Submitted:
11 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Population and Imaging
2.2. Procedural Details
2.3. Endpoint and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics: Clinical and Echocardiography
3.2. Outcomes and Correlates
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- El-Said, H.G.; Moore, J.W. Erosion by the Amplatzer Septal Occluder: Experienced Operator Opinions at Odds with Manufacturer Recommendations? Catheter. Cardiovasc. Interv. 2009, 73, 925–930. [Google Scholar] [CrossRef]
- Schoen, S.P.; Boscheri, A.; Lange, S.A.; Braun, M.U.; Fuhrmann, J.; Kappert, U.; Strasser, R.H. Incidence of Aortic Valve Regurgitation and Outcome after Percutaneous Closure of Atrial Septal Defects and Patent Foramen Ovale. Heart 2008, 94, 844–847. [Google Scholar] [CrossRef]
- Zahn, E.M.; Wilson, N.; Cutright, W.; Latson, L.A. Development and Testing of the Helex Septal Occluder, a New Expanded Polytetrafluoroethylene Atrial Septal Defect Occlusion System. Circulation 2001, 104, 711–716. [Google Scholar] [CrossRef]
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Louis Mas, J.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; et al. European Position Paper on the Management of Patients with Patent Foramen Ovale. General Approach and Left Circulation Thromboembolism. Eur. Heart J. 2019, 40, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Thaman, R.; Faganello, G.; Gimeno, J.R.; Szantho, G. V.; Nelson, M.; Curtis, S.; Martin, R.P.; Turner, M.S. Efficacy of Percutaneous Closure of Patent Foramen Ovale: Comparison among Three Commonly Used Occluders. Heart 2011, 97, 394–399. [Google Scholar] [CrossRef] [PubMed]
