1. Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease affecting 0.5–2% of the general population[
1]. Although BAV can be an isolated disorder, it is often associated with serious valvular disorders, including aortic stenosis (AS), aortic regurgitation (AR), as well as aortic wall abnormalities such as aortopathy, aneurysm, and dissection. BAV can anatomically vary in terms of the number of commissures, cusps, and raphes. The association between variability of the phenotype and valvular and aortic morbidity has been discussed but remains controversial in many studies[
2]. Accurate assessment of the BAV valve morphology, particularly the presence of raphe and the burden of calcification, as well as the aorta morphology are clinically important for selecting an appropriate treatment and minimizing complications[
3].
Transthoracic echocardiography (TTE) is the standard diagnostic imaging modality for evaluating BAV patients[
4]. However, the operator’s skill, poor acoustic window, extensive calcifications in the valve, and patient body habitus may challenge the diagnostic accuracy of TTE[
4]. Retrospective electrocardiography (ECG)-gated multidetector computed tomography (MDCT) is considered to be more accurate than TTE for evaluating aortic valve morphology and the associated aortopathy by providing morphological information and functional cine imaging[
5]. Therefore, when TTE fails or is uncertain, cardiac computed tomography (CCT) is considered an alternative diagnostic imaging tool for the evaluation of aortic valve[
5].
To the best of our knowledge, most studies on the association between BAV morphology and patterns of valvular dysfunction as well as bicuspid aortopathy were based on the position and number of cusps as well as commissures [
6,
7], which were performed mostly using TTE in the Western population [
8]. Therefore, this study aimed to determine whether the presence or absence of a raphe is associated with various degrees of valvular dysfunction and different types of aortopathy using TTE and CCT in the Korean population, and to identify whether the presence or absence of the raphe is a determinant of valvular dysfunction.
