1. Introduction
Coronaviridae is a large family of RNA–containing pathogens infecting humans and other mammals [
1]. Their virions have a complex immunologic composition, including three antigenically distinct subunits. Until the beginning of the 21st century, four seasonal coronaviruses (HCoV-229E, HCoV-OC43, HCoV-NL63 and HCoV-HKU-1) were described in the human population. [
1]. In 2002 severe acute respiratory syndrome (caused by SARS-CoV-1), as well as in 2012 - Middle East respiratory syndrome (MERS, caused by MERS-CoV virus) were described, hence first couple of highly pathogenic members of the family appeared in the scope of medical attention, causing life-treating pneumonia and terminal respiratory failure.
In December 2019, the previously unknown coronavirus SARS-CoV-2 was evaluated [
2], causing a pandemic of the novel highly pathogenic respiratory infection [
3].
Novel coronavirus infection and autoimmunity
The respiratory system is the one to be the most impacted by the SARS-CoV-2 virus, which results in pulmonary complications such as alveolar damage, fibrosis in the interstitial tissue, and infiltration of the lung [
4]. The ACE2 receptors and other molecules that allow the virus to enter cells are described in various human tissues, leading to a wide range of complications. The ACE2 enzyme can also exist in a soluble form, which may facilitate the binding of SARS-CoV-2 spike proteins and the formation of neoantigens [
5].
SARS-CoV-2 is notorious for inducing an exaggerated response from the innate immune system and the production of pro-inflammatory substances, causing breaches in localized inflammation barriers and leading to the development of a cytokine storm and autoimmune complications [
4,
6]. A molecular mimicry can be the possible explanation for this hyperactive immune response, where similar sequences between foreign and self peptides can act as a trigger [
7,
8]. A study by Kanduc et al. identified a high degree of similarity between SARS-CoV-2 spike glycoprotein and human surfactant-related proteins, suggesting that this mechanism could explain the immunopathological processes in the lungs associated with coronavirus infection. Further investigation revealed extensive overlap between the spike glycoprotein of SARS-CoV-2 and the proteomes of humans and mice, both of which are known to experience prolonged health issues after acute SARS-CoV-2 infection [
9]. Supporting this hypothesis, Kanduc identified more than 400 specific human peptides that match immunoreactive viral epitopes [
10].
Small fiber neuropathy and the post-COVID-19 condition
Post-COVID syndrome is caused by a combination of immunologic, cardiovascular, and neurological issues. The dysfunction in both the central and peripheral nervous systems in these patients is evaluated [
11]. While there have been cases of encephalitis or encephalomyelitis linked to the viral infection [
12], it is still unclear, whether the coronavirus persists in the central nervous system without destructing the blood-brain barrier [
13,
14,
15].
Autoimmune damage to the peripheral nervous system is also important in the post-COVID-19 complications development. A series of cases of Guillain-Barré syndrome and necrotizing autoimmune myositis as the post-viral sequel were described [
11]. However, a less studied type of peripheral nervous system damage, known as small fiber neuropathy (SFN), can lead to numerous complications. SFN occurs when the smallest nerve fibers (A-delta and C-types) that penetrate almost all organs and tissues are damaged due to immunological inflammation. Standard electroneuromyography may not detect these changes, permitting the SFN to progress unrecognized.
