1. Introduction
A growing number of epidemiological studies have found that diabetes mellitus increases the risk of developing many types of cancer [
1]. The association between these two diseases was first time discussed over 75 years ago. Recent and multiple evidence had shown that diabetes is associated with an increased risk of cancer and a higher mortality rate in cancer patients [
2]. An epidemiologic study that enrolled 420 patients with type 2 diabetes mellitus concluded that the most frequent association between diabetes and neoplasia was for lung, breast, pancreatic, and colorectal cancers [
3]. Lactate dehydrogenase (LDH) levels, complete blood count, carcinoembryonic antigen (CEA), glycated hemoglobin (HbA1c), and C-reactive protein (CRP) are accessible biomarkers, forming part of the standard patient assessment; it could be predictive factors for cancer in diabetic patients and can guide the oncologist in terms of the patient prognosis at the onset of treatment [
4]. We believe that these biomarkers can also be used by a diabetes doctor to be able to suspect the presence of cancer, especially when an unexplained glycemic imbalance occurs. Developing colorectal cancer is 27% more possible in patients with type 2 diabetes mellitus than in non-diabetic patients [
5]. Various studies have also demonstrated that the risk of colon cancer recurrence is higher in diabetic patients [
5].
One study of 47 patients with diabetes and colorectal cancer showed an average HbA1c value of 6.0%, and 45% of them had an HbA1c of at least 7% [
5]. There is insufficient information on CEA in colorectal cancer patients with diabetes. Several studies showed a statistically significant correlation between elevated CEA levels and diabetes as well as a correlation between serum CEA levels and HbA1c levels [
5]. Insulin resistance and relative insulin deficiency are characteristic of type 2 diabetic patients, they are often overweight and elderly with diabetes oncoming than type 1 diabetic subjects. Obesity promotes insulin resistance and is considered one of the main reasons for the current diabetes epidemic [
5]. Albumin is a major protein synthesized in the liver. Energy intake is a very important factor in the normal physiology of albumin production. In fact, reduced serum albumin levels are observed in diseases associated with malnutrition, while high serum albumin levels are associated with metabolic syndrome, an indicator of obesity and overeating. In addition, a link between serum albumin and insulin resistance has recently been suggested [
6].
Long-term type 2 diabetes mellitus is associated with about 1.5 increased risk of pancreatic cancer. [
7] A causal relationship between diabetes and pancreatic cancer is also supported by prediagnosis measurements of glucose and insulin levels in prospective studies [
7]. Insulin resistance and associated hyperglycemia, hyperinsulinemia, and inflammation are the underlying mechanisms contributing to the development of diabetes-associated pancreatic cancer. The mechanism of the relationship between diabetes and pancreatic cancer is elusive and could include metabolic, hormonal, and immunologic changes that influence tumor growth. Insulin resistance and compensatory hyperinsulinemia are maybe the most suspected mechanisms underlying the relationship between type 2 diabetes mellitus and pancreatic cancer [
7].
Diabetes was associated with a significantly more pronounced risk of lung cancer compared with patients without diabetes when the analysis was restricted to studies that accounted for smoking status. In contrast, this association disappeared when the analysis was restricted to studies that did not consider smoking status [
8]. Subjects with type 1 and type 2 diabetes had a higher level of fasting plasma lactate, versus the group without diabetes [
9].
Chronic hyperlactatemia maintained by increased lactate formation from adipocytes in obese individuals has been found to precede the onset of diabetes [
10] and might contribute to the appearance of the disease. Taken together, these data suggest that chronic hyperlactatemia may indicate the early stages of insulin resistance. Several epidemiological analyses have shown that high lactate levels may predict the onset of diabetes.
LDH’s prognostic value in patients with lung cancer was investigated by some researchers, but the findings were inconclusive. According to some studies, patients with lung cancer have worse prognoses when their LDH levels are higher. Some researchers discovered that this correlation was insignificant [
11]. Higher pretreatment LDH levels have been associated with poorer overall survival in subjects with lung cancer [
11].
Anemia is the most common hematologic change in patients with malignancies [
12]. It may be the first diagnostic clue for underlying malignant disease and contribute to symptomatology and treatment decisions. Cytokine production associated with the tumor is an important factor in the appearance of anemia in cancer patients. A malignant tumor can also affect bone marrow – fibrosis -, which can also result in anemia. Bone marrow is known for its rich blood supply and is, therefore, a common site for metastasis to develop [
13]. Bone marrow fibrosis, originally characterized by Wartofsky and Burman, was originally called "low T3 syndrome" [
13]. Breast malignancy, prostate, and lung are the most involved types of cancer, although almost all types of cancers can lead to this complication. Normocytic, normochromic anemia occurs frequently in patients with a variety of inflammatory disorders, with many contributing factors.
The objectives of the study were to assess the utility of CEA, CRP, serum albumin level, hemoglobin, and LDH as biomarkers of cancer risk and the biological implications of diabetes on the evolution and prognosis of oncological patients.
2. Materials and Methods
We performed a retrospective, longitudinal, observational study, over a period of 2 years, between 2016 and 2017, on two equal groups of patients, 217 already having type 2 diabetes and recently diagnosed with breast, lung, colorectal, or pancreatic cancer (the most frequent types of tumors associated with type 2 diabetes), and the other 217, without diabetes. All of them were hospitalized in the Oncology Medical Clinic of the Clinical County Emergency Hospital Craiova, Romania.
Criteria for inclusion: patients with a maximum performance status of 2, hospitalized in the Oncology Medical Clinic of Emergency Clinical County Hospital Craiova (Romania) at the initiation of oncologic treatment, with the diagnosis of lung, breast, colon, or pancreatic cancer, who signed an informed consent. These patients were divided into two subgroups: subgroup 1 with type 2 diabetes, and subgroup 2 without diabetes.
Criteria for exclusion – both patients with other types of tumors and patients with other types of inflammatory, acute, or chronic conditions were excluded; also, patients for whom insufficient data was recorded or who did not sign an informed consent.
The study group was represented by 217 patients, already with type two diabetes, who were hospitalized in the Oncology Medical Clinic of the Emergency Clinical County Hospital Craiova (ECCHC), in the period 2016 – 2017, with a performance status of a maximum of 2, with the intention of beginning oncologic specific treatment: 81 patients with lung cancer, 45 patients with colon cancer, 38 patients with pancreatic cancer, and 53 patients with breast cancer). After that, another equal group was formed, composed of 217 patients – a group without type 2 diabetes. All data, for all 434 patients, were collected from the medical records of patients hospitalized in the Oncology Medical Clinic of ECCHC.
