Submitted:
19 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Salonization in Cotton
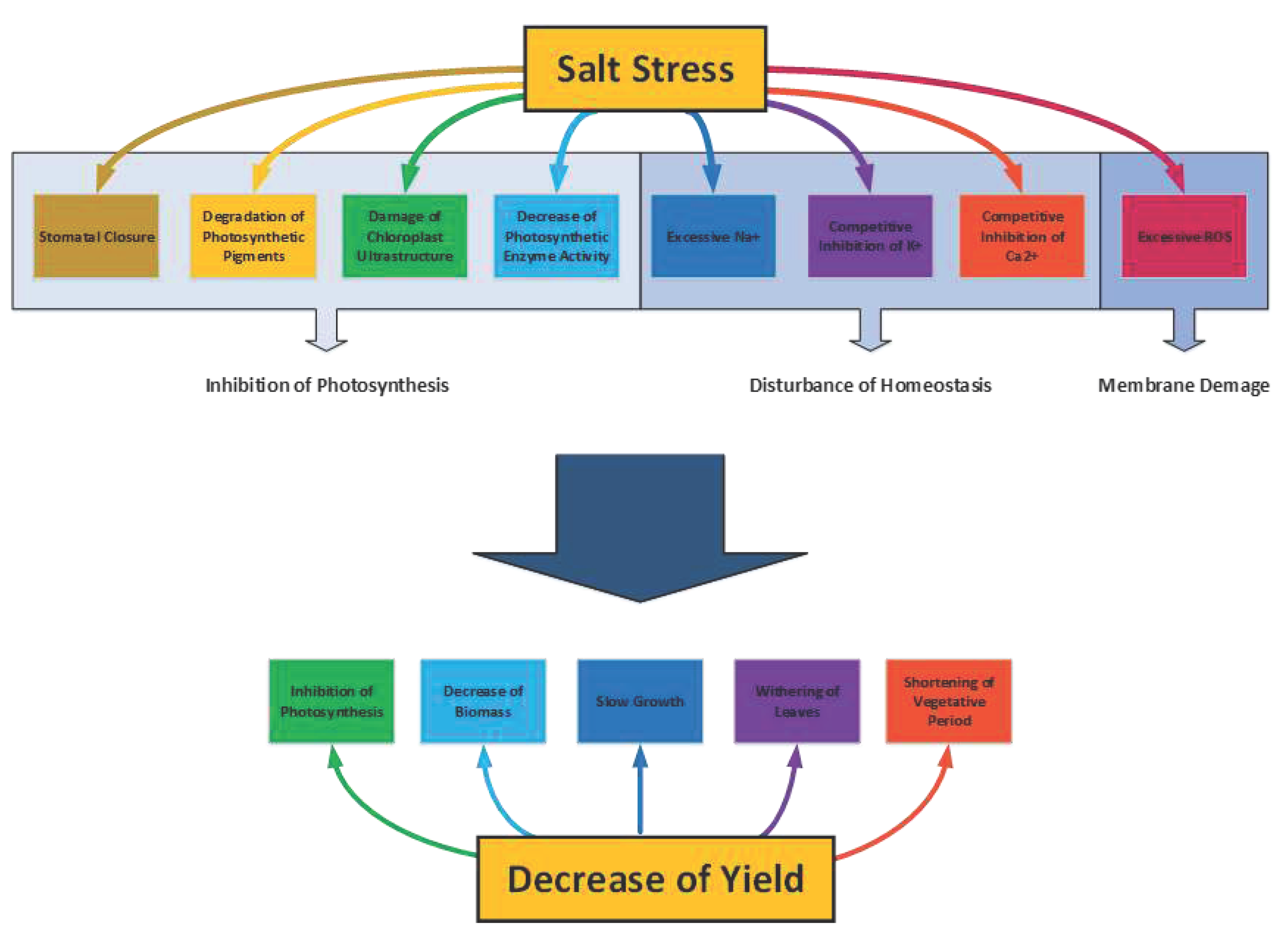
3. Salonization and Photosynthetic Activity in Cotton
3.1. Effect of Salinity on Stomatal Conductance
3.2. Salinity Influence on Total Chlorophyll Contents
3.3. Osmotic Pressure Affected by Salinity
3.4. Salt Stress Harms the Components of Photosynthesis
3.5. Salt stress Influence on Photosynthetic Enzyme Activities
4. Ion Homeostasis Disruption
5. Salt stress and Membrane Permeability
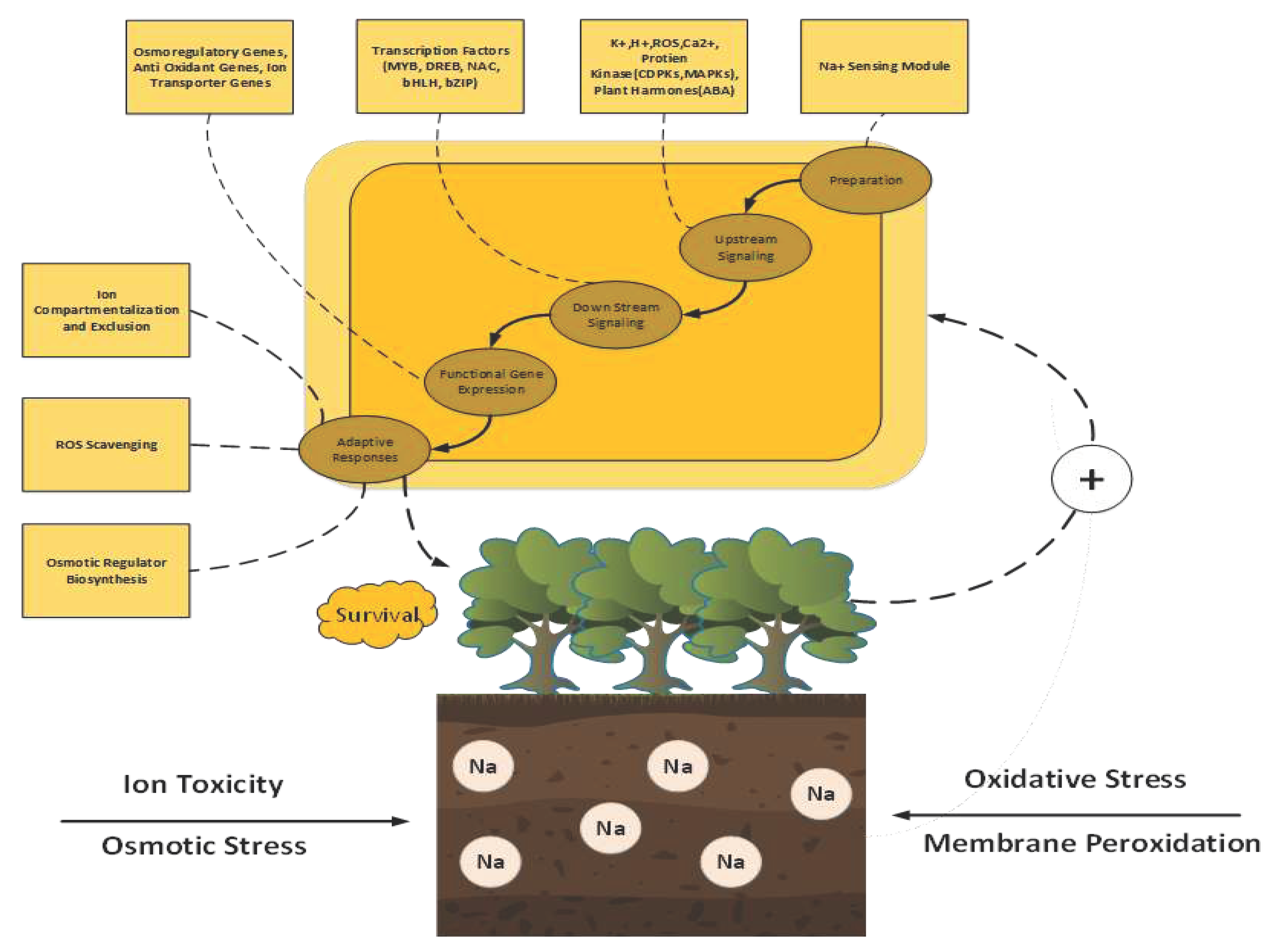
6. Characteristics of Salt Stress in Cotton
7. Cotton Tolerance to Salt Stress: Physiological and Biochemical Mechanisms
7.1. Osmotic and Salinity
7.2. Organic Substances
7.3. Inorganic Ions
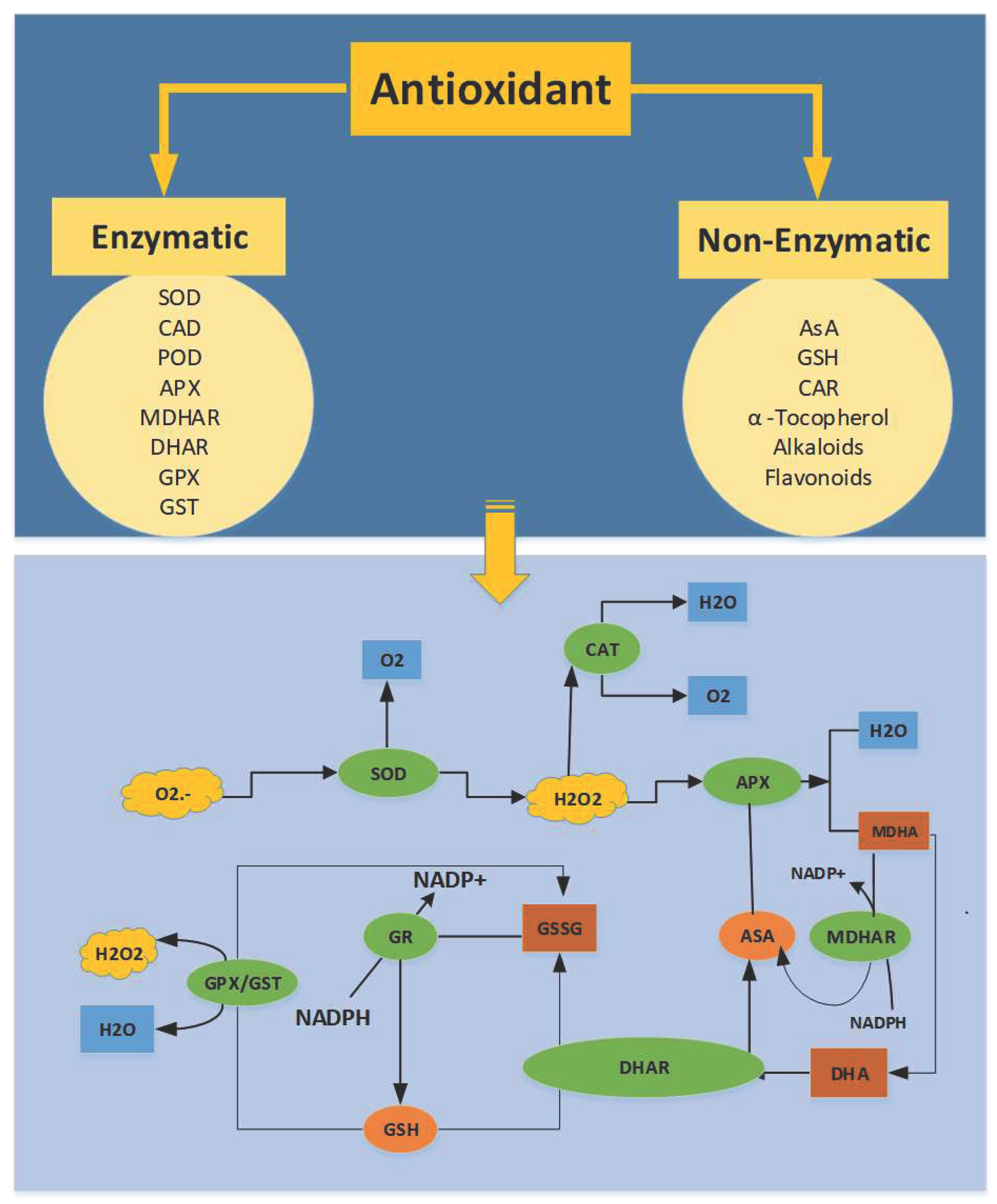
7.4. Antioxidants Enzymatic System against Oxidation
7.5. Non-Enzymatic Antioxidants
8. Additional Physiological Control in Salt Stress
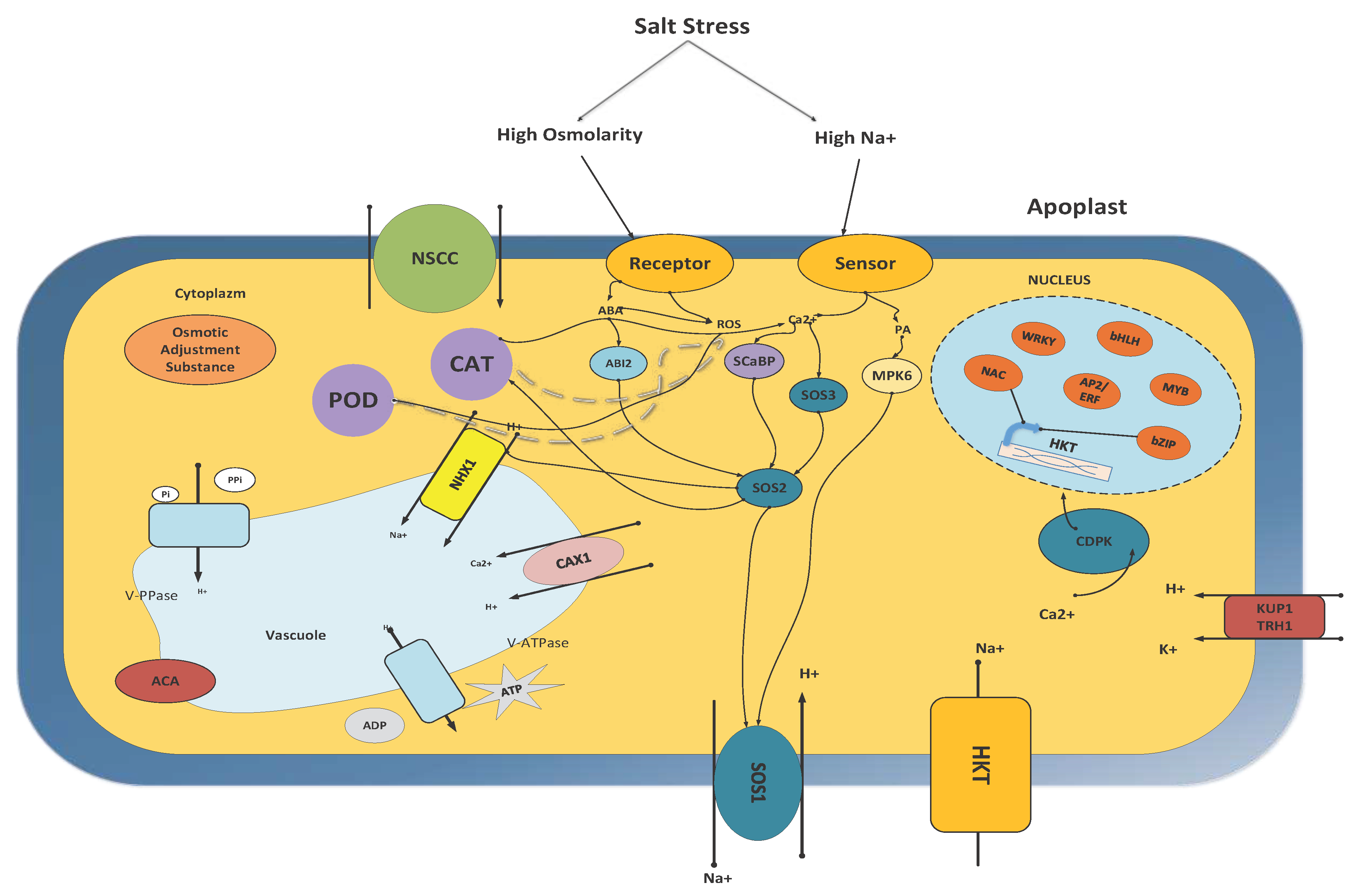
8.1. Ca2+-Dependent Signalling Pathways Pathway
8.2. SOS Pathway
8.3. CDPK
8.4. Ca2+- (MAPKs Cascade)
9. Genes Involved in Salt Tolerance
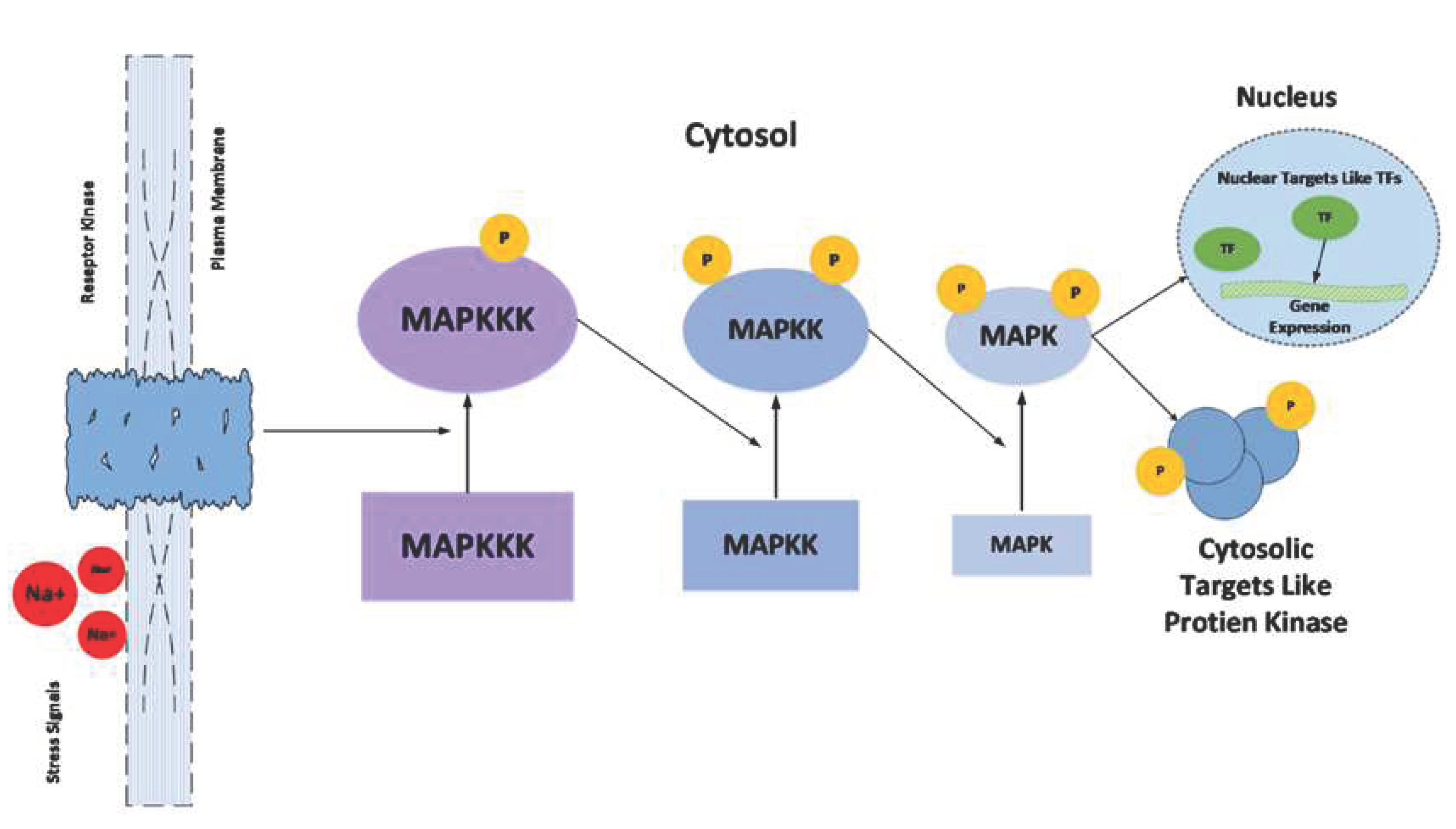
9.1. Genes Involved in Osmotic Adjustment
9.2. Genes Involved in Antioxidants
9.3. Plant Reaction to Salinity Stress in Genes Upregulating and Downregulating
9.4. Genes Related to Signal Transduction
9.5. Regulatory of Several Genes
10. Techniques for Non-Genetic Improvement
Genetic Improvement Techniques
11. Conclusions Future Research Perspectives
Author Contributions
Funding
Acknowledgments
References
- Abbas, W.; Ashraf, M.; Akram, N.A. Alleviation of salt-induced adverse effects in eggplant (Solanum melongena L.) by glycinebetaine and sugarbeet extracts. Scientia horticulturae 2010, 125, 188–195. [Google Scholar] [CrossRef]
- Abobatta, W.F. Plant responses and tolerance to extreme salinity. In Salt and Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 177–210. [Google Scholar]
- Adhikari, S.; Anuragi, H.; Chandra, K.; Tarte, S.H.; Dhaka, S.R.; Jatav, H.S.; Hingonia, K. Molecular basis of plant nutrient use efficiency-concepts and challenges for its improvement. In Sustainable Plant Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; pp. 107–151. [Google Scholar]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein Ibrahim, M.E.; Ibrahim Salih, E.G.; Hussain, S.; Younas, M.U. Pivotal role of phytohormones and their responsive genes in plant growth and their signaling and transduction pathway under salt stress in cotton. International Journal of Molecular Sciences 2022, 23, 7339. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. International Journal of Plant Production 2012, 2, 353–366. [Google Scholar]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant abiotic stress tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–205. [Google Scholar]