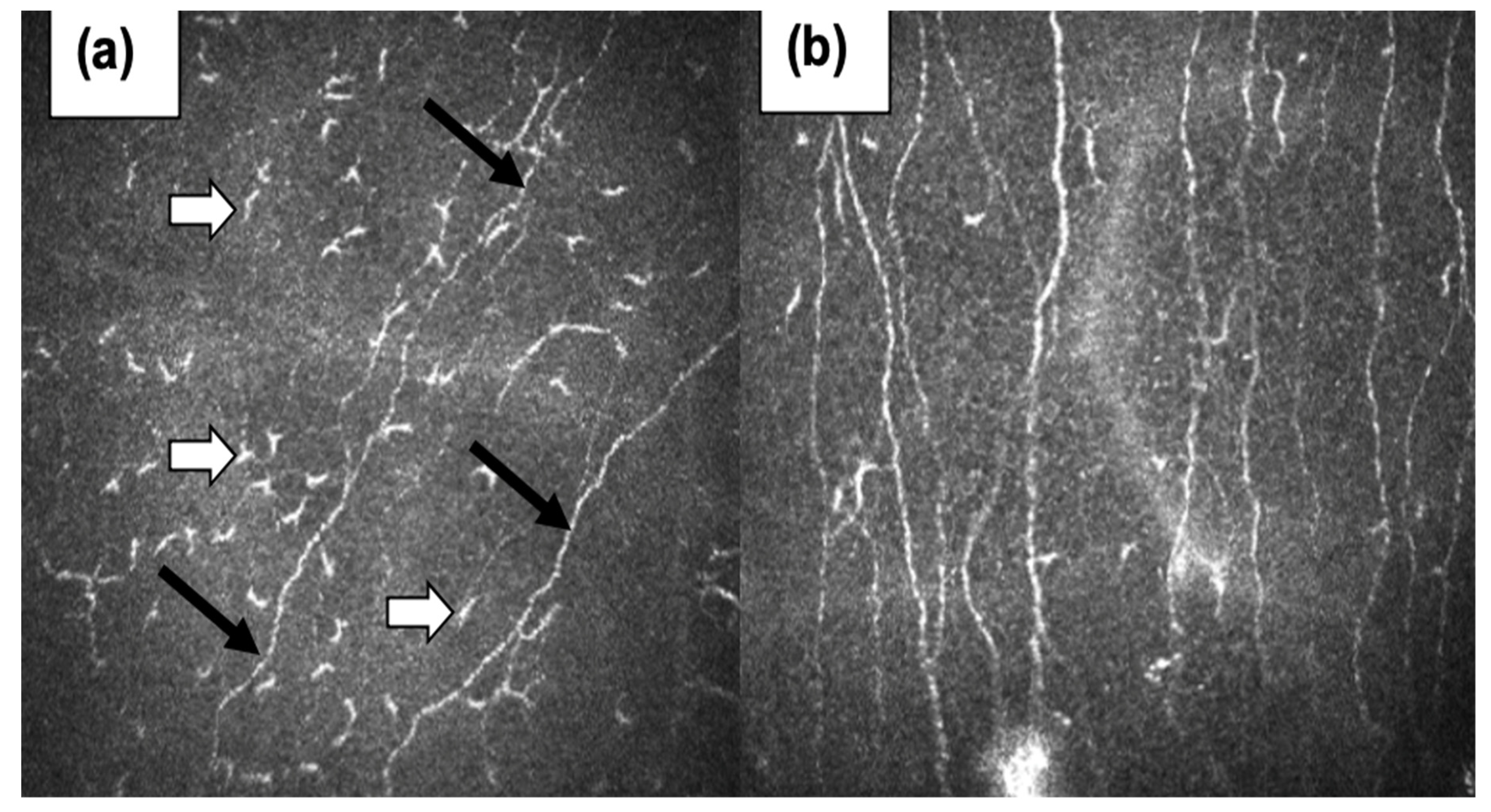
The widespread presence of these nerve fibers in the body contributes to a diverse range of symptoms. Common manifestations include sensory dysfunction and dysautonomia, affecting the cardiovascular, urinary, gastrointestinal, skin, and mucous membrane systems. Due to the varying symptoms, the diagnosis of SFN can be challenging and may require comprehensive evaluation by specialists, such as neurologists. Given the importance of dysautonomia and polyneuropathy in the post-COVID-19 clinical evaluation, the presence of molecular mimicry between the antigens of small nerve fibers and different coronaviruses may lead to the new insights in this field. Moreover, our evaluation of small nerve fibers by confocal microscopy of the cornea shows the correctness of this approach (
Figure 1).