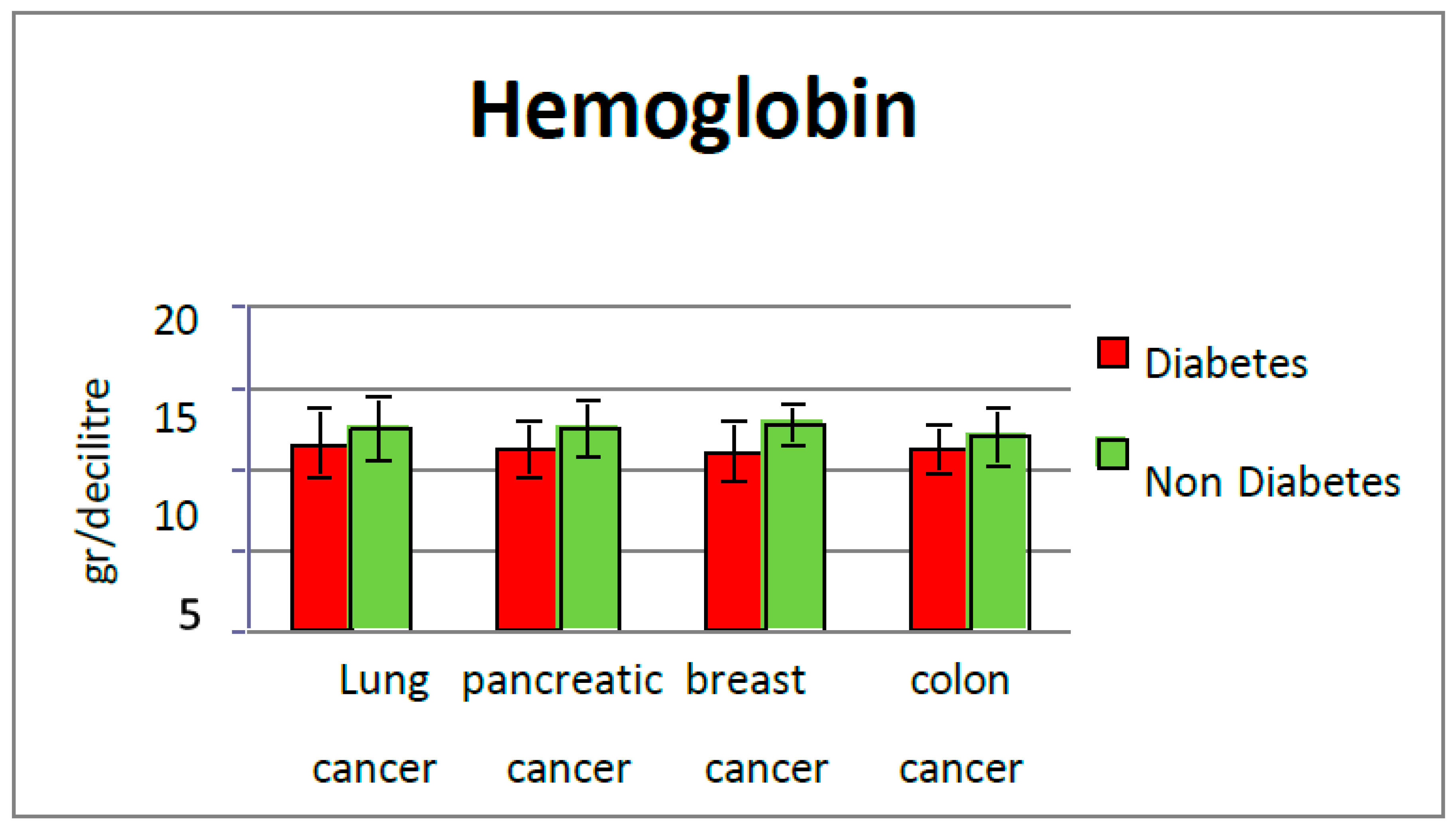
In this study, at the moment of the first admission, we evaluated the values of hemoglobin, CRP, HbA1c, LDH, alkaline phosphatase (FAL), serum albumin, and CEA in subgroup 1, with diabetes, and we compared them with the values found in subgroup 2, without diabetes. The HbA1c values in subgroup 2 were less than 5.7%, so type 2 diabetes and prediabetes were excluded [
5,
7], not only through HbA1c but also through personal history.
There were used the following ECCHC laboratory analyzers: COULTER DXH for hemoglobin, MINDRAY BS 800 for CRP and LDH levels, ARCHITECT C8000 for serum albumin, COBAS E411-2 for CEA and HbA1c.
Statistical analysis was realized using Microsoft Office Pack - Microsoft Excel 2000 to collect data and obtain graphics and average values, and the MATLAB v.9.0 (2016) Student T-Test program, to test the normality of data and to expose the statistically significant differences between the two analyzed groups, the average values in the study group is compared with those of patients in the group without diabetes, a p-value < 0.05 being considered statistically significant. A confidence coefficient was also used, ‘r’ value close to 1 considering a positive correlation.
Ethical concerns. The protocol of the study was approved by the Ethics Committee of the University of Medicine and Pharmacy of Craiova, approval number 39/20.01.2023.
4. Discussion
This study was designed to assess the utility of CEA, CRP, serum albumin level, hemoglobin, and LDH as biomarkers of cancer risk (colon, lung, breast, pancreatic cancer) in obese and/or diabetic patients, and for studying correlations between them and the prognosis of cancer patients with associated type 2 diabetes, being at the onset of treatment.
In our study, it was found that anemia, hypoalbuminemia, elevated lactate dehydrogenase, glycated hemoglobin, and C-reactive protein levels are more pronounced in subjects with type 2 diabetes and cancer (p = 0.002). These biomarkers may be an indicator of a patient's inflammatory state for diabetic patients, and a neoplasm can be sought.
Hematologic markers that reflect systemic inflammation can also be used to predict the prognosis of cancer. Systemic inflammatory responses play a significant role in carcinogenesis, cancer progression influencing tumoral responses under oncologic treatment, and survival [
4].
