Submitted:
17 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Chemistry of Lipid Peroxidation
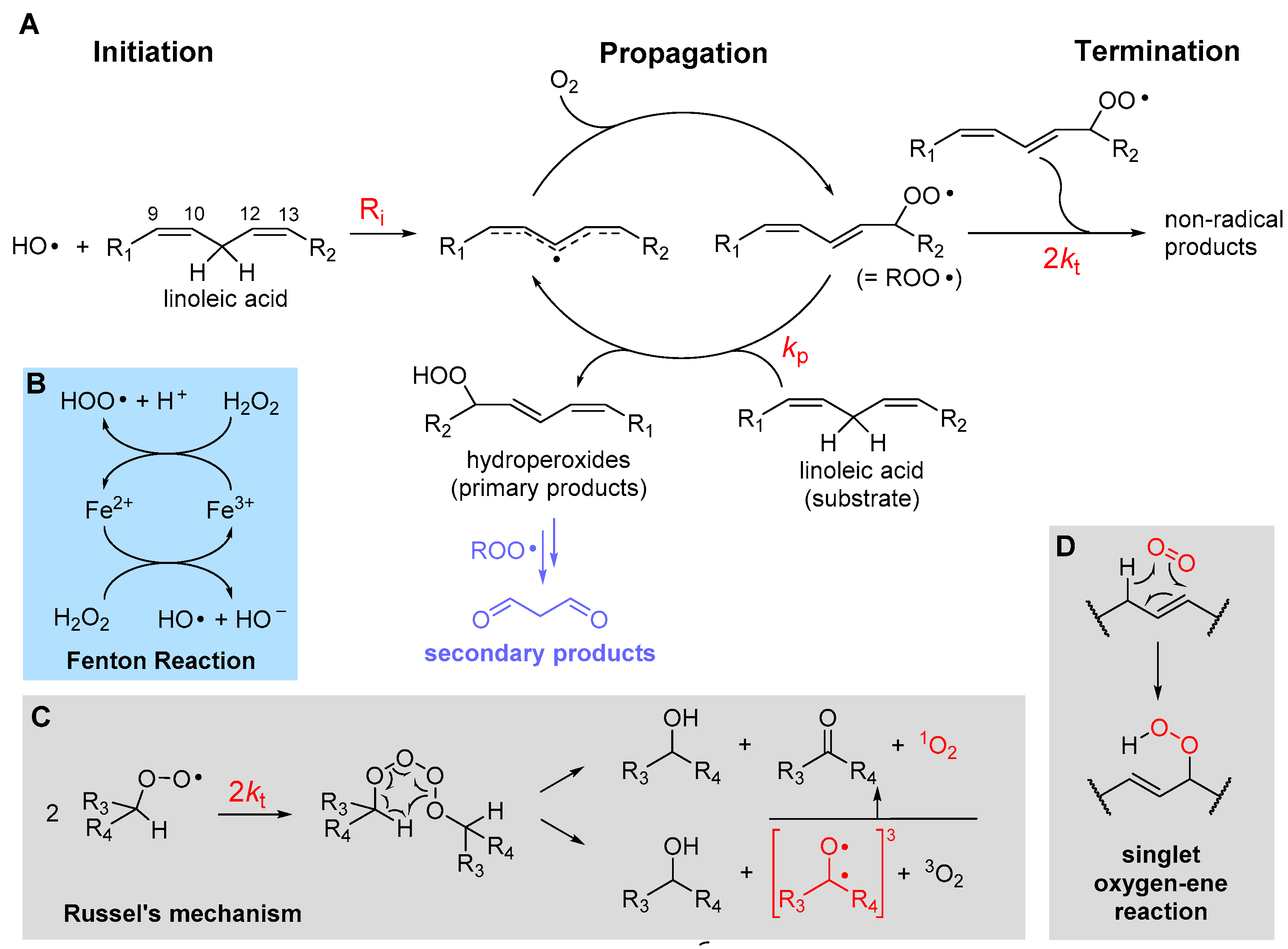
2.1. The Three Stages of Lipid Peroxidation
2.2. The Rate of Lipid Peroxidation
2.3. Light as a Product of Lipid Peroxidation
2.4. Further Insights Into Chain-Propagation Reactions
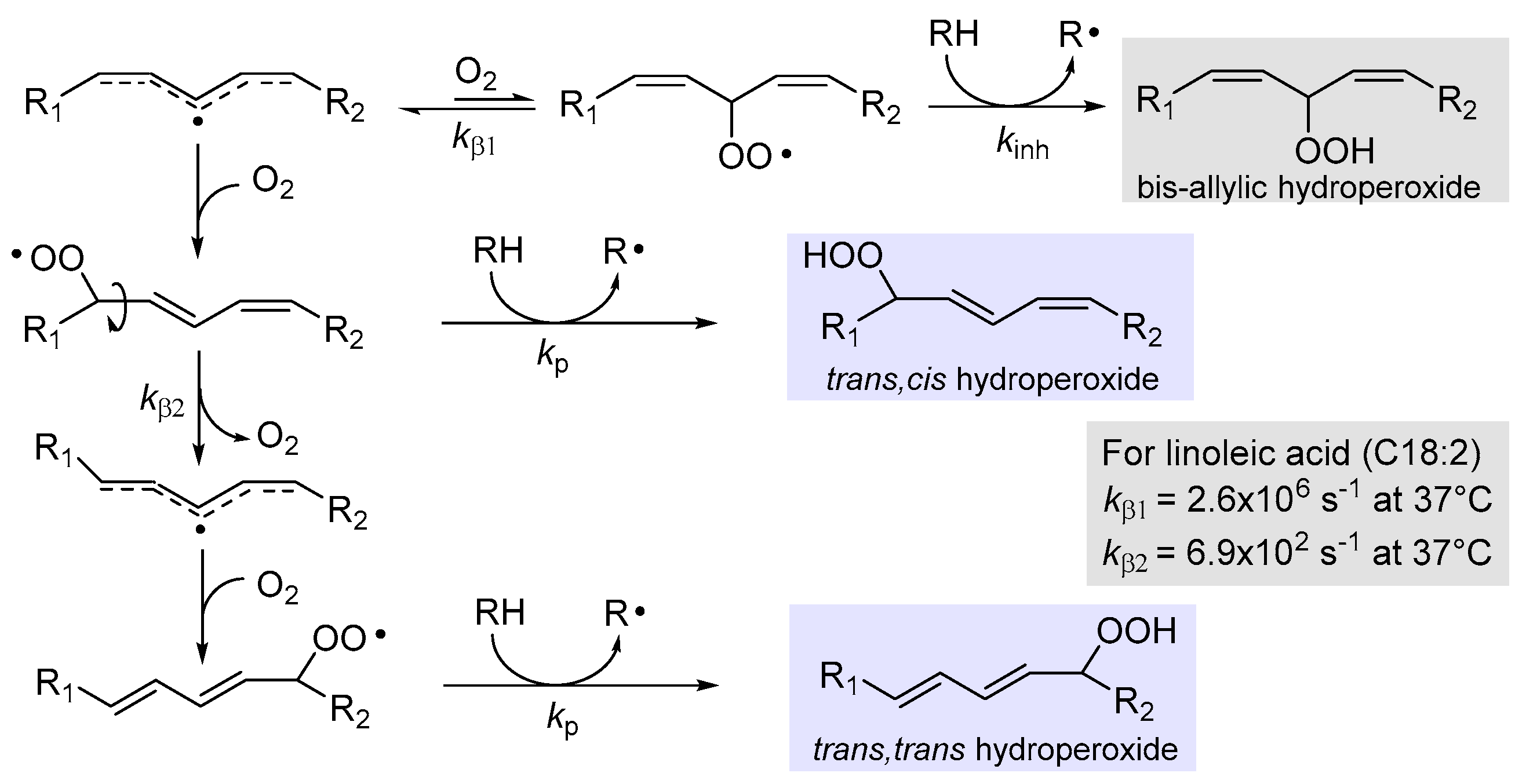
2.4.1. β-Fragmentation of the Peroxyl Radical
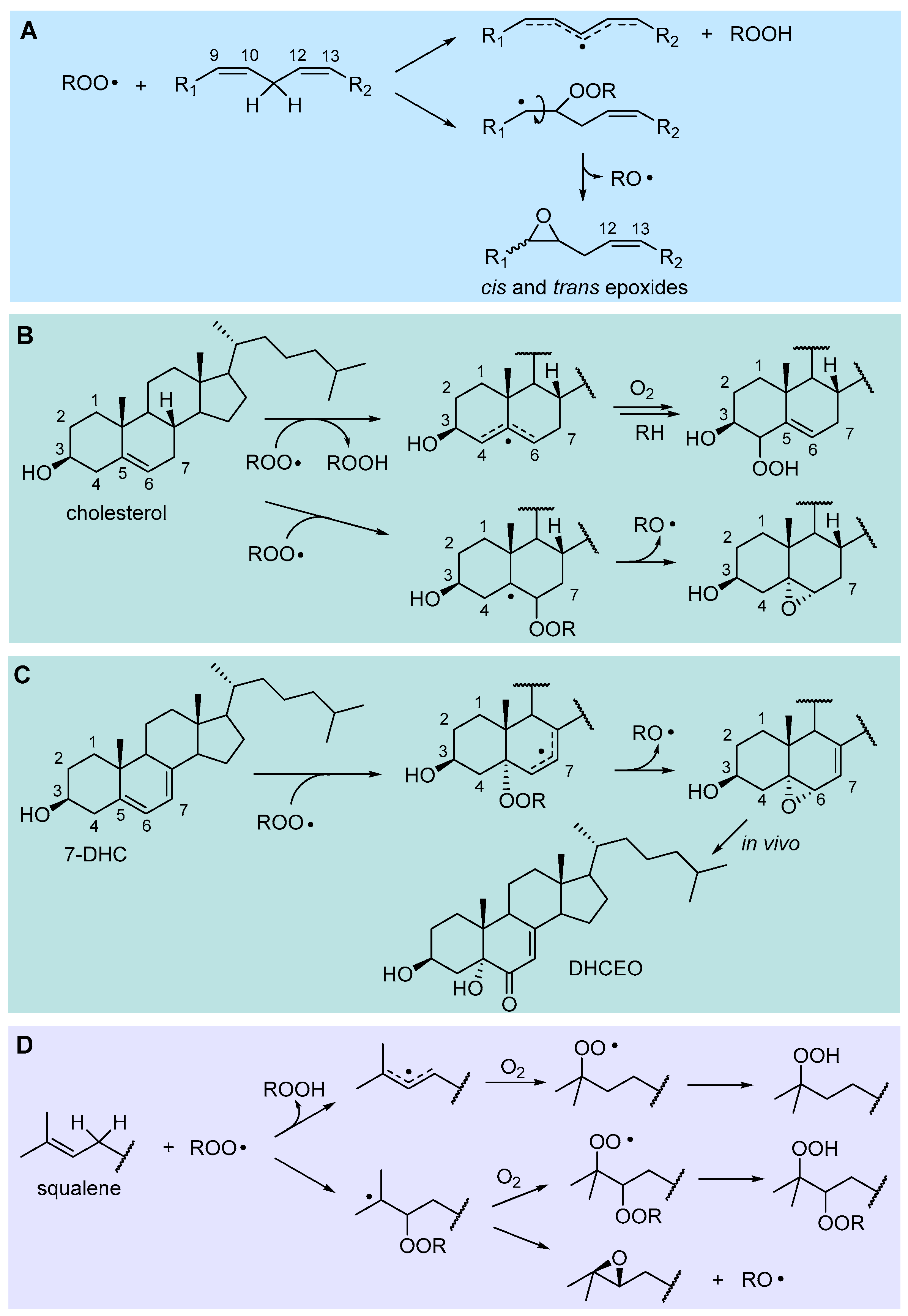
2.4.2. Hydrogen-Atom Abstraction vs Radical Addition: Formation of Primary Epoxides
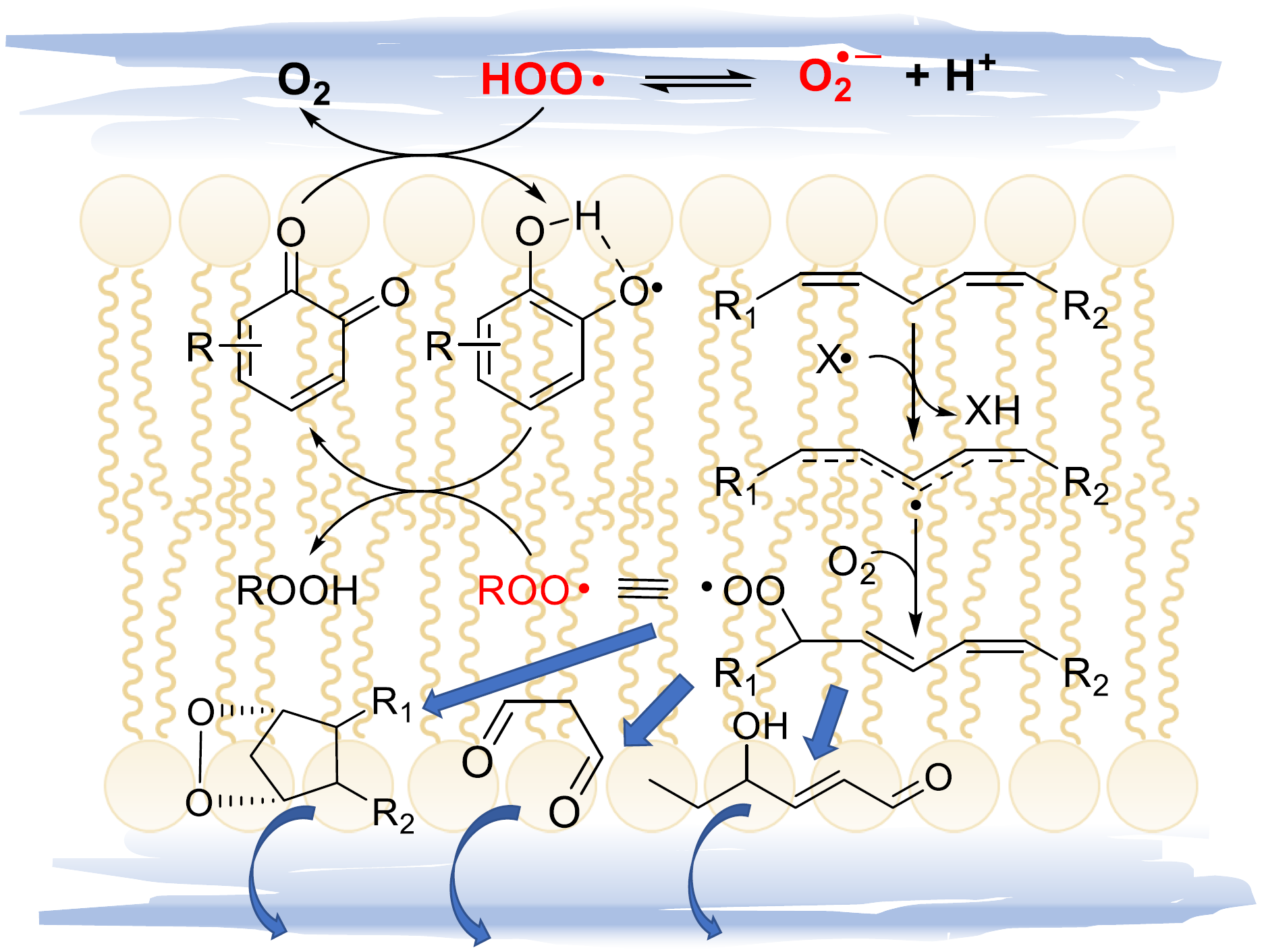
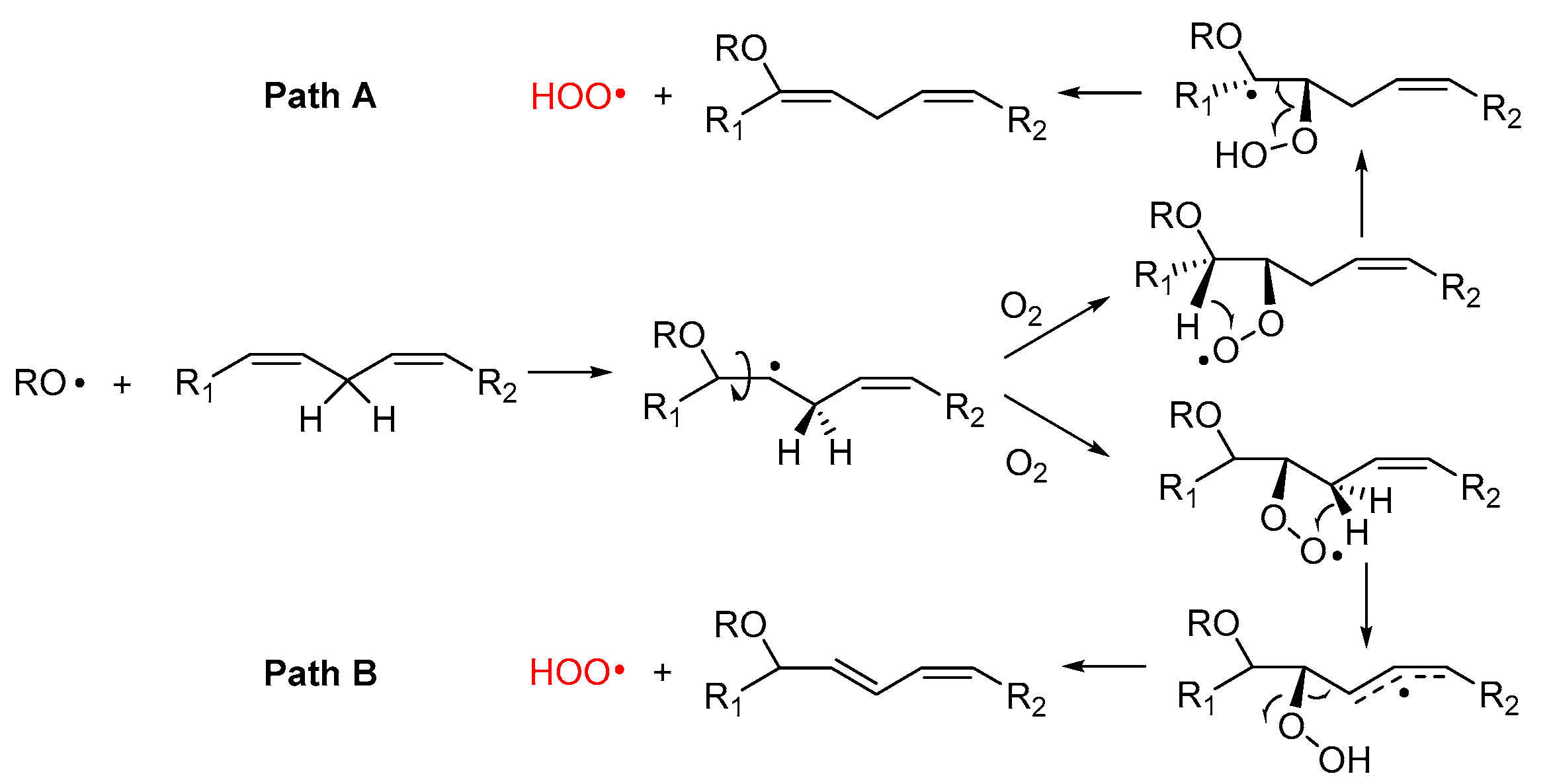
2.4.3. Release of HOO• and Chain-Transfer Processes
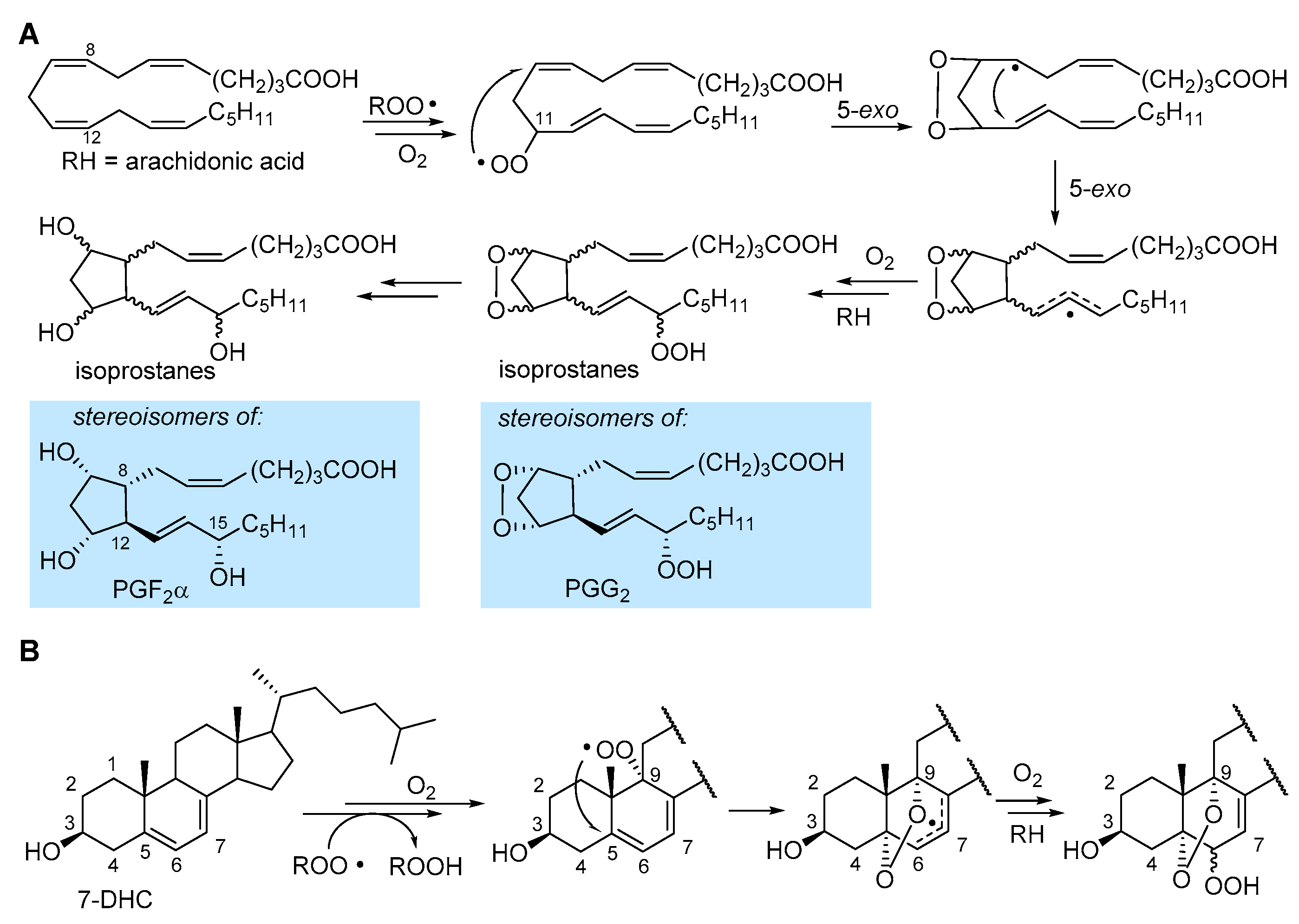
2.4.4. Formation of Endoperoxides
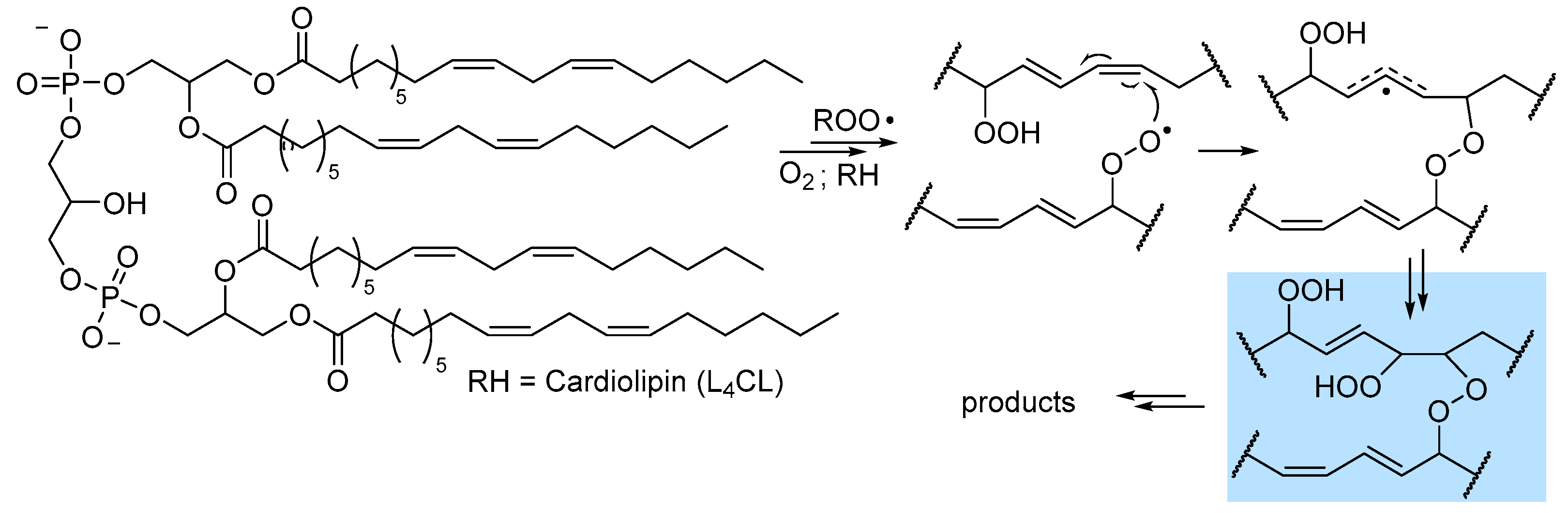
2.5. Peroxidation of Intact Triglycerides and Phospholipids
3. Secondary and Late Products of Lipid Peroxidation
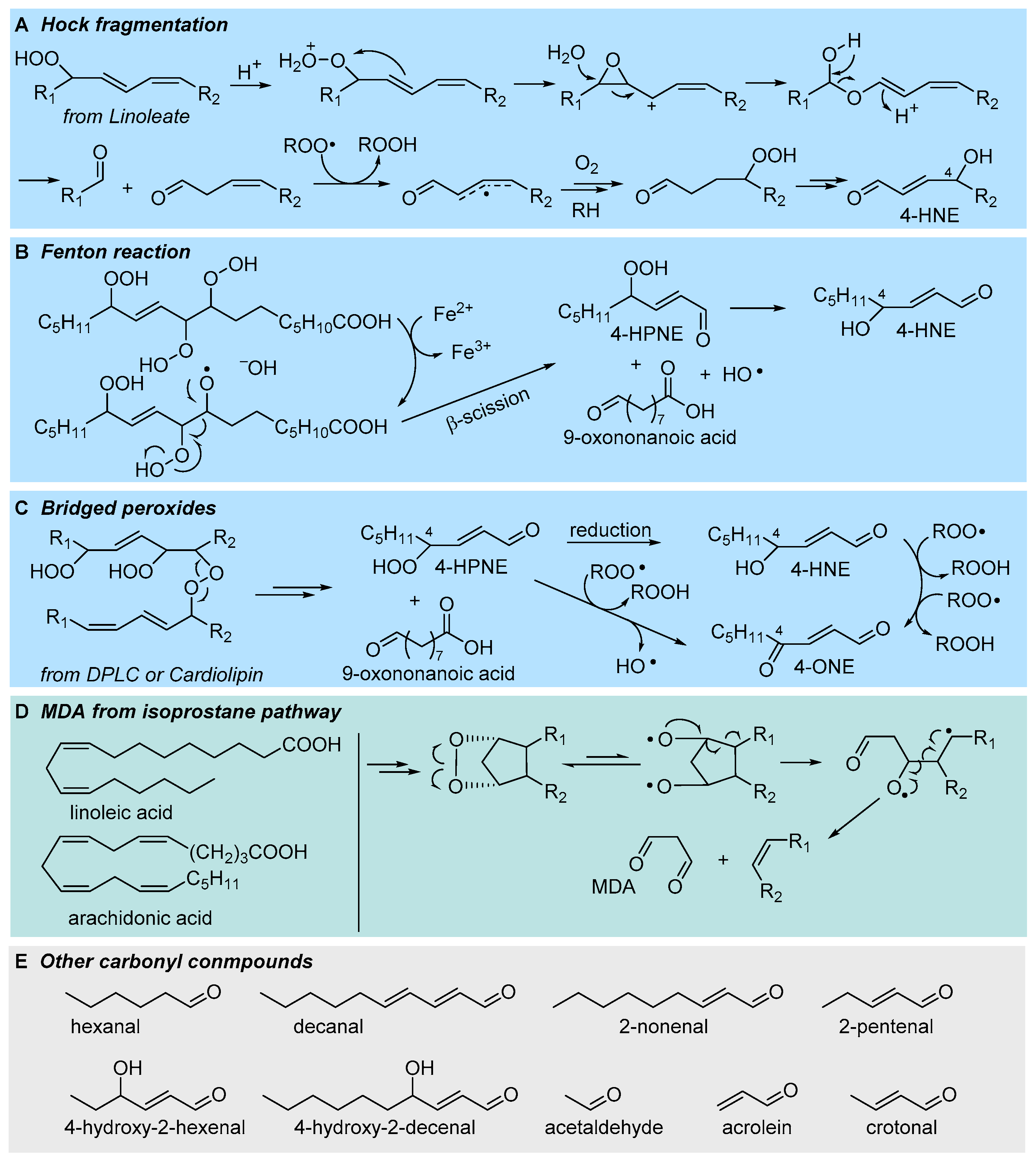
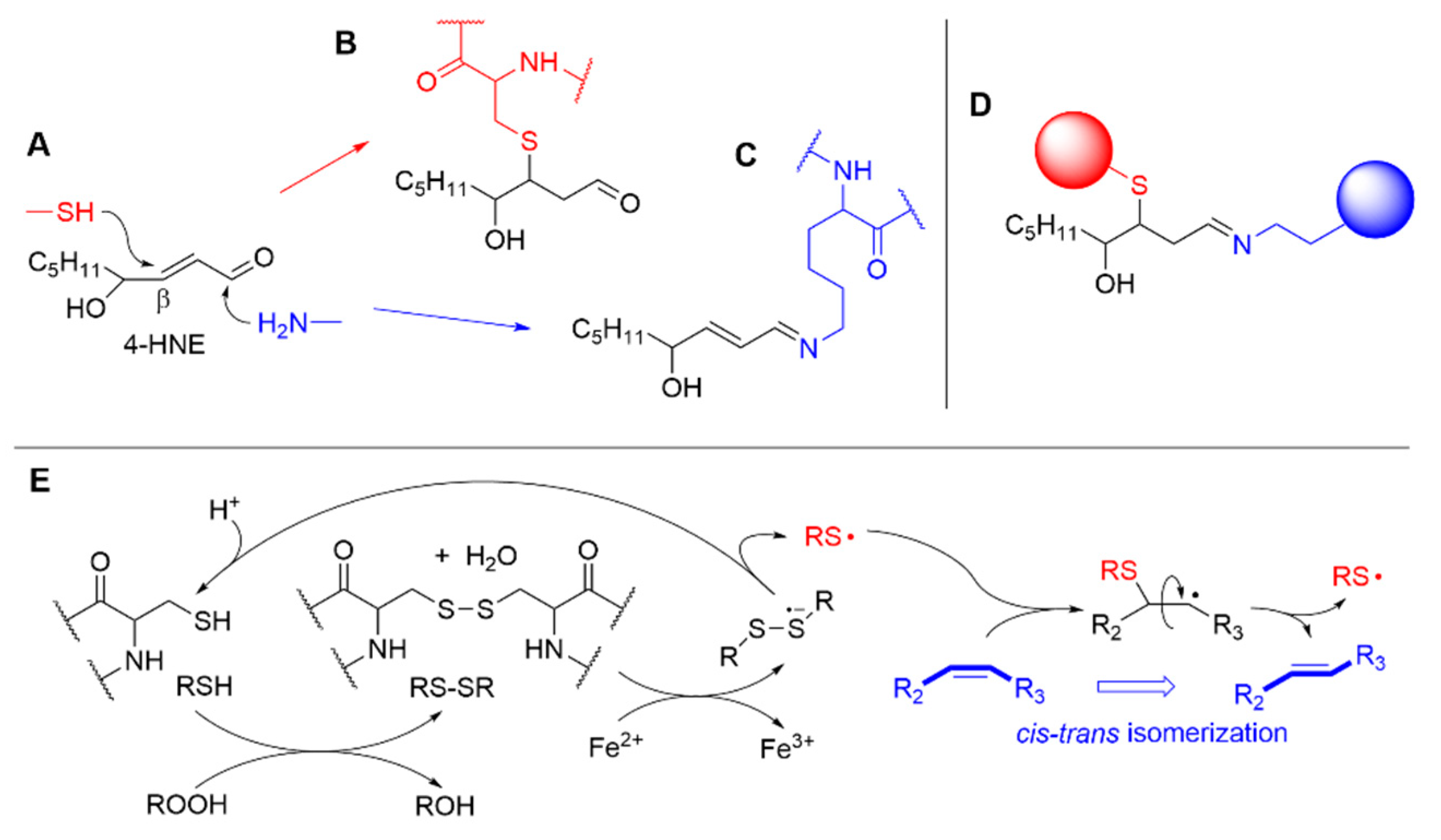
3.1. Formation of Electrophilic Carbonyl Compounds
3.2. Formation of Isoprostanes
3.3. Interaction of LP Products with Aminoacids and Proteins
4. Biological Consequences of Lipid Peroxidation
4.1. LP and Membranes Integrity and Functions
4.2. LP and Cell Signaling
4.3. LP Association with Cancer and Apoptosis
4.4. LP and Neurological Disorders
4.5. LP and Ferroptosis
5. Antioxidants
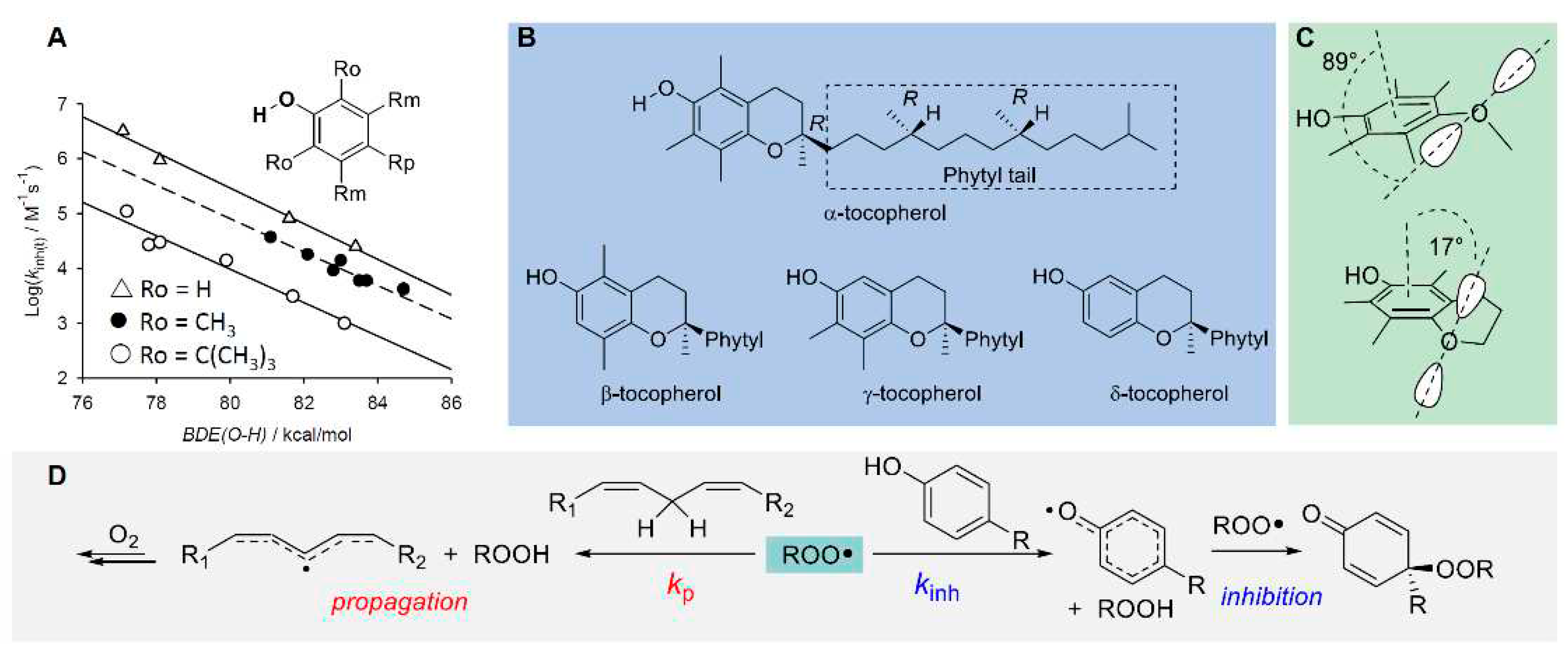
5.1. Preventive Antioxidants
5.2. Chain-Breaking Antioxidants
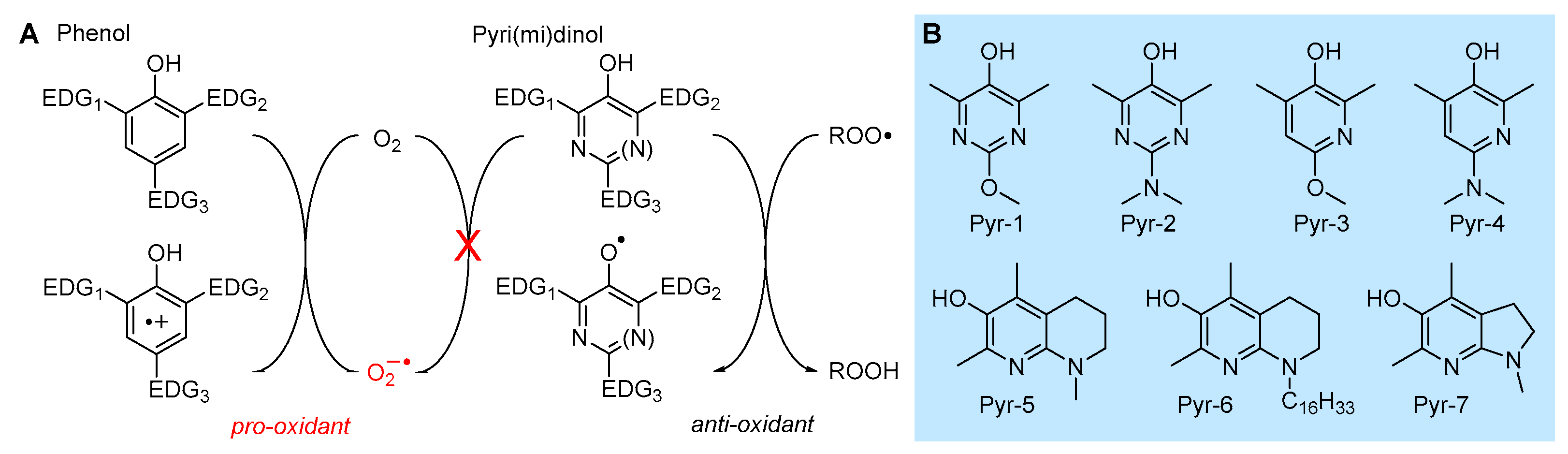
5.2.1. Insertion of N(s) in the Phenolic Ring: 3-Pyridinols and 5-Pyrimidinols
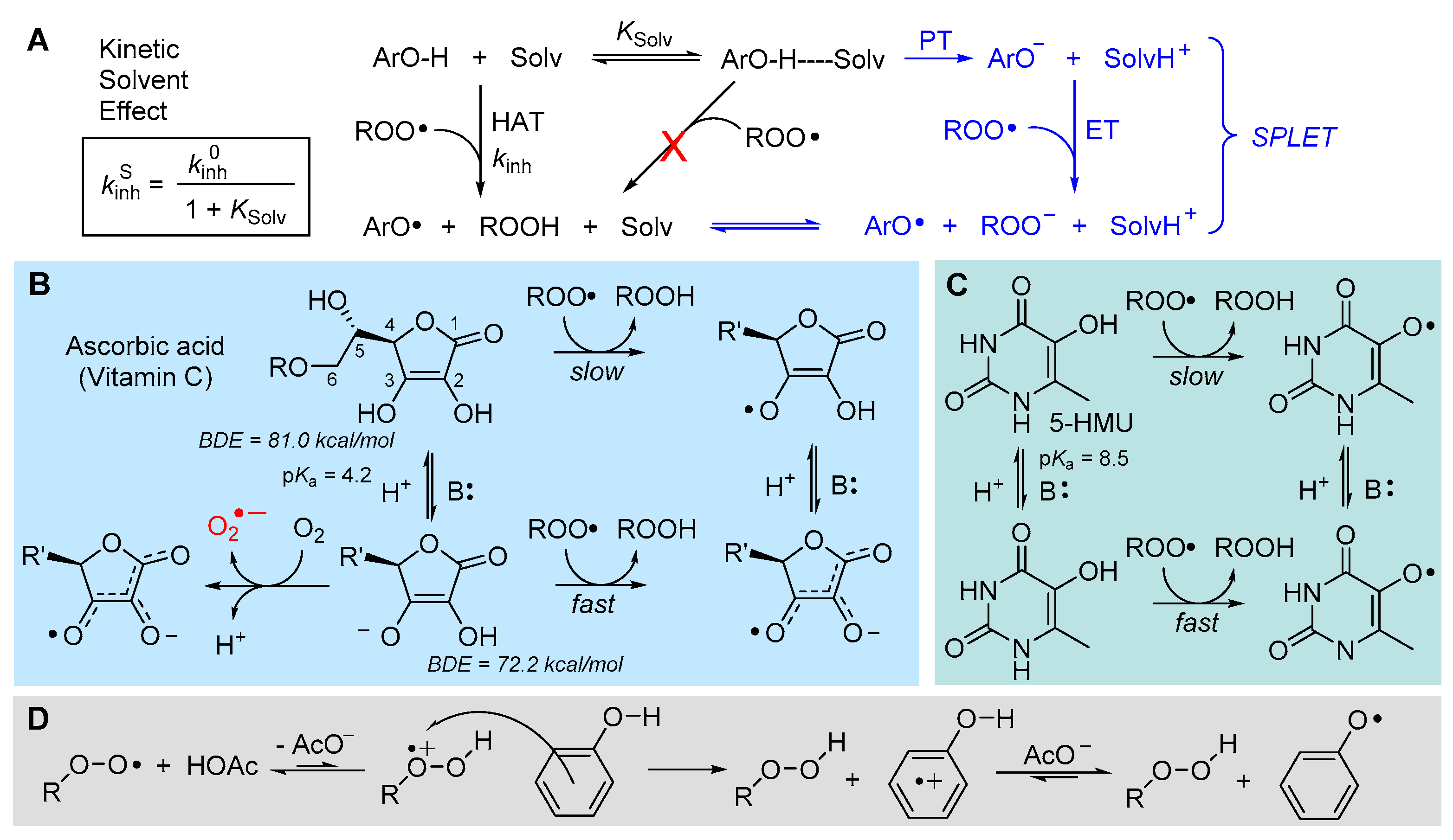
5.2.2. Solvent and Medium Effects in Chain-Breaking Antioxidant Activity
5.2.3. Polyphenols and Flavonoids
5.2.4. Synergy among Antioxidants and Tocopherol-Mediated-Peroxidation (TMP)
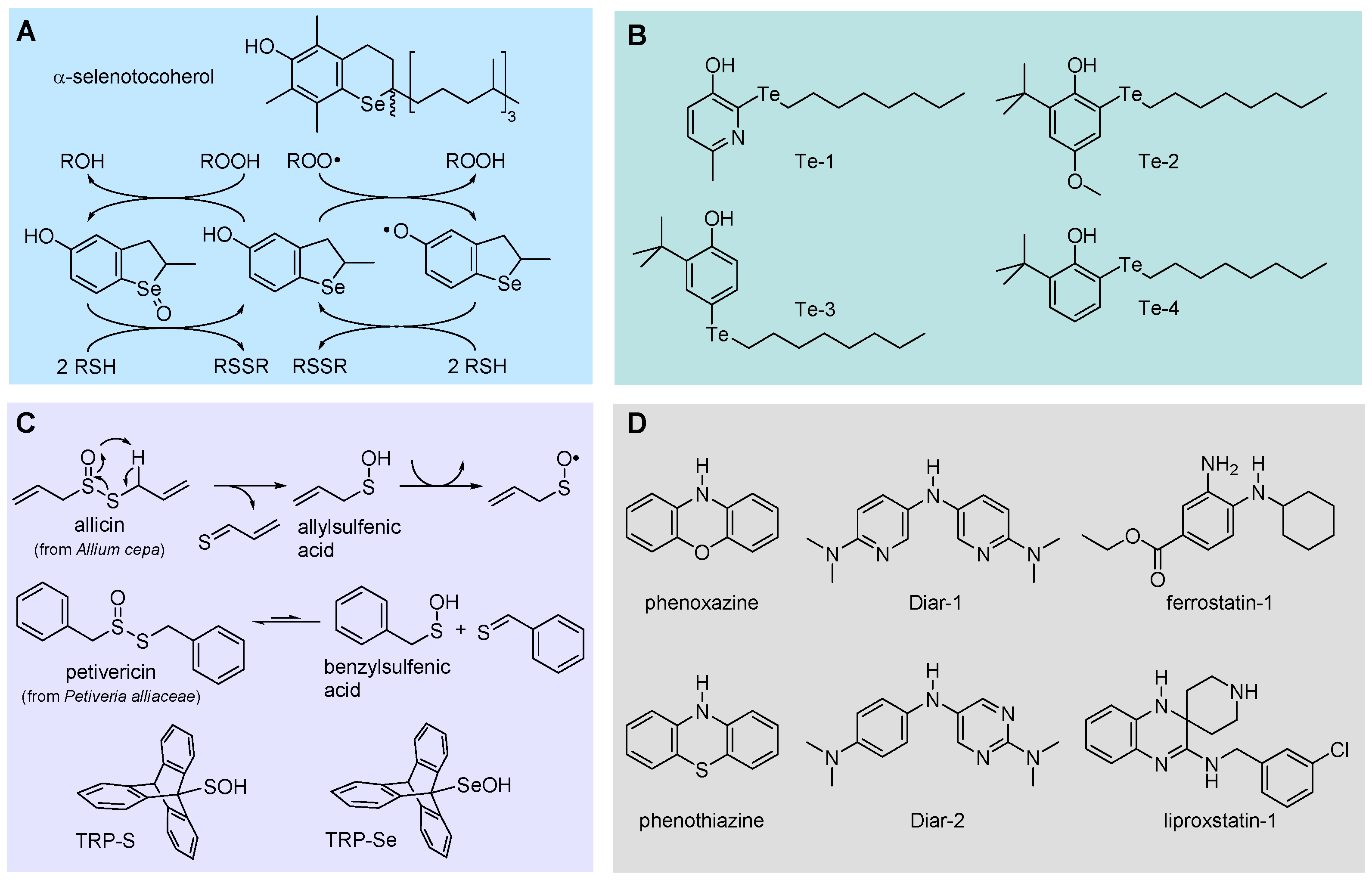
5.2.5. Phenols Bearing Organochalcogen Substituents
5.2.6. Sulfenic and Selenenic Acids
5.2.7. Aromatic Amines and Diarylamines as RTAs
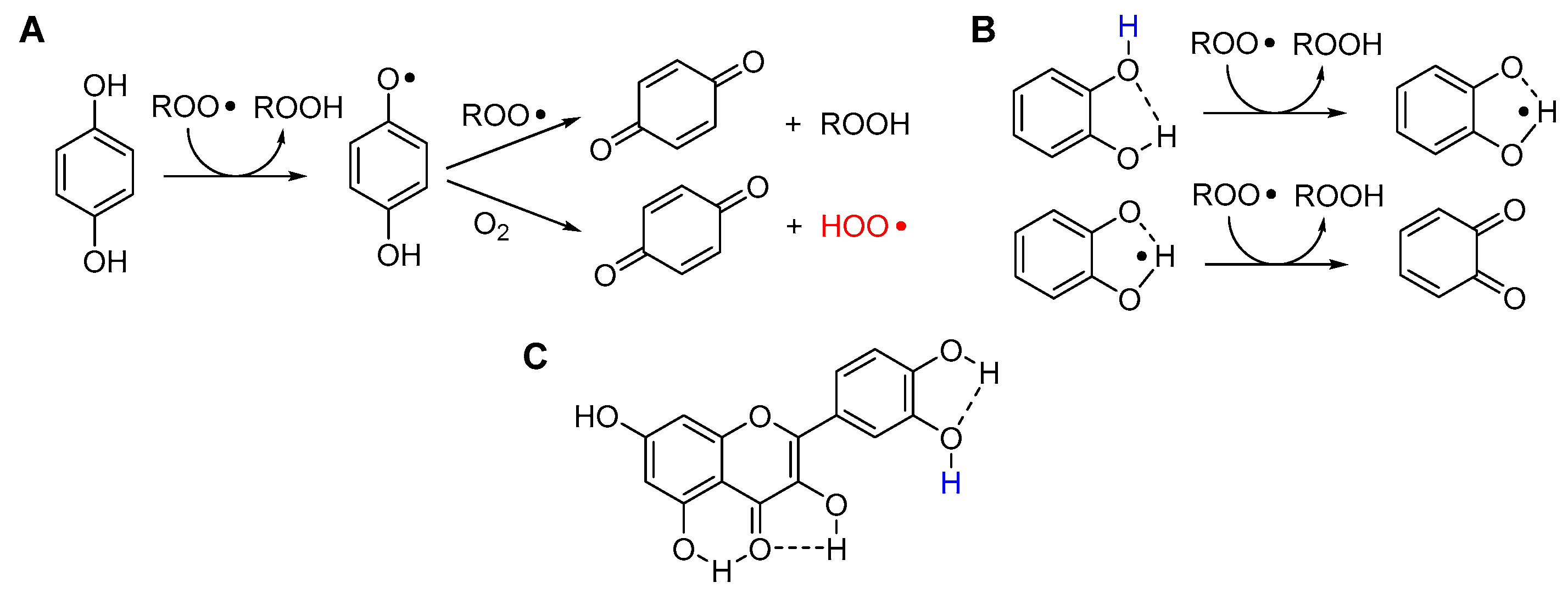
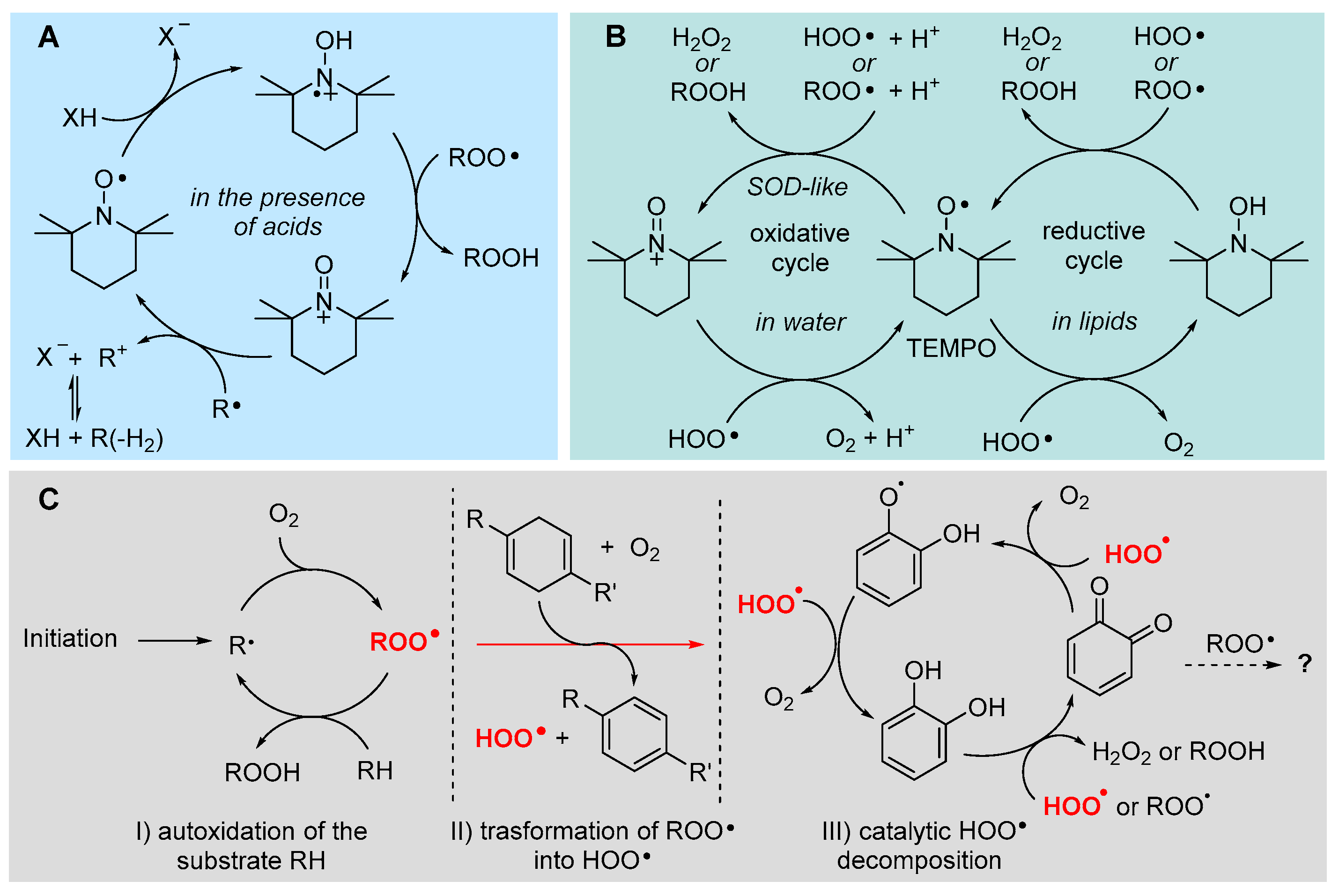
5.2.8. Unconventional Antioxidant Mechanisms and HOO• as Co-Antioxidant
5.3. Termination-Enhancing Antioxidants
5.4. Indirect Antioxidants
6. Conclusive Remarks and Future Perspective
Funding
Conflicts of Interest
References
- Porter, N.A. Mechanism for the Autoxidation of Polyunsaturated Lipids. Acc. Chem. Res. 1986, 19, 262-268. https://doi.org/10.1021/ar00129a001. [CrossRef]
- Ingold, K.U. Peroxyl radicals. Acc. Chem. Res. 1969, 2, 1-9. https://doi.org/10.1021/ar50013a001. [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E: Application of the Principles of Physical Organic Chemistry to the Exploration of Its Structure and Function. Acc. Chem. Res. 1986, 19, 194-201. https://doi.org/10.1021/ar00127a001. [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. https://doi.org/10.3390/antiox8030072. [CrossRef]
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Angela Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharma. Res. Per., 2014, 2, e00023. https://doi.org/10.1002/prp2.23. [CrossRef]
- Foret, M.K; Lincoln, R.; Do Cormo, S.; Cuello, A.C.; Cosa, G. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chem. Rev. 2020, 120, 12757−12787. https://doi.org/10.1021/acs.chemrev.0c00761. [CrossRef]
- Canistro, D.; Boccia, C.; Falconi, R.; Bonamassa, B.; Valgimigli, L.; Vivarelli, F.; Soleti, A.; Genova, M.L.; Lenaz, G.; Sapone, A.; Zaccanti, F.; Abdel-Rahman, S.Z.; Paolini, M. Redox-Based Flagging of the Global Network of Oxidative Stress Greatly Promotes Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015 70, 936-43. https://doi.org/10.1093/gerona/glu160. [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; Morrison, B.; Stockwell, B.R. Ferroptosis: An Iron Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060−1072. [CrossRef]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.E.; Dupont, M.; Hirschhorn, T.; Shchepinov, M.S.; Min, W.; Woerpel, K.A.; Stockwell, B.R. Identification of essential sites of lipid peroxidation in ferroptosis. Nature Chem. Biol. 2023, 19, 719–730. https://doi.org/10.1038/s41589-022-01249-3. [CrossRef]
- Zhang, L.; Jia, R.; Li, H.; Yu, H.; Ren, K.; Jia, S.; Li, Y.; Wang, Q. Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 2021, 11, 1790. https://doi.org/10.3390/biom11121790. [CrossRef]
- Shah, R.; Margison, K.; Pratt, D. A. The Potency of Diarylamine Radical-Trapping Antioxidants as Inhibitors of Ferroptosis Underscores the Role of Autoxidation in the Mechanism of Cell Death. ACS Chem. Biol. 2017, 12, 2538−2545. [CrossRef]
- Poon, J.-F.; Zilka, O.; Pratt, D. A. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J. Am. Chem. Soc. 2020, 142, 14331−14342. https://doi.org/10.1021/jacs.0c06379. [CrossRef]
