Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods and Results
Database
Population of Interest
Analysis
Variables and Significance
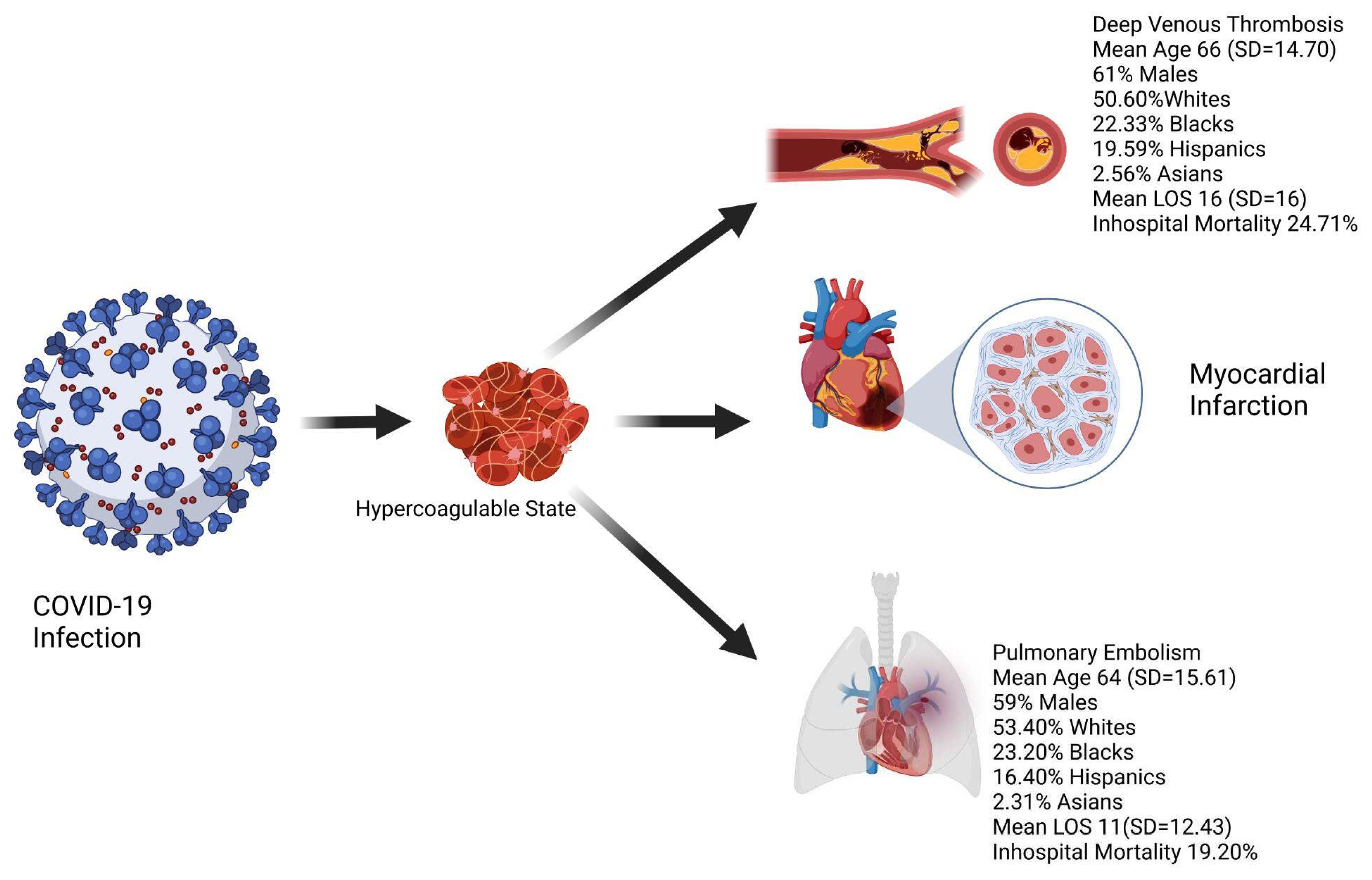
Results
Discussion
Limitations of the Study
Conclusion
Author contribution
Source(s) of fund support
Disclaimers
Regulatory Approval or Research Subject Protection Requirements
Ethical approval
Data Availability
Conflict of interest
References
- Umakanthan S, Sahu P, Ranade A, v., et al., Origin, transmission, diagnosis, and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753-758. [CrossRef]
- Weekly Review. https://covid.cdc.gov/covid-data-tracker.