2. Materials and Methods
This retrospective, single-institutional study was approved by the Institutional Review Board and Local Ethics Committee (KUMC-2020-02-014). The requirement for written informed consent was waived.
2.1. Patients
This study cohort included 312 BAV patients who underwent both CCT and TTE within four weeks from January 2008 to April 2019, without an intervening change in clinical status or cardiovascular events. Baseline clinical characteristics, cardiovascular risk factors, and cardiac evaluation results were collected from the medical and radiological records. CCT was performed for 2 different reasons. First, CCT was performed to evaluate coronary artery disease in patients with chest pain. Second, CCT was performed as a planned imaging study after echocardiography in patients with suspected or known aortic valvular heart disease. The purpose of CCT in these patients was to evaluate the preoperative coronary artery anatomy and stenosis, aortic valve morphology, aortic valve cusp calcification, and ascending aortic dimensions.
2.2. CT Imaging Protocols
A dual-source CT scanner (Somatom Definition; Siemens Medical Solutions, Forchheim, Germany) was used at our institution. To acquire good-quality cardiac imaging, patients with a pre-scan heart rate (HR) of > 65 beats per minute (bpm) without contraindications for beta-blockers were administered 50–100 mg of metoprolol orally for 1 hour to control the HR. All patients received 0.6-mg nitroglycerin sublingually 1 minute before examination to dilate the coronary arteries.
CCT scans were performed from 2 cm above the carina to the diaphragm, without including the entire aortic arch. The detector collimation was 2×32×0.6 mm, with a slice acquisition of 2×64×0.6 mm; the gantry rotation time was 330 ms, the pitch of 0.20–0.43 was adapted to the HR, and the tube voltages were 100 or 120 kV. A tube current-time product of 100–140 mAs per rotation was used for