The purpose of this article is to consider the possibility of implementing the phenomenon of molecular mimicry between autoantigens of the human nervous system and immunoreactive epitopes of human coronaviruses, which causes the emergence and development of immunological complications.
3. Results
In addition,
Table 1,
Table 2 and
Table 3 represent the number of immunoreactive epitopes containing pentapeptides in human coronavirus antigens and human autoantigens, where the IEDB database was used.
All ID numbers of immunoreactive epitopes in the IEDB database containing shared pentapeptides between human autoantigens and human coronaviruses were posted at this link (
https://github.com/muslimb/Epitope).
Analysis of the data presented in
Table 1 allows us to conclude that:
1. A Shared pentapeptide NLREF was found for Fibroblast growth factor receptor 3 and for 26 immunoreactive epitopes of the SARS-CoV-2 spike glycoprotein. This shared pentapeptide is located outside the immunoreactive epitopes of Fibroblast growth factor receptor 3.
2. A Shared pentapeptide NLNES was found for Plexin-D1 and for 32 immunoreactive epitopes of the SARS-CoV-2 spike glycoprotein. This Shared pentapeptide is located outside the immunoreactive epitopes of Plexin D1.
3. A Shared pentapeptide NLNES was found for Plexin-D1 and for two immunoreactive epitopes of the SARS-CoV spike glycoprotein. This Shared pentapeptide is located outside the immunoreactive epitopes of Plexin D1.
4. Shared pentapeptides ALALC, LALCV, LDVLE were discovered for Fibroblast growth factor receptor 3 and spike glycoprotein of the MERS-CoV. Shared pentapeptides ALALC, LALCV are located outside the immunoreactive epitopes in both spike glycoprotein of the MERS-CoV and Fibroblast growth factor receptor 3. The Shared pentapeptide LDVLE is located within the 14 of immunoreactive epitopes of the MERS-CoV spike glycoprotein and within immunoreactive epitope of Fibroblast growth factor receptor 3.
5. A shared pentapeptide EYSLY was discovered for myelin protein P2 and immunoreactive epitope of the MERS-CoV spike glycoprotein. The shared pentapeptide EYSLY is located within immunoreactive epitope of myelin protein P2.
6. Shared pentapeptides KLSDV, IILFT, ILFTG, KLSDF were discovered for sodium channel protein type 9 subunit alpha and HCoV-HKU-1 spike glycoprotein. Shared pentapeptides KLSDV and ILFTG are located within immunoreactive epitopes of HCoV-HKU-1 spike glycoprotein and outside the immunoreactive epitopes of sodium channel protein type 9 subunit alpha. The common pentapeptide IILFT is located outside the immunoreactive epitopes in both spike glycoprotein of the HCoV-HKU-1 and sodium channel protein type 9 subunit alpha. The common pentapeptide KLSDF is located within the 15 of immunoreactive epitopes of the HCoV-HKU-1 spike glycoprotein and within one immunoreactive epitope of sodium channel protein type 9 subunit alpha.
7. Common pentapeptides YVLPL, SDLQL, DLQLG were discovered for autoantigens of Plexin-D1 and immunoreactive epitopes of HCoV-HKU-1 spike glycoprotein. The common pentapeptide YVLPL is located within one immunoreactive epitope of HCoV-HKU-1 spike glycoprotein and within 7 immunoreactive epitopes of Plexin-D1. Common pentapeptides SDLQL and DLQLG are located within immunoreactive epitopes of HCoV-HKU-1 spike glycoprotein, but outside immunoreactive epitopes of Plexin-D1.
8. A Shared pentapeptide KLSDV was discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes of HCoV-ОС43 spike glycoprotein. A Shared pentapeptide is located within 10 immunoreactive epitopes of HCoV-ОС43 spike glycoprotein, but outside of immunoreactive epitopes of sodium channel protein type 9 subunit alpha.