- Albaladejo, I.; Egea, I.; Morales, B.; Flores, F.B.; Capel, C.; Lozano, R.; Bolarin, M.C. Identification of key genes involved in the phenotypic alterations of res (restored cell structure by salinity) tomato mutant and its recovery induced by salt stress through transcriptomic analysis. BMC plant biology 2018, 18, 1–19. [Google Scholar] [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Ansari, H.H.; Siddiqui, A.; Wajid, D.; Tabassum, S.; Umar, M.; Siddiqui, Z.S. Profiling of energy compartmentalization in photosystem II (PSII), light harvesting complexes and specific energy fluxes of primed maize cultivar (P1429) under salt stress environment. Plant Physiology and Biochemistry 2022, 170, 296–306. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiology and Biochemistry 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Ashraf, J.; Zuo, D.; Wang, Q.; Malik, W.; Zhang, Y.; Abid, M.A.; Cheng, H.; Yang, Q.; Song, G. Recent insights into cotton functional genomics: progress and future perspectives. Plant biotechnology journal 2018, 16, 699–713. [Google Scholar] [CrossRef]
- Ashraf, M.; Wu, L. Breeding for salinity tolerance in plants. Critical Reviews in Plant Sciences 1994, 13, 17–42. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Askari, S.H.; Iqbal, M.; Rasheed, R.; Hussain, I. Recent advances in abiotic stress tolerance of plants through chemical priming: an overview. Advances in seed priming 2018, 51–79. [Google Scholar]
- Ashrafi, N.; Rezaei Nejad, A. Lisianthus response to salinity stress. Photosynthetica 2018, 56, 487–494. [Google Scholar] [CrossRef]
- Atzori, G.; Guidi Nissim, W.; Mancuso, S.; Palm, E. Intercropping Salt-Sensitive Lactuca sativa L. and Salt-Tolerant Salsola soda L. in a Saline Hydroponic Medium: An Agronomic and Physiological Assessment. Plants 2022, 11, 2924. [Google Scholar] [CrossRef]
- Azad, K.; Kaminskyj, S. A fungal endophyte strategy for mitigating the effect of salt and drought stress on plant growth. Symbiosis 2016, 68, 73–78. [Google Scholar] [CrossRef]
- Azevedo Neto, A.D.D.; Prisco, J.T.; Enéas-Filho, J.; Lacerda, C.F.D.; Silva, J.V.; Costa, P.H.a.D. , and Gomes-Filho, E. Effects of salt stress on plant growth, stomatal response and solute accumulation of different maize genotypes. Brazilian Journal of Plant Physiology 2004, 16, 31–38. [Google Scholar] [CrossRef]
- Aziz, A.; Ashraf, M.; Sikandar, S.; Asif, M.; Akhtar, N.; Shahzad, S.M.; Wasaya, A.; Raza, A.; Babar, B.H. Optimizing sulfur for improving salt tolerance of sunflower (Helianthus annuus L.). Soil Environ 2019, 38, 222–233. [Google Scholar] [CrossRef]
- Azooz, M.; Ismail, A.; Elhamd, M.A. Growth, lipid peroxidation and antioxidant enzyme activities as a selection criterion for the salt tolerance of maize cultivars grown under salinity stress. Int. J. Agric. Biol 2009, 11, 21–26. [Google Scholar]
- Babayev, D.; Rahimova, N.; Arzyamova, O.; Yollibayev, A. EFFECT OF FERTILIZERS INTO PHYSIOLOGICAL AND BIOCHEMICAL INDICATORS OF COTTON IN SALINE SOILS.
