1. Introduction
Chile is number three worldwide in tailings impoundments, with a total of 757 in 2022, 112 of which are active, 467 inactive, 173 abandoned, and 5 under construction [
1]. The management of abandoned tailings, which in some cases are close to towns, is completely necessary to mitigate the risks associated with heavy metals pollution. In the case of Chile, this task oversees Municipalities which have a limited budget to handle this problem. Emblematic examples of problematic mine tailings impoundments are Andacollo, Illapel, and La Higuera where the population suffers from irreversible pollution [
2].
Copper mine tailings are the residual waste material produced during the extraction and processing of copper ore and often contain significant amounts of copper, as well as other potentially harmful substances such as heavy metals (Ni, Hg, Zn, Pb, Cr), metalloids (As) and chemicals used in the mining process [
3,
4]. Every year is discharged more than 10 billion tons of tailings from mining activities [
5].
Among the biological treatment, one with low cost is phytoremediation. It is a process that uses plants to remove, transfer, stabilize, or destroy pollutants in the soil and/or water. In Chile, where copper mining is a significant industry, native or endemic flora can be used for phytoremediation to address pollution. Several studies have shown the usefulness of this technique to achieve successful results [
6,
7]. Some studies related to the ability of certain Chilean species to phytoremediate tailings and proposals for the management of abandoned mine tailings in Chile have been carried out by [
8,
9,
10,
11].
Mining tailings contain several heavy metals, among other contaminants, which pose significant environmental and health risks, as they can contaminate surrounding soil, water, and air, and eventually make their way into the food chain. Pollutants can travel long distances in the environment, and they persist for many years. In the case of copper and lead, the primary and big concern is their leaching into nearby soil, groundwater, and surface water sources [
12] when they are improperly stored or managed. Lead can enter the human body through dust ingestion and wildlife via dust and food chain transfer [
13], it is a neurotoxic metal and can produce several harmful effects, especially in children. The Canadian soil quality guidelines for lead establish a concentration of 140 mg·kg
-1 for residential lands, and the Dutch standard establishes 85 mg·kg
-1 as the target value and 530 mg·kg
-1 as the intervention value [
14,
15]. Copper is a metal that can be toxic in high concentrations to aquatic life and in humans, exposure to high levels of copper can cause a range of health problems, including gastrointestinal distress, liver, and kidney damage. Long-term exposure to low levels of copper may also contribute to the development of chronic diseases, such as Alzheimer's and Parkinson's [
16]. Dutch Soil Standards established 36 mg·kg
-1 as the target value and 190 mg·kg
-1 as the intervention value for copper [
14].
Efforts to remediate contaminated sites, such as re-vegetating tailings storage areas to reduce erosion and the leaching of contaminants, can help to mitigate the risks associated with heavy metals’ contamination from mine tailings [
17,
18]. Some species as
Schinus polygamus and
Atriplex deserticola have been studied to determine their ability to accumulate copper, both species can accumulate over 1.2 g·kg
-1 in leaves [
19]. Studies in Chilean soil related to lead phytoremediation have used the species
Atriplex halinus reaching concentrations around 360 mg·kg
-1 in roots and between 32 and 42 mg·kg
-1 in aerial parts [
20]. Other native plants studied for phytoremediation of an industrial area in Valparaíso were
Oenothera picensis,
Sphaeralcea velutina, and
Argemone subfusiformis, all of them present phytoremediation factors which suggest their potential for removal Pb [
21].
This work studies the stabilization of copper and lead from copper mine tailings through ex-situ experiments using two species Lycium chilense and Haplopappus foliosus and it complements previous research with the same species and Ni, Zn and Cr. The experiments with a duration of seven months are focused on the determination of phytoremediation factors as bioconcentration and translocation factors with removal efficiency, in tailings enriched with liquid organic fertilizer.
2. Materials and Methods
2.1. Plants
The experiments will be carried out with two species:
Haplopappus foliosus and
Lycium chilense; which can be found in the central zone of Chile. Both species have ornamental value and no frost resistance.
Haplopappus foliosus is an endemic shrub distributed between Atacama and O’Higgins Regions.