calcium scoring and 100–280 mAs per rotation for CCT. A non-enhanced ECG-gated CCT scan, prospectively triggered at 75% of the R-R interval, measured the coronary artery and aortic valve calcium scores. Except for patients with mean HRs > 80 bpm or those with arrhythmia, ECG-based tube current modulation was used for CCT. A full dose window of 20–70% of the cardiac cycle was used in patients with an HR ≤ 80 bpm.
The contrast agent was administered using the bolus-tracking technique. For all CT examinations, a dual-head power injector (Stellant D; Medrad, Indianola, PA, USA) administered the three-phase bolus at a rate of 4.5 mL/s. First, 70–80 mL of iopromide (Ultravist 370®; Bayer Healthcare, Berlin, Germany) was administered. Thereafter, 45 mL of a 70-to-30% blend of contrast medium and saline was added. Finally, 45 mL of saline was administered.
2.3. CT Image Reconstruction and Analysis
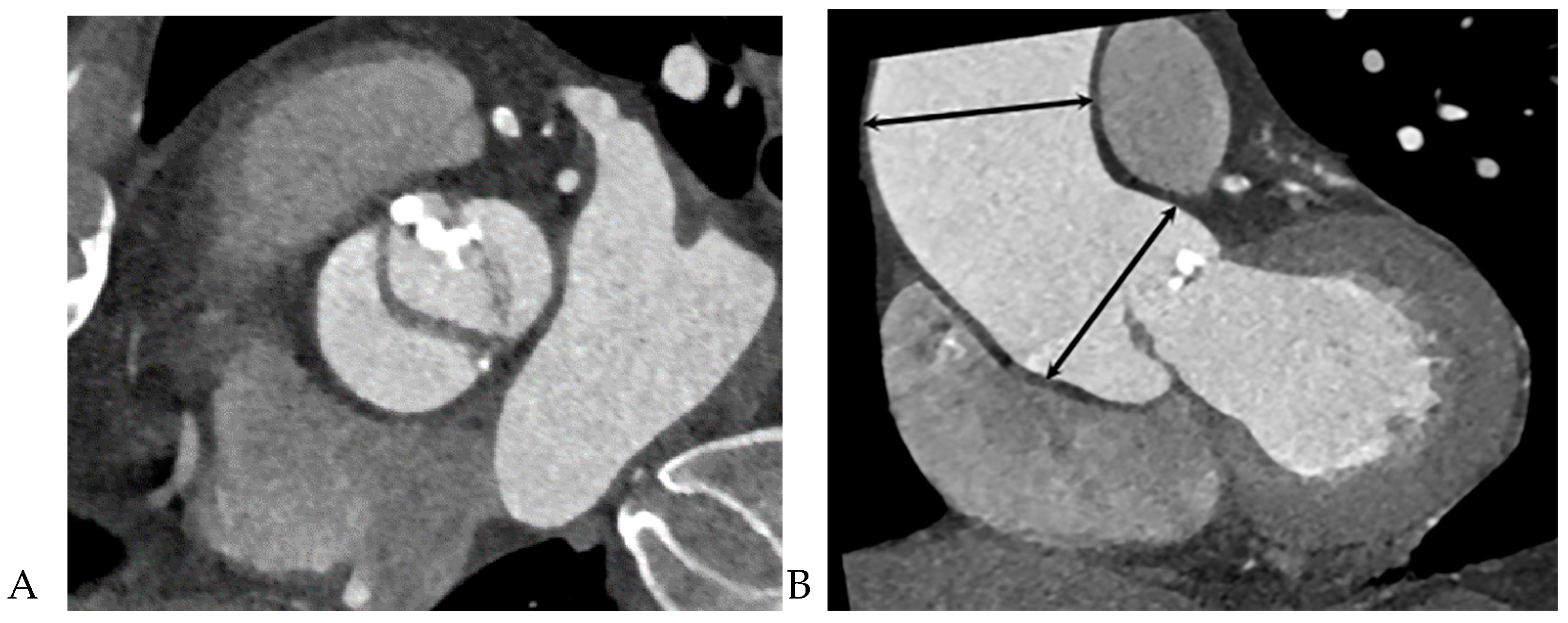
To assess BAV morphology and function, images were reconstructed parallel to the aortic valve plane with retrospective ECG gating every 10% of the cardiac cycle, from 0% to 90% of the R-R interval. The images were reconstructed with a slice thickness of 0.75 mm and an increment of 0.4 mm. Non-enhanced CCT images were reconstructed with a section thickness of 3 mm and a reconstruction interval of 1.5 mm to quantify the coronary artery and aortic valve calcium score. Oblique coronal and oblique sagittal planes of the aortic valve during the entire cardiac cycle were reconstructed for the morphological and functional aspects of the BAV, and double-oblique transverse images were reconstructed to measure the dimensions of the ascending aorta. Once the reconstruction was completed, all datasets were transferred to a post-processing workstation (Vitrea 2, Vital Images, Plymouth, MN, USA) to evaluate the phenotype and function of the aortic valve and to measure the ascending aortic dimensions.
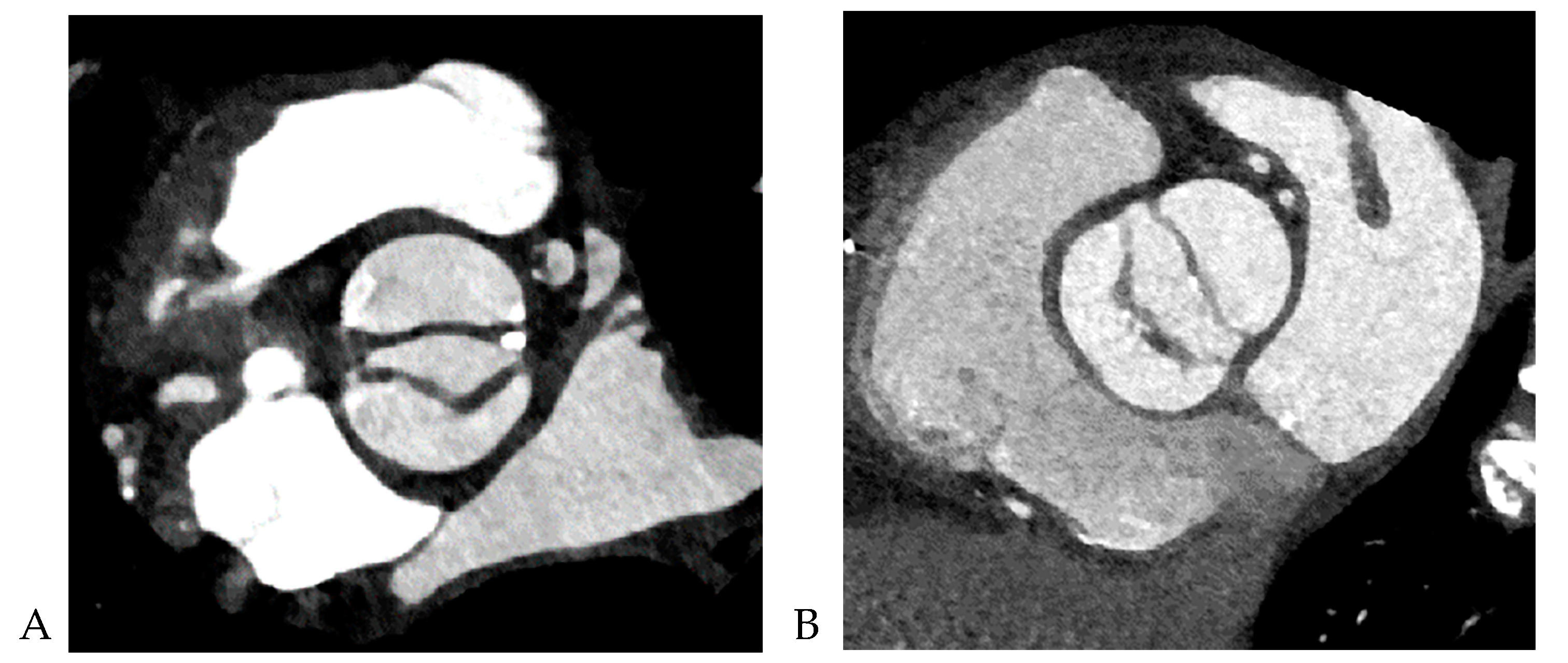
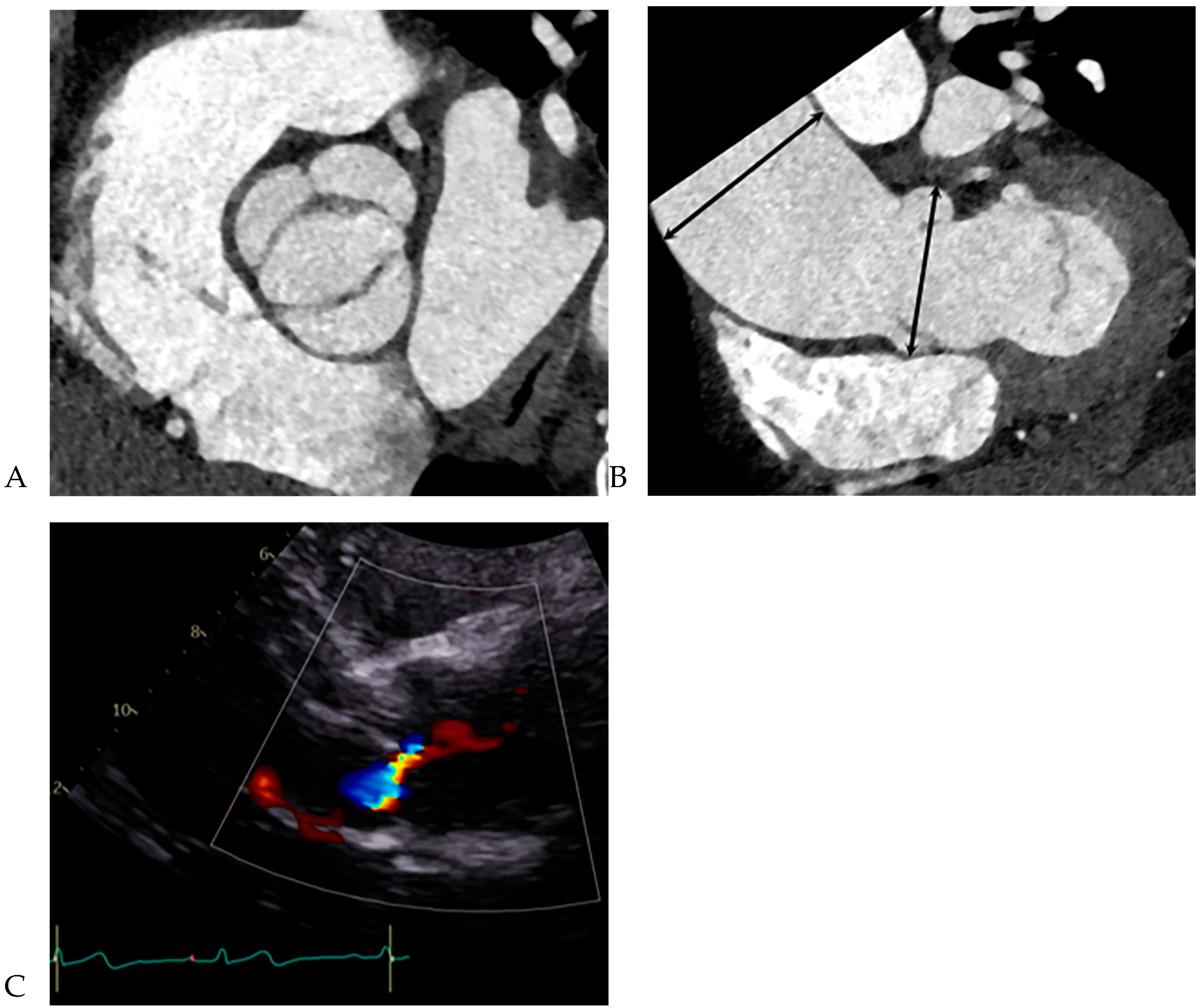