9. Shared pentapeptides AEAEA, GTCPA, DLQLG were discovered for autoantigen Plexin-D1 and immunoreactive epitopes of HCoV-ОС43 spike glycoprotein. Shared pentapeptides AEAEA, GTCPA are located within immunoreactive epitopes of both HCoV-ОС43 spike glycoprotein (6 - AEAEA and 11 - GTCPA) and Plexin-D1. A common pentapeptide DLQLG is located outside immunoreactive epitopes of Plexin-D1.
10. A Shared pentapeptide YSDNG was discovered for Myelin protein P0 and HCoV-NL63 spike glycoprotein. A Shared pentapeptide YSDNG is located outside immunoreactive epitopes in both HCoV-NL63 spike glycoprotein and Myelin protein P0.
11. Shared pentapeptides TIVGA, IVGAL, GRSAL were discovered for sodium channel protein type 9 subunit alpha and immunoreactive epitopes HCoV-NL63 spike glycoprotein. Shared pentapeptides are located outside and immunoreactive epitopes of sodium channel protein type 9 subunit alpha.
12. Shared pentapeptides LSNAS, VTATF were discovered for Plexin-D1 and immunoreactive epitopes HCoV-NL63 spike glycoprotein. Shared pentapeptide VTATF is located within immunoreactive epitope in both HCoV-NL63 spike glycoprotein (one epitope) and Plexin-D1 (one epitope).
13. A Shared pentapeptide FVDVL was discovered for autoantigen of Ubiquitin carboxyl-terminal hydrolase isozyme L1 and immunoreactive epitopes of HCoV-NL63 spike glycoprotein. Shared pentapeptide FVDVL is located within immunoreactive epitope in both HCoV-NL63 spike glycoprotein (2 epitopes) and autoantigen of Ubiquitin carboxyl-terminal hydrolase isozyme L1 (3 epitopes).
14. Shared pentapeptides TLANV, LANVS were discovered for Fibroblast growth factor receptor 3 and immunoreactive epitopes of HCoV-229E spike glycoprotein. Shared pentapeptides are located within immunoreactive epitope of HCoV-229E spike glycoprotein, but outside immunoreactive epitopes in autoantigen of Fibroblast growth factor receptor 3.
15. Shared pentapeptides TFIVL, SYDSV were discovered for Sodium channel protein type 9 subunit alpha and immunoreactive epitopes of HCoV-229E spike glycoprotein. Shared pentapeptides are located outside immunoreactive epitopes in autoantigen of Fibroblast growth factor receptor 3.
Analysis of the data presented in
Table 2 allows us to conclude that:
1. A Shared pentapeptide TVEEL was discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes of membrane protein of SARS- CoV-2. Shared pentapeptide TVEEL is located within immunoreactive epitopes (25 epitopes) of SARS-CoV-2, but outside immunoreactive epitopes of sodium channel protein type 9 subunit alpha.
2. A Shared pentapeptide TVEEL was discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes of membrane protein of SARS- CoV. Shared pentapeptide TVEEL is located within immunoreactive epitopes (4 epitopes) of SARS-CoV, but outside immunoreactive epitopes of sodium channel protein type 9 subunit alpha.
3. A Shared pentapeptide IISIV was discovered for sodium channel protein type 9 subunit alpha and membrane protein of HCoV-HKU-1. A shared pentapeptide IISIV is located outside immunoreactive epitopes in both membrane protein of HCoV-HKU-1 and sodium channel protein type 9 subunit alpha.
4. A Shared pentapeptide ILTVF was discovered for sodium channel protein type 9 subunit alpha and immunoreactive epitope of membrane protein of HCoV-NL63. A shared pentapeptide ILTVF is located outside immunoreactive epitopes of sodium channel protein type 9 subunit alpha.