- von Bardeleben, R.S.; Richter, C.; Otto, J.; Himmrich, L.; Schnabel, R.; Kampmann, C.; Rupprecht, H.J.; Marx, J.; Hommel, G.; Münzel, T.; et al. Long Term Follow up after Percutaneous Closure of PFO in 357 Patients with Paradoxical Embolism: Difference in Occlusion Systems and Influence of Atrial Septum Aneurysm. Int. J. Cardiol. 2009, 134, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, P.; Rondi, M.; Pavone, A.; Aiolfi, E.; Facchinetti, A.; Funaro, A.; Negrini, P. Clinical Performance of the New Gore Septal Occluder in Patent Foramen Ovale Closure: A Single-Center Experience. J. Invasive Cardiol. 2015, 27, 430–434. [Google Scholar] [PubMed]
- Butera, G.; Saracino, A.; Danna, P.; Sganzerla, P.; Chessa, M.; Carminati, M. Transcatheter PFO Closure with GORE® Septal Occluder: Early and Mid-Term Clinical Results. Catheter. Cardiovasc. Interv. 2013, 82, 944–949. [Google Scholar] [CrossRef]
- Søndergaard, L.; Kasner, S.E.; Rhodes, J.F.; Andersen, G.; Iversen, H.K.; Nielsen-Kudsk, J.E.; Settergren, M.; Sjöstrand, C.; Roine, R.O.; Hildick-Smith, D.; et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N. Engl. J. Med. 2017, 377, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.S.; Shapiro, L.M.; McCarthy, K.P.; Ho, S.Y. Three-Dimensional Imaging of the Atrial Septum and Patent Foramen Ovale Anatomy: Defining the Morphological Phenotypes of Patent Foramen Ovale. Eur. J. Echocardiogr. 2010, 11, 19–25. [Google Scholar] [CrossRef]
- Rana, B.S.; Thomas, M.R.; Calvert, P.A.; Monaghan, M.J.; Hildick-Smith, D. Echocardiographic Evaluation of Patent Foramen Ovale Prior to Device Closure. JACC Cardiovasc. Imaging 2010, 3, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Kavinsky, C.J.; Szerlip, M.; Goldsweig, A.M.; Amin, Z.; Boudoulas, K.D.; Carroll, J.D.; Coylewright, M.; Elmariah, S.; MacDonald, L.A.; Shah, A.P.; et al. SCAI Guidelines for the Management of Patent Foramen Ovale. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100039. [Google Scholar] [CrossRef]