All CCT images were reviewed by a consensus of two radiologists with 16 and 5 years of experience, respectively, who were blinded to the clinical and surgical data. BAV was defined as the presence of two cusps and commissures in both systole and diastole. The following morphological variables were assessed: (1) presence or absence of raphe and (2) ascending aortic diameter. The term “raphe” defines the conjoined or “fused” area of two adjacent undeveloped leaflets that turn into a malformed commissure between both leaflets [
9]. BAV morphology was classified as raphe+ or raphe- according to the presence or absence of raphe (
Figure 1) [
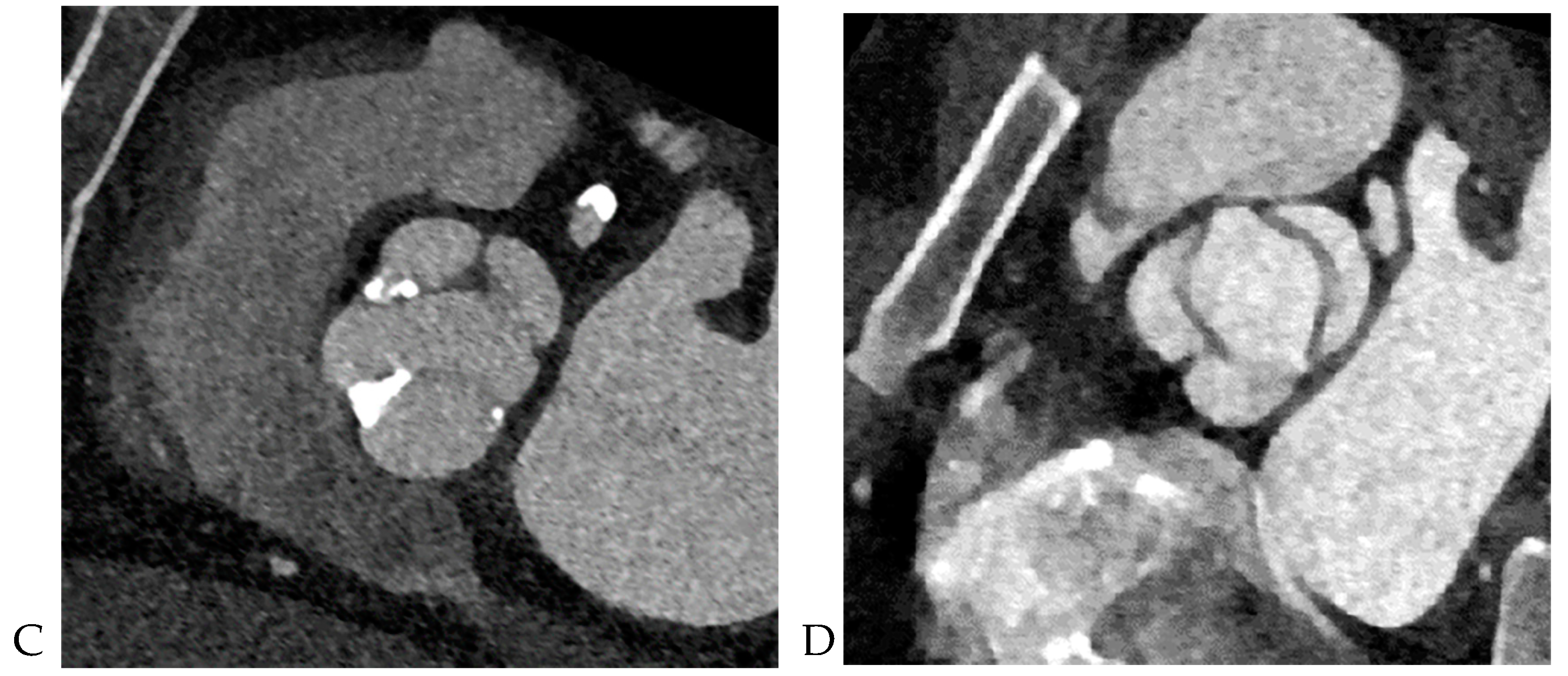
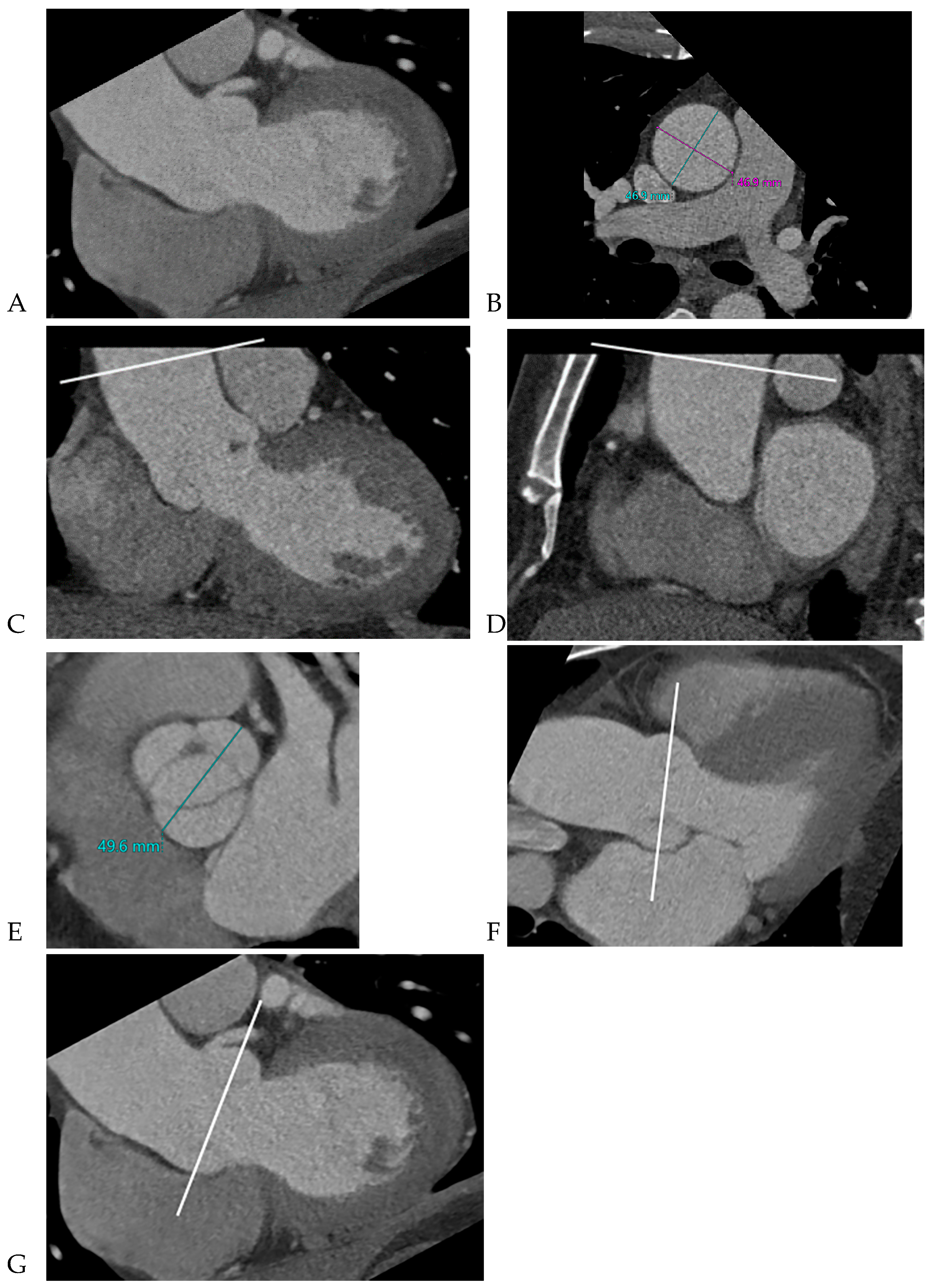
10]. The dimensions of the ascending aorta were measured at the sinus of Valsalva and tubular portion. Double-oblique coronal images of the ascending aorta were reconstructed at 10 % or 20 % of the cardiac cycle (early to mid-systole) to measure the tubular portion of the ascending aorta dimensions. The measurement of the maximum dimension of the aortic sinuses of Valsalva was performed using a double oblique transverse view of the aortic root at 10 % or 20 % of the cardiac cycle (
Figure 2). Aortopathy refers to progressive dilatation of the ascending aorta and is defined as indexed maximal aortic diameter exceeding 21 mm/m
2 of the body surface area (BSA) [
11]. Ascending aortic dilation configurations were slightly modified from the Fazel classification, which was assigned to four types depending on whether the segment of the vessel was exclusively or predominantly involved in dilatation: normal aorta, type 1, isolated dilation of the ascending aorta root at the level of the sinus of Valsalva [
12]; type 2, middle ascending dilatation at the level of the tubular ascending portion; and type 3, combined dilatation of the aortic root and mid-ascending aorta (
Figure 3) [
12]. Aortic aneurysms are defined as those with dimensions greater than 50 mm. The association between the presence of raphe and the risk of aortic valve dysfunction and aortopathy was also analyzed.