Recent studies show that type 2 diabetes is an independent risk factor for the progression of several cancers. Although these two diseases have an important number of common risk factors, the connection between them is still not well understood, posing a challenge for clinical management [
9].
The incidence of diabetes has been increasing in the past two decades (464.237 million in 2019) and is expected to increase further, estimated at more than 700 million in 2045 [
14,
15].
Given the increasing interest of the European Community and the Romanian government in implementing comprehensive prevention programs against the spread of cancer and type 2 diabetes [
31] and the fact that many diabetic patients develop a malignancy during their lifetime [
4], we wanted also to investigate the impact of type 2 diabetes on the oncological patient and how predictive factors can be used. Diabetes is a concomitant disease that can also influence the therapeutic response to cancer therapy [
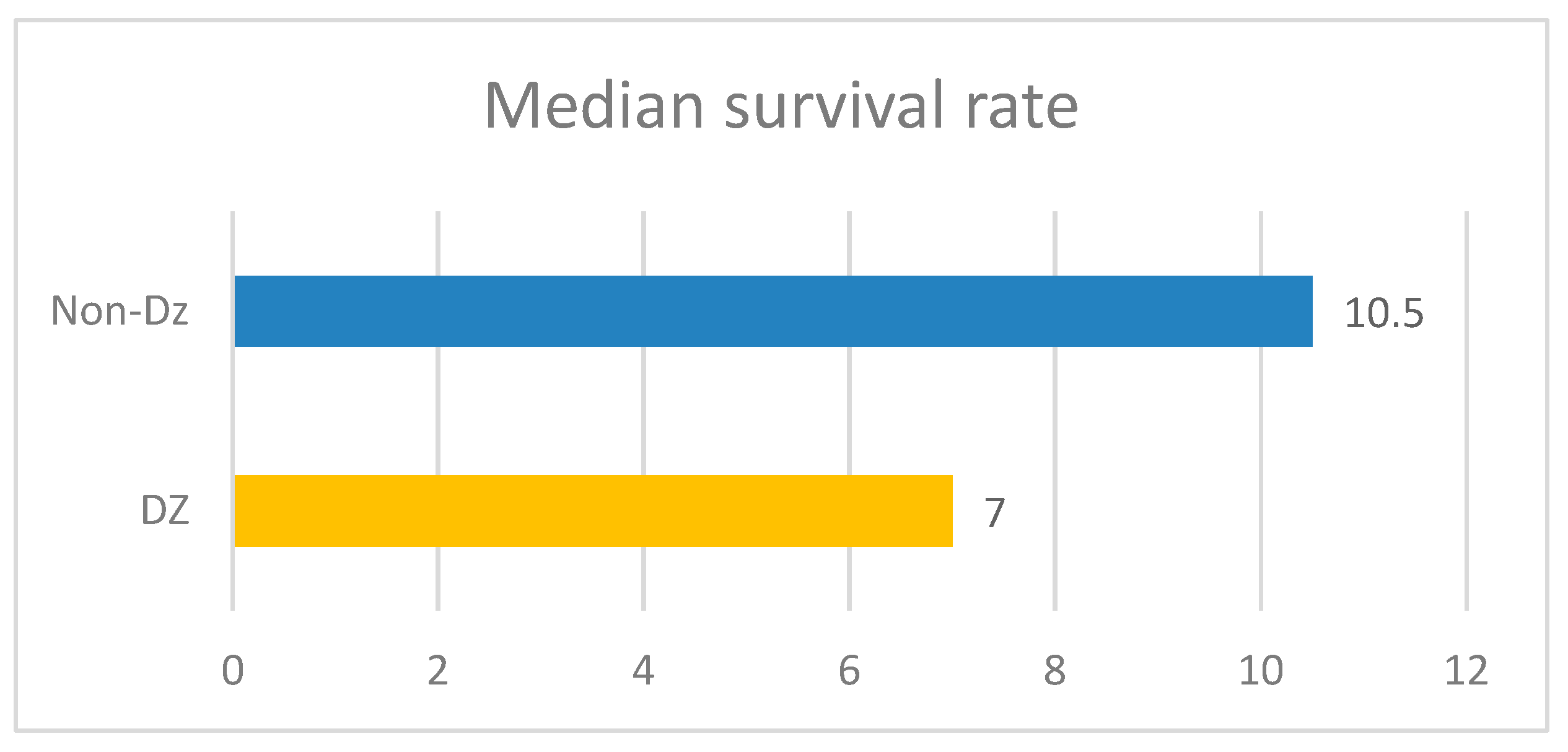
4]. The duration of treatment in a patient who also has diabetes was shorter compared to those patients without diabetes, which also has an impact on survival, its median is 7 months in the diabetic group, compared to 10 months in cancer patients without diabetes.