- Baghour, M.; Gálvez, F.J.; Sánchez, M.E.; Aranda, M.N.; Venema, K.; Rodríguez-Rosales, M.P. Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant Physiology and Biochemistry 2019, 135, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Chen, J.; Gao, T.; Tang, Z.; Li, H.; Gong, S.; Du, Y.; Yu, Y.; Wang, W. A Na+/H+ antiporter localized on the Golgi-to-vacuole transport system from Camellia sinensis, CsNHX6, plays a positive role in salt tolerance. Scientia Horticulturae 2023, 309, 111704. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef] [PubMed]
- Bakala, H.S.; Devi, J.; Sarao, L.K.; Kaur, S. Utilization of Wheat and Maize Waste as Biofuel Source. In Agroindustrial Waste for Green Fuel Application; Springer: Berlin/Heidelberg, Germany, 2023; pp. 27–66. [Google Scholar]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. Journal of Plant Physiology 2009, 166, 146–156. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, R.; Li, F.; Tang, W.; Han, L. Simultaneous expression of Spinacia oleracea chloroplast choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH) genes contribute to dwarfism in transgenic Lolium perenne. Plant Molecular Biology Reporter 2011, 29, 379–388. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Critical reviews in plant sciences 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Coppa, E.; Brunori, E.; Cristofori, V.; Rugini, E.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Nawaz Shah, M.K. Response of olive shoots to salinity stress suggests the involvement of sulfur metabolism. Plants 2021, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Hasanuzzaman, M.; Li, Y.; Akhtar, K.; Zhang, C.; Zhao, T. Seed germination behavior, growth, physiology and antioxidant metabolism of four contrasting cultivars under combined drought and salinity in soybean. Antioxidants 2022, 11, 498. [Google Scholar] [CrossRef]
- Bernstein, L.; Hayward, H. Physiology of salt tolerance. Annual review of plant physiology 1958, 9, 25–46. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of Low Temperature Stress on Photosynthesis and Allied Traits: A Review. Physiological Processes in Plants Under Low Temperature Stress 2022, 199–297. [Google Scholar]
- Bibi, N.; Jan, G.; Jan, F.G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Rehman, H.; Tawab, A.; Khushdil, F. Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant physiology and biochemistry 2019, 139, 459–469. [Google Scholar] [CrossRef]
- Billah, M.; Aktar, S.; Brestic, M.; Zivcak, M.; Khaldun, A.B.M.; Uddin, M.S.; Bagum, S.A.; Yang, X.; Skalicky, M.; Mehari, T.G. Progressive genomic approaches to explore drought-and salt-induced oxidative stress responses in plants under changing climate. Plants 2021, 10, 1910. [Google Scholar] [CrossRef]
- Bor, M.; Özdemir, F. Manipulating metabolic pathways for development of salt-tolerant crops. In Salinity Responses and Tolerance in Plants, Volume 1; Springer: Berlin/Heidelberg, Germany, 2018; pp. 235–256. [Google Scholar]
- Brenya, E. (2020). Elucidating thigmomorphogenesis: An epigenetic phenomenon of mechanical stress acclimation in plants. University of Western Sydney.