Lycium chilense Miers ex Bertero is a native shrub that can be found between Valparaíso and Maule Regions [
22].
2.2. Tailings samples preparation
The tailings samples were provided by Minera Las Cenizas (32°28′16.1″ S, 71°05′00.2″ W). Their characterization is presented in
Table 1. They were ground and homogenized using a ball mill and ASTM N°18 mesh. The pH was determined according to 9045D EPA Method [
23].
2.3. Process parameters
The translocation and bioconcentration factors were obtained to evaluate the process. The translocation factor (TF) indicates the ability to mobilize the target element from the roots to the shoots. The bioconcentration factor (BCF)
roots indicate the ability to accumulate the target element in the roots, and (BCF)
shoots indicate the ability to accumulate it in shoots.
A TF<1 indicates an excluder character of the plant for the target metal and a TF>1 corresponds to a plant with the ability to translocate the target metal from roots to shoots. In the case of BCF, a value higher than 1 corresponds to a plant with the ability to accumulate the target metal in a determined part of the plant [
24,
25]. The removal efficiency will also be calculated to obtain the total mass of the target metal removed from tailings according to Equation (3).
where C
i and C
f are the initial and final concentrations of the element in the tailings.
2.4. Phytoremediation tests
Ex-situ experiments were carried out at the Universidad Técnica Federico Santa María (33°02′05” S, 71°35′43″ W). The plants were planted in 3,100 g of dry tailings contained in pots and liquid commercial organic stimulant from the algae
Ascophyllum nodosum and water was supplied weekly. After seven months, the plants were analyzed for copper, lead, arsenic, and molybdenum following the same procedure described by Lazo et al., 2023 [
26].
2.5. Statistical treatment of the data
Five specimens of each species were analyzed for copper and lead content. Three samples of roots, aerial parts, and tailings from each pot were taken for each specimen. The mean value and standard deviation were calculated and the comparison between species was made using an analysis of variance (p < 005). Tukey’s test was carried out to compare different species.
3. Results
The concentration of copper in tailings is higher than the intervention value of 190 mg·kg
-1 indicated in the Dutch list, the concentration detected in the sample is eight times higher. In the case of lead, its concentration in the tailings sample doubles the value in the Dutch list [
14].
The final concentration of copper, lead, arsenic, and molybdenum was determined in both species as can be seen from
Table 1. Blank samples were used in all cases, they correspond to the species planted in natural ground with the addition of water and organic fertilizer. The initial and final concentrations of lead and copper do not show significant differences.
Kumar et al., 2021 [
27] indicate as “satisfactory” a maximum concentration of copper in plant tissues of 30 mg·kg
-1 dry matter. For its part, Oorts, 2013 [
28] indicates the onset of Cu toxicity in aerial parts between 4 and 15 mg·kg
-1 dry matter. In roots, the critical concentrations are between 100 and 400 mgCu·kg
-1 dry mater [
29,
30]. Moreover, Kumar et al., 2019 [
31] suggest a permissible limit in soil a value of 20 mg·kg
-1, and the Dutch List [
14] indicates a target value for copper in soils of 36 mg·kg
-1 and an intervention value of 190 mg·kg
-1. All these values are surpassed in the aerial parts and roots of both species, as can be seen in
Table 1.
Haplopappus foliosus exhibits a concentration of Cu in roots (847 mg·kg
-1 dry matter) and aerial parts (262 mg·kg
-1 dry matter) higher than the concentrations measured in
Lycium chilense. The same trend was observed for Pb and Mo. However, the concentration of lead in roots is almost ten times its concentration in aerial parts. The concentration of lead in the sample exceeds the levels established for the Dutch list [
14] and Canadian standards [
15]. Arsenic concentrations were under the detection limit of the instrument in all cases. The value of molybdenum concentration is within the normal range.
4. Discussion
After seven months of plant growth, the concentration of metals in roots is higher than in aerial parts in both species, but higher ratios of roots/aerial concentrations were obtained with Lycium chilense compared to Haplopappus foliosus.