- Friedmann Angeli, J.P.; Shah, R.; Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci., 2017, 38, 489-498. https://doi.org/10.1016/j.tips.2017.02.005. [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027. [CrossRef]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. https://doi.org/10.1002/med.21712. [CrossRef]
- Gabbanini, S.; Matera, R.; Valvassori, A.; Valgimigli, L. Rapid liquid chromatography–tandem mass spectrometry analysis of 4-hydroxynonenal for the assessment of oxidative degradation and safety of vegetable oils. Anal. Chim. Acta, 2015, 869, 50–58. https://doi.org/10.1016/j.aca.2015.02.046. [CrossRef]
- Yang, S.; Takeuchi, M.; Friedrich, H.; van Duynhoven, J.P.M.; Hohlbein, J. Unravelling mechanisms of protein and lipid oxidation in mayonnaise at multiple length scales. Food Chem. 2023, 402, 134417. https://doi.org/10.1016/j.foodchem.2022.134417. [CrossRef]
- Gęgotek, A.; Skrzydlewska, A. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lip. 2019, 221, 46–52. https://doi.org/10.1016/j.chemphyslip.2019.03.011. [CrossRef]
- Christensen, H.; Sehested, K. HO2 and O2 Radicals at Elevated Temperatures. J. Phys. Chem. 1988, 92, 3007-3011. https://doi.org/10.1021/j100321a060. [CrossRef]
- Maillard, B.; Ingold, K.U.; Scaiano, J.C. Rate constants for the reactions of free radicals with oxygen in solution. J. Am. Chem. Soc. 1983, 105, 5095-5099. https://doi.org/10.1021/ja00353a039. [CrossRef]
- Valgimigli, L.; Pratt, D.A. Antioxidants in Chemistry and Biology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C.; Studer, A., Eds.; John Wiley & Sons, Ltd., Chirchester, UK, 2012; Volume 3, pp. 1623-1678. https://doi.org/10.1002/9781119953678.rad055. [CrossRef]
- Howard, J.A.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. VI. Alkyl aromatic and olefinic hydrocarbons. Can. J. Chem. 1967, 45, 793-802. [CrossRef]
- Xu, L.; Davis, T.A.; Porter, N.A. Rate Constants for Peroxidation of Polyunsaturated Fatty Acids and Sterols in Solution and in Liposomes. J. Am. Chem. Soc. 2009, 131, 13037–13044. https://doi.org/10.1021/ja9029076. [CrossRef]
- Russell, G. A. Deuterium-Isotope Effects in the Autoxidation of Aralkyl Hydrocarbons. Mechanism of the Interaction of Peroxy Radicals1. J. Am. Chem. Soc. 1957, 79, 3871−3877. [CrossRef]
- Miyamoto, S.; Martinez, G. R.; Medeiros, M. H.; Di Mascio, P. Singlet Molecular Oxygen Generated by Biological Hydroperoxides. J. Photochem. Photobiol., B 2014, 139, 24−33. https://doi.org/10.1016/j.jphotobiol.2014.03.028. [CrossRef]
- Noguchi, N.; Nakada, A.; Itoh, Y.; Watanabe, A.; Niki, E. Formation of Active Oxygen Species and Lipid Peroxidation Induced by Hypochlorite. Arch. Biochem. Biophys. 2002, 397, 440-7. https://doi.org/10.1006/abbi.2001.268. [CrossRef]
- Miyamoto, S.; Martinez, G.R.; Rettori, D.; Augusto, O.; Medeiros, M.H.G.; Di Mascio, P. Linoleic acid hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen. Proc. Natl. Acad. Sci. USA, 2006, 103, 293–298. https://doi.org/10.1073/pnas.0508170103. [CrossRef]
- Miyamoto, S.; Nantes, I.L.; Faria, P.A.; Cunha, D.; Ronsein, G.E.; Medeiros, M.H.G.; Di Mascio, P. Cytochrome c-promoted cardiolipin oxidation generates singlet molecular oxygen. Photochem. Photobiol. Sci. 2012, 11, 1536–1546. [CrossRef]
- Zielinski, Z. A. M.; Pratt, D. A. Lipid Peroxidation: Kinetics, Mechanisms, and Products. J. Org. Chem. 2017, 82, 2817−2825. [CrossRef]
- Baschieri, A.; Pizzol, R.; Guo, Y.;Amorati, R.; Valgimigli, L. Calibration of Squalene, p-Cymene, and Sunflower Oil as Standard Oxidizable Substrates for Quantitative Antioxidant Testing. J. Agric. Food Chem. 2019, 67, 6902−6910. https://doi.org/10.1021/acs.jafc.9b01400. [CrossRef]
- Miranda-Apodaca, J.; Hananya, N.; Velázquez-Campoy, A.; Shabat, D.; Arellano, J.B. Emissive Enhancement of the Singlet Oxygen Chemiluminescence Probe after Binding to Bovine Serum Albumin. Molecules 2019, 24, 2422. https://doi.org/10.3390/molecules24132422. [CrossRef]
- Lissi, E.A.; Caceres, T.; Videla, L.A. Visible chemiluminescence from rat brain homogenates undergoing autoxidation. II Kinetics of theluminescence decay. Free Radic. Biol. Med. 1988, 4, 93-97. https://doi.org/10.1016/0891-5849(88)90069-x. [CrossRef]
- Timmins, G.S.; dos Santos, R.E.; Whitwood, A.C.; Catalani, L.H.; Di Mascio, P.; Gilbert, B.C.; Bechara, E.J.H. Lipid peroxidation-dependent chemiluminescence from the cyclization of alkylperoxyl radicals to dioxetane radical intermediates. Chem. Res. Toxicol. 1997, 10, 1090-1096. https://doi.org/10.1021/tx970075p. [CrossRef]
- Fedorova, G. F.; Menshov, V. A.; Trofimov, A. V.; Vasil'Ev, R. F. Facile chemiluminescence assay for antioxidative properties of vegetable lipids: Fundamentals and illustrative examples. Analyst 2009, 134, 2128–2134. https://doi.org/10.1039/B905059K. [CrossRef]
- Vasil’ev, R.F.; Veprintsev, T.L.; Dolmatova, L.S.; Naumov, V.V.; Trofimov, A.V.; Tsaplev, Y.B. Kinetics of ethylbenzene oxy-chemiluminescence in the presence of antioxidants from tissues of the marine invertebrate Eupentacta fraudatrix: Estimating the concentration and reactivity of the natural antioxidants. Kinet. Catal., 2014, 55, 148–153. https://doi.org/10.1134/S0023158414020153. [CrossRef]