- Brugnoli, E.; Björkman, O. Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 1992, 187, 335–347. [Google Scholar] [CrossRef]
- Çakır, B.; Kılıçkaya, O. Mitogen-activated protein kinase cascades in Vitis vinifera. Frontiers in plant science 2015, 6, 556. [Google Scholar] [CrossRef]
- Chai, C.; Wang, Y.; Valliyodan, B.; Nguyen, H.T. Comprehensive analysis of the soybean (Glycine max) GmLAX auxin transporter gene family. Frontiers in plant science 2016, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Bhaduri, D.; Meena, H.N.; Kalariya, K. External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiology and Biochemistry 2016, 103, 143–153. [Google Scholar] [CrossRef]
- Chauhan, A.; Abuamarah, B.A.; Kumar, A.; Verma, J.; Ghramh, H.A.; Khan, K.A.; Ansari, M.J. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi journal of biological sciences 2019, 26, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Jing, R.; Blair, M.W.; Mao, X.; Wang, S. Cloning and genetic diversity analysis of a new P5CS gene from common bean (Phaseolus vulgaris L.). Theoretical and applied genetics 2010, 120, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Yao, L.; Li, B.; Ma, X.; Si, E.; Yang, K.; Li, C.; Shang, X.; Meng, Y. Combined proteomic and metabolomic analysis of the molecular mechanism underlying the response to salt stress during seed germination in barley. International Journal of Molecular Sciences 2022, 23, 10515. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, N.; Oukarroum, A.; Strasser, R.J.; Schansker, G. Salt stress effects on the photosynthetic electron transport chain in two chickpea lines differing in their salt stress tolerance. Photosynthesis research 2018, 136, 291–301. [Google Scholar] [CrossRef]
- Corpas, F.J.; Fernandez-Ocana, A.; Carreras, A.; Valderrama, R.; Luque, F.; Esteban, F.J.; Rodríguez-Serrano, M.; Chaki, M.; Pedrajas, J.R.; Sandalio, L.M. The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves. Plant and cell physiology 2006, 47, 984–994. [Google Scholar] [CrossRef]
- Dai, J.; Duan, L.; Dong, H. Comparative effect of nitrogen forms on nitrogen uptake and cotton growth under salinity stress. Journal of Plant Nutrition 2015, 38, 1530–1543. [Google Scholar] [CrossRef]
- Das, M.; Chauhan, H.; Chhibbar, A.; Haq, R.; Mohd, Q.; Khurana, P. High-efficiency transformation and selective tolerance against biotic and abiotic stress in mulberry, Morus indica cv. K2, by constitutive and inducible expression of tobacco osmotin. Transgenic research 2011, 20, 231–246. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Wylie, S.J. Plant–fungi association: role of fungal endophytes in improving plant tolerance to water stress. In Plant-microbe interactions in agro-ecological perspectives; Springer: Berlin/Heidelberg, Germany, 2017; pp. 143–159. [Google Scholar]
- Del Amor, F.M.; Cuadra-Crespo, P. Alleviation of salinity stress in broccoli using foliar urea or methyl-jasmonate: analysis of growth, gas exchange, and isotope composition. Plant Growth Regulation 2011, 63, 55–62. [Google Scholar] [CrossRef]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.-T. , and Figueroa, C.R. Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. International Journal of Molecular Sciences 2021, 22, 3082. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of cell science 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, Q.; Cao, R.; Ren, Y.; Wang, G.; Guo, H.; Bu, S.; Liu, J.; Ma, P. Overexpression of SmMYC2 enhances salt resistance in Arabidopsis thaliana and Salvia miltiorrhiza hairy roots. Journal of Plant Physiology 2023, 280, 153862. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yao, Q.; Zhu, D.; Dong, B. Hydrogen-rich water maintains the quality of Rosa roxburghii by regulating AsA biosynthesis and regeneration. Postharvest Biology and Technology 2023, 195, 112136. [Google Scholar] [CrossRef]
- Dong, X.; Wang, F. Edited by: Loredana F. Ciarmiello, University of Campania Luigi Vanvitelli, Italy Reviewed by. Salt Tolerance: Molecular and Physiological Mechanisms and Breeding Applications.
- Ejaz, S.; Fahad, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ahmad, S. Role of osmolytes in the mechanisms of antioxidant defense of plants. In Sustainable Agriculture Reviews 39; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–117. [Google Scholar]
- El-Banna, M.; Abdelaal, K.A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. Journal of Plant Production 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- El-Katony, T.M.; El-Bastawisy, Z.M.; El-Ghareeb, S.S. Timing of salicylic acid application affects the response of maize (Zea mays L.) hybrids to salinity stress. Heliyon 2019, 5, e01547. [Google Scholar] [CrossRef]
- Elkeilsh, A.; Awad, Y.M.; Soliman, M.H.; Abu-Elsaoud, A.; Abdelhamid, M.T.; El-Metwally, I.M. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. Journal of plant research 2019, 132, 881–901. [Google Scholar] [CrossRef]
- Etesami, H.; Noori, F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants. In Saline soil-based agriculture by halotolerant microorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–22. [Google Scholar]
- Finkina, E.; Melnikova, D.; Bogdanov, I. Lipid transfer proteins as components of the plant innate immune system: structure, functions, and applications. Acta Naturae (англoязычная версия) 2016, 8, 47–61. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytologist.
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Gaafar, R.M.; Seyam, M.M. Ascorbate–glutathione cycle confers salt tolerance in Egyptian lentil cultivars. Physiology and Molecular Biology of Plants 2018, 24, 1083–1092. [Google Scholar] [CrossRef]
- Galić, V.; Mazur, M.; Šimić, D.; Zdunić, Z.; Franić, M. Plant biomass in salt-stressed young maize plants can be modelled with photosynthetic performance. Photosynthetica 2020, 58, 194–204. [Google Scholar] [CrossRef]
- Gao, H.; Yu, C.; Liu, R.; Li, X.; Huang, H.; Wang, X.; Zhang, C.; Jiang, N.; Li, X.; Cheng, S. The Glutathione S-Transferase PtGSTF1 Improves Biomass Production and Salt Tolerance through Regulating Xylem Cell Proliferation, Ion Homeostasis and Reactive Oxygen Species Scavenging in Poplar. International Journal of Molecular Sciences 2022, 23, 11288. [Google Scholar] [CrossRef] [PubMed]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Science 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology and biochemistry 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Wen, D.; Vandenlangenberg, K.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Scientia Horticulturae 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Goodman, A.; Ennos, A. A comparative study of the response of the roots and shoots of sunflower and maize to mechanical stimulation. Journal of Experimental Botany 1996, 47, 1499–1507. [Google Scholar] [CrossRef]
- Graus, D.; Li, K.; Rathje, J.M.; Ding, M.; Krischke, M.; Müller, M.J.; Cuin, T.A.; Al-Rasheid, K.A.; Scherzer, S.; Marten, I. Tobacco leaf tissue rapidly detoxifies direct salt loads without activation of calcium and SOS signaling. New Phytologist 2022. [Google Scholar] [CrossRef]
- Gross, E.; Sevier, C.S.; Heldman, N.; Vitu, E.; Bentzur, M.; Kaiser, C.A.; Thorpe, C.; Fass, D. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proceedings of the National Academy of Sciences 2006, 103, 299–304. [Google Scholar] [CrossRef]
- Gupta, S.; Goyal, M.R.; Singh, A. Physiological and biochemical changes in plants under soil salinity stress: a review. Engineering practices for management of soil salinity 2018, 159–200. [Google Scholar]
- Hafez, E.M.; Gowayed, S.M.; Nehela, Y.; Sakran, R.M.; Rady, A.M.; Awadalla, A.; Omara, A.E.-D. , and Alowaiesh, B.F. Incorporated biochar-based soil amendment and exogenous glycine betaine foliar application ameliorate rice (Oryza sativa L.) tolerance and resilience to osmotic stress. Plants 2021, 10, 1930. [Google Scholar] [CrossRef]
- Hamza, O.M.; Al-Taey, D.K. A study on the effect of Glutamic acid and benzyl adenine application upon growth and yield parameters and active components of two broccoli hybrids. Int. J. Agricult. Stat. Sci 2020, 16, 1163–1167. [Google Scholar]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Haro, R.; Bañuelos, M.A.; Senn, M.E.; Barrero-Gil, J.; Rodríguez-Navarro, A. HKT1 mediates sodium uniport in roots. Pitfalls in the expression of HKT1 in yeast. Plant Physiology 2005, 139, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and responses of plants under salt stress; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–87. [Google Scholar]
- Hashemi, S.E.; Madahhosseini, S.; Pirasteh-Anosheh, H.; Sedaghati, E.; Race, M. The Role of Nitrogen in Inducing Salt Stress Tolerance in Crocus sativus L.: Assessment Based on Plant Growth and Ions Distribution in Leaves. Sustainability 2023, 15, 567. [Google Scholar] [CrossRef]
- Hatam, Z.; Sabet, M.S.; Malakouti, M.J.; Mokhtassi-Bidgoli, A.; Homaee, M. Zinc and potassium fertilizer recommendation for cotton seedlings under salinity stress based on gas exchange and chlorophyll fluorescence responses. South African Journal of Botany 2020, 130, 155–164. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: a review. Plant signaling & behavior 2012, 7, 1456–1466. [Google Scholar]
- Hirt, H.; Shinozaki, K. Plant responses to abiotic stress; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Hniličková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant, Soil and Environment 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Horváth, E.; Bela, K.; Holinka, B.; Riyazuddin, R.; Gallé, Á.; Hajnal, Á.; Hurton, Á.; Fehér, A.; Csiszár, J. The Arabidopsis glutathione transferases, AtGSTF8 and AtGSTU19 are involved in the maintenance of root redox homeostasis affecting meristem size and salt stress sensitivity. Plant Science 2019, 283, 366–374. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. International Journal of Molecular Sciences 2022, 23, 5186. [Google Scholar] [CrossRef]
- Hou, C.; Cheng, X.; Zhang, X.; Zhu, X.; Xu, J.; Luo, X.; Wu, D.; Liang, H. Effect of ferrous-activated calcium peroxide oxidation on forward osmosis treatment of algae-laden water: Membrane fouling mitigation and mechanism. Science of The Total Environment 2023, 858, 160100. [Google Scholar] [CrossRef]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.; Shabala, L.; Ahmed, H.a.I. , Deng, S.; Zhang, H.; Song, P. Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Dickmann, L.J.; Satterlee, J.S.; Sussman, M.R. Characterization of eight new members of the calmodulin-like domain protein kinase gene family from Arabidopsis thaliana. Plant molecular biology 1996, 31, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. Journal of plant physiology 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria journal of medicine 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ilyas, M.; Ahad, A.; Batool, T.; Jabbar, W.; Ejaz, M.; Gul, A.; Ozturk, M. Salinity induced stress in wheat. Special Issue on “Cereals and Stress Tolerance” 2022, 126891, 67. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Changes in growth, photosynthetic capacity and ionic relations in spring wheat (Triticum aestivum L.) due to pre-sowing seed treatment with polyamines. Plant Growth Regulation 2005, 46, 19–30. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: many unanswered questions remain. Frontiers in plant science 2019, 10, 80. [Google Scholar] [CrossRef]
- Jadidi, O.; Etminan, A.; Azizi-Nezhad, R.; Ebrahimi, A.; Pour-Aboughadareh, A. Physiological and molecular responses of barley genotypes to salinity stress. Genes 2022, 13, 2040. [Google Scholar] [CrossRef]
- Jia, C.; Zhao, S.; Bao, T.; Zhao, P.; Peng, K.; Guo, Q.; Gao, X.; Qin, J. Tomato BZR/BES transcription factor SlBZR1 positively regulates BR signaling and salt stress tolerance in tomato and Arabidopsis. Plant Science 2021, 302, 110719. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, X.; Li, M.; Hou, J.; Tong, X.; Gao, Z.; Chen, G. Exogenous spermidine enhances salt-stressed rice photosynthetic performance by stabilizing structure and function of chloroplast and thylakoid membranes. Photosynthetica 2020, 58, 61–71. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, Q.; Wu, Z.; Zhang, Q.; Wang, L.; Liu, J.; Hu, X.; Guo, D.; Wang, X.; Zhang, Z. Analysis of CAT Gene Family and Functional Identification of OsCAT3 in Rice. Genes 2023, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S. (2016). The Mechanisms of drought stress tolerance in the crop Sorghum Bicolor. Durham University.