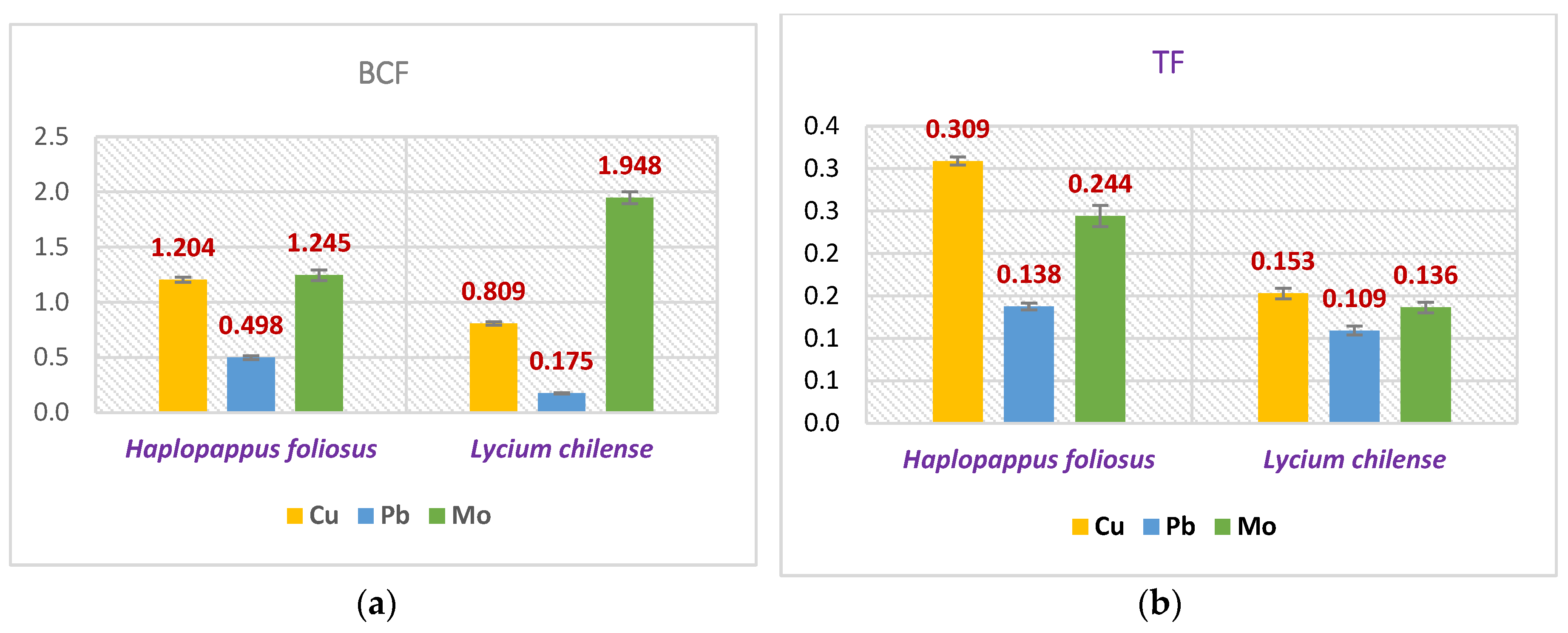
Figure 1a and b presents the phytoremediation factors which allow the evaluation of the usefulness of both species for remediation.
Figure 1 shows a BCF higher than 1 in the case of Cu and
Halopappus foliosus and Cu and Mo, and for Mo with
Lycium chilense. Neither of the two shows significant values for TF.
Removal efficiencies for Mo are 14.5% in the case of Haplopappus foliosus and 31.8% for Lycium chilense, in the same order the values for Pb are 18.1% and 14.7%, and for Cu 55.5% and 53.19%, respectively. In the case of Cu, the results have no significative difference, however, Lycium chilense doubles the removal efficiency for Mo and it presents a lower value (four percentage points) in comparison to Haplopappus foliosus.
In general, Haplopappus foliosus presents better performance, with the ability to concentrate the Cu and Pb in higher concentrations than Lycium chilense. Moreover, this species presents a bioconcentration factor higher than 1 for Cu.
