Continuo Nacional). INEGI, México, 2005.
- World Health Organization. Chapter 3.1.12. Leptospirosis. In OIE Terrestrial Manual 2021. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.01.12_LEPTO.pdf (accessed on 18, March 2023).
- Ahasan, M.S.; Rahman, M.S.; Rahman, A.K.M.A.; et al. Bovine and Caprine Brucellosis in Bangladesh: Bayesian evaluation of four serological tests, true prevalence, and associated risk factors in household animals. Trop. Anim. Health Prod. 2017, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.A.; Pinho, F.A.; Batista, J.F. , et al. Risk factors and detection of Brucella ovis in naturally infected rams and ewes. Rev. Bras. Cienc. Vet. 2021,28:48-52.
- Xavier, M.N.; Sant'Anna, F.M.; Silva, T.M.A.; Costa, E.A.; Moustacas, V.S.; Merlo, F.A.; Carvalho Júnior, C.A.; Dasso, M.G.; Mathias, L.A.; Gouveia, A.M.G.; Lage, A.P.; Santos, R.L. A comparison of two agar gel immunodiffusion methods and a complement fixation test for serologic diagnosis of Brucella ovis infection in experimentally infected rams. Arq. Bras. Med. Vet. Zootec. 2011, 63, 1026–1021. [Google Scholar] [CrossRef]
- Wilson, K.; Livingstone, M.; Longbottom, D. Comparative evaluation of eight serological assays for diagnosing Chlamydophila abortus infection in sheep. Vet. Microbiol. 2009, 135, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A. Building and Applying Logistic Regression Models. In: An Introduction to Categorical Data Analysis. Hoboken, NJ: John Wiley & Sons, 2002, pp. 137–172.
- Alemayehu, G.; Mamo, G.; Alemu, B.; Desta, H.; Tadesse, B.; Benti, T.; Bahiru, A.; Yimana, M. and Wieland, B. Causes and Flock Level Risk Factors of Sheep and Goat Abortion in Three Agroecology Zones in Ethiopia. Front. Vet. Sci. 2021, 8, 615310. [Google Scholar] [CrossRef]
- Azócar-Aedo, L. Basic Aspects and Epidemiological Studies on Leptospirosis Carried Out in Animals in Chile: A Bibliographic Review. Trop. Med. Infect. Dis. 2023, 8, 97. [Google Scholar] [CrossRef]
- Guesmi, K.; Kalthoum, S.; Mamlouk, A.; et al. Seroprevalence of zoonotic abortive diseases and their associated risk factors in Tunisian sheep. BMC Vet. Res. 2023, 19, 50. [Google Scholar] [CrossRef]
- Shawaqfah, M. ; Almomani. F. Forecast of the outbreak of COVID-19 using artificial neural network: Case study Qatar, Spain, and Italy. Results Phys. 2021,27,104484. [CrossRef]
- Wang, L.; Zhang, Y.; Wang, D.; Tong, X.; Liu, T.; Zhang, S.; Huang, J.; Zhang, L.; Chen, L.; Fan, H. and Clarke, M. Artificial Intelligence for COVID-19: A Systematic Review. Front. Med. 2021,8,704256. [CrossRef]
- Abnaroodheleh, F.; Emadi, A.; Dadar, M. Seroprevalence of brucellosis and chlamydiosis in sheep and goats with history of abortion in Iran. Small Ruminant Research 2021, 202, 106459. [Google Scholar] [CrossRef]
- Abdelfattah, S.; Kotb, A.; Eman, R.; Yaser, M.H.; Alamery, S. Seroprevalence and molecular characterization of Brucella species in naturally infected cattle and sheep. Prev. Vet. Med. 2019, 171, 104756. [Google Scholar] [CrossRef]
- Ebid, M.; El Mola, A.; Salib, F. Seroprevalence of brucellosis in sheep and goats in the Arabian Gulf region. Vet. World 2020, 13, 1495–1509. [Google Scholar] [CrossRef]
- Almeida, D.S.; Paz, L.N.; de Oliveira, D.S.; Silva, D.N.; Ristow, P.; Hamond, C. , et al. Investigation of chronic infection by Leptospira spp. in asymptomatic sheep slaughtered in slaughterhouse. PLoS ONE 2019, 14, e0217391. [Google Scholar] [CrossRef]
- Haji Hajikolaei, M.R.; Rezaei, S.; Ghadrdan Mashhadi, A.R.; et al. The role of small ruminants in the epidemiology of leptospirosis. Sci. Rep. 2022, 12, 2148. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Turchi, B.; Pinzauti, P.; Cerri, D.; & Fratini, F. Epidemiology of leptospirosis in North-Central Italy: Fifteen years of serological data (2002–2016). Comparative Immunology, Microbiology and Infectious Diseases 2019,65, 14–22. [CrossRef]
- Karpagam, K.B.; Ganesh, B. Leptospirosis: a neglected tropical zoonotic infection of public health importance-an updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Mexican Ministry of Health. Ministry of Health, General Directorate of Epidemiology. Mexico SUIVE Epidemiology Bolletin. https://www.gob.mx/salud/acciones-y-programas/historico.boletinepidemiologico. (Accessed 24 March 2023). 24 March.