5. A Shared pentapeptide VGQLP was discovered for Plexin-D1 and two immunoreactive epitopes of membrane protein of HCoV-NL63. A shared pentapeptide VGQLP is located outside immunoreactive epitopes of Plexin-D1.
Analysis of the data presented in
Table 3 allows us to conclude that:
1. Shared pentapeptides YFYYL, LALLL were discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes of nucleocapsid protein of SARS-CoV-2. Shared pentapeptides YFYYL, LALLL are located within immunoreactive epitopes of SARS-CoV-2 (YFYYL – 10 epitopes, LALLL – 4 epitopes), but outside immunoreactive epitopes of sodium channel protein type 9 subunit alpha.
2. A shared pentapeptide ALLLL was discovered for autoantigen of Ubiquitin carboxyl-terminal hydrolase isozyme L1 and 12 immunoreactive epitopes of SARS-CoV-2. Shared pentapeptide outside immunoreactive epitopes of Ubiquitin carboxyl-terminal hydrolase isozyme L1.
3. Shared pentapeptides YFYYL, LALLL were discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes of nucleocapsid protein of SARS-CoV. Shared pentapeptides YFYYL, LALLL are located within immunoreactive epitopes of SARS-CoV (YFYYL – 1 epitope, LALLL – 5 epitopes), but outside immunoreactive epitopes of autoantigen sodium channel protein type 9 subunit alpha.
4. A shared pentapeptide ALLLL was discovered for autoantigen of Ubiquitin carboxyl-terminal hydrolase isozyme L1 and 5 immunoreactive epitopes of SARS-CoV. Shared pentapeptide ALLLL is located outside immunoreactive epitopes of Ubiquitin carboxyl-terminal hydrolase isozyme L1.
5. A shared pentapeptide TDAPS was discovered for autoantigen of Fibroblast growth factor receptor 3 and 7 immunoreactive epitopes of MERS-CoV. Shared pentapeptide TDAPS is located outside immunoreactive epitopes of autoantigen of Fibroblast growth factor receptor 3.
6. Shared pentapeptides YFYYL, GKDSK were discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes (two and one, respectively) of HCoV-HKU-1. Shared pentapeptides YFYYL, GKDSK are located outside immunoreactive epitopes of autoantigen of sodium channel protein type 9 subunit alpha.
7. A shared pentapeptide LGKDS was discovered for autoantigen of Plexin-D1 and two immunoreactive epitopes of HCoV-HKU-1. Shared pentapeptide LGKDS is located outside immunoreactive epitopes of autoantigen of Plexin-D1.
8. Shared pentapeptides YFYYL, PIAPG were discovered for autoantigen of sodium channel protein type 9 subunit alpha and immunoreactive epitopes (two and 6, respectively) of HCoV-OC43. Shared pentapeptides YFYYL, PIAPG are located outside immunoreactive epitopes of autoantigen of sodium channel protein type 9 subunit alpha.
9. Shared pentapeptides VLNAS, VAAVT, AAVTL were discovered for autoantigen of Fibroblast growth factor receptor 3 and immunoreactive epitopes (4, 4 and 4, respectively) of HCoV-NL63. Shared pentapeptides VLNAS, VAAVT, AAVTL are located outside immunoreactive epitopes of autoantigen of Fibroblast growth factor receptor 3.
Based on the results, summarized in
Table 3, an assumption can be made, that the most important targets for the molecular similarities are the sodium channel subunits and fibroblast growth factor receptor 3, both for seasonal and highly pathogenic coronaviruses.
As a result, we found 50 common shared pentapeptides between human autoantigens of the small nerve fibers and antigens of human coronaviruses. We found 30 mimicking pentapeptides in spike proteins of human coronaviruses, 5 pentapeptides in membrane proteins of human coronaviruses and 15 shared pentapeptides in nucleocapsid proteins of human coronaviruses.