- Kancheva, V.D.; Dettori, M.A.; Fabbri, D.; Alov, P.; Angelova, S.E.; Slavova-Kazakova, A.K.; Carta, P.; Menshov, V.A.; Yablonskaya, O.I.; Trofimov, A.V.; et al. Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress. Antioxidants 2021, 10, 624. https://doi.org/10.3390/antiox10040624. [CrossRef]
- Porter, N. A. A Perspective on Free Radical Autoxidation: The Physical Organic Chemistry of Polyunsaturated Fatty Acid and Sterol Peroxidation. J. Org. Chem. 2013, 78, 3511−3524. https://doi.org/10.1021/jo4001433. [CrossRef]
- Pratt, D.A. Tallman, K.A.; Porter, N.A. Free Radical Oxidation of Polyunsaturated Lipids: New Mechanistic Insights and the Development of Peroxyl Radical Clocks. Acc. Chem. Res. 2011, 44, 458–467. https://doi.org/10.1021/ar200024c. [CrossRef]
- Howard, JA, Ingold, KU. Absolute rate constants for hydrocarbon oxidation. XII. Rate constants for secondary peroxy radicals. Can. J. Chem. 1968, 46, 2661-2666. [CrossRef]
- Maerker, G, Haeberer, E.T., Ault, W.C. Epoxidation of methyl linoleate. I. The question of positional selectivity in monoepoxidation. J. Am. Oil Chem. Soc. 1966, 43 100-104. [CrossRef]
- Aliwarga, T.; Raccor, B. S.; Lemaitre, R. N.; Sotoodehnia, N.; Gharib, S. A.; Xu, L.; Totah, R. A. Enzymatic and free radical formation of cis- and trans-epoxyeicosatrienoic acids in vitro and in vivo. Free Radical Biol. Med. 2017, 112, 131-140. https://doi.org/10.1016/j.freeradbiomed.2017.07.015. [CrossRef]
- Zielinski, Z.A.M.; Pratt, D.A. H-Atom Abstraction vs Addition: Accounting for the Diverse Product Distribution in the Autoxidation of Cholesterol and Its Esters. J. Am. Chem. Soc. 2019, 141, 3037−3051. https://doi.org/10.1021/jacs.8b11524. [CrossRef]
- Do, Q.; Lee, D.D.; Dinh, A.N.; Seguin, R.P.; Zhang, R.; Xu, L. Development and Application of a Peroxyl Radical Clock Approach for Measuring Both Hydrogen-Atom Transfer and Peroxyl Radical Addition Rate Constants. J. Org. Chem. 2021, 86, 153−168. https://doi.org/10.1021/acs.joc.0c01920. [CrossRef]
- Denisov, E.T. The role of triplet repulsion in alkyl radical addition to a 7π-C-O bond and alkoxy radical addition to a π-C-C bond. Kinet. Catal. 2000, 41, 293–297. https://doi.org/10.1007/BF02755363. [CrossRef]
- Degirmenci, I.; Coote, M.L. Comparison of Thiyl, Alkoxyl, and Alkyl Radical Addition to Double Bonds: The Unusual Contrasting Behavior of Sulfur and Oxygen Radical Chemistry. J. Phys. Chem. A 2016, 120, 1750−1755. https://doi.org/10.1021/acs.jpca.6b00538. [CrossRef]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO• to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. https://doi.org/10.1002/anie.202101033. [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. https://doi.org/10.1021/jf020993f. [CrossRef]
- Harrison, K.A.; Haidasz, E.A.; Griesser, M.; Pratt, D.A. Inhibition of hydrocarbon autoxidation by nitroxide-catalyzed cross-dismutation of hydroperoxyl and alkylperoxyl radicals. Chem. Sci. 2018, 9, 6068–6079. https://doi.org/10.1039/c8sc01575a. [CrossRef]
- Antunes, F.; Barclay, L. R. C.; Vinqvist, M. R.; Pinto, R. E. Int. J. Chem. Kinet. 1998, 30, 753–767.
- Liu, W.; Porter, N. A.; Schneider, C.; Brash, A. R.; Yin, H. Formation of 4-Hydroxynonenal from Cardiolipin Oxidation: Intramolecular Peroxyl Radical Addition and Decomposition. Free Radical Biol. Med. 2011, 50, 166−178. https://doi.org/10.1016/j.freeradbiomed.2010.10.709. [CrossRef]
- Bowry, V.W. Arm-to-Arm Autoxidation in a Triglyceride: Remote Group Reaction Kinetics. J. Org. Chem. 1994,59, 2250-2252. [CrossRef]
- Esterbauer, H.; Schaur, R. J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 1991, 11, 81-128. https://doi.org/10.1016/0891-5849(91)90192-6. [CrossRef]
- Ullery, J. C.; Marnett, L. J. Protein modification by oxidized phospholipids and hydrolytically released lipid electrophiles: Investigating cellular responses. Biochim. Biophys. Acta, Biomembr. 2012, 1818, 2424- 2435. https://doi.org/10.1016/j.bbamem.2012.04.014. [CrossRef]
- Codreanu, S. G.; Ullery, J. C.; Zhu, J.; Tallman, K. A.; Beavers, W. N.; Porter, N. A.; Marnett, L. J.; Zhang, B.; Liebler, D. C. Alkylation Damage by Lipid Electrophiles Targets Functional Protein Systems. Mol. Cell. Proteomics 2014, 13, 849-859. https://doi.org/10.1074/mcp.M113.032953. [CrossRef]
- Spickett, C.M. The lipidperoxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol., 2013, 2013, 145–152. https://doi.org/10.1016/j.redox.2013.01.007. [CrossRef]
- Liang, X.; Qian, R.; Ou, Y.; Wang, D.; Lin, X.; Sun , C. Lipid peroxide-derived short-chain aldehydes promote programmed cell death in wheat roots under aluminum stress. J. Hazard. Mater. 2023, 443, 130142. https://doi.org/10.1016/j.jhazmat.2022.130142. [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798−8888. https://doi.org/10.1021/acs.chemrev.7b00698. [CrossRef]