- Global Situation Situation in REGION Russian Federation. https://covid19.who.int.
- Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26(8):1212-1217. [CrossRef]
- Potere N, Valeriani E, Candeloro M, et al., Acute complications and mortality in hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Crit Care. 2020;24(1). [CrossRef]
- Driggin E, Madhavan M v., Bikdeli B, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75(18):2352-2371. [CrossRef]
- Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and Cardiovascular Disease. Circulation. 2020;141(20):1648-1655. [CrossRef]
- Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5(7):831-840. [CrossRef]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [CrossRef]
- Bikdeli B, Madhavan M, v. Jimenez D, et al., COVID-19, and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-2973. [CrossRef]
- Bikdeli B, Madhavan M, v. Jimenez D, et al., COVID-19, and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-2973. [CrossRef]
- Goeijenbier M, van Wissen M, van de Weg C et al. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680-1696. [CrossRef]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [CrossRef]
- Baccellieri, D., Bertoglio, L., Apruzzi, L., Ardita, V., D'Angelo, A., Bossi, M., Rinaldi, E., Bilman, V., Calvisi, S., Castagna, A., Galli, L., Landoni, G., Melissano, G., Querini, P. R., Tresoldi, M., De Cobelli, F., Zangrillo, A., Ciceri, F., & Chiesa, R. (2021). Incidence of deep venous thrombosis in COVID-19 hospitalized patients during the first peak of the Italian outbreak. Phlebology, 36(5), 375–383. [CrossRef]
- Loo, J., Spittle, D. A., & Newnham, M. (2021). COVID-19 immunothrombosis and venous thromboembolism: biological mechanisms. Thorax, 76(4), 412–420. [CrossRef]
- Erben, Y., Franco-Mesa, C., Gloviczki, P., Stone, W., Quinones-Hinojoas, A., Meltzer, A. J., Lin, M., Greenway, M. R. F., Hamid, O., Devcic, Z., Toskich, B., Ritchie, C., Lamb, C. J., De Martino, R. R., Siegel, J., Farres, H., Hakaim, A. G., Sanghavi, D. K., Li, Y., Rivera, C., … Meschia, J. F. (2021). Deep vein thrombosis and pulmonary embolism among hospitalized coronavirus disease 2019-positive patients predicted for higher mortality and prolonged intensive care unit and hospital stay in a multisite healthcare system. Journal of Vascular Surgery. Venous and lymphatic disorders, 9(6), 1361–1370.e1. [CrossRef]
- Ameri, P., Inciardi, R. M., Di Pasquale, M., Agostoni, P., Bellasi, A., Camporotondo, R., Canale, C., Carubelli, V., Carugo, S., Catagnano, F., Danzi, G., Dalla Vecchia, L., Giovinazzo, S., Gnecchi, M., Guazzi, M., Iorio, A., La Rovere, M. T., Leonardi, S., Maccagni, G., Mapelli, M., … Metra, M. (2021). Pulmonary embolism in patients with COVID-19: characteristics and outcomes in the Cardio-COVID Italy multicenter study. Clinical research in cardiology : official journal of the German Cardiac Society, 110(7), 1020–1028. [CrossRef]
- Fauvel, C., Weizman, O., Trimaille, A., Mika, D., Pommier, T., Pace, N., Douair, A., Barbin, E., Fraix, A., Bouchot, O., Benmansour, O., Godeau, G., Mecheri, Y., Lebourdon, R., Yvorel, C., Massin, M., Leblon, T., Chabbi, C., Cugney, E., Benabou, L., … Critical Covid-19 France Investigators (2020). Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. European heart journal, 41(32), 3058–3068. [CrossRef]