- López-Robles, G.; Córdova-Robles, F.N.; Sandoval-Petris, E.; & Montalvo-Corral, M. Leptospirosis at human-animal-environment interfaces in Latin-America: drivers, prevention, and control measures. Biotecnia 2021,23,89-100. [CrossRef]
- Tique, V.; Mattar, S.; Miranda, J.; Oviedo, M.; Noda, A.; Montes, E.; Rodriguez, V. Clinical and Epidemiological Status of Leptospirosis in a Tropical Caribbean Area of Colombia. Biomed. Res. Int. 2018,29:6473851. [CrossRef]
- Franc, K.A.; Krecek, R.C.; Häsler, B.N.; Arenas-Gamboa, A.M. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health 2018, 11, 18. [Google Scholar] [CrossRef]
- Harran, E.; Pinot, A.; Kodjo, A.; Djelouadji, Z.; Le Gudayer, M.; Sionfoungo Daouda, S.; Groud, K.; Lattard, V.; Ayral, F. Identification of Pathogenic Leptospira kirschneri Serogroup Grippotyphosa in Water Voles (Arvicola terrestris) from Ruminant Pastures in Puy-de-Dôme, Central France. Pathogens 2023, 12, 260. [Google Scholar] [CrossRef]
- Viana, M.; Mancy, R.; Biek, R.; Cleaveland, S.; Cross, P.C.; Lloyd-Smith, J.O.; Haydon, D.T. Assembling Evidence for Identifying Reservoirs of Infection. Trends Ecol. Evol. 2014, 29, 270–279. [Google Scholar] [CrossRef]
- Luna, A.; Moles, C.; Salazar, G.; Nava, V. and Gavaldón, R. La Leptospirosis canina y su problemática en México. Revista de Salud Animal 2008,30, 1-11.
- Martínez-Barbabosa, I.; Alpizar-Sosa, E.A.; Gavaldón-Rosas, D.G.; Moles-Cervantes, L.P.; Cárdenas, M.G.; García-González, R.; Shea, M. and Fernández-Presas, A. M. Canine Leptospirosis Serology in Southern Mexico City. Open Journal of Medical Microbiology 2016, 6, 171–180. [Google Scholar] [CrossRef]
- Miotto, B.A.; Guilloux, A.G.A.; Tozzi, B.F.; Moreno, L.Z.; da Hora, A.S.; Días, R.A.; Heinemann, M.B.; Moreno, A.M.; Filho, A.F.S.; Lilenbaum, W.; Hagiwara, M.K. Prospective study of canine leptospirosis in shelter and stray dog populations: Identification of chronic carriers and different Leptospira species infecting dogs. PLoS One 2018, 13, e0200384. [Google Scholar] [CrossRef]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; Iqbal Yatoo, M.; Gupta, V.K.; Dhama, K.; Sah, R.; Chaicumpa, W. Bovine brucellosis - a comprehensive review. Vet. Q. 2021, 1, 41, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Gomard, Y.; Dellagi, K.; Goodman, S.M.; Mavingui, P.; Tortosa, P. Tracking Animal Reservoirs of Pathogenic Leptospira: The Right Test for the Right Claim. Trop. Med. Infect. Dis. 2021,30,6,205. [CrossRef] [PubMed]
- Guedes, I.B. , Souza, G.O., Castro, J.F., Cavalini, M.B., de Souza Filho, A.F. and Heinemann, M.B. Usefulness of the Ranking Technique in the Microscopic Agglutination Test (MAT) to Predict the Most Likely Infecting Serogroup of Leptospira. Front. Vet. Sci. 2021,8,654034. [CrossRef]