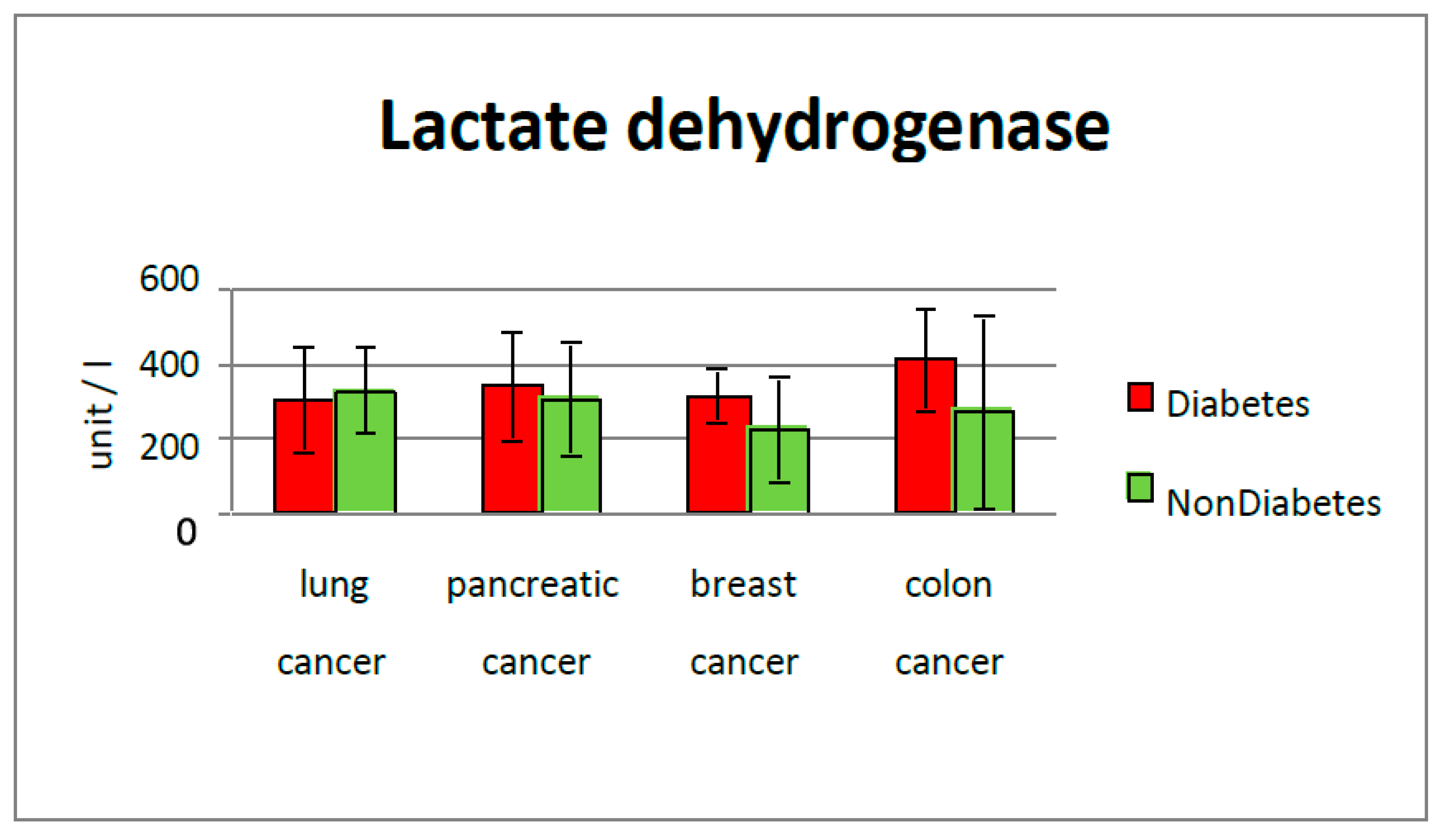
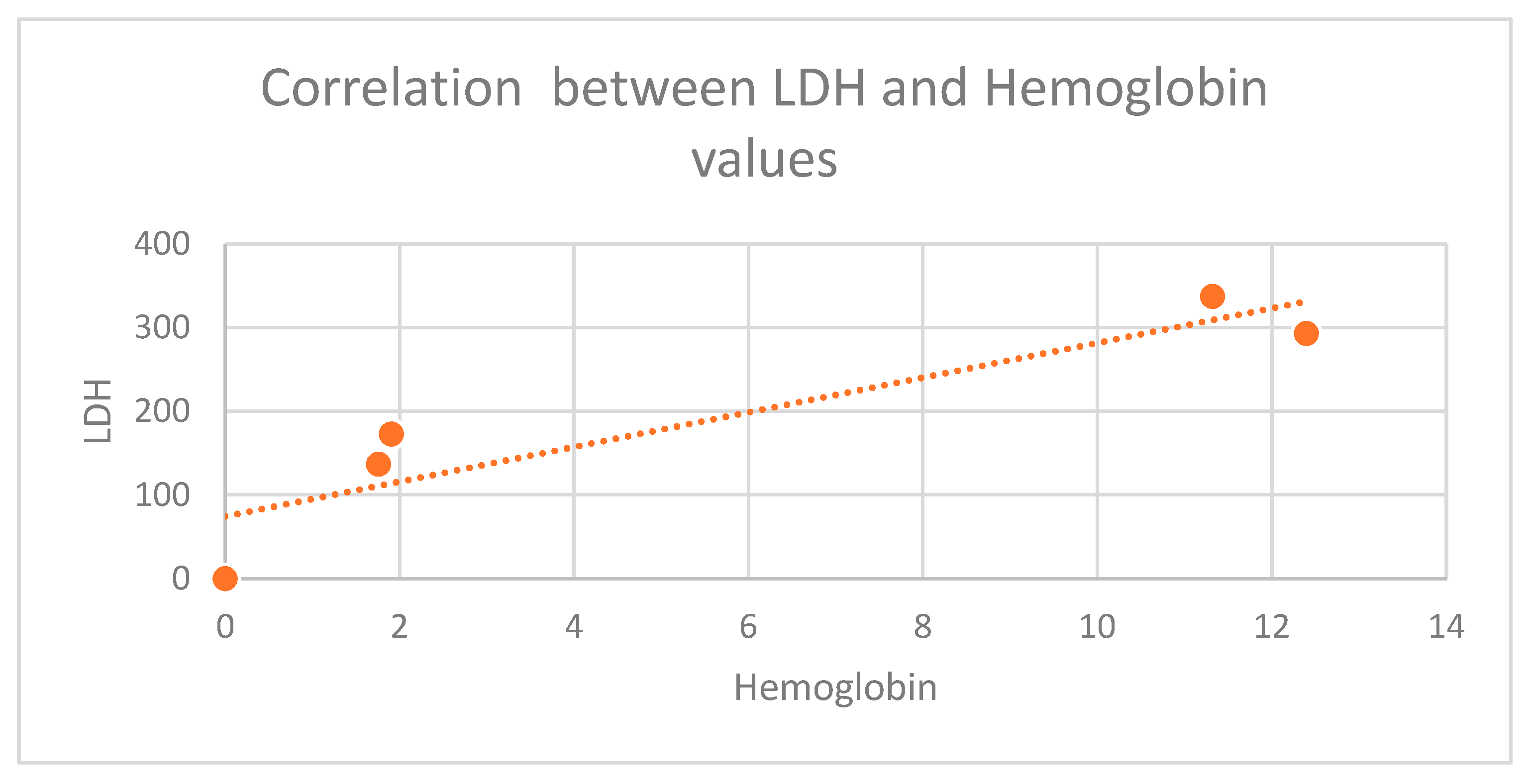
LDH level in the diabetic group for each type of cancer that was studied, but the most significant difference was in colorectal cancer (411 mg/dL vs. 273 mg/dL in the group without diabetes), and breast cancer sub-group (312 mg/dL vs. 223 mg/dL in non-diabetic cases). Increased lactate alters the microenvironment, provides nutrients to cancer cells, and leads to acidosis, inflammation, angiogenesis, immunosuppression, and radiation resistance. Bonuccelli et al (2010) showed that ketones and lactate promote tumor growth and metastatic disorders, which may state why diabetic subjects have increased cancer incidence and poor prognosis due to increased lactate production [
16]. The body of a human contains LDH in a variety of tissues. In order for lactic acid and pyruvic acid to react, an enzyme called LDH is required. Usually, anaerobic environments like the intratumoral environment are where the reaction between lactic acid and pyruvic acid takes place. The environment around tumors contains high levels of LDH reflex anaerobic glycolytic metabolism. The patients in the high metastatic score group had significantly higher serum LDH concentrations than those in the low metastatic score group, according to other studies [
16].