- Schopfer, F. J.; Cipollina, C.; Freeman, B. A. Formation and Signaling Actions of Electrophilic Lipids. Chem. Rev. 2011, 111, 5997−6021. [CrossRef]
- Yang, J.; Tallman, K. A.; Porter, N. A.; Liebler, D. C. Quantitative Chemoproteomics for Site-Specific Analysis of Protein Alkylation by 4-Hydroxy-2-Nonenal in Cells. Anal. Chem. 2015, 87, 2535−2541. [CrossRef]
- Perkovic, M.N.; Jaganjac, M.; Milkovic, L.; Horvat, T.; Rojo, D.; Zarkovic, K.; C´ oric´, M.; Hudolin, T.; Waeg, G.; Orehovec, B.; et al. Relationship between 4-Hydroxynonenal (4-HNE) as Systemic Biomarker of Lipid Peroxidation and Metabolomic Profiling of Patients with Prostate Cancer. Biomolecules 2023, 13, 145. https://doi.org/10.3390/bi om13010145. [CrossRef]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E879–E886. https://doi.org/10.1152/ajpendo.00508.2010. [CrossRef]
- Onyango, A.N.; Baba, N. New hypotheses on the pathways of formation of malondialdehyde and isofurans. Free Radical Biol. Med. 2010, 49, 1594–1600. https://doi.org/10.1016/j.freeradbiomed.2010.08.012. [CrossRef]
- Takeuchi, C.; Galvé, R.; Nieva, J.; Witter, D. P.; Wentworth, A.D.; Troseth, R. P.; Lerner, R. A.; Wentworth, P. Proatherogenic Effects of the Cholesterol Ozonolysis Products, Atheronal-A and Atheronal-B. Biochemistry 2006, 45, 7162 –7170. https://doi.org/10.1021/bi0604330. [CrossRef]
- Nieva, J.; Song, B.-D.; Rogel, J. K.; Kujawara, D.; Altobel, L., III; Izharrudin, A.; Boldt, G. E.; Grover, R. K.; Wentworth, A. D.; Wentworth, P., Jr. Cholesterol Secosterol Aldehydes Induce Amyloidogenesis and Dysfunction of Wild-Type Tumor Protein p53. Chem. Biol. 2011, 18, 920-927. https://doi.org/10.1016/j.chembiol.2011.02.018. [CrossRef]
- Usui, K.; Hulleman, J. D.; Paulsson, J. F.; Siegel, S. J.; Powers, E.T.; Kelly, J. W. Site-specific modification of Alzheimer's peptides by cholesterol oxidation products enhances aggregation energetics and neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 18563-18568. https://doi.org/10.1073/pnas.080475810. [CrossRef]
- Wójcik, P.; Žarkovi´, N.; Gegotek, A.; Skrzydlewska, E. Involvement of Metabolic Lipid Mediators in the Regulation of Apoptosis. Biomolecules 2020, 10, 402;. https://doi.org/10.3390/biom10030402. [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.-J.; Hemmendinger, M.; Guseva Canu, I.; Hopf, N.B. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol. Lett. 2020, 328, 19-27. https://doi.org/10.1016/j.toxlet.2020.04.006. [CrossRef]
- Gao, X.; Brenner, H.; Holleczek, B.; Cuk, K.; Zhang, Y.; Anusruti, A.; Xuan, Y.; Xu, Y.; Schottker, B. Urinary 8-isoprostane levels and occurrence of lung, colorectal, prostate, breast and overall cancer: Results from a large, population-based cohort study with 14 years of follow-up. Free Radic. Biol. Med., 2018, 123, 20-26. https://doi.org/10.1016/j.freeradbiomed.2018.05.065. [CrossRef]
- Yang, J.; Tallman, K. A.; Porter, N. A.; Liebler, D. C. Quantitative Chemoproteomics for Site-Specific Analysis of Protein Alkylation by 4-Hydroxy-2-Nonenal in Cells. Anal. Chem. 2015, 87, 2535−2541. [CrossRef]
- Ferreri, C.; Ferocino, A.; Batani, G.; Chatgilialoglu, C.; Randi, V.; Riontino, M.V.; Vetica, F.; Sansone, A. Plasmalogens: Free Radical Reactivity and Identification of Trans Isomers Relevant to Biological Membranes. Biomolecules 2023, 13, 730. https://doi.org/10.3390/biom13050730. [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. https://doi.org/10.1021/cr4002287. [CrossRef]
- Torreggiani, A.; Tinti, A.; Jurasekova, Z.; Capdevila, M.; Saracino, M.; Foggia, M.D. Structural Lesions of Proteins Connected to Lipid Membrane Damages Caused by Radical Stress: Assessment by Biomimetic Systems and Raman Spectroscopy. Biomolecules 2019, 9, 794. https://doi.org/10.3390/biom9120794. [CrossRef]
- Chatgilialoglu, C.; Ferreri, C. Reductive Stress of Sulfur-Containing Amino Acids within Proteins and Implication of Tandem Protein–Lipid Damage. Int. J. Mol. Sci. 2021, 22, 12863. [CrossRef]
- Hirata, Y.; Ferreri, C.; Yamada, Y.; Inoue, A.; Sansone, A.; Vetica, F.; Suzuki, W.; Takano, S.; Noguchi, T.; Matsuzawa, A.; Chatgilialoglu, C. Geometrical isomerization of arachidonic acid during lipid peroxidation interferes with ferroptosis. Free Radical Biol. Med. 2023, 204, 374–384. https://doi.org/10.1016/j.freeradbiomed.2023.05.026. [CrossRef]
- Itri, R.; Junqueira, H. C.; Mertins, O.; Baptista, M. S. Membrane Changes under Oxidative Stress: The Impact of Oxidized Lipids. Biophys. Rev. 2014, 6, 47−61. https://doi.org/10.1007/s12551-013-0128-9. [CrossRef]
- Weber, G.; Charitat, T.; Baptista, M. S.; Uchoa, A. F.; Pavani, C.; Junqueira, H. C.; Guo, Y.; Baulin, V. A.; Itri, R.; Marques, C. M.; Schroder, A.P. Lipid Oxidation Induces Structural Changes in Biomimetic Membranes. Soft Matter 2014, 10, 4241−4247. https://doi.org/10.1039/c3sm52740a. [CrossRef]