- Ju, F.; Pang, J.; Sun, L.; Gu, J.; Wang, Z.; Wu, X.; Ali, S.; Wang, Y.; Zhao, W.; Wang, S. Integrative transcriptomic, metabolomic and physiological analyses revealed the physiological and molecular mechanisms by which potassium regulates the salt tolerance of cotton (Gossypium hirsutum L.) roots. Industrial Crops and Products 2023, 193, 116177. [Google Scholar] [CrossRef]
- Ju, Y.-L. , Yue, X.-F., Min, Z.; Wang, X.-H., Fang, Y.-L., and Zhang, J.-X. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiology and Biochemistry 2020, 146, 98–111. [Google Scholar] [PubMed]
- Kader, M.A.; Seidel, T.; Golldack, D.; Lindberg, S. Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. Journal of Experimental Botany 2006, 57, 4257–4268. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, M.; Sevim, G.; Bor, M. The role of proline, glycinebetaine, and trehalose in stress-responsive gene expression. In osmoprotectant-mediated abiotic stress tolerance in plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 241–256. [Google Scholar]
- Kaushal, M. Insights into microbially induced salt tolerance and endurance mechanisms (STEM) in plants. Frontiers in Microbiology 2020, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Sonmez, O.; Aydemir, S.; Ashraf, M.; Dikilitas, M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). Journal of plant interactions 2013, 8, 234–241. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environmental and Experimental Botany 2007, 60, 397–403. [Google Scholar] [CrossRef]
- Ke, D.; He, Y.; Fan, L.; Niu, R.; Cheng, L.; Wang, L.; Zhang, Z. The soybean TGA transcription factor GmTGA13 plays important roles in the response to salinity stress. Plant Biology 2022, 24, 313–322. [Google Scholar] [CrossRef]
- Khalvandi, M.; Amerian, M.; Pirdashti, H.; Keramati, S. Does co-inoculation of mycorrhiza and Piriformospora indica fungi enhance the efficiency of chlorophyll fluorescence and essential oil composition in peppermint under irrigation with saline water from the Caspian Sea? PloS one 2021, 16, e0254076. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: a life cycle study. Environmental Science and Pollution Research 2021, 28, 13712–13724. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Al-Mas’oudi, R.S.; Al-Said, F.; Khan, I. Salinity effects on growth, electrolyte leakage, chlorophyll content and lipid peroxidation in cucumber (Cucumis sativus L.). In International Conference on Food and Agricultural Sciences Malaysia; IACSIT Press: Singapore, 2013; pp. 28–32. [Google Scholar]
- Khan, M.N.; Fu, C.; Li, J.; Tao, Y.; Li, Y.; Hu, J.; Chen, L.; Khan, Z.; Wu, H.; Li, Z. Seed nanopriming: How do nanomaterials improve seed tolerance to salinity and drought? Chemosphere 2022, 136911. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Mazid, M.; Mohammad, F. A review of ascorbic acid potentialities against oxidative stress induced in plants. Journal of agrobiology 2011, 28, 97. [Google Scholar] [CrossRef]
- Kong, X.; Pan, J.; Zhang, M.; Xing, X.; Zhou, Y.; Liu, Y.; Li, D.; Li, D. ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant, cell & environment 2011, 34, 1291–1303. [Google Scholar]
- Krasensky, J.; Broyart, C.; Rabanal, F.A.; Jonak, C. The redox-sensitive chloroplast trehalose-6-phosphate phosphatase AtTPPD regulates salt stress tolerance. Antioxidants & redox signaling 2014, 21, 1289–1304. [Google Scholar]
- Kumar, S.; Singh, T.B.; Agnihotri, R.K.; Chaturvedi, P. Comparative effect of nacl and peg on physiological and biochemical attributes during vegetative stage of tomato. Res J Agric Sci 2021, 12, 955–961. [Google Scholar]
- Kumari, S.; Chhillar, H.; Chopra, P.; Khanna, R.R.; Khan, M.I.R. Potassium: A track to develop salinity tolerant plants. Plant Physiology and Biochemistry 2021, 167, 1011–1023. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytologist 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Zhang, K.; Sun, Y.; Cui, H.; Cao, S.; Yan, L.; Xu, M. Growth, physiology, and transcriptional analysis of two contrasting Carex rigescens genotypes under salt stress reveals salt-tolerance mechanisms. Journal of plant physiology 2018, 229, 77–88. [Google Scholar] [CrossRef]
- Li, P.; Yang, H.; Liu, G.; Ma, W.; Li, C.; Huo, H.; He, J.; Liu, L. PpSARK regulates moss senescence and salt tolerance through ABA related pathway. International journal of molecular sciences 2018, 19, 2609. [Google Scholar] [CrossRef]
- Li, Q.-L. , Gao, X.-R., Yu, X.-H., Wang, X.-Z., and An, L.-J. Molecular cloning and characterization of betaine aldehyde dehydrogenase gene from Suaeda liaotungensis and its use in improved tolerance to salinity in transgenic tobacco. Biotechnology letters 2003, 25, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, R.; Li, Z.; Fan, H.; Song, J. Positive effects of NaCl on the photoreaction and carbon assimilation efficiency in Suaeda salsa. Plant Physiology and Biochemistry 2022, 177, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Q.; Nan, H.; Li, X.; Lu, S.; Zhao, X.; Liu, B.; Guo, C.; Kong, F.; Cao, D. Overexpression of GmFDL19 enhances tolerance to drought and salt stresses in soybean. PLoS One 2017, 12, e0179554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Liang, S.; Chen, X.; Liu, J.; Zhang, Y.; Wang, A. Identification of CDPK Gene Family in Solanum habrochaites and Its Function Analysis under Stress. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef]
- Li, Z.; Palmer, W.M.; Martin, A.P.; Wang, R.; Rainsford, F.; Jin, Y.; Patrick, J.W.; Yang, Y.; Ruan, Y.-L. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. Journal of experimental botany 2012, 63, 1155–1166. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochemical and biophysical research communications 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, S.; Wang, K.; Tian, H.; Gao, J.; Zhao, Q.; Du, C. A leucine-rich repeat receptor-like kinase, OsSTLK, modulates salt tolerance in rice. Plant Science 2020, 296, 110465. [Google Scholar] [CrossRef]
- Liu, A.; Xiao, Z.; Li, M.W.; Wong, F.L.; Yung, W.S.; Ku, Y.S.; Wang, Q.; Wang, X.; Xie, M.; Yim, A.K.Y. Transcriptomic reprogramming in soybean seedlings under salt stress. Plant, Cell & Environment 2019, 42, 98–114. [Google Scholar]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2023, 12. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, Y.; Liu, J.; Barrett, S.C.; Wang, Y. A New Type of Cell for Organ Movement in Plants. Available at SSRN 416 4238. [CrossRef]
- Liu, X.; Xu, X.; Ji, M.; Amombo, E.; Fu, J. Growth, Photosynthesis, and Gene Expression of Bermudagrass in Response to Salinity and Shade Stress. Journal of the American Society for Horticultural Science 2023, 148, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Zeng, S.; Patra, B.; Yuan, L.; Wang, Y. Isolation and characterization of a salt stress-responsive betaine aldehyde dehydrogenase in Lycium ruthenicum Murr. Physiologia plantarum 2018, 163, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium-and salt-stress signaling in plants: shedding light on SOS pathway. Archives of biochemistry and biophysics 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, P.; Munns, R.; Colmer, T.; Condon, A.; Peltonen-Sainio, P. Effect of foliar applications of glycinebetaine on stomatal conductance, abscisic acid and solute concentrations in leaves of salt-or drought-stressed tomato. Functional Plant Biology 1998, 25, 655–663. [Google Scholar] [CrossRef]
- Malik, A. Groundwater hydro-geochemistry, irrigation and drinking quality, and source apportionment in the intensively cultivated area of Sutlej sub-basin of main Indus basin. Environmental Earth Sciences 2022, 81, 1–26. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Siddique, M.H.; Anwar, F.; Shahzad, T. Unlocking the potential of co-applied biochar and plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture under stress conditions. Chemical and biological technologies in agriculture 2022, 9, 1–29. [Google Scholar] [CrossRef]
- Manandhar, A. Making space for the pore: Structural characterization of stomatal movements when guard cells and epidermal cells interact. 2022.
- Mansour, M.M.F. Role of Vacuolar Membrane Transport Systems in Plant Salinity Tolerance. Journal of Plant Growth Regulation 2022, 1–38. [Google Scholar] [CrossRef]
- Masuda, M.S.; Azad, M.a.K. , Hasanuzzaman, M.; Arifuzzaman, M. Evaluation of salt tolerance in maize (Zea mays L.) at seedling stage through morphological characters and salt tolerance index. Plant Physiology Reports 2021, 26, 419–427. [Google Scholar] [CrossRef]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity responses and tolerance in plants, volume 1; Springer: Berlin/Heidelberg, Germany, 2018; pp. 85–136. [Google Scholar]
- Meloni, D.A.; Oliva, M.A.; Ruiz, H.A.; Martinez, C.A. Contribution of proline and inorganic solutes to osmotic adjustment in cotton under salt stress. Journal of Plant Nutrition 2001, 24, 599–612. [Google Scholar] [CrossRef]
- Mitra, G. Molecular approaches to nutrient uptake and cellular homeostasis in plants under abiotic stress. In Plant nutrients and abiotic stress tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 525–590. [Google Scholar]
- Mittal, S.; Kumari, N.; Sharma, V. Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiology and Biochemistry 2012, 54, 17–26. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends in plant science 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.K.S.; Qayyum, M.F.; Abdel-Hadi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Archives of Agronomy and Soil Science 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Nadeem, M.; Anwar-Ul-Haq, M.; Saqib, M.; Maqsood, M.; He, Z. Ameliorative effect of silicic acid and silicates on oxidative, osmotic stress, and specific ion toxicity in spring wheat (Triticum aestivum L.) genotypes. Journal of Soil Science and Plant Nutrition.