- Poissy, J., Goutay, J., Caplan, M., Parmentier, E., Duburcq, T., Lassalle, F., Jeanpierre, E., Rauch, A., Labreuche, J., Susen, S., & Lille ICU Haemostasis COVID-19 Group (2020). Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation, 142(2), 184–186. [CrossRef]
- Badr, O. I., Alwafi, H., Elrefaey, W. A., Naser, A. Y., Shabrawishi, M., Alsairafi, Z., & Alsaleh, F. M. (2021). Incidence and Outcomes of Pulmonary Embolism among Hospitalized COVID-19 Patients. International journal of environmental research and public health, 18(14), 7645. [CrossRef]
- Miró, Ò., Jiménez, S., Mebazaa, A., Freund, Y., Burillo-Putze, G., Martín, A., Martín-Sánchez, F. J., García-Lamberechts, E. J., Alquézar-Arbé, A., Jacob, J., Llorens, P., Piñera, P., Gil, V., Guardiola, J., Cardozo, C., Mòdol Deltell, J. M., Tost, J., Aguirre Tejedo, A., Palau-Vendrell, A., LLauger García, L., … Spanish Investigators on Emergency Situations TeAm (SIESTA) network (2021). Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. European heart journal, 42(33), 3127–3142. [CrossRef]
- Benito, N., Filella, D., Mateo, J., Fortuna, A. M., Gutierrez-Alliende, J. E., Hernandez, N., Gimenez, A. M., Pomar, V., Castellvi, I., Corominas, H., Casademont, J., & Domingo, P. (2020). Pulmonary Thrombosis or Embolism in a Large Cohort of Hospitalized Patients With Covid-19. Frontiers in medicine, 7, 557. [CrossRef]
- Franco-Moreno, A., Herrera-Morueco, M., Mestre-Gómez, B., Muñoz-Rivas, N., Abad-Motos, A., Salazar-Chiriboga, D., Duffort-Falcó, M., Medrano-Izquierdo, P., Bustamante-Fermosel, A., Pardo-Guimera, V., Ulla-Anés, M., Torres-Macho, J., & Infanta Leonor Thrombosis Research Group (2021). Incidence of Deep Venous Thrombosis in Patients With COVID-19 and Pulmonary Embolism: Compression Ultrasound COVID Study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine, 40(7), 1411–1416. [CrossRef]
- Cai, C., Guo, Y., You, Y., Hu, K., Cai, F., Xie, M., Yang, L., Ling, K., Ye, D., Misra, S., Wang, W., & Li, Y. (2020). Deep Venous Thrombosis in COVID-19 Patients: A Cohort Analysis. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, 26, 1076029620982669. [CrossRef]
- Yu, Y., Tu, J., Lei, B., Shu, H., Zou, X., Li, R., Huang, C., Qu, Y., & Shang, Y. (2020). Incidence and Risk Factors of Deep Vein Thrombosis in Hospitalized COVID-19 Patients. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, 26, 1076029620953217. [CrossRef]
- Xu, H., Martin, A., Singh, A., Narasimhan, M., Lau, J., Weinberg, M., Jauhar, R., & Rao, G. (2020). Pulmonary Embolism in Patients Hospitalized With COVID-19 (From a New York Health System). The American journal of cardiology, 133, 148–153. [CrossRef]
- Pereira de Godoy, J. M., Russeff, G. J. D. S., Costa, C. H., Sato, D. Y., Silva, D. F. D. F., Guerreiro Godoy, M. F., Pereira de Godoy, H. J., & Espada, P. C. (2021). Mortality of Patients Infected by COVID-19 with and without Deep-Vein Thrombosis. Medicines (Basel, Switzerland), 8(12), 75. [CrossRef]