- Wang, G., Wang, J., Fan, X., Ansari, G.A.S., Khan, M.F. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose–and time–response studies in female MRL +/+ mice. Toxicology, 2012, 292, 113–122. [CrossRef]
- Yamada, T., Place, N., Kosterina, N., Östberg, T., Zhang, S.J., Grundtman, C., Erlandsson-Harris, H.; Lundberg, I.E.; Glenmark, B.; Bruton, J.D.; Westerblad, H.. Impaired myofibrillar function in the soleus muscle of mice with collagen-induced arthritis. Arthritis Rheumat. 2009, 60, 3280–3289. https://doi.org/10.1002/art.24907. [CrossRef]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta, 2015, 1851, 308-30. https://doi.org/10.1016/j.bbalip.2014.10.002. [CrossRef]
- Yang, W. S.; Kim, K. J.; Gaschler, M. M.; Patel, M.; Shchepinov, M. S.; Stockwell, B. R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E4966−4975. https://doi.org/10.1073/pnas.1603244113. [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429;. https://doi.org/10.3390/biom10101429. [CrossRef]
- Peña-Bautista, C.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Lipid peroxidation in neurodegeneration. Clin. Chim. Acta, 2019, 497, 178–188. https://doi.org/10.1016/j.cca.2019.07.037. [CrossRef]
- da Santana, L.N.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.P.; Freitas Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; do Maia, C.S.F.; Lima, R.R. et al., J. Trace Elem. Med. Biol. 2019, 51, 19–27. https://doi.org/10.1016/j.jtemb.2018.09.004. [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer's disease: Lipid peroxidation biomarkers. Clin. Chim. Acta, 2019, 491, 85–90. https://doi.org/10.1016/j.cca.2019.01.021. [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.;, Walch, A.; Eggenhofer, E.; Basavarajappa, D. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell. Biol. 2014, 16, 1180–1191. https://doi.org/10.1038/ncb3064. [CrossRef]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis. Nature Chem. Biol. 2019, 15, 1137–1147. https://doi.org/10.1038/s41589-019-0408-1. [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; Henkelmann, B.; Yamada, K.; Wanninger, J.; Zilka, O.; Sato, E.; Feederle, R.; Hass, D.; Adriano Maida, A.; Mourão, A.S.D.; Linkermann, A.; Geissler, E. K.; Nakagawa, K.; Abe, T.; Fedorova, M.; Proneth, B.; Pratt, D.A.; Conrad. M. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature, 2022, 608, 778-783. https://doi.org/10.1038/s41586-022-05022-3. [CrossRef]
- Shah, R.; Shchepinov, M. S.; Pratt, D. A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387−396. https://doi.org/10.1021/acscentsci.7b00589. [CrossRef]
- Soula, M.; Weber, R.A.; Zilka, O.Z.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nature Chem. Biol. 2020, 16, 1351-1360. https://doi.org/10.1038/s41589-020-0613-y. [CrossRef]
- Friedmann Angeli, J.P.; Shah, R.; Derek A. Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489-498. https://doi.org/10.1016/j.tips.2017.02.005. [CrossRef]
- Zhang, L.; Jia, R.; Li, H.; Yu, H.; Ren, K.; Jia, S.; Li, Y.; Wang, Q. Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 2021, 11, 1790. https://doi.org/10.3390/biom11121790. [CrossRef]
- Amorati, R.; Valgimigli, L. Methods to measure the antioxidant activity of phytochemicals and plant extracts. J. Agric. Food Chem., 2018, 66, 3324–3329. https://doi.org/10.1021/acs.jafc.8b01079. [CrossRef]
- Baschieri, A.; Ajvazi, M. D.; Tonfack, J. L. F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. https://doi.org/10.1016/j.foodchem.2017.04.036. [CrossRef]
- Lucarini, M.; Pedulli, G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev., 2010, 39, 2106-2119. https://doi.org/10.1039/B901838G. [CrossRef]
- Cariola, A.; El Chami, M.; Granatieri, J.; Valgimigli, L. Anti-tyrosinase and antioxidant activity of meroterpene bakuchiol from Psoralea corylifolia (L.). Food Chem. 2023, 405, 134953. https://doi.org/10.1016/j.foodchem.2022.134953. [CrossRef]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants – the fight for Forever. Chem. Soc. Rev., 2021, 50, 7343–7358. https://doi.org/10.1039/d1cs00265a. [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022−9046. https://doi.org/10.1021/cr500226n. [CrossRef]
- Valgimigli, L.; Pratt, D.A. Maximizing the Reactivity of Phenolic and Aminic Radical-Trapping Antioxidants: Just Add Nitrogen! Acc. Chem. Res. 2015, 48, 966−975. https://doi.org/10.1021/acs.accounts.5b00035. [CrossRef]
- Pratt, D. A.; DiLabio, G. A.; Brigati, G.; Pedulli, G. F.; Valgimigli, L. 5-Pyrimidinols: Novel Chain-Breaking Antioxidants More Effective Than Phenols. J. Am. Chem. Soc. 2001, 123, 4625−4626. https://doi.org/10.1021/ja005679l. [CrossRef]
- Valgimigli, L.; Brigati, G.; Pedulli, G. F.; DiLabio, G. A.; Mastragostino, M.; Arbizzani, C.; Pratt, D. A. The Effect of Ring Nitrogen Atoms on the Homolytic Reactivity of Phenolic Compounds: Understanding the Radical-Scavenging Ability of 5-Pyrimidinols. Chem. Eur. J. 2003, 9, 4997−5010. https://doi.org/10.1002/chem.200304960. [CrossRef]