A major reason for the development of cancer is that the immune system loses its ability to effectively eliminate aberrant cells. High levels of lactate have a deleterious effect on immune cells infiltrating the tumor. Finally, lactate is an inflammatory mediator [
18] and could be a biomarker of inflammatory processes promoting tumor development [
19]. This specific inflammatory microenvironment also promotes tumor metastasis [
4], leading to a negative prognosis [
20].
Malignancies can also attack the bone marrow (bone marrow fibrosis), which can also lead to anemia. The bone marrow has a rich blood supply and is, therefore, a frequent site for the development of metastases [
13]. Breast cancer, prostate, and lung malignancies are most frequently associated with anemia, although anemia may be encountered in almost all cancers. Normocytic normochromic anemia is also common in patients with several inflammatory diseases. Iron may be plentiful in the bone marrow but is not absorbed and does not enter the bloodstream, making it unavailable for erythropoiesis [
12]. Due to diabetes mellitus, nephropathy may occur, which further undermines renal production of erythropoietin, contributing to anemia [
21]. Anemia in diabetic patients affects the quality of life and is associated with disease progression and the development of comorbidities [
22].
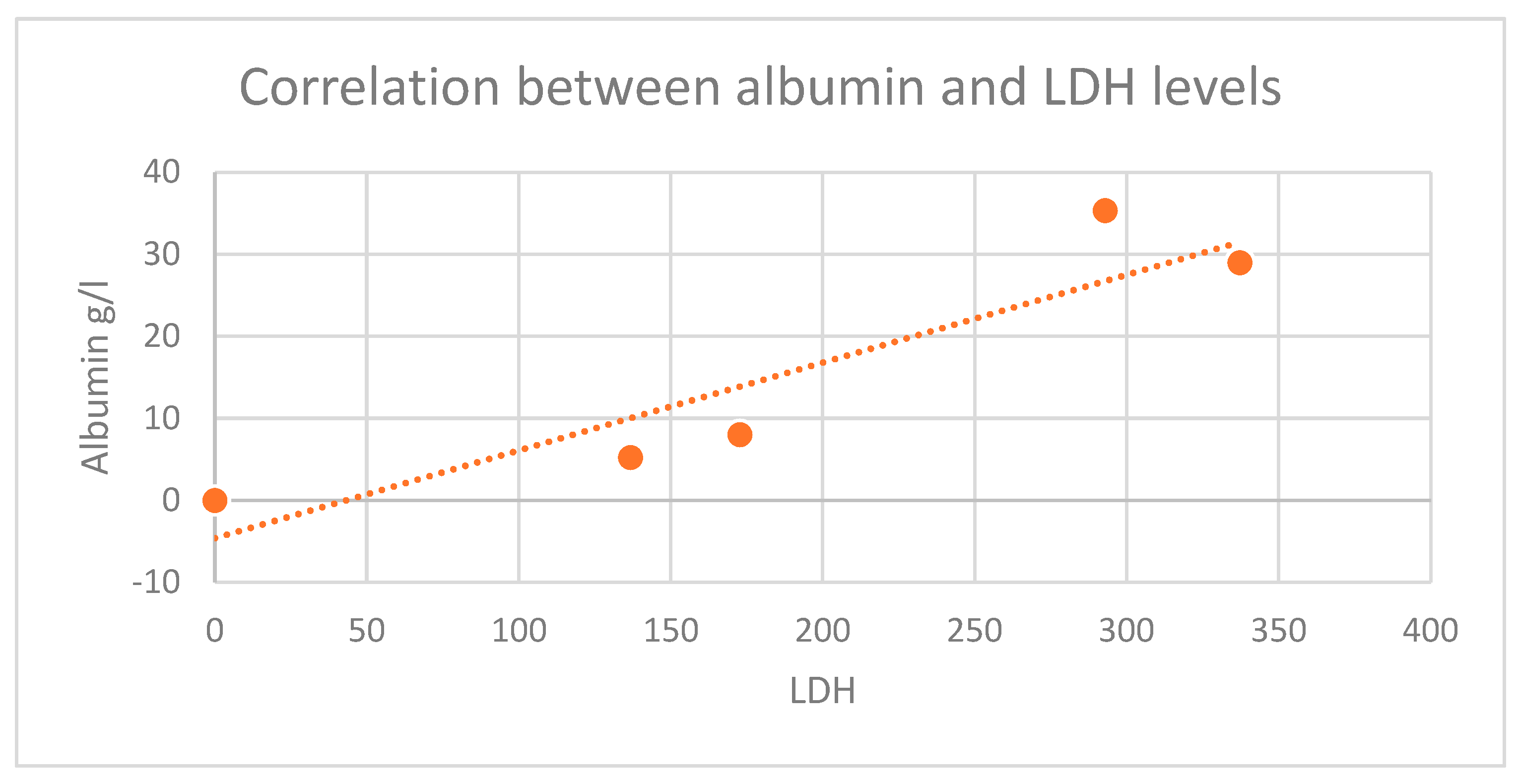
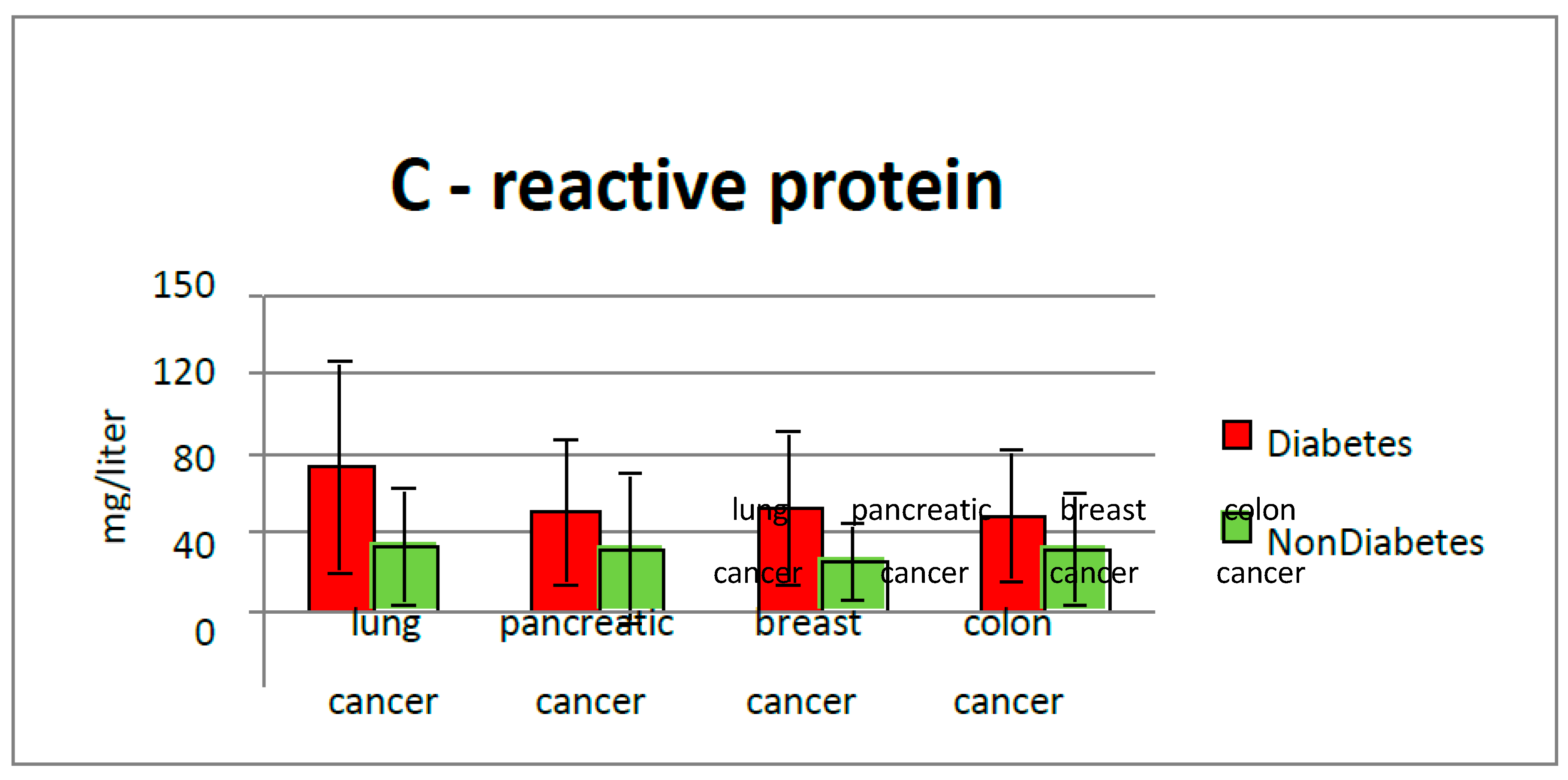
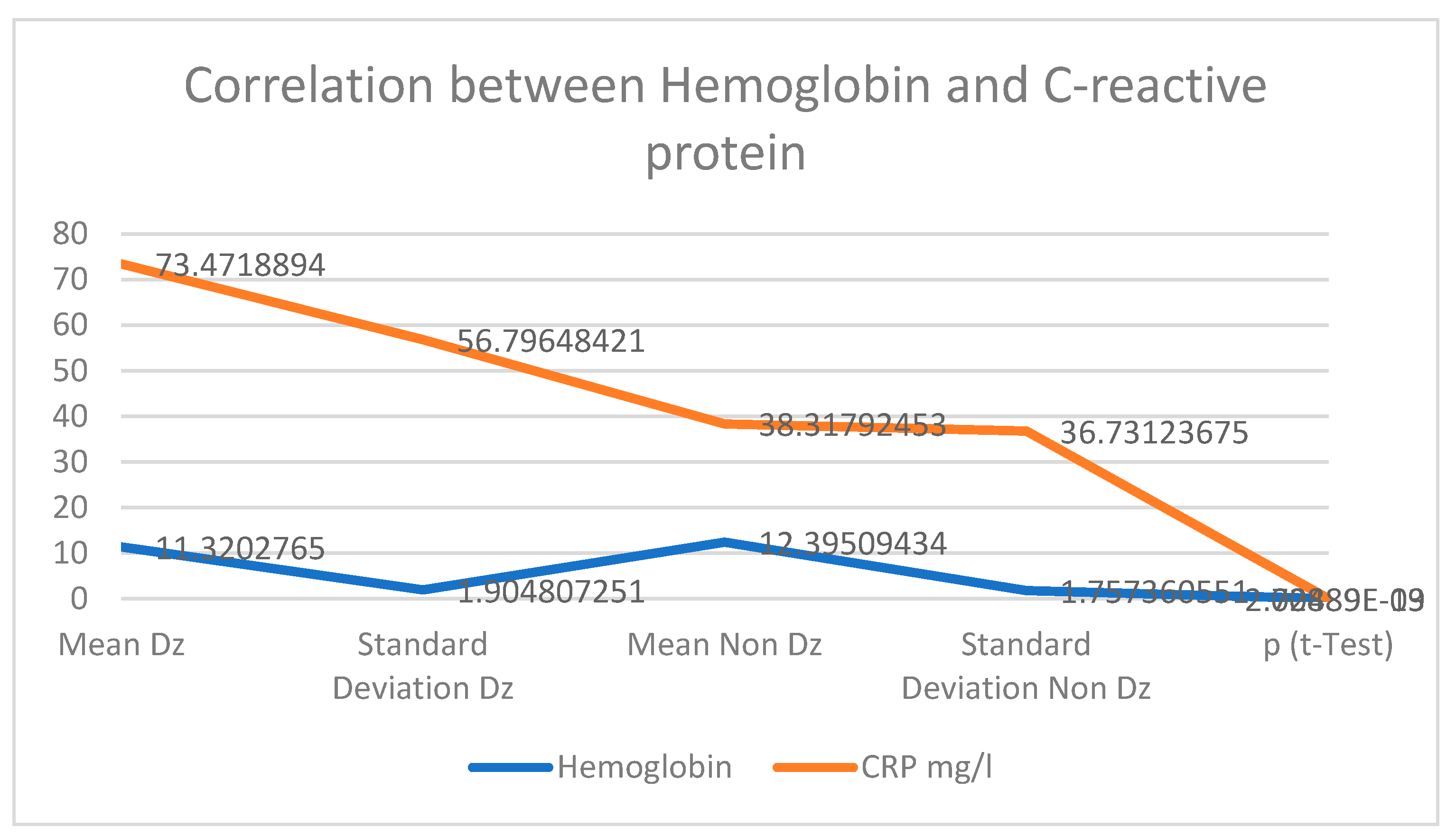
The increase in pro-inflammatory cytokines plays a major role in insulin resistance and leads to the occurrence of microvascular and macrovascular diabetic complications. Increasing IL -6 results in an anti-erythropoietic effect, as this cytokine alters the sensitivity of precursor cells to erythropoietin and promotes cellular death of immature erythrocytes, leading to a further decrease in the number of circulating erythrocytes and a decrease in circulating hemoglobin. Our study highlighted that higher CRP values were found in diabetics and cancer patients, (Average value 73.47 g/L) compared with nondiabetic patients (average CRP value 38.3 g/L). Positive correlations between inflammatory biomarkers, serum albumin and hemoglobin values were found, suggesting a more sustained inflammatory response, as the nutritional and biologic status is more balanced.