It is necessary to look for how to improve these results by considering the species Haplopappus foliosus as an alternative for phytoremediation. The main advantage is to achieve a better chance of adaptation to the environmental conditions of north and central Chile because it is an endemic Chilean species.
5. Conclusions
Phytoremediation with native and endemic flora can be a low-cost alternative to the management of abandoned tailings in Chile. In this work two species Haplopappus foliosus and Lycium chilense were evaluated to determine their ability to remediate copper mine tailings, specifically, to stabilize or translocate lead and copper. The first of them presents a better performance because it can accumulate higher concentrations of Pb and Cu than the second one. The concentration of both metals exceeds the concentration established in the Dutch standard as the target value, however, has not the character of a hyperaccumulator species. A bioconcentration factor higher than 1 for Cu is obtained only with Haplopappus foliosus.
Author Contributions
Conceptualization, A.L. and P.L.; methodology, A.L and C.G..; formal analysis, A.L. and C.G..; investigation, A.L. and P.L.; resources, A.L.; data curation, R.O.; writing—original draft preparation, A.L.; writing—review and editing, H.H.; visualization, R.O.; supervision, A.L.; project administration, A.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID Fondecyt de Iniciación en Investigación 2020, grant [N°11200189, 2020], and the APC was funded by ANID Fondecyt de Iniciación en Investigación 2020, grant [N°11200189, 2020].
Acknowledgments
Andrea Lazo thanks ANID Fondecyt de Iniciación en Investigación 2020 N°11200189 and Grupo Minero Las Cenizas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sernageomin. Servicio Nacional de Geología y Minería (Chile). Available online: https://www.sernageomin.cl/datos-publicos-deposito-de-relaves/ (accessed on 18 September 2023).
- Cacciuttolo, C.; Atencio, E. Past, Present, and Future of Copper Mine Tailings Governance in Chile (1905–2022): A Review in One of the Leading Mining Countries in the World. Int. J. Environ. Res. Public Health 2022, 19, 13060. [Google Scholar] [CrossRef]
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean Prod. 2020, 263. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Zeyad, A.M.; De Azevedo, A.R.G.; Ahmed, H. U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16. [Google Scholar] [CrossRef]
- Menaga, D.; Saravanan, S. 7- Application of artificial intelligence in the perspective of data mining. Artificial Intelligence in Data Mining. Theories and Applications 2021, 133–154. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: a sustainable environmental technology for heavy metals decontamination. SN Appl. Sci 2021, 2021. 3, 286. [Google Scholar] [CrossRef]
- Azizi, M.; Faz, A.; Zornoza, R.; Martinez-Martinez, S.; Acosta, J.A. Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loids) and Rare Earth Elements, Plants 2023, 12. 2023, 12. [Google Scholar] [CrossRef]
- Lam, E.J. , Cánovas, M. , Gálvez, M.E., Montofré, I.L., Keith, B.F., Faz, A. Evaluation of the phytoremediation potential of native plants growing on a copper mine tailing in northern Chile, J. Geochem. Explor. 2017, 182. [Google Scholar] [CrossRef]
- Lam E.J., Montofré, I.; Ramírez, Y. Chapter 6: Mine tailings phytoremediation in arid and semiarid environments. In book: Phytorestoration of Abandoned Mining and Oil Drilling Sites 2021, Elsevier, pp 115-166. [CrossRef]
- Lazo, P.; Lazo, A. Assessment of Native and Endemic Chilean Plants for Removal of Cu, Mo and Pb from Mine Tailings. Minerals 2020, 10, 1020. [Google Scholar] [CrossRef]
- Lazo, A.; Lazo, P.; Urtubia, A.; Lobos, M.G.; Hansen, H.K.; Gutiérrez, C. An Assessment of the Metal Removal Capability of Endemic Chilean Species. Int. J. Environ. Res. Public Health 2022, 19, 3583. [Google Scholar] [CrossRef]
- Wang, P. , Sun, Z., Hu, Y., Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695. [Google Scholar]
- Kumar, A.; Kumar, A. ; M. M.S., C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Dutch Target and Intervention Values, version 2000 (the New Dutch List).
- Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health, Lead, Canadian Environmental Quality Guidelines, Canadian Council of Ministers of the Environment, 1999.
- Sereni, L.; Guenet, B.; Lamy, I. Mapping risks associated with soil copper contamination using availability and bio-availability proxies at the European scale. Environ. Sci. Pollut. Res. 2003, 30, 19828–19844. [Google Scholar] [CrossRef] [PubMed]
- Al-Lami, M.K.; Oustriere, N.; Gonzales, E. , Burken, J.G. Amendment-assisted revegetation of mine tailings: improvement of tailings quality and biomass production. Int J Phytoremediation 2019, 21, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Xie, L. , Van Zyl, D. Distinguishing reclamation, revegetation and phytoremediation, and the importance of geochemical processes in the reclamation of sulfidic mine tailings: A review. Chemosphere 2020, 252. [Google Scholar] [CrossRef]
- Ortiz-Calderon, C.; Alcaide, O.; Li Kao, J. Copper distribution in leaves and roots of plants growing on a copper mine-tailing storage facility in northern Chile. Rev. chil. de hist. nat. 2008, 81, 489–499. [Google Scholar] [CrossRef]
- Acuña, E. , Castillo, B., Queupuan, M. et al. Assisted phytoremediation of lead contaminated soil using Atriplex halimus and its effect on some soil physical properties. Int. J. Environ. Sci. Technol. 2021, 18, 1925–1938. [Google Scholar] [CrossRef]
- Salmani-Ghabeshi, S.; Fadic-Ruiz, X.; Miró-Rodríguez, C.; Pinilla-Gil, E.; Cereceda-Balic, F. Trace Element Levels in Native Plant Species around the Industrial Site of Puchuncaví-Ventanas (Central Chile): Evaluation of the Phytoremediation Potential. Appl. Sci. 2021, 11, 713. [Google Scholar] [CrossRef]
- Chileflora. Available online: https://www.chileflora.com/Shome.htm (accessed on 10 June 2023).
- U.S. Environmental Protection Agency. SW-846 Test Method 9045D: Soil and Waste pH. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-9045d-soil-and-waste-ph (accessed on 01 February 2023).
- Mellem, J.J.; Baijnath, H.; Odhav, B. Translocation and accumulation of Cr, Hg, As, Pb, Cu and Ni byAmaranthus dubius (Amaranthaceae) from contaminated sites. J. Environ. Sci. Health Part A 2009, 44, 568–575. [Google Scholar] [CrossRef]
- Nirola, R.; Megharaj, M.; Palanisami, T.; Aryal, R.; Venkateswarlu, K.; Naidu, R. Evaluation of metal uptake factors of native trees colonizing an abandoned copper mine—A quest for phytostabilization. J. Sustain. Min. 2015, 14, 115–123. [Google Scholar] [CrossRef]
- Lazo, P.; Lazo, A.; Hansen, H.K.; Ortiz-Soto, R.; Hansen, M.E.; Arévalo, F.; Gutiérrez, C. Removal of Heavy Metals from Mine Tailings in Central Chile Using Solidago chilensis Meyen, Haplopappus foliosus DC, and Lycium chilense Miers ex Bertero. Int. J. Environ. Res. Public Health 2023, 20, 2749. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Singh Sidhu, G. P.; Sharma, A.; Kjanna, K.; Kaur, P.; Shreeya Bali, A.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262. [Google Scholar] [CrossRef]
- Oorts, K. Copper. In: Alloway, B. (eds) Heavy Metals in Soils. Environmental Pollution; Springer, Dordrecht, 2013; Volume 22. [CrossRef]
- Pedersen, M. B.; Kjaer, C.; Elmegaard, N. Toxicity and bioaccumulation of copper to black bindweed (Fallopia convolvulus) in relation to bioavailability and the age of soil contamination. Arch. Environ. Contam. Toxicol. 2000, 39, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, A. R.; Menzies, N. W. The effect of copper toxicity on the growth and root morphology of Rhodes grass (Chloris gayana Knuth.) in resin buffered solution culture. Plant Soil 2005, 278, 341–349. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).