- Martín, F.; Sánchez, P.L.; Doherty, E.; Colon-Hernandez, P.J.; Delgado, G.; Inglessis, I.; Scott, N.; Hung, J.; King, M.E.E.; Buonanno, F.; et al. Percutaneous Transcatheter Closure of Patent Foramen Ovale in Patients with Paradoxical Embolism. Circulation 2002, 106, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Vitarelli, A.; Mangieri, E.; Capotosto, L.; Tanzilli, G.; D’Angeli, I.; Toni, D.; Azzano, A.; Ricci, S.; Placanica, A.; Rinaldi, E.; et al. Echocardiographic Findings in Simple and Complex Patent Foramen Ovale before and after Transcatheter Closure. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.C.; Lock, J.E. Structural and Compliant Anatomy of the Patent Foramen Ovale in Patients Undergoing Transcatheter Closure. Am. Heart J. 2000, 140, 303–307. [Google Scholar] [CrossRef]
- Vizzari, G.; Pizzino, F.; Zwicke, D.; Tajik, A.J.; Carerj, S.; Di Bella, G.; Micari, A.; Khandheria, B.K.; Zito, C. Patent Foramen Ovale: Anatomical Complexity and Long-Tunnel Morphology Related Issues. Am. J. Cardiovasc. Dis. 2021, 11, 316–329. [Google Scholar]
- Calvert, P.A.; Rana, B.S.; Kydd, A.C.; Shapiro, L.M. Patent Foramen Ovale: Anatomy, Outcomes, and Closure. Nat. Rev. Cardiol. 2011, 8, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Greutmann, M.; Greutmann-Yantiri, M.; Kretschmar, O.; Senn, O.; Roffi, M.; Jenni, R.; Luescher, T.F.; Eberli, F.R. Percutaneous PFO Closure with Amplatzer PFO Occluder: Predictors of Residual Shunts at 6 Months Follow-Up. Congenit. Heart Dis. 2009, 4, 252–257. [Google Scholar] [CrossRef]
- Giordano, M.; Gaio, G.; Santoro, G.; Palladino, M.T.; Sarubbi, B.; Golino, P.; Russo, M.G. Patent Foramen Ovale with Complex Anatomy: Comparison of Two Different Devices (Amplatzer Septal Occluder Device and Amplatzer PFO Occluder Device 30/35). Int. J. Cardiol. 2019, 279, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Geis, N.A.; Pleger, S.T.; Katus, H.A.; Hardt, S.E. Using the GORE® Septal Occluder (GSO) in Challenging Patent Foramen Ovale (PFO) Anatomies. J. Interv. Cardiol. 2015, 28, 190–197. [Google Scholar] [CrossRef]