2.4. Echocardiographic evaluation
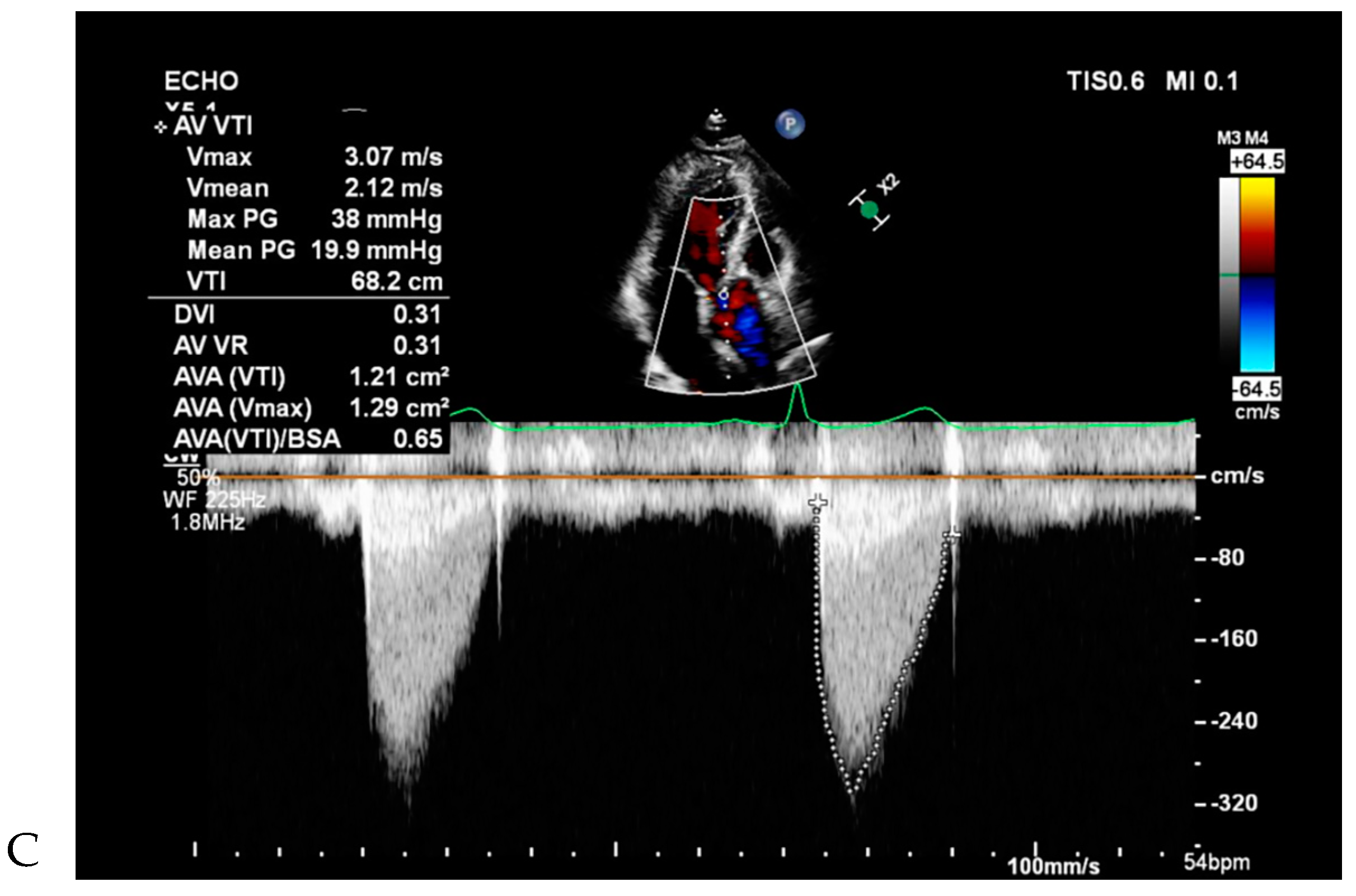
All patients underwent two-dimensional (2D) TTE using a Vivid 7 device (GE Healthcare, Wauwatosa, WI, USA) and an Acuson Sequoia C512 apparatus (Siemens, Erlangen, Germany) with 2.5–3.5 MHz imaging transducers. Aortic valve morphology and severity of aortic valve dysfunction were reviewed by cardiologists. Aortic valve function was recorded as normal, AR, AS, and combined AS and AR (ASR) using TTE [
13], and their severity was graded as none, mild, moderate, or severe, based on current recommendations [
14]. Left ventricular functional parameters were measured on 2D-TTE using a modified Simpson’s method.
2.5. Statistical Analyses
For descriptive statistical analysis, continuous variables are presented as mean ± standard deviation (SD) and categorical variables are presented as numbers or percentages. The chi-square or Fisher’s exact test for categorical data and unpaired two-tailed t-test for continuous data examined the significant differences between BAV patients with raphe+ vs. raphe-. The independent association between the presence of raphe and the severity and type of aortic valve dysfunction was evaluated using binary logistic regression analysis, with AR and AS being dependent variables and sex, age, and cardiovascular risk factors as independent variables. Statistical significance was set at P < 0.05. All data analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
A total of 312 BAV patients (mean [SD] age, 52.7 [14.3] years) were identified and enrolled in this study. No patient had two raphes. Of these, 227 were male (72.8%) and 185 (53.9%) were BAV-raphe+.