- Naikwade, P.V. Plant Responses to Drought Stress: Morphological, Physiological, Molecular Approaches, and Drought Resistance. In Plant Metabolites under Environmental Stress; Apple Academic Press: Cambridge, MA, USA, 2023; pp. 149–183. [Google Scholar]
- Naz, F. Plant nutrition, transport, mechanism and sensing in plants. In Sustainable Plant Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; pp. 209–228. [Google Scholar]
- Nguyen, N.H.; Jung, C.; Cheong, J.-J. Chromatin remodeling for the transcription of type 2C protein phosphatase genes in response to salt stress. Plant Physiology and Biochemistry 2019, 141, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Shiblawi, A.; Razzaq, F.; Sentenac, H. Roles and transport of sodium and potassium in plants. In The alkali metal ions: Their role for life; Springer: Berlin/Heidelberg, Germany, 2016; pp. 291–324. [Google Scholar]
- Noble, C.; Rogers, M. Arguments for the use of physiological criteria for improving the salt tolerance in crops. Plant and soil 1992, 146, 99–107. [Google Scholar] [CrossRef]
- Panda, S.; Zhou, K. Engineering microbes to overproduce natural products as agrochemicals. Synthetic and Systems Biotechnology 2023, 8, 79–85. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Bok, V.V.; Brkanac, S.R.; Strnad, M.; Salopek-Sondi, B. Early Brassica crops responses to salinity stress: a comparative analysis between Chinese cabbage, white cabbage, and kale. Frontiers in plant science 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Li, H.; Zhou, Y.; Li, W.; Jiang, Y.; Li, H. Exogenous glycinebetaine application contributes to abiotic stress tolerance in maize. Journal of Plant Biology 2020, 1–13. [Google Scholar] [CrossRef]
- Peng, J.; Liu, J.; Zhang, L.; Luo, J.; Dong, H.; Ma, Y.; Zhao, X.; Chen, B.; Sui, N.; Zhou, Z. Effects of soil salinity on sucrose metabolism in cotton leaves. PLoS One 2016, 11, e0156241. [Google Scholar] [CrossRef]
- Phour, M.; Sindhu, S.S. Soil Salinity and Climate Change: Microbiome-Based Strategies for Mitigation of Salt Stress to Sustainable Agriculture. In Climate Change and Microbiome Dynamics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 191–243. [Google Scholar]
- Pigeot-Rémy, S.; Simonet, F.; Atlan, D.; Lazzaroni, J.; Guillard, C. Bactericidal efficiency and mode of action: a comparative study of photochemistry and photocatalysis. Water research 2012, 46, 3208–3218. [Google Scholar] [CrossRef]
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: environment-plants-molecules; Springer: Berlin/Heidelberg, Germany, 2002; pp. 3–20. [Google Scholar]
- Prodjinoto, H.; Irakoze, W.; Gandonou, C.; Lepoint, G.; Lutts, S. Discriminating the impact of Na+ and Cl− in the deleterious effects of salt stress on the African rice species (Oryza glaberrima Steud.). Plant Growth Regulation 2021, 94, 201–219. [Google Scholar] [CrossRef]
- Qi, Z.; Spalding, E.P. Protection of plasma membrane K+ transport by the salt overly sensitive1 Na+-H+ antiporter during salinity stress. Plant Physiology 2004, 136, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, H.; Li, J.; Zhu, Y.; Jiao, G.; Wang, C.; Wu, S. Down-regulation of GhADF1 in cotton (Gossypium hirsutum) improves plant drought tolerance and increases fiber yield. The Crop Journal 2022, 10, 1037–1048. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Al Mahmud, J.; Hasanuzzaman, M.; Hossain, M.S.; Fujita, M. Salt stress tolerance in rice: Emerging role of exogenous phytoprotectants. Advances in international rice research 2017, 9, 139–174. [Google Scholar]
- Rajappa, S.; Krishnamurthy, P.; Kumar, P.P. Regulation of AtKUP2 expression by bHLH and WRKY transcription factors helps to confer increased salt tolerance to Arabidopsis thaliana plants. Frontiers in plant science 2020, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Kumari, A. Harish. Singh, RK.
- Rauf, M.; Shahzad, K.; Ali, R.; Ahmad, M.; Habib, I.; Mansoor, S.; Berkowitz, G.A.; Saeed, N.A. Cloning and characterization of Na+/H+ antiporter (LfNHX1) gene from a halophyte grass Leptochloa fusca for drought and salt tolerance. Molecular Biology Reports 2014, 41, 1669–1682. [Google Scholar] [CrossRef]
- Raziq, A.; Din, A.M.U.; Anwar, S.; Wang, Y.; Jahan, M.S.; He, M.; Ling, C.G.; Sun, J.; Shu, S.; Guo, S. Exogenous spermidine modulates polyamine metabolism and improves stress responsive mechanisms to protect tomato seedlings against salt stress. Plant Physiology and Biochemistry 2022, 187, 1–10. [Google Scholar] [CrossRef]
- Ren, W.; Chen, L.; Peng, X. Combined transcriptome and metabolome analysis revealed pathways involved in improved salt tolerance of Gossypium hirsutum L. seedlings in response to exogenous melatonin application. BMC Plant Biology 2022, 22, 1–19. [Google Scholar] [CrossRef]
- Rengel, Z. The role of calcium in salt toxicity. Plant, Cell & Environment 1992, 15, 625–632. [Google Scholar]
- Riley, J.J. Review of halophyte feeding trials with ruminants. In Halophytic and Salt-Tolerant Feedstuffs; CRC Press: Boca Raton, FL, USA, 2015; pp. 177–217. [Google Scholar]
- Rios-Gonzalez, K.; Erdei, L.; Lips, S.H. The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Science 2002, 162, 923–930. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. Journal of Experimental Botany 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Islam, M.S.; Iqbal, M.A.; Hossain, A.; Mubeen, M.; Jabeen, T.; Waleed, M.; Wasaya, A.; Rahman, M.H.U.; Ratnasekera, D. Salinity Stress in Cotton: Adverse Effects, Survival Mechanisms and Management Strategies. In Engineering Tolerance in Crop Plants Against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021; pp. 59–80. [Google Scholar]
- Sadau, S.B.; Mehari, T.G.; Ahmad, A.; Tajo, S.M.; Ibrahim, S.; Iqbal, M.S.; Elasad, M.; Zhang, J.; Wei, H.; Yu, S. Genome wide identification and characterization of MAPK genes reveals their potential in enhancing drought and salt stress tolerance in Gossypium hirsutum. Journal of Cotton Research 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci 2019, 17, 34–40. [Google Scholar]
- Salimath, S.S.; Romsdahl, T.B.; Konda, A.R.; Zhang, W.; Cahoon, E.B.; Dowd, M.K.; Wedegaertner, T.C.; Hake, K.D.; Chapman, K.D. Production of tocotrienols in seeds of cotton (Gossypium hirsutum L.) enhances oxidative stability and offers nutraceutical potential. Plant Biotechnology Journal 2021, 19, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Vivek, S. Salinity Stress in Plants and Role of Microbes in Its Alleviation. In Agricultural Biocatalysis; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 175–214. [Google Scholar]
- Schobert, B.; Tschesche, H. Unusual solution properties of proline and its interaction with proteins. Biochimica et Biophysica Acta (BBA)-General Subjects 1978, 541, 270–277. [Google Scholar] [CrossRef]
- Sghaier, D.B.; Pedro, S.; Duarte, B.; Caçador, I.; Sleimi, N. Photosynthetic Responses of Two Salt-Tolerant Plants, Tamarix gallica and Arthrocnemum indicum Against Arsenic Stress: A Case Study. Arsenic in Plants: Uptake, Consequences and Remediation Techniques.
- Shah, W.H.; Rasool, A.; Saleem, S.; Mushtaq, N.U.; Tahir, I.; Hakeem, K.R.; Rehman, R.U. Understanding the integrated pathways and mechanisms of transporters, protein kinases, and transcription factors in plants under salt stress. International Journal of Genomics 2021. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Critical reviews in plant sciences 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Shi, H.; Lee, B.-H. , Wu, S.-J., and Zhu, J.-K. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature biotechnology 2003, 21, 81–85. [Google Scholar] [CrossRef]
- Shi, Q.; Ding, F.; Wang, X.; Wei, M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant physiology and biochemistry 2007, 45, 542–550. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi journal of biological sciences 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Sikder, R.K.; Wang, X.; Jin, D.; Zhang, H.; Gui, H.; Dong, Q.; Pang, N.; Zhang, X.; Song, M. Screening and evaluation of reliable traits of upland cotton (Gossypium hirsutum L.) genotypes for salt tolerance at the seedling growth stage. Journal of Cotton Research 2020, 3, 1–13. [Google Scholar] [CrossRef]
- Singh, A. Potassium (K+) transporters in plants: regulation and functional role in K+ uptake and homeostasis. In Cation Transporters in Plants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–47. [Google Scholar]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc Oxide Nanoparticles Improve Salt Tolerance in Rice Seedlings by Improving Physiological and Biochemical Indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, V.; Sharma, J.; Saini, S.; Sharma, P.; Kumar, S.; Sinhmar, Y.; Kumar, D.; Sharma, A. Silicon supplementation alleviates the salinity stress in wheat plants by enhancing the plant water status, photosynthetic pigments, proline content and antioxidant enzyme activities. Plants 2022, 11, 2525. [Google Scholar] [CrossRef]
- Solebo, O.R. (2021). The Biochemical Functionality and Cellular Importance of the Vacuolar H+ Translocating Pyrophosphatase 1, PfVP1, in Plasmodium Falciparum. Drexel University.