- Wijtmans, M.; Pratt, D. A.; Valgimigli, L.; DiLabio, G. A.; Pedulli, G. F.; Porter, N. A. 6-Amino-3-Pyridinols: Towards Diffusion-Controlled Chain-Breaking Antioxidants. Angew. Chem., Int. Ed. 2003, 42, 4370−4373. https://doi.org/10.1002/anie.200351881. [CrossRef]
- Wijtmans, M.; Pratt, D. A.; Brinkhorst, J.; Serwa, R.; Valgimigli, L.; Pedulli, G. F.; Porter, N. A. Synthesis and Reactivity of Some 6-Substituted-2,4-Dimethyl-3-Pyridinols, a Novel Class of Chain-Breaking Antioxidants. J. Org. Chem. 2004, 69, 9215−9223. https://doi.org/10.1021/jo048842u. [CrossRef]
- Kim, H.-Y.; Pratt, D. A.; Seal, J. R.; Wijtmans, M.; Porter, N. A. Lipid-Soluble 3-Pyridinol Antioxidants Spare α-Tocopherol and Do Not Efficiently Mediate Peroxidation of Cholesterol Esters in Human Low-Density Lipoprotein. J. Med. Chem. 2005, 48, 6787−6789. https://doi.org/10.1021/jm0507173. [CrossRef]
- Nam, T.-G.; Nara, S. J.; Zagol-Ikapitte, I.; Cooper, T.; Valgimigli, L.; Oates, J. A.; Porter, N. A.; Boutaud, O.; Pratt, D. A. Pyridine and Pyrimidine Analogs of Acetaminophen as Inhibitors of Lipid Peroxidation and Cyclooxygenase and Lipoxygenase Catalysis. Org. Biomol. Chem. 2009, 7, 5103−5112. https://doi.org/10.1039/B912528K. [CrossRef]
- Serwa, R.; Nam, T.-g.; Valgimigli, L.; Culbertson, S.; Rector, C.L.; Jeong, B.-S.; Pratt, D.A.; Porter, N.A. Preparation and Investigation of Vitamin B 6-Derived Aminopyridinol Antioxidants. Chem. Eur. J. 2010, 16, 14106 – 14114. https://doi.org/10.1002/chem.201001382. [CrossRef]
- Valgimigli, L.; Bartolomei, D.; Amorati, R.; Haidasz, E.; Hanthorn, J. J.; Nara, S. J.; Brinkhorst, J.; Pratt, D. A. 3-Pyridinols and 5-Pyrimidinols: Tailor-Made for Use in Synergistic Radical-Trapping Co-antioxidant Systems. Beilstein J. Org. Chem. 2013, 9, 2781−2792. [CrossRef]
- Valgimigli, L.; Ingold, K.U.; Lusztyk, J. Antioxidant activities of vitamin E analogues in water and a Kamlet - Taft β-value for water. J. Am. Chem. Soc. 1996, 118, 3545 – 3549. https://doi.org/10.1021/ja954030r. [CrossRef]
- Valgimigli, L.; Ingold, K.U.; Lusztyk, J. Solvent effects on the reactivity and free spin distribution of 2,2-diphenyl-1-picrylhydrazyl radicals. J. Org. Chem. 1996, 61, 7947-7950. https://doi.org/10.1021/jo9608578. [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L. Do Peroxyl Radicals Obey the Principle That Kinetic Solvent Effects on H-Atom Abstraction Are Independent of the Nature of the Abstracting Radical? J. Org. Chem. 1988, 63, 4497 – 449926. https://doi.org/10.1021/jo971944i. [CrossRef]
- Abraham, M. H.; Grellier, P. L.; Prior, D. V.; Taft, R. W.; Morris, J. J.; Taylor, P. J.; Laurence, C.; Berthelot, M.; Doherty, R. M.; Kamlet, M. J.; Abboud, J. L. M.; Sraidi, K.; Guiheneuf, G. A general treatment of hydrogen bond complexation constants in tetrachloromethane. J. Am. Chem. Soc. 1988, 110, 8534–8536. https://doi.org/10.1021/ja00233a034. [CrossRef]
- Abraham, M. H.; Grellier, P. L.; Prior, D. V.; Morris, J. J.; Taylor, P. J. Hydrogen bonding. Part 10. A scale of solute hydrogen-bond basicity using log K values for complexation in tetrachloromethane. J. Chem. Soc., Perkin Trans. 2 1990, 521–529. https://doi.org/10.1039/p29900000521. [CrossRef]
- Snelgrove, D.W.; Lusztyk, J.; Banks, J.T.; Mulder, P.; Ingold, K.U. Kinetic Solvent Effects on Hydrogen-Atom Abstractions: Reliable, Quantitative Predictions via a Single Empirical Equation. J. Am. Chem. Soc. 2001, 123, 469-477. https://doi.org/10.1021/ja002301e. [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. https://doi.org/10.1021/ar0682029. [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem. Eur. J. 2016, 22, 7924-7934. https://doi.org/10.1002/chem.201504492. [CrossRef]
- Amorati, R.; Valgimigli, L.; Pedulli, G.F.; Grabovskiy, S.A.; Kabalnova, N.N.; Chatgilialoglu, C. Base-promoted reaction of 5-hydroxyuracil derivatives with peroxyl radicals. Org. Lett. 2010, 12, 4130-4133. https://doi.org/10.1021/ol1017245. [CrossRef]
- Amorati, R., Pedulli, G.F., Valgimigli, L. Kinetic and thermodynamic aspects of the chain-breaking antioxidant activity of ascorbic acid derivatives in non-aqueous media. Org. Biomol. Chem. 2011, 9, 3792-3800. https://doi.org/10.1039/c1ob05334e. [CrossRef]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced antioxidant activity under biomimetic settings of ascorbic acid included in halloysite nanotubes. Antioxidants 2019, 8, 30. https://doi.org/10.3390/antiox8020030. [CrossRef]
- Valgimigli, L.; Amorati, R.; Petrucci, S.; Pedulli, G.F.; Hu, D.; Hanthorn, J.J.; Pratt, D.A. Unexpected acid catalysis in reactions of peroxyl radicals with phenols. Angew. Chem. Int. Ed. 2009, 48, 8348-8351. https://doi.org/10.1002/anie.200903360. [CrossRef]