Genetic syndromes, inflammatory bowel diseases, history of abdominal radiation therapy, dietary factors (red meat, alcohol, high-fat/low-fiber diet), and tobacco use are the most incriminated factors for developing digestive tube malignancies [
23,
24]. There are risk factors (obesity and physical inactivity) common to those with diabetes mellitus. Inflammation associated with diabetes may also contribute to the development and progression of colorectal cancer [
25]. CRP levels were more significant in the diabetic group than in the non-diabetic group with colorectal cancer (49.9 mg/L vs. 39.1 mg/dL), supporting this fact. In a study, the researchers reported that Increased lactate dehydrogenase release in HT-29 colon cells has been linked to the biological mechanisms of Diclofenac-induced cell death while chrysin alleviated this effect [
26,
27].
There was no difference between patients with colorectal cancer who had diabetes and those who did not, according to a recent case-control study on the relationship between type 2 diabetes and colorectal cancer. The same study discovered that diabetic patients with colorectal cancer had a higher CEA than the group without diabetes and that their value decreased much more quickly under specific treatment than the group of patients without type 2 diabetes. This finding may have an impact on treatment choices [
22].
For pancreatic cancer, there is increasing evidence that inflammation plays an important role in its development [
28]. Our study found that inflammatory markers are higher in diabetic patients (CRP average value 59.96 mg/dL vs. 39.2 mg/dL in the non-diabetic group). Inflammatory pathways are frequently activated by obesity and macronutrient intake. Glucose and fat intake can trigger inflammation by increasing oxidative stress and activating transcription factors such as nuclear factor-κB, activating protein-1, and early growth response-1 [