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Somaddar, U.; Dey, H.C.; Mim, S.K.; Sarker, U.K.; Uddin, M.R.; Ahmed, N.U.; Mostofa, M.G.; Saha, G. Assessing Silicon-Mediated Growth Performances in Contrasting Rice Cultivars under Salt Stress. Plants 2022, 11, 1831. [Google Scholar] [CrossRef]
- Song, Y.; Ci, D.; Tian, M.; Zhang, D. Comparison of the physiological effects and transcriptome responses of Populus simonii under different abiotic stresses. Plant molecular biology 2014, 86, 139–156. [Google Scholar] [CrossRef]
- Soori, N.; Bakhshi, D.; Rezaei, N.A.; Faizian, M. Effect of salinity stress on some physiological characteristics and photosynthetic parameters of several Iranian commercial pomegranate genotypes. 2019.
- Sottosanto, J.B.; Gelli, A.; Blumwald, E. DNA array analyses of Arabidopsis thaliana lacking a vacuolar Na+/H+ antiporter: impact of AtNHX1 on gene expression. The Plant Journal 2004, 40, 752–771. [Google Scholar] [CrossRef]
- Stigter, T.; Ribeiro, L.; Dill, A. Evaluation of an intrinsic and a specific vulnerability assessment method in comparison with groundwater salinisation and nitrate contamination levels in two agricultural regions in the south of Portugal. Hydrogeology journal 2006, 14, 79–99. [Google Scholar] [CrossRef]
- Suman, S. Plant tissue culture: A promising tool of quality material production with special reference to micropropagation of banana. Biochem. Cell. Arch 2017, 17, 1–26. [Google Scholar]
- Sun, H.; Sun, X.; Wang, H.; Ma, X. Advances in salt tolerance molecular mechanism in tobacco plants. Hereditas 2020, 157, 1–6. [Google Scholar] [CrossRef]
- Sun, L.J.; Zhao, X.Y.; Ren, J.; Yan, S.P.; Zhao, X.Y.; Song, X.S. Overexpression of Cerasus humilis ChAOX2 improves the tolerance of Arabidopsis to salt stress. 3 Biotech 2021, 11, 1–11. [Google Scholar]
- Talaat, N.B.; Mostafa, A.A.; El-Rahman, S.N.A. A novel plant growth–promoting agent mitigates salt toxicity in barley (Hordeum vulgare L.) by activating photosynthetic, antioxidant defense, and methylglyoxal detoxification machineries. Journal of Soil Science and Plant Nutrition.
- Tanveer, M.; Ahmed, H.a.I. ROS signalling in modulating salinity stress tolerance in plants. In Salt and drought stress tolerance in plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 299–314. [Google Scholar]
- Tian, C.; Feng, G.; Li, X.; Zhang, F. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Applied Soil Ecology 2004, 26, 143–148. [Google Scholar] [CrossRef]
- Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regulation 2015, 77, 343–356. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: a review. Amino acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Vivaldi, G.A.; Camposeo, S.; Romero-Trigueros, C.; Pedrero, F.; Caponio, G.; Lopriore, G.; Álvarez, S. Physiological responses of almond trees under regulated deficit irrigation using saline and desalinated reclaimed water. Agricultural Water Management 2021, 258, 107172. [Google Scholar] [CrossRef]
- Wall, S.; Vialet-Chabrand, S.; Davey, P.; Van Rie, J.; Galle, A.; Cockram, J.; Lawson, T. Stomata on the abaxial and adaxial leaf surface contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytologist 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fu, X.; Zhao, W.; Zhang, M.; Chen, F. Ectopic Expression of Maize Plastidic Methionine Sulfoxide Reductase ZmMSRB1 Enhances Salinity Stress Tolerance in Arabidopsis thaliana. Plant Molecular Biology Reporter 2022, 40, 284–295. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.-H. , Zhang, J.-Y., and Cheng, Z.-M.M. Cloning, molecular and functional characterization by overexpression in Arabidopsis of MAPKK genes from grapevine (Vitis vinifera). BMC plant biology 2020, 20, 1–15. [Google Scholar]
- Wang, G.; Zhang, L.; Zhang, S.; Li, B.; Li, J.; Wang, X.; Zhang, J.; Guan, C.; Ji, J. The combined use of a plant growth promoting Bacillus sp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system, enhancing photosynthesis and improving soil enzyme activities. Microbiological Research 2023, 266, 127225. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Liu, X.; Dong, X.; Zhang, Z.; Fan, H.; Zhang, L.; Wang, J.; Shi, S.; Tu, P. Salinity stress induces the production of 2-(2-phenylethyl) chromones and regulates novel classes of responsive genes involved in signal transduction in Aquilaria sinensis calli. BMC plant biology 2016, 16, 1–20. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant physiology and biochemistry 2019, 135, 385–394. [Google Scholar]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. The Crop Journal 2016, 4, 162–176. [Google Scholar]
- Wei, X.; Huang, X.; Yang, W.; Wang, X.; Guan, T.; Kang, Z.; Liu, J. A Chloroplast-Localized Glucose-6-Phosphate Dehydrogenase Positively Regulates Stripe Rust Resistance in Wheat. International Journal of Molecular Sciences 2022, 24, 459. [Google Scholar] [PubMed]
- Wei, Y.; Xu, Y.; Lu, P.; Wang, X.; Li, Z.; Cai, X.; Zhou, Z.; Wang, Y.; Zhang, Z.; Lin, Z. Salt stress responsiveness of a wild cotton species (Gossypium klotzschianum) based on transcriptomic analysis. PLoS One 2017, 12, e0178313. [Google Scholar]
- Williams, L.; Baeza, P.; Vaughn, P. Midday measurements of leaf water potential and stomatal conductance are highly correlated with daily water use of Thompson Seedless grapevines. Irrigation Science 2012, 30, 201–212. [Google Scholar]
- Wu, L.; Huo, W.; Yao, D.; Li, M. Effects of solid matrix priming (SMP) and salt stress on broccoli and cauliflower seed germination and early seedling growth. Scientia horticulturae 2019, 255, 161–168. [Google Scholar]
- Xu, F.; Xu, S.; Wiermer, M.; Zhang, Y.; Li, X. The cyclin L homolog MOS12 and the MOS4-associated complex are required for the proper splicing of plant resistance genes. The Plant Journal 2012, 70, 916–928. [Google Scholar]
- Xu, H.; Lu, Y.; Tong, S. Effects of arbuscular mycorrhizal fungi on photosynthesis and chlorophyll fluorescence of maize seedlings under salt stress. Emirates Journal of Food and Agriculture.
- Yadav, S.P.; Bharadwaj, R.; Nayak, H.; Mahto, R.; Singh, R.K.; Prasad, S.K. Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. International Journal of Chemical Studies 2019, 7, 1793–1798. [Google Scholar]
- Yan, K.; Mei, H.; Dong, X.; Zhou, S.; Cui, J.; Sun, Y. Dissecting photosynthetic electron transport and photosystems performance in Jerusalem artichoke (Helianthus tuberosus L.) under salt stress. Frontiers in Plant Science 2022, 13, 905100. [Google Scholar]
- Yang, G.; Yu, L.; Zhang, K.; Zhao, Y.; Guo, Y.; Gao, C. A ThDREB gene from Tamarix hispida improved the salt and drought tolerance of transgenic tobacco and T. hispida. Plant Physiology and Biochemistry 2017, 113, 187–197. [Google Scholar]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. "Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 2021, 7–50. [Google Scholar]
- Yang, Y.; Xie, J.; Li, J.; Zhang, J.; Zhang, X.; Yao, Y.; Wang, C.; Niu, T.; Bakpa, E.P. Trehalose alleviates salt tolerance by improving photosynthetic performance and maintaining mineral ion homeostasis in tomato plants. Frontiers in Plant Science 2022, 13, 974507. [Google Scholar] [PubMed]
- Yang, Y.M.; Zhu, Y.; Naseer, M.; Wang, Q.; Li, G.; Tao, H.Y.; Zhu, S.G.; Wang, B.Z.; Wang, W.; Xiong, Y.C. Rhizosphere effect of nanoscale zero-valent iron on mycorrhiza-dependent maize assimilation. Plant, Cell & Environment 2023, 46, 251–267. [Google Scholar]
- Yao, X.; Horie, T.; Xue, S.; Leung, H.-Y. , Katsuhara, M.; Brodsky, D.E.; Wu, Y.; Schroeder, J.I. Differential sodium and potassium transport selectivities of the rice OsHKT2; 1 and OsHKT2; 2 transporters in plant cells. Plant physiology 2010, 152, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ağar, G.; Ors, S.; Dursun, A.; Kul, R.; Akgül, G. Physiological and Biochemical Changes of Pepper Cultivars Under Combined Salt and Drought Stress. Gesunde Pflanzen 2022, 1–9. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Turkan, I. Flavonoid naringenin alleviates short-term osmotic and salinity stresses through regulating photosynthetic machinery and chloroplastic antioxidant metabolism in Phaseolus vulgaris. Frontiers in plant science 2020, 11, 682. [Google Scholar]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta physiologiae plantarum 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, D.-D. , Min, D.-H., Cao, T.; Ning, L.; Jiang, Q.-Y., Sun, X.-J., Zhang, H.; Tang, W.-S., and Gao, S.-Q. (2022). Foxtail millet MYB-like transcription factor SiMYB16 confers salt tolerance in transgenic rice by regulating phenylpropane pathway. Plant Physiology and Biochemistry.