- Fu, Z., Bai, G., Song, B., Wang, Y., Song, H., Ma, M., Zhu, J., Zhang, Z., & Kang, Q. (2022). Risk factors and mortality of pulmonary embolism in COVID-19 patients: Evidence based on fifty observational studies. Medicine, 101(45), e29895. [CrossRef]
- Ohsfeldt, R. L., Choong, C. K., Mc Collam, P. L., Abedtash, H., Kelton, K. A., & Burge, R. (2021). Inpatient Hospital Costs for COVID-19 Patients in the United States. Advances in therapy, 38(11), 5557–5595. [CrossRef]
- Madjid, M., Safavi-Naeini, P., Solomon, S. D., & Vardeny, O. (2020). Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA cardiology, 5(7), 831–840. [CrossRef]
- Giustino, G., Croft, L. B., Oates, C. P., Rahman, K., Lerakis, S., Reddy, V. Y., & Goldman, M. (2020). Takotsubo Cardiomyopathy in COVID-19. Journal of the American College of Cardiology, 76(5), 628–629. [CrossRef]
- Das, M., Bristow, M. R., & Chung, M. K. (2021). The Essential Vulnerability of Human Cardiac Myocytes to SARS-CoV-2. JACC. Basic to translational science, 6(4), 346–349. [CrossRef]
- Libby, P., Loscalzo, J., Ridker, P. M., Farkouh, M. E., Hsue, P. Y., Fuster, V., Hasan, A. A., & Amar, S. (2018). Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. Journal of the American College of Cardiology, 72(17), 2071–2081. [CrossRef]
- Tersalvi, G., Vicenzi, M., Calabretta, D., Biasco, L., Pedrazzini, G., & Winterton, D. (2020). Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. Journal of cardiac failure, 26(6), 470–475. [CrossRef]
- Majeed, H., Gangu, K., Sagheer, S., Garg, I., Khan, U., Shuja, H., Bobba, A., Chourasia, P., Shekhar, R., Avula, S. R., & Sheikh, A. B. (2022). COVID-19 and NSTEMI Outcomes among Hospitalized Patients in the United States and Racial Disparities in Mortality: Insight from National Inpatient Sample Database. Vaccines, 10(12), 2024. [CrossRef]
- Case, B. C., Yerasi, C., Forrestal, B. J., Shea, C., Rappaport, H., Medranda, G. A., Zhang, C., Satler, L. F., Ben-Dor, I., Hashim, H., Rogers, T., & Waksman, R. (2021). Comparison of Characteristics and Outcomes of Patients With Acute Myocardial Infarction With Versus Without Coronarvirus-19. The American journal of cardiology, 144, 8–12. [CrossRef]
- Rodriguez-Leor, O., Cid Alvarez, A. B., Pérez de Prado, A., Rossello, X., Ojeda, S., Serrador, A., López-Palop, R., Martin-Moreiras, J., Rumoroso, J. R., Cequier, A., Ibáñez, B., Cruz-González, I., Romaguera, R., & Moreno, R. (2021). In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 16(17), 1426–1433. [CrossRef]
- Choudry, F. A., Hamshere, S. M., Rathod, K. S., Akhtar, M. M., Archbold, R. A., Guttmann, O. P., Woldman, S., Jain, A. K., Knight, C. J., Baumbach, A., Mathur, A., & Jones, D. A. (2020). High Thrombus Burden in Patients With COVID-19 Presenting With ST-Segment Elevation Myocardial Infarction. Journal of the American College of Cardiology, 76(10), 1168–1176. [CrossRef]
- Saad, M., Kennedy, K. F., Imran, H., Louis, D. W., Shippey, E., Poppas, A., Wood, K. E., Abbott, J. D., & Aronow, H. D. (2021). Association Between COVID-19 Diagnosis and In-Hospital Mortality in Patients Hospitalized With ST-Segment Elevation Myocardial Infarction. JAMA, 326(19), 1940–1952. [CrossRef]