- Lockhart, C.J.; Johnston, N.G.; Spence, M.S. Experience Using the New GORE® Septal Occluder at the Margins. Catheter. Cardiovasc. Interv. 2013, 81, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
| Patient population [n] | 118 |
| Male gender [n] | 60 (50,8%) |
| Age [years] | 55,4 ± 12,7 |
| Current smoker (n, %) | 19 (16,1%) |
| Indication for closure | |
| Stroke (n,%) | 68 (57,6%) |
| Transient Ischemic Attack (n,%) | 50 (42,4%) |
| Head CT or MRI de-novo lesion (n,%) | 101 (85,6%) |
| TEE Doppler study | |
| Small shunt (n,%) | 15 (12,7%) |
| Moderate-to-severe shunt (n,%) | 37 (31,4%) |
| Anatomical setting | |
| PFO only (n,%) | 105 (89%) |
| PFO+ASA (n,%) | 13 (11%) |
| Median procedure time [minutes] | 37 (range 33-45) |
| Median fluoroscopic time [minutes] | 7.3 (range 5.5-10.2) |
| Device used | |
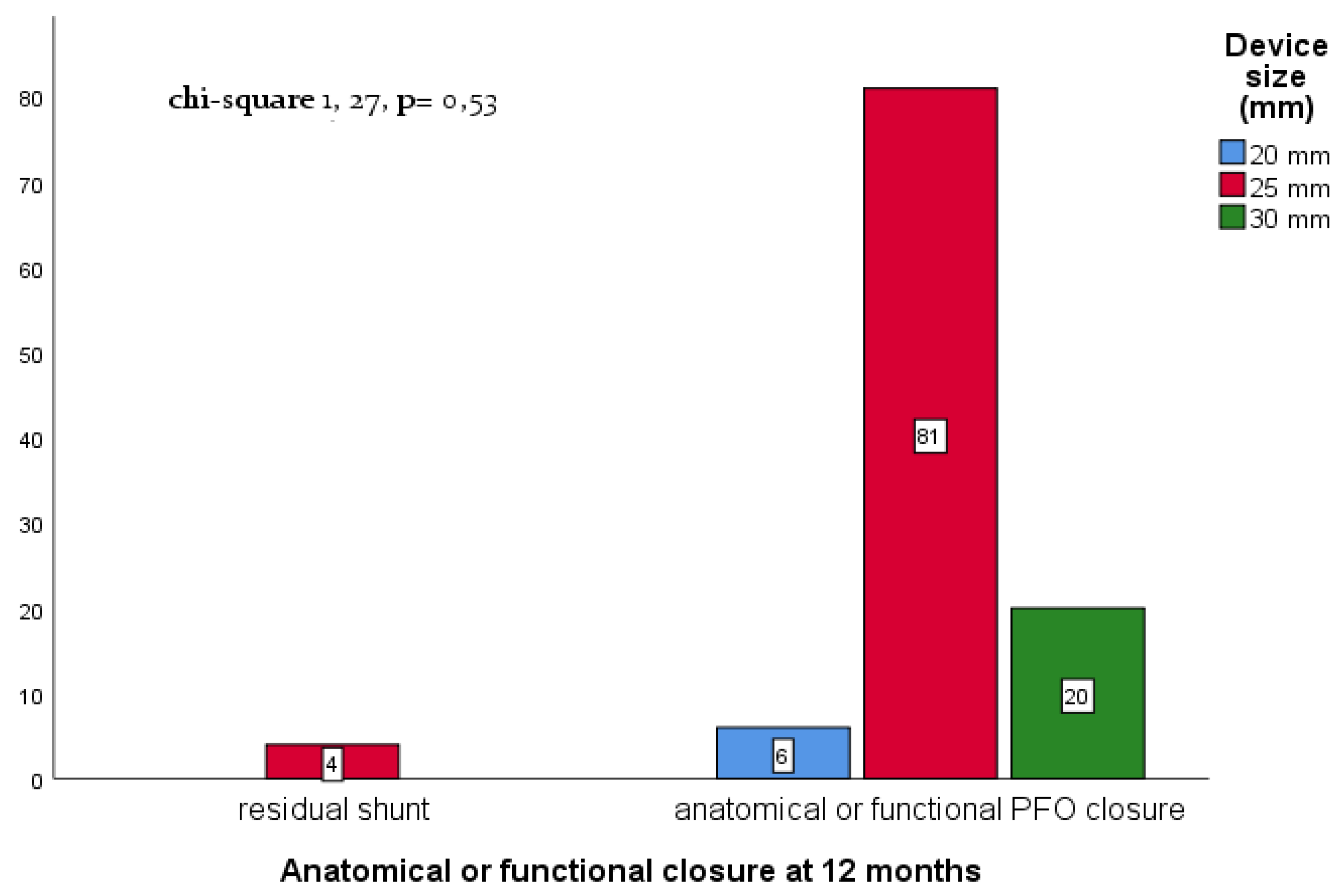
| 20 mm (n,%) | 6 (5,0%) |
| 25 mm (n,%) | 92 (78%) |
| 30 mm (n,%) | 20 (17%) |
| Median contrast administration [ml] | 36 (range 25-54) |
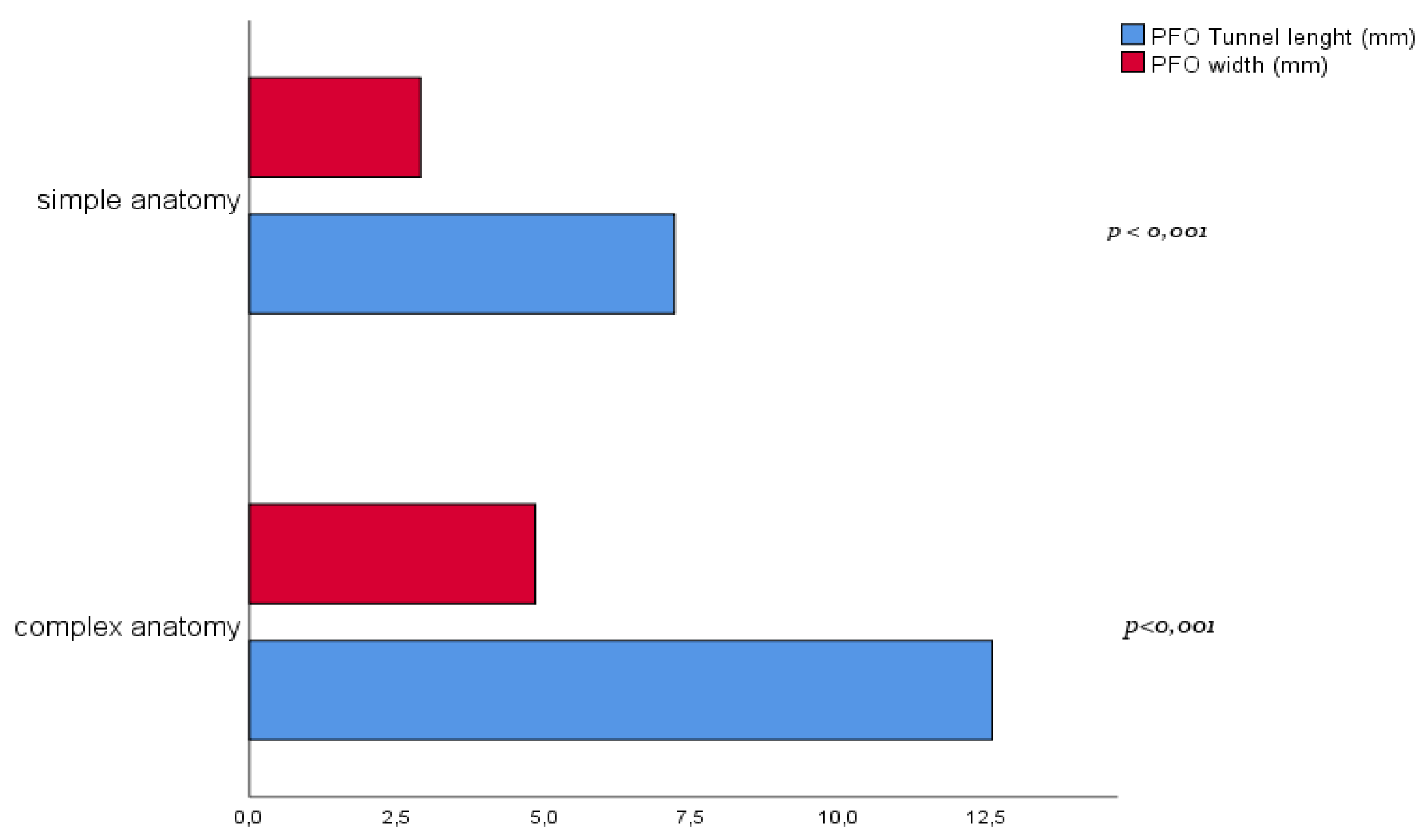
| Complex anatomy (n. 57) | Simple anatomy (n. 54) | p-value | |
| Age | 53.9 ± 12.4 | 56.6 ± 13.0 | 0.279 |
| Procedure time (min) | 39.6 ± 12.1 | 41.5 ± 15.2 | 0.502 |
| Fluoroscopy time (min) | 10.1 ± 16.5 | 9.4 ± 5.8 | 0.748 |
| Contrast dye (ml) | 38.2 ± 26.1 | 47.1 ± 26.1 | 0.090 |
| SS thickness (mm) | 5.7 ± 2.3 | 5.7 ± 1.9 | 0.995 |
| Tunnel length (mm) | 12.6 ± 3.8 | 7.2 ± 2.0 | <0.001 |
| PFO width (mm) | 4.9 ± 1.8 | 2.9 ± 1.1 | <0.001 |
| Predictor | Anatomical or functional closure at 12-m (%) | OR | C.I. 95% | p-value |
|---|---|---|---|---|
| Complex vs simple anatomy¶ | 107/111 (96,4%) | 0,36 | 0,04 – 3,6 | 0,39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