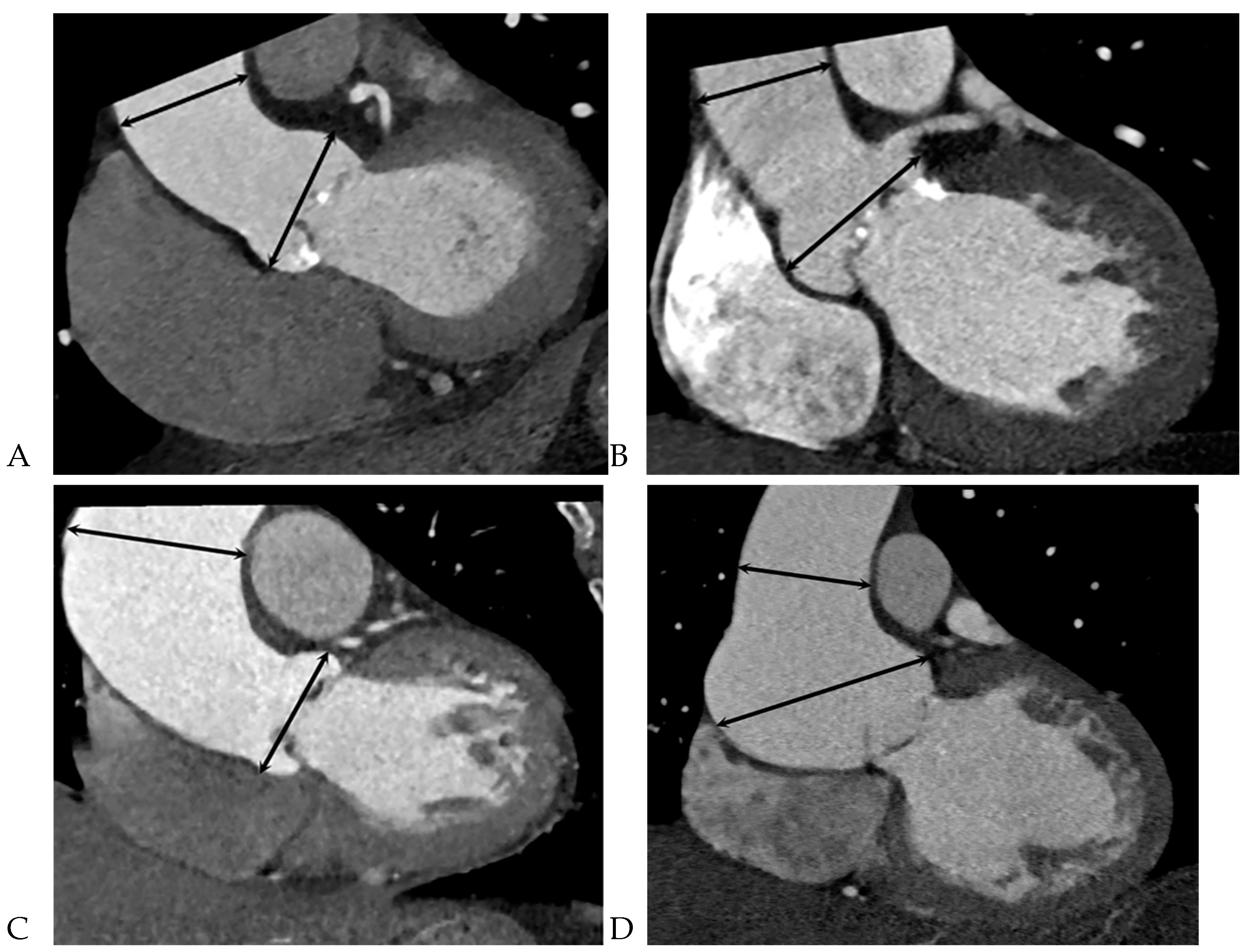
Table 1 summarizes the differences between BAV patients with raphe+ and raphe-. The BAV-raphe+ male patients had more complications (mean [SD], 156 [84.3] vs. 71 [55.9] years; P < 0.001), a larger BSA (1.8±0.2 vs. 1.7±0.2; P = 0.001), and were smokers (mean [SD], 80 [43.2] vs. 37 [29.1]; P = 0.011) as compared with BAV-raphe- patients. A larger aortic annulus, a larger sinus of Valsalva diameter, and smaller tubular portion were more often observed in BAV-raphe+ patients (29.8±4.3 mm vs. 26.7±3.8 mm, 40.2±6.4 mm vs. 38.7±5.6 mm,42.1±7 mm vs. 44.3±8.3 mm, respectively. P < 0.05). The difference persisted only in the tubular portion after indexing the proximal aortic diameter for BSA (26.6±5.6 mm vs. 24.2±5 mm, P = 0.000). Although morphology of the BAV was not associated with the aortopathy, the distribution of various aorta types was significantly different between the two groups (P = 0.021). In particular, isolated dilation of the aortic root (type 1) was the predominant form for BAV patients with raphe+ (13.5% [25 of 185] vs. 6.3% [8 of 127] in raphe-) (
Figure 4), but the prevalence of combined dilation of the ascending aorta and aortic root (type 3) was most common in BAV patients with raphe- (72.4% [92 of 127] in raphe- vs. 55.7% [103 of 185] in raphe+) (
Table 2,
Figure 5).
The presence of raphe was significantly correlated with the pattern of valvular dysfunction (P < 0.001). AS and severe AR were not the most common in BAV patients with raphe+, whereas severe AS and no AR were the most common patterns in BAV patients with raphe-. Dominant AR (defined as moderate to severe AR with no or mild AS) was present in 73 patients with raphe+ (39.5%) and 8 patients with raphe- (6.3%); dominant AS (defined as moderate to severe AS with no or mild AR) was present in 70 patients with raphe+ (37.8%) and 82 patients with raphe- (64.6%); balanced ASR (defined as moderate to severe both AS and AR) was present in 10 patients with raphe+ (5.4%) and 9 patients with raphe- (7.1%) (
Table 1).
In multivariate analysis, female sex (OR: 0.2, 95% CI: 0.1–0.4, P = 0.000), aortic valve calcium score (OR: 1, 95% CI: 1–1, P < 0.001), and end-diastole volume (EDV) (OR: 1, 95% CI: 1–1, P = 0.021) remained independently associated with AS. The presence of raphe was significantly associated with AS in the simple logistic regression analysis (P < 0.001) but not in the multiple logistic regression analysis (P > 0.5). In contrast, younger age (OR: 1, 95% CI: 0.9–1, P = 0.003), EDV (OR: 1.1, 95% CI: 1.0–1.1, P < 0.001), end-systole volume (ESV) (OR: 1, 95% CI: 0.9–1, P = 0.025), and the presence of raphe (OR: 3.7, 95% CI: 1.4–9.6, P = 0.007) remained independently associated with AR in both the simple and multivariable analyses (
Table 3).
4. Discussion
In this study, the presence of raphe in BAV patients was relatively lower than that in the Western population and was significantly associated with a higher prevalence of AR and isolated dilatation of the aortic root and middle ascending aorta. In addition, the presence of raphe was an independent determinant of AR. The absence of a raphe is associated with a high prevalence of AS and combined dilatation of the aortic root and middle ascending aorta.
Kong et al.[
15,
16]showed that the presence of raphe is of clinical and prognostic importance and was significantly correlated with valve dysfunction and aortic dilation. Most studies have focused on the specific BAV morphology according to the orientation of leaflet fusion and the number of commissures[
1,
15]; however, only few have focused on the presence or absence of raphe.
In previous Western studies, raphe has been described in almost 90% of BAV patients [
15]. In this study, the prevalence of BAV with raphe was only 60%, and interestingly, the absence of raphe was relatively more often observed than in other studies[
15,
16]. BAV in Korean patients with raphe was 52%, as indicated by Kang et al.[
16]. The hypothesis that the distribution of BAV morphologies differs between Korean and Western populations could be considered for the explanation of differences in study results[
17].