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The unusual reaction of semiquinone radicals with molecular oxygen. J. Org. Chem. 2008, 73, 1830-1841. https://doi.org/10.1021/jo7024543. [CrossRef]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; D'Ischia, M. 5-S-lipoylhydroxytyrosol, a multidefense antioxidant featuring a solvent-tunable peroxyl radical-scavenging 3-thio-1,2-dihydroxybenzene motif. J. Org. Chem., 2013, 78, 9857-9864. https://doi.org/10.1021/jo401522q. [CrossRef]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The Antioxidant Activity of Quercetin in Water Solution. Biomimetics 2017, 2, 9. https://doi.org/10.3390/biomimetics2030009. [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L.; Amorati,R.; Minisci, F. Thermochemical and kinetic studies of a bisphenol antioxidant. J. Org. Chem. 2001, 66, 5456 – 546210.. https://doi.org/10.1021/jo015653s. [CrossRef]
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated anthocyanins from sprouts of Raphanus sativus cv. Sango: Isolation, structure elucidation and antioxidant activity. Food Chem. 2015, 166, 397-406. https://doi.org/10.1016/j.foodchem.2014.06.056. [CrossRef]
- Niki, E.; Saito, T.; Kawakami, A.; Kamiya, Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J. Biol. Chem. 1984, 259, 4177–4182. https://doi.org/10.1016/S0021-9258(17)43026-2. [CrossRef]
- Bowry, V.W.; Ingold, K.U. The Unexpected Role of Vitamin E (α-Tocopherol) in the Peroxidation of Human Low-Density Lipoprotein. Acc. Chem. Res. 1999, 32, 27-34. https://doi.org/10.1021/ar950059o. [CrossRef]
- Valgimigli, L.; Lucarini, M.; Pedulli, G.F.; Ingold, K.U. Does β-carotene really protect vitamin E from oxidation? J. Am. Chem. Soc. 1997, 119, 8095-8096. https://doi.org/10.1021/ja971351p. [CrossRef]
- Alfieri, M.L.; Panzella, L.; Amorati, R.; Cariola, A.; Valgimigli, L.; Napolitano, A. Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds. Biomolecules 2022, 12, 90. https://doi.org/10.3390/biom12010090. [CrossRef]
- Amorati, R.; Pedulli, G.F.; Valgimigli, L.; Johansson, H.; Engman, L. Organochalcogen substituents in phenolic antioxidants. Org. Lett. 2010, 12, 2326-2329. https://doi.org/10.1021/ol100683u. [CrossRef]
- Kumar, S.; Johansson, H.; Engman, L.; Valgimigli, L.; Amorati, R., Fumo, M.G.; Pedulli, G.F. Regenerable chain-breaking 2,3-dihydrobenzo[b]selenophene-5-ol antioxidants. J. Org. Chem. 2007, 72, 2583-2595. https://doi.org/10.1021/jo0700023. [CrossRef]
- Kumar, S.; Johansson, H.; Kanda, T.; Engman, L.; Muller, T.; Jonsson, M.; Pedulli, G.F.; Petrucci, S.; Valgimigli, L. Catalytic chain-breaking pyridinol antioxidants. Org. Lett., 2008, 10, 4895-4898. https://doi.org/10.1021/ol801983n. [CrossRef]
- Johansson, H.; Shanks, D.; Engman, L.; Amorati, R.; Pedulli, G.F.; Valgimigli, L. Long-lasting antioxidant protection: A regenerable BHA analogue. J. Org. Chem. 2010, 75, 7535-7541. https://doi.org/10.1021/jo101239c. [CrossRef]
- Amorati, R.; Valgimigli, L.; Dinér, P.; Bakhtiari, K.; Saeedi, M.; Engman, L. Multi-faceted reactivity of alkyltellurophenols towards peroxyl radicals: Catalytic antioxidant versus thiol-depletion effect. Chem. Eur. J. 2013, 19, 7510-7522. https://doi.org/10.1002/chem.201300451. [CrossRef]
- McGrath, A.J.; Garrett, G.E.; Valgimigli, L.; Pratt, D.A. The redox chemistry of sulfenic acids. J. Am. Chem. Soc. 2010, 132, 16759-16761. https://doi.org/10.1021/ja1083046. [CrossRef]
- Amorati, R., Lynett, P.T., Valgimigli, L., Pratt, D.A. The reaction of sulfenic acids with peroxyl radicals: Insights into the radical-trapping antioxidant activity of plant-derived thiosulfinates. Chem. Eur. J. 2012, 18, 6370-6379. https://doi.org/10.1002/chem.201103459. [CrossRef]
- Zielinski, Z.; Presseau, N.; Amorati, R.; Valgimigli, L.; Pratt, D.A. Redox chemistry of selenenic acids and the insight it brings on transition state geometry in the reactions of peroxyl radicals. J. Am. Chem. Soc. 2014, 136, 1570-1578. https://doi.org/10.1021/ja411493t. [CrossRef]
- Hanthorn, J.J.; Valgimigli, L.; Pratt, D.A. Preparation of highly reactive pyridine- and pyrimidine-containing diarylamine antioxidants. J. Org. Chem. 2012, 77, 6908-6916. https://doi.org/10.1021/jo301013c. [CrossRef]
- Hanthorn, J.J.; Amorati, R.; Valgimigli, L.; Pratt, D.A. The reactivity of air-stable pyridine- and pyrimidine-containing diarylamine antioxidants. J. Org. Chem. 2012, 77, 6895-6907. https://doi.org/10.1021/jo301012x. [CrossRef]
- Shah, R.; Haidasz, E.A.; Valgimigli, L.; Pratt, D.A. Unprecedented inhibition of hydrocarbon autoxidation by diarylamine radical-trapping antioxidants. J. Am. Chem. Soc. 2015, 137, 2440-2443. https://doi.org/10.1021/ja5124144. [CrossRef]
- Vaz, S. M.; Augusto, O. Reactive Oxygen Species Special Feature: Inhibition of myeloperoxidase-mediated protein nitration by tempol: Kinetics, mechanism, and implications. Proc. Natl. Acad. Sci. U.S.A., 2008, 105, 8191- 8196. https://doi.org/10.1073/pnas.0708211105. [CrossRef]
- Bi, W.; Bi, Y.; XGao, X.; Li, P.; Hou, S.; Zhang, Y.; Bammert, C.; Jockusch, S.; Legalley, T.D.; Gibson, K.M.; Bi, L. Indole-TEMPO conjugates alleviate ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function. Bioorg. Med. Chem. 2017, 5, 2545-2568. https://doi.org/10.1016/j.bmc.2017.03.033. [CrossRef]