- Rajapakse, S.; Rodrigo, C.; Handunnetti, S.M.; Fernando, S.D. Current immunological and molecular tools for leptospirosis: diagnostics, vaccine design, and biomarkers for predicting severity. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 2. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Status | Zone 1 (slopes with elevations up to 2600 masl) |
Zone 2 (slopes with elevations between 2600 to 2800 masl) |
Zone 3 (slopes with elevations above 2800 masl) |
|||
| Prev. (%) | 95% CI | Prev. (%) |
95% CI | Prev. (%) |
95% CI | ||
| Leptospira spp. | Abortion | 38.6 | 32.9-44.3 | 13.7 | 9.7-17.7 | 5.3 | 2.7-7.9 |
| No abortion | 36.7 | 24.5-48.9 | 18.3 | 8.5-28.1 | 5 | 0.1-10.5 | |
| Smooth Brucella spp.* | Abortion | 48.4 | 42.6-54.2 | 18.6 | 14.1-23.1 | 7 | 4.0-10.0 |
| No abortion | 75.0 | 64.0-86.0 | 26.7 | 15.5-37.9 | 5 | 0.1-10.5 | |
| B. ovis | Abortion | 6.7 | 3.8-9.6 | 5 | 2.5-7.5 | 0 | N.D. |
| No abortion | 6.7 | 3.8-9.6 | 1.7 | 0.1-3.2 | 0 | N.D. | |
| C. abortus | Abortion | 17.5 | 13.1-21.9 | 4.6 | 2.2-7.0 | .7 | 0.1-1.6 |
| No abortion | 8.3 | 1.3-15.3 | 10 | 2.4-17.6 | 5.0 | 0.1-10.5 | |
|
Leptospira-C. abortus dual reactivity |
Abortion | 15.6 | 11.4-19.8 | 10.4 | 2.6-18.1 | 6.7 | 3.8-9.6 |
| No abortion | 16.7 | 7.3-26.1 | 14.3 | 5.4-23.2 | 20 | 9.9-30.1 | |
| Leptospira-smooth Brucella spp. dual reactivity | Abortion | 55.1 | 49.3-60.9 | 50.7 | 44.3-55.9 | 50 | 44.2-55.8 |
| No abortion | 66.7 | 54.8-78.6 | 47.6 | 35-60.2 | 40 | 27.6-52.4 | |
| Leptospira-B. ovis dual reactivity | Abortion | 5.3 | 2.6-7.9 | .7 | .67-.73 | 0 | N.D. |
| No abortion | 6.7 | .4-13 | 1.7 | .1-4.9 | 0 | N.D. | |
| Smooth Brucella spp.-B. ovis dual reactivity | Abortion | 5.6 | 2.9-8.3 | .7 | .67-.73 |
0 | N.D. |
| No abortion | 6.7 | .4 - 13 | 1.7 | .1-4.9 | 0 | N.D. | |
| Smooth Brucella spp.-C. abortus dual reactivity | Abortion | 12.3 | 8.5-16.1 | 3.5 | 1.4-5.6 | .7 | .67-.73 |
| No abortion | 8.3 | 1.3-15.3 | 5 | .1-10.5 | 1.7 | .1-4.9 | |
| B. ovis-C. abortus dual reactivity | Abortion | 1.1 | .1-2.3 | 0 | N.D. | 0 | N.D. |
| No abortion | .4 | .1-8.9 | 0 | N.D. | 0 | N.D. | |
| Leptospira-smooth Brucella spp.-B. ovis-C. abortus multi-reactivity | Abortion | .7 | .67-.73 | 0 | N.D. | 0 | N.D. |
| No abortion | 1.7 | .1-4.9 | 0 | N.D. | 0 | N.D. | |
| Serovars (type strain) | Status | Zone 1 (slopes with elevations up to 2600 masl) |
Zone 2 (slopes with elevations between 2600 to 2800 masl) |
Zone 3 (slopes with elevations above 2800 masl) |
|||
| Prev. (%) | 95% CI | Prev. (%) |
95% CI | Prev. (%) |
95% CI | ||
| Icterohaemorrhagiae (Palo Alto) |