Clinically, the association between the presence of a raphe and aortic valve dysfunction is more relevant[
15]. However, few studies[
2,
18] have been conducted, particularly in Korea. A previous study showed that the prevalence of AS (44% vs. 56%) and AR (19% vs. 21%) was similar in BAV patients with raphe+ and raphe-[
16,
19]. However, some studies have indicated that the presence or absence of raphe is significantly associated with the type of valvular dysfunction[
15]. In this study, AR was more common in BAV patients with raphe+ and AS was more common in raphe-, the dominant AR was more common in BAV patients with raphe+ and the dominant AS was more common in raphe-. This result was confirmed in a previous study and was consistent with the study conducted by Lee et al. in Korea[
20]. A raphe is an avascular fibrous mass of connective tissue that protrudes from the aortic surface. Significant redundancy of a conjoined leaflet-associated prolapse may lead to AR, whereas relatively little redundancy of valve margins with restricted motion tends to lead to AS[
20]. The reasons for the discrepancies with Seviers et al.[
16]may be influenced by the type of patient and regional (ethnic) differences included in this study. In addition, severe AR was significantly higher among BAV patients with raphe+, followed by those with moderate AR. A previous study[
21]indicated that degenerative aortic valve disease occurs earlier in BAV patients than in patients with tricuspid aortic valves. Valvular motional fatigue and blood abnormalities with aging can cause damage to collagen fibers, calcification, as well as fiber thickening, and calcification progressing along the raphe can form a small coaptation defect, which causes AR.
The findings in this study are similar to those of previous studies, which showed that the female sex was significantly associated with BAV patients with AS, and AR was more common in the early period of a BAV patient’s life than AS[
20]. Moreover, we demonstrated that BAV along with raphe occurs more frequently in men than in women, whereas Kong et al.[
15,
16]indicated no such relationship. The small proportion of female patients and inter-ethnic differences may account for this discrepancy in the results.
Progressive dilatation of aorta may cause aortopathy, which is a risk factor associated with an increased risk of catastrophic aortic prognosis (such as dissection and rupture) [
22]. However, the relationship between BAV morphology and the presence or absence of raphe as well as aortopathy remains unclear. A larger multicenter, collaborative BAV registry has shown no difference in the prevalence of aortopathy between BAV-raphe+ and BAV-raphe-[
15]. In this study, the relationship between raphe and aortopathy was statistically significant. Genetic and hemodynamic factors are considered the underlying mechanisms of aortopathy in BAV patients[
23]. The four-dimensional flow cardiac magnetic resonance imaging confirmed that diverse BAV fusion patterns were associated with the direction of the post-valvular blood jet, flow displacement, flow angles, and regional wall shear stress, which correspond to the aortic wall and jet impingement positions, resulting in the dilation of different planes[
2]. However, no relationship between rotational flow and wall shear stress was found between BAV with or without raphe. Further studies should be conducted to identify these factors. In addition, aortic dimensions in this study were measured by cardiac CT, whereas the study measured TTE when the TTE measurement of the ascending aortic dimensions, especially the aortic root, was lower than that measured by ECG-gated CT angiography[
24] and phenotypic classification was impossible in up to 20% of patients with BAV using TTE alone[
16,
25].
This study had several limitations. First, the study was conducted in a single, tertiary medical center (institution) and was retrospective in design. Second, the enrolled population was heterogeneous due to different reasons for CCT examination. Accordingly, our CCT protocol did not scan the distal ascending aorta and aortic arch because the coronary arteries or/and aortic valve were the main sites of concern. Third, only 85 women (27.2%) were included and selection bias existed. Finally, the lack of tissue samples and hemodynamic data limited our ability to describe the relative influence of genetic factors on flow disturbances. Further prospective, multi-institutional studies with larger sample sizes are warranted.
5. Conclusions
In conclusion, the presence or absence of a raphe was significantly associated with the type of aortopathy and degree of valve dysfunction. Raphe+ BAV patients were independent determinants of AR.
Author Contributions
Conceptualization: Bo Hwa Choi, Hyun Keun Chee, Jun Seok Kim, Sung Min Ko; Data curation: Hyun Keun Chee, Jun Seok Kim, Sung Min Ko; Formal analysis: Bo Hwa Choi, Sung Min Ko; Funding acquisition: None; Investigation: Bo Hwa Choi, Hyun Keun Chee, Jun Seok Kim, Sung Min Ko; Methodology: Yu Zhang, Bo Hwa Choi, Hyun Keun Chee, Sung Min Ko; Project administration: Hyun Keun Chee, Jun Seok Kim, Sung Min Ko; Resources: Hyun Keun Chee, Jun Seok Kim, Sung Min Ko; Software: Bo Hwa Choi, Sung Min Ko; Supervision: Hyun Keun Chee, Jun Seok Kim; Validation: Bo Hwa Choi, Hyun Keun Chee, Jun Seok Kim; Visualization: Bo Hwa Choi, Sung Min Ko; Writing-original draft: Yu Zhang, Bo Hwa Choi, Sung Min Ko; Writing-review & editing: Hyun Keun Chee, Jun Seok Kim, Sung Min Ko
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board and Local Ethics Committee (KUMC-2020-02-014).
Informed Consent Statement
The requirement for written informed consent was waived (this is retrospective study).
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.