- Kite, T. A., Ludman, P. F., Gale, C. P., Wu, J., Caixeta, A., Mansourati, J., Sabate, M., Jimenez-Quevedo, P., Candilio, L., Sadeghipour, P., Iniesta, A. M., Hoole, S. P., Palmer, N., Ariza-Solé, A., Namitokov, A., Escutia-Cuevas, H. H., Vincent, F., Tica, O., Ngunga, M., Meray, I., … International COVID-ACS Registry Investigators (2021). International Prospective Registry of Acute Coronary Syndromes in Patients With COVID-19. Journal of the American College of Cardiology, 77(20), 2466–2476. [CrossRef]
- Dadras, O., SeyedAlinaghi, S., Karimi, A., Shamsabadi, A., Qaderi, K., Ramezani, M., Mirghaderi, S. P., Mahdiabadi, S., Vahedi, F., Saeidi, S., Shojaei, A., Mehrtak, M., Azar, S. A., Mehraeen, E., & Voltarelli, F. A. (2022). COVID-19 mortality and its predictors in the elderly: A systematic review. Health science reports, 5(3), e657. [CrossRef]
- Stefanini, G. G., Montorfano, M., Trabattoni, D., Andreini, D., Ferrante, G., Ancona, M., Metra, M., Curello, S., Maffeo, D., Pero, G., Cacucci, M., Assanelli, E., Bellini, B., Russo, F., Ielasi, A., Tespili, M., Danzi, G. B., Vandoni, P., Bollati, M., Barbieri, L., … Chieffo, A. (2020). ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation, 141(25), 2113–2116. [CrossRef]
- Kaur, P., Patel, P., Singh, B., Guragai, N., Vasudev, R., Virk, H. S., Shamoon, F., & Bikkina, M. (2021). ST-Segment Elevation in Patients with COVID-19: A Late Complication. The American journal of the medical sciences, 361(3), 403–405. [CrossRef]
- Muñoz-Price, L. S., Nattinger, A. B., Rivera, F., Hanson, R., Gmehlin, C. G., Perez, A., Singh, S., Buchan, B. W., Ledeboer, N. A., & Pezzin, L. E. (2020). Racial Disparities in Incidence and Outcomes Among Patients With COVID-19. JAMA network open 3(9), e2021892. [CrossRef]
- Dai, C. L., Kornilov, S. A., Roper, R. T., Cohen-Cline, H., Jade, K., Smith, B., Heath, J. R., Diaz, G., Goldman, J. D., Magis, A. T., & Hadlock, J. J. (2021). Characteristics and Factors Associated With Coronavirus Disease 2019 Infection, Hospitalization, and Mortality Across Race and Ethnicity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 73(12), 2193–2204. [CrossRef]
- Bangalore, S., Sharma, A., Slotwiner, A., Yatskar, L., Harari, R., Shah, B., Ibrahim, H., Friedman, G. H., Thompson, C., Alviar, C. L., Chadow, H. L., Fishman, G. I., Reynolds, H. R., Keller, N., & Hochman, J. S. (2020). ST-Segment Elevation in Patients with Covid.
- A Case Series. The New England journal of medicine, 382(25), 2478–2480. [CrossRef]
- Hamadeh, A., Aldujeli, A., Briedis, K., Tecson, K. M., Sanz-Sánchez, J., Al Dujeili, M., Al-Obeidi, A., Diez, J. L., Žaliūnas, R., Stoler, R. C., & McCullough, P. A. (2020). Characteristics and Outcomes in Patients Presenting With COVID-19 and ST-Segment Elevation Myocardial Infarction. The American journal of cardiology, 131, 1–6. [CrossRef]
- Evbayekha E, Okorare O, Okobi O. A Year Retrospective Study on the Morbidity and Mortality Pattern of Covid-19 Patients in an Isolation Facility in Benin City, Nigeria -. IJSCIA. Published August 27, 2021. Accessed May 10, 2023. https://www.ijscia.com/full-text-volume-2-issue-4-jul-sep-2021-659-663/.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