Abortion | 37.2 | 31.5-42.9 | 12.3 | 8.5-16.1 | 5.3 | 2.7-7.9 |
| No abortion | 20 | 9.1-30.9 | 10 | 1.8-18.2 | 2 | .1-5.8 | |
| Bratislava (Jez-Bratislava) |
Abortion | 27 | 21.8-32.2 | 8.4 | 5.2-11.6 | 3.5 | 1.3-5.6 |
| No abortion | 14 | 4.5-23.4 | 8 | .6-15.4 | 2 | .1-5.8 | |
| Canicola (Hond Utrecht IV) |
Abortion | 12.3 | 8.5-16.1 | 4.2 | 2.5-7.5 | 3.2 | 1.1-5.2 |
| No abortion | 10 | 1.8-18.2 | 3 | .01-7.6 | 1 | .1-3.7 | |
| Tarassovi (Mitis Johnson) |
Abortion | 10.2 | 6.7-13.7 | 2.5 | 2.2-7.0 | 1.4 | .02-2.8 |
| No abortion | 9 | 1.2-16.8 | 3 | .01-7.6 | 2 | .1-5.8 |
|
| Pyrogenes (Salinem) |
Abortion | 3.5 | 1.4-5.6 | 1.8 | 2.6-18.1 | .4 | .1-1.1 |
| No abortion | 4 | .01-9.3 | 0 | N.D. | 0 | N.D. | |
| Pomona (Pomona) |
Abortion | 2.8 | .8-4.7 | 1.1 | .1-2.3 | .4 | .1-1.1 |
| No abortion | 4 | .01-9.3 | 2 | .1-5.8 | 0 | N.D. | |
| Canicola (Portland-vere) |
Abortion | 2.5 | .7-4.3 | .7 | .67-.73 | .4 | .1-1.1 |
| No abortion | 2 | .1-5.8 | 0 | N.D. | 0 | N.D. | |
| Hardjo (H-89) |
Abortion | 6 | .4-3.8 | 2 | .67-.73 |
0 | N.D. |
| No abortion | 0 | N.D. | 0 | N.D. | 5 | .1-10.9 | |
| Grippotyphosa* (Moskva V) |
Abortion | 1.4 | .02-2.8 | 1.4 | .02-2.8 | .4 | .1-1.1 |
| No abortion | 2 | .1-5.8 | 0 | N.D. | 2 | .1-5.8 | |
| Covariable | S.E. | (95%CI) | P value | |
| Intercept | -4.63 | .42 | -20.1 – 10.8 | .56 |
| Grippotyphosa serovar. | -1.98 | .05 | -3.7 – -.27 | .02 |
| Ewe gave birth on a bed of straw. | -4.77 | .02 | -7.1 – -2.46 | <.001 |
| Lerma municipality. | -17.31 | .19 | -24 – -10.32 | <.001 |
| Santiago Tianguistenco. | -8.57 | .11 | -12.4 – -4.7 | <.001 |
| Hardjo serovar. | 18.95 | .03 | 17.9 – 20 | <.001 |
| Leptospira-Brucella ovis co-infection. | -18.27 | .08 | -21 – -15.5 | <.001 |
| The water supply for animals. | 7.43 | .08 | 4.5 – 10.31 | <.001 |
| Sheep pens constructed with metal sheets and untreated wood. | 2.21 | .05 | .29 – 4.14 | .024 |
| Suffolk breed. | -7.6 | .13 | -12.42 – -2.79 | .002 |
| Slopes with elevations above 2800 masl. | -9.2 | .12 | -13.7 – -4.8 | <.001 |
| Slopes with elevations between 2600 to 2800 masl. | 7.9 | .1 | 4.2 – 11.7 | <.001 |
| Municipality of Chapultepec. | -8.7 | .12 | -12.9 – -4.5 | <.001 |
| Dirt and concrete pen flooring. | 9.6 | .12 | 5.4 – 13.9 | <.001 |
| Municipality of Texcalyacac. | -1.8 | .05 | -3.6 – -.001 | .05 |
| Municipality of Calpulhuac | -16.5 | .19 | -23.4 – -9.53 | <.001 |
| Brucella ovis. | 17.8 | .07 | 3 – 10.29 | <.001 |
| Tarassovi serovar. | -1.4 | .03 | -2.5 – -.42 | .006 |
| Municipality of Xalatlaco. | 29.1 | .11 | 24.9 – 33.2 | <.001 |
| Agglomeration of excreta near the housing pen. | 6.7 | .1 | 3.0 – 10.3 | <.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