- Yue, J.-Y. , Jiao, J.-L., Wang, W.-W., and Wang, H.-Z. The Calcium-Dependent Protein Kinase TaCDPK27 Positively Regulates Salt Tolerance in Wheat. International Journal of Molecular Sciences 2022, 23, 7341. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiology and Biochemistry 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, S.; Pan, J.; Kong, X.; Zhou, Y.; Liu, Y.; Li, D. The overexpression of a maize mitogen-activated protein kinase gene (Z m MPK 5) confers salt stress tolerance and induces defence responses in tobacco. Plant biology 2014, 16, 558–570. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Huang, J.; Xia, J. OsNAC45 is involved in ABA response and salt tolerance in rice. Rice 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Xu, X.; Cai, C.; Guo, W. Genome-wide identification of mitogen-activated protein kinase gene family in Gossypium raimondii and the function of their corresponding orthologs in tetraploid cultivated cotton. BMC plant biology 2014, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+/K+ balance and transport regulatory mechanisms in weedy and cultivated rice (Oryza sativa L.) under salt stress. BMC plant biology 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. The Crop Journal 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K. , and Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. The innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Zhao, W.; Jung, S.; Schubert, S. Transcription profile analysis identifies marker genes to distinguish salt shock and salt stress after stepwise acclimation in Arabidopsis thaliana and Zea mays. Plant Physiology and Biochemistry 2019, 143, 232–245. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, T.; Guo, S.; Hu, J.; Zhan, Y. Ectopic Expression of AeNAC83, a NAC Transcription Factor from Abelmoschus esculentus, Inhibits Growth and Confers Tolerance to Salt Stress in Arabidopsis. International Journal of Molecular Sciences 2022, 23, 10182. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, G.; Zhou, S.; Ren, Y.; Wang, W. The improvement of salt tolerance in transgenic tobacco by overexpression of wheat F-box gene TaFBA1. Plant Science 2017, 259, 71–85. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, Y.; Zhou, H.; Hong, Y.; Zhao, C.; Shabala, S.; Lv, C.; Guo, B.; Zhou, M.; Xu, R. Genome wide association study and haplotype analysis reveals the role of HvHKT1; 5 in potassium retention but not Na+ exclusion in barley (Hordeum vulgare L.). Environmental and Experimental Botany 2022, 201, 104973. [Google Scholar] [CrossRef]
- Zou, J.; Hu, W.; Li, Y.; Zhu, H.; He, J.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Wang, S. Leaf anatomical alterations reduce cotton’s mesophyll conductance under dynamic drought stress conditions. The Plant Journal 2022. [Google Scholar] [CrossRef]
| Name of Genes | Function | Upregulated/ Downregulated | References |
|---|---|---|---|
| ETR1/ ETR2/ EIN4 | Increase salt tolerance | Downregulated | (Arif et al., 2020) |
| B-ARR, CRE1 | Improve plant development with ion homeostasis | Downregulated | (Song et al., 2014) |
| LHC | Adapt the light-harvesting system | Downregulated | (Ansari et al., 2022) |
| BRI1/2 | Ion homeostasis improves plant development | Downregulated | (Brenya, 2020) |
| MPK3, MEKK2 | Ion homeostasis, osmoprotectant | Downregulated | (Billah et al., 2021) |
| NPR1 | Reduce the input, exclusion, and sequestration of salt | Upregulated | (Bor and Özdemir, 2018) |
| GmST1 | Exclusion of salt and improvement of the immune and antioxidant systems | Upregulated | (Kaushal, 2020) |
| SAUR, ARF | Promotes plant growth and balance | Upregulated | (Zhao et al., 2022) |
| CaM, CDPK, CML, CBL | Ion homeostasis enhances plant development and growth | Upregulated | (Shah et al., 2021) |
| MYC2, TGA | Increase the production of jasmonates and activate plant defenses | Upregulated | (Delgado et al., 2021) |
| CCOMT | Promote the biosynthesis of lignin and suberin. | Upregulated | (Yu et al., 2022) |
| NRT1/PTR, CAX1 | Enhance the antioxidant system and control calcium homeostasis | Upregulated | (Mitra, 2018) |
| SOS3/,SOS2/ CIPK24 | Salt exclusion and sequestration | Upregulated | (Mahajan et al., 2008) |
| SDIR1 | Salt exclusion and sequestration | Upregulated | (Zhao et al., 2017) |
| SDIR1 | Salt homeostasis, decrease senescence | Upregulated | (Johnson, 2016) |
| BAK1 | Exclusion and sequestration of salt | Upregulated | (Lin et al., 2020) |
| NCED | Osmoprotectant, closure of the stomata, development of lateral roots, and salt acclimation | Upregulated | (Wani et al., 2016) |
| LAX | Vascular development | Upregulated | (Chai et al., 2016) |
| SWEET15 | Modify vacuolar transit and sugar storage | Upregulated | (Zhao et al., 2019) |
| RCA1, AOX1A | Modulate photosynthesis | Upregulated | (Albaladejo et al., 2018) |
| LRR-RLK, CRR-RLK, | Plant development and growth | Upregulated | (Wang et al., 2016) |
| NHX1 | Transport of salt from the cytosol to the vacuole | Upregulated | (Graus et al., 2022) |
| SOS, VDAC | Salt exclusion, ion homeostasis, and homeostasis | Upregulated | (Dong and Wang, 2022) |
| AtNHX1 | Enhanced germination and increased biomass | Upregulated | (Ren et al., 2022) |
| OsNHX1 | Enhanced biomass | Upregulated | (Mansour, 2022) |
| GutD | Enhanced biomass | Upregulated | (Khan et al., 2022) |
| BADH | Better resiliency to salt stress and development | Upregulated | (Chen et al., 2022) |
| AtNHX1 | Improved germination and increased biomass | Upregulated | (Deng et al., 2023) |
| Bt | Improved chlorophyll growth and stability | Upregulated | (Talaat et al., 2022) |
| CAX1 | Enhance the antioxidant system and control calcium homeostasis | Upregulated | (Wei et al., 2017) |
| ZEP, ABA 8′- OH, ABI5 | Osmoprotectant, closure of the stomata, development of lateral roots, and salt acclimation | Upregulated | (Bartels and Sunkar, 2005) |
| Gene name | Role in Gene | Origin Gene | References |
|---|---|---|---|
| VvNAC17 | An osmotic regulation-related tonoplast intrinsic protein | Glycine max | (Yang et al., 2021) |
| VvNAC17 | Increases ABA and stress-related gene expression | Vitis vinifera | (Ju et al., 2020) |
| PpSARK | ABA-related senescence-associated receptor-like kinase | Physcomitrella patens | (Li et al., 2018b) |
| AtGSTU19 | Effectively fine-tune redox homeostasis at the root | Arabidopsis thaliana | (Horváth et al., 2019) |
| AtGSTF8 | A root-level process that fine-tunes the redox homeostasis | Arabidopsis thaliana | (Wang et al., 2022) |
| SlMYB102 | A transcriptional regulator of stress reactions | Solanum Lycopersicum | (Jia et al., 2021) |
| ThDREB | A transcription factor that plays a role in stress responses | Tamarix his pida | (Yang et al., 2017) |
| AtMYB20 | Negatively controls serine/threonine protein phosphatases of type 2C | Arabidopsis thaliana | (Nguyen et al., 2019) |
| AtWRKY33 | Regulated salt responsive gene improved salt tolerance AtKUP2 | Arabidopsis thaliana | (Rajappa et al., 2020) |
| AtbHLH122 | Enhanced salt sensitivity via controlling the salt-sensitive gene AtKUP2 | Arabidopsis thaliana | (Singh, 2022) |
| GmbZIP2 | A gene transcription factor that plays a role in the response to salt stress. | Glycine max | (Ke et al., 2022) |
| GhMPK2 | MAPK signaling via protein kinase | Gossypium herbaceum | (Zhang et al., 2014b) |
| GhMAP3K40 | In MAPK signaling, protein kinase | Gossypium herbaceum | (Ashraf et al., 2018a) |
| ZmMPK5 | MAPK signaling via protein kinase | Zea mays | (Zhang et al., 2014a) |
| ZmMKK4 | Protein kinase in MAPKs signaling | Zea mays | (Kong et al., 2011) |
| VvMKK4 | MAPK signaling protein kinase | Vitis vinifera | (Wang et al., 2020) |
| VvMKK2 | protein kinase in MAPKs signaling | Vitis vinifera | (Çakır and Kılıçkaya, 2015) |
| PtMAPKK4 | protein kinase in MAPKs signaling | Populus trichocarpa | (Sun et al., 2020) |
| AtCPK12 | protein kinase in CDPKs signaling | Arabidopsis thaliana | (Hrabak et al., 1996) |
| AtCDPK27 | CDPK signaling involves a membrane-localized protein kinase | Arabidopsis thaliana | (Yue et al., 2022) |
| OsMADS25 | A transcription factor that participates in ABA-mediated regulatory pathways | Oryza sativa | (Hussain et al., 2021) |
| OsNAC45 | A transcription factor is involved in several stress and ABA signaling responses | Oryza sativa | (Zhang et al., 2020) |
| ChVDE | Violaxanthin de-epoxidase catalyzes the transformation | Cerasus humilis | (Sun et al., 2021) |
| AVP1 | Vacuolar H+ phosphorylase | Arabidopsis thaliana | (Solebo, 2021) |
| TaTVP1 | Vacuolar H+ phosphorylase | Triticum aestivuml | (Hao et al., 2021) |
| PtVP1.1 | vacuolar H+ phosphorylase | Populus trichocarpa | (Gao et al., 2022) |
| VrNHX1 | Na+/H+ vacuolar antiporter | Vigna radiata | (Bai et al., 2023) |
| P5CS1 | A protein that regulates the rate of proline biosynthesis | Phaseolus vulgaris | (Hosseinifard et al., 2022) |
| P5CS2 | The enzyme responsible for limiting the rate of proline biosynthesis | Phaseolus vulgaris | (Chen et al., 2010) |
| LrAMADH1 | Betaine aldehyde to betaine converting catalyzed | Lycium ruthenicum Murr | (Liu et al., 2018) |
| SlBADH | Promote the transformation of betaine aldehyde to betaine | Suaeda liaodonggensis | (Li et al., 2003) |
| AtTPPD | The dephosphorylation of trehalose 6-phosphate to form trehalose is instigated | Arabidopsis thaliana | (Krasensky et al., 2014) |
| HvHKT1;1 | When the external K+ level is low, transports Na+ and mediates Na+ absorption. | Hordeum vulgare | (Haro et al., 2005) |
| HvHKT1;5 | Negatively transport Na+ in Barley | Hordeum vulgare | (Zhu et al., 2022) |
| OsHKT1 | Transport Na+ | Oryza sativa | (Zhang et al., 2018) |
| OsHKT2 | Transport Na+ | Oryza sativa | (Yao et al., 2010) |
| OsVHA | Transport Na+ | Oryza sativa | (Kader et al., 2006) |
| AtNHX1 | Vacuolar Na+/H+ antiporter | Arabidopsis thaliana | (Sottosanto et al., 2004) |
| LfNHX1 | Na+/H+ vacuolar | Leptochloa fusca | (Rauf et al., 2014) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