- Goldstein, S.; Merenyi, G.; Russo, A.; Samuni, A. The Role of Oxoammonium Cation in the SOD-Mimic Activity of Cyclic Nitroxides. J. Am. Chem. Soc. 2003, 125, 789−795. https://doi.org/10.1021/ja028190. [CrossRef]
- Goldstein, S.; Samuni, A. Kinetics and Mechanism of Peroxyl Radical Reactions with Nitroxides. J. Phys. Chem. A 2007, 111, 1066−1072. https://doi.org/10.1021/jp0655975. [CrossRef]
- Amorati, R.; Pedulli, G. F.; Pratt, D. A.; Valgimigli, L. TEMPO reacts with oxygen-centered radicals under acidic conditions. Chem. Commun. 2010, 46, 5139−5141. https://doi.org/10.1039/c0cc00547a. [CrossRef]
- Haidasz, E. A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K. U.; Valgimigli, L.; Pratt, D. A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290−5298. https://doi.org/10.1021/jacs.6b00677. [CrossRef]
- Baschieri, A.; Valgimigli, L.; Gabbanini, S.; DiLabio, G.A.; Romero-Montalvo, E.; Amorati, R. Extremely Fast Hydrogen Atom Transfer between Nitroxides and HOO· Radicals and Implication for Catalytic Coantioxidant Systems J. Am. Chem. Soc. 2018, 140, 10354 – 10362. https://doi.org/10.1021/jacs.8b06336. [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345,128468. https://doi.org/10.1016/j.foodchem.2020.128468. [CrossRef]
- Baschieri, A.; Daci Ajvazi, M.; Folifack Tonfack, J.L.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. https://doi.org/10.1016/j.foodchem.2017.04.036. [CrossRef]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules 2021, 26, 5237. https://doi.org/10.3390/molecules2617523. [CrossRef]
- Khoobchandani, M., Ganesh, N., Gabbanini, S., Valgimigli, L., Srivastava, M.M. Phytochemical potential of Eruca sativa for inhibition of melanoma tumor growth. Fitoterapia, 2011, 82, 647-653. https://doi.org/10.1016/j.fitote.2011.02.004. [CrossRef]
- Cores, Á.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. https://doi.org/10.3390/biom10060904. [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. https://doi.org/10.3390/molecules28020901. [CrossRef]
- Zamanian, M.Y.; Soltani, A.; Khodarahmi, Z.; Alameri, A.A.; Alwan, A.M.R.; Ramírez-Coronel, A.A.; Obaid, R.F.; Abosaooda, M.; Heidari, M.; Golmohammadi, M.; Anoush, M. Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundam. Clin. Pharmacol. 2023, 1-15. https://doi.org/10.1111/fcp.12926. [CrossRef]
| Lipid |
kp/(2kt)1/2 10-5 M-1/2s-1/2 |
kp M-1s-1 |
2kt 105 M-1s-1 |
Ref. |
|---|---|---|---|---|
| Methyl Stearate (18:0) 1 | ~0.8 | ~0.01 | 15 | 21 |
| Methyl Oleate (18:1) | 89.0 | 0.89 | 10 | 22 |
| Methyl Linoleate (18:2) | 2100 | 62.0 | 88 | 22 |
| Methyl linolenate (18:3) | 3900 | 236.0 | 360 | 22 |
| Linoleic acid (18:2) | - | 62 | - | 23 |
| Arachidonic acid (20:4) | - | 197 | - | 23 |
| Eicosapentaenoic ac. (20:5) | - | 249 | - | 23 |
| Docosahexaenoic ac. (22:6) | - | 334 | - | 23 |
| Cholesterol | - | 11 | - | 23 |
| 7-Dehydrocholesterol | - | 2260 | - | 23 |
| Squalene | 2500 | 68.0 | 74.0 | 30 |
| Sunflower oil (60% of 18:2) | 3600 | 66.9 | 34.5 | 30 |
| PLPC 2 | - | 16.6 | 1.27 | 49 |
| DLPC 3 | - | 13.6 4 | 1.02 | 49 |
| Entry | Compound | BDE kcal/mol |
kinh M-1s-1 |
n | Ref. |
|---|---|---|---|---|---|
| 1 | α-Tocopherol | 77.1 | 3.2×106 | 2.0 | 3,21 |
| 2 | β-Tocopherol | - | 1.3×106 | 2.0 | 3,21 |
| 3 | γ-Tocopherol | - | 1.4×106 | 2.0 | 3,21 |
| 4 | δ-Tocopherol | - | 4.4×105 | 2.0 | 3,21 |
| 5 | 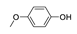 |
81.7 | 2.7×105 | 2.0 | 21,94 |
| 6 | 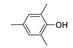 |
81.6 | 8.5×104 | 2.0 | 21,94 |
| 7 | 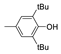 |
79.9 | 1.4×104 | 2.0 | 21,94 |
| 8 | 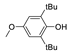 |
77.2 | 1.1×105 | 2.0 | 21,94 |
| 9 | 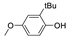 |
80.3 | 6.4×105 | 1.8 | 21,94 |
| 10 | 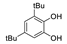 |
78.2 | 1.1×106 | 2.0 | 94,120 |
| 11 |  |
79.2 | 1.6×106 1 | 0.3 | 119 |
| 12 | Quercetin | - | 5.5×105 | 2.1 | 120 |
| 13 | Pyr-1 2 | 81.4 | 2.1×105 | 2.0 | 100 |
| 14 | Pyr-2 2 | 77.1 | 8.6×106 | 2.0 | 100 |
| 15 | Pyr-3 | 78.9 | 4.4×105 | 2.1 | 106 |
| 16 | Pyr-4 | 75.9 | 1.6×107 | 2.0 | 101 |
| 17 | Pyr-5 | 75.2 | 8.8×107 | 1.3-2.0 | 101 |
| 18 | Pyr-6 | 75.2 | 8.8×107 | ~2 | 103 |
| 19 | Pyr-7 | 74.3 | 2.8×108 | ~2 | 101 |
| 20 | α-Selenotocopherol | 78.1 | 1.2×106 | 1.9 | 127 |
| 21 | 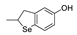 |
81,6 | 3.8×105 | 2.0 | 129 |
| 22 | Te-1 | - | 9.2×106 | 0.4 6 | 130 |
| 23 | Te-2 | 78.9 | 1.0×107 | 0.4 6 | 131 |
| 24 | Te-3 | - | 1.6×106 | 0.3 6 | 132 |
| 25 | Te-4 | - | 1.0×107 | 0.4 6 | 132 |
| 26 | Phenoxazine 2,3 | 76.1 | 2.9×107 | 5 | 94 |
| 27 | Phenothiazine 2,4 | 78.2 | 8.8×106 | 1.8 | 94 |
| 28 | Dia-1 | 78.8 | 3.4×107 | >2 | 137 |
| 29 | Dia-2 | 79.0 | 3.7×107 | >2 | 137 |
| 30 | Ferrostatin-1 5 | - | 3.5×105 | 2.0 | 11 |
| 31 | Liproxstatin-1 5 | - | 2.4×105 | 1.9 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