25]. Some adipocytokines are key compounds involved in innate immunity, inflammation, apoptosis, and metabolism. Significant levels of proinflammatory cytokines promote angiogenesis, tumor progression, and metastasis [
7]. A high level of glycemia is directly related to the development of an inflammatory state, as evidenced by the increased expression of proinflammatory cytokines such as IL -6, TNF-α, and NFκB. Studies show that the longer the disease persists and/or the poorer the glycemic control, the more severe the inflammatory process. The increase in pro-inflammatory cytokines plays a major role in insulin resistance and leads to the occurrence of diabetic macrovascular cardiovascular and microvascular complications and anemia.
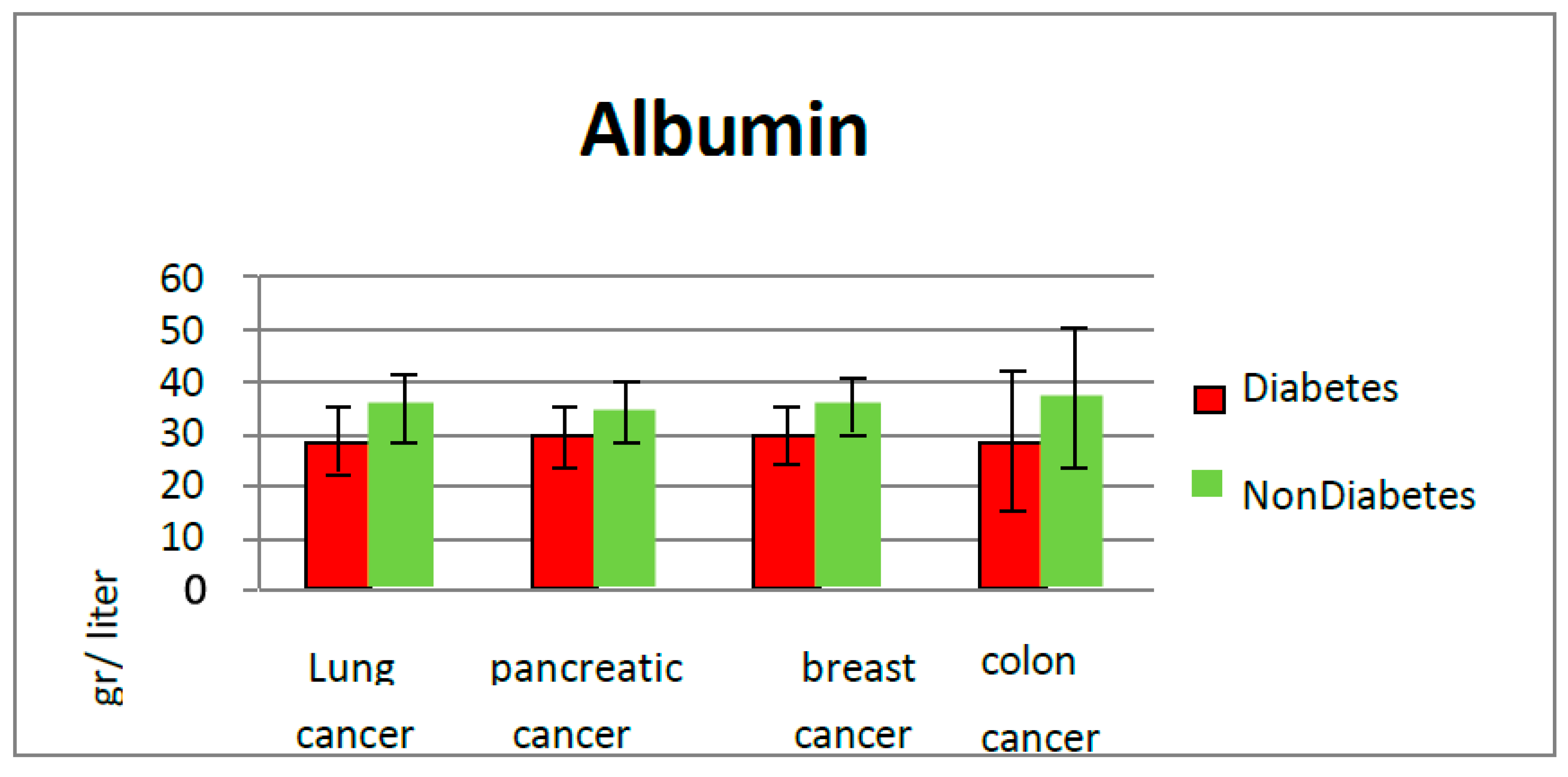
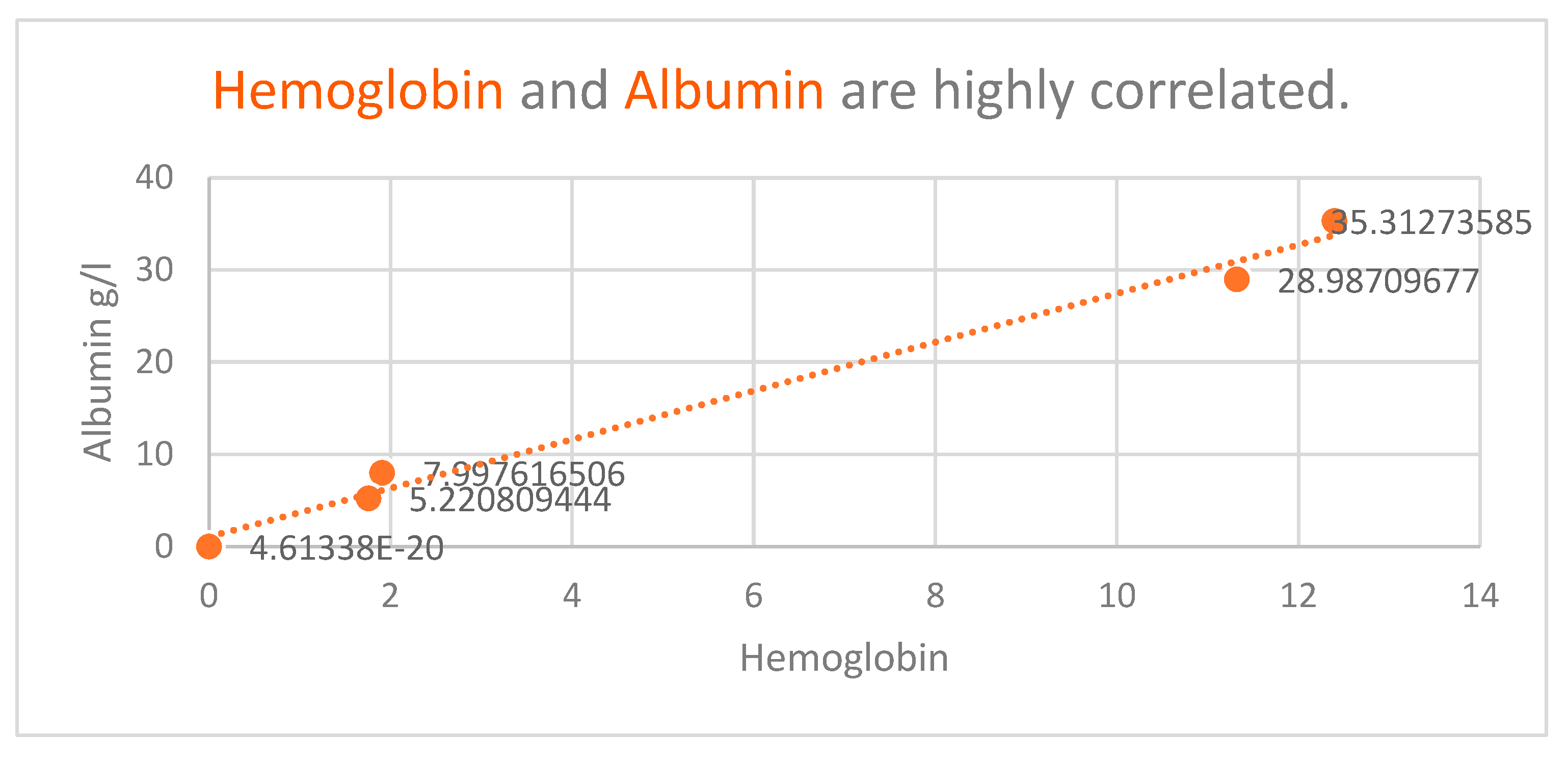
Denutrition is a known major problem both for diabetics and cancer patients. This study revealed that low plasmatic albumin levels were found in diabetic patients (average value of albumin 28.9 g/L), compared with non-diabetic patients (35.3 g/L).
Uncontrolled type 2 diabetes in cancer patients was also specific in our study, confirmed by an average value of HbA1c of 8.57%.
Several studies suggest an important association between the depth of anemia and HbA1c levels in patients with diabetes, underlying the hypothesis that anemia is more common in poorly controlled diabetics [
12,
29]. In our study, hemoglobin and HbA1c levels were not correlated.
Cell proliferation and tissue damage are induced by chronic inflammation [
30]. Diabetes and cancer are in a vicious cycle with each other, and lactate plays a central role in this interaction. Insulin resistance/diabetes and cancer lead to high lactate levels; conversely, high lactate levels promote the development and progression of diabetes and cancer [
9].
The study limitations are represented by the limited biological tests available, with few options of state-settled biomarkers for the basic biological assessment of the hospitalized patient, which can be used for this purpose.