Acknowledgments
The authors thank the patients who participated in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.; Deng, W.; Lv, Q.; Li, Y.; Liu, T.; Xie, M. Aortic Dilatation in Patients With Bicuspid Aortic Valve. Frontiers in Physiology 2021, 12. [Google Scholar] [CrossRef]
- Hardikar, A.; Harle, R. Does the Leaflet Fusion Subtype Affect Pattern and Rate of Growth in BAV Aortopathy?: A Study of 102 BAV Aortopathy Cases With A Literature Review. Heart, Lung and Circulation 2021, 30, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.K.F.; Regeer, M.V.; Poh, K.K.; Yip, J.W.; van Rosendael, P.J.; Yeo, T.C.; Tay, E.; Kamperidis, V.; van der Velde, E.T.; Mertens, B.; et al. Inter-ethnic differences in valve morphology, valvular dysfunction, and aortopathy between Asian and European patients with bicuspid aortic valve. European heart journal 2018, 39, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.D.; Mann, R.D.; Kristenson, S.D.; Buck, R.M.; Mendoza, J.D.; Reese, J.M.; Grant, D.W.; Roberge, E.A. Transthoracic Echocardiography: Beginner's Guide with Emphasis on Blind Spots as Identified with CT and MRI. Radiographics 2021, 41, 1022–1042. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Agarwal, V.; Williams, M.L.; Wang, D.D.; Reardon, M.J.; Cavalcante, J.L.; Makkar, R.; Forrest, J.K. Imaging, Treatment Options, Patient Selection, and Outcome Considerations for Patients With Bicuspid Aortic Valve Disease. Journal of the Society for Cardiovascular Angiography & Interventions 2022, 1, 100506. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur J Cardiothorac Surg 2021, 60, 448–476. [Google Scholar] [CrossRef]
- Sievers, H.H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. The Journal of thoracic and cardiovascular surgery 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; III, J.H.B.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. Journal of the American College of Cardiology 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Ayşe Inci, Y.; Aysu Türkmen, K. Structural Insufficiency Anomalies in Cardiac Valves. Kaan, K., Ed. IntechOpen: Rijeka, 2018. p. Ch. 5. [CrossRef]
- Kang, J.W.; Song, H.G.; Yang, D.H.; Baek, S.; Kim, D.H.; Song, J.M.; Kang, D.H.; Lim, T.H.; Song, J.K. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC. Cardiovascular imaging 2013, 6, 150–161. [Google Scholar] [CrossRef]
- Fazel, S.S.; Mallidi, H.R.; Lee, R.S.; Sheehan, M.P.; Liang, D.; Fleischman, D.; Herfkens, R.; Mitchell, R.S.; Miller, D.C. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. The Journal of thoracic and cardiovascular surgery 2008, 135, 901–907. [Google Scholar] [CrossRef]
- Nappi, F.; Giacinto, O.; Lusini, M.; Garo, M.; Caponio, C.; Nenna, A.; Nappi, P.; Rousseau, J.; Spadaccio, C.; Chello, M. Patients with Bicuspid Aortopathy and Aortic Dilatation. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Shin, J.K.; Chee, H.K.; Kim, J.S.; Ko, S.M. Characteristics of aortic valve dysfunction and ascending aorta dimensions according to bicuspid aortic valve morphology. European radiology 2015, 25, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Suwa, K.; Rahman, O.A.; Bollache, E.; Rose, M.J.; Rahsepar, A.A.; Carr, J.C.; Collins, J.D.; Barker, A.J.; Markl, M. Effect of Aortic Valve Disease on 3D Hemodynamics in Patients With Aortic Dilation and Trileaflet Aortic Valve Morphology. Journal of magnetic resonance imaging : JMRI 2020, 51, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.K.; Delgado, V.; Poh, K.K.; Regeer, M.V.; Ng, A.C.; McCormack, L.; Yeo, T.C.; Shanks, M.; Parent, S.; Enache, R.; et al. Prognostic Implications of Raphe in Bicuspid Aortic Valve Anatomy. JAMA cardiology 2017, 2, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Guan, L.; Mu, Y. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction: A meta-analysis. Clin Cardiol 2021, 44, 1683–1691. [Google Scholar] [CrossRef]
- Sun, B.J.; Oh, J.K. Mid-term Clinical Outcomes in a Cohort of Asymptomatic or Mildly Symptomatic Korean Patients with Bicuspid Aortic Valve in a Tertiary Referral Hospita. 2019, 27, 105–118. [Google Scholar] [CrossRef]
- Koenraadt, W.M.; Grewal, N.; Gaidoukevitch, O.Y.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; Bartelings, M.M.; Holman, E.R.; Klautz, R.J.; Schalij, M.J.; Jongbloed, M.R. The extent of the raphe in bicuspid aortic valves is associated with aortic regurgitation and aortic root dilatation. Netherlands heart journal 2016, 24, 127–133. [Google Scholar] [CrossRef]
- Sievers, H.H.; Stierle, U.; Mohamed, S.A.; Hanke, T.; Richardt, D.; Schmidtke, C.; Charitos, E.I. Toward individualized management of the ascending aorta in bicuspid aortic valve surgery: the role of valve phenotype in 1362 patients. The Journal of thoracic and cardiovascular surgery 2014, 148, 2072–2080. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shim, C.Y.; Kim, D.; Cho, I.; Hong, G.R.; Ha, J.W.; Chung, N. Factors Determining Aortic Valve Dysfunction in Korean Subjects With a Bicuspid Aortic Valve. Am J Cardiol 2017, 119, 2049–2055. [Google Scholar] [CrossRef]
- Yang, L.T.; Lo, H.Y.; Lee, C.C.; Takeuchi, M.; Hsu, T.C.; Tsai, C.M.; Michelena, H.I.; Enriquez-Sarano, M.; Chen, Y.S.; Chen, W.J.; et al. Comparison Between Bicuspid and Tricuspid Aortic Regurgitation: Presentation, Survival, and Aorta Complications. JACC Asia 2022, 2, 476–486. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Prakash, S.K.; Milewicz, D.M.; Evangelista, A.; Enriquez-Sarano, M. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. International journal of cardiology 2015, 201, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Junco-Vicente, A.; del Río-García, Á.; Martín, M.; Rodríguez, I. Update in Biomolecular and Genetic Bases of Bicuspid Aortopathy. International Journal of Molecular Sciences 2021, 22, 5694. [Google Scholar] [CrossRef] [PubMed]
- Frazao, C.; Tavoosi, A.; Wintersperger, B.J.; Nguyen, E.T.; Wald, R.M.; Ouzounian, M.; Hanneman, K. Multimodality Assessment of Thoracic Aortic Dimensions: Comparison of Computed Tomography Angiography, Magnetic Resonance Imaging, and Echocardiography Measurements. J Thorac Imaging 2020, 35, 399–406. [Google Scholar] [CrossRef]
- Kang, J.-W.; Song, H.G.; Yang, D.H.; Baek, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Lim, T.-H.; Song, J.-K. Association Between Bicuspid Aortic Valve Phenotype and Patterns of Valvular Dysfunction and Bicuspid Aortopathy: Comprehensive Evaluation Using MDCT and Echocardiography. JACC: Cardiovascular Imaging 2013, 6, 150–161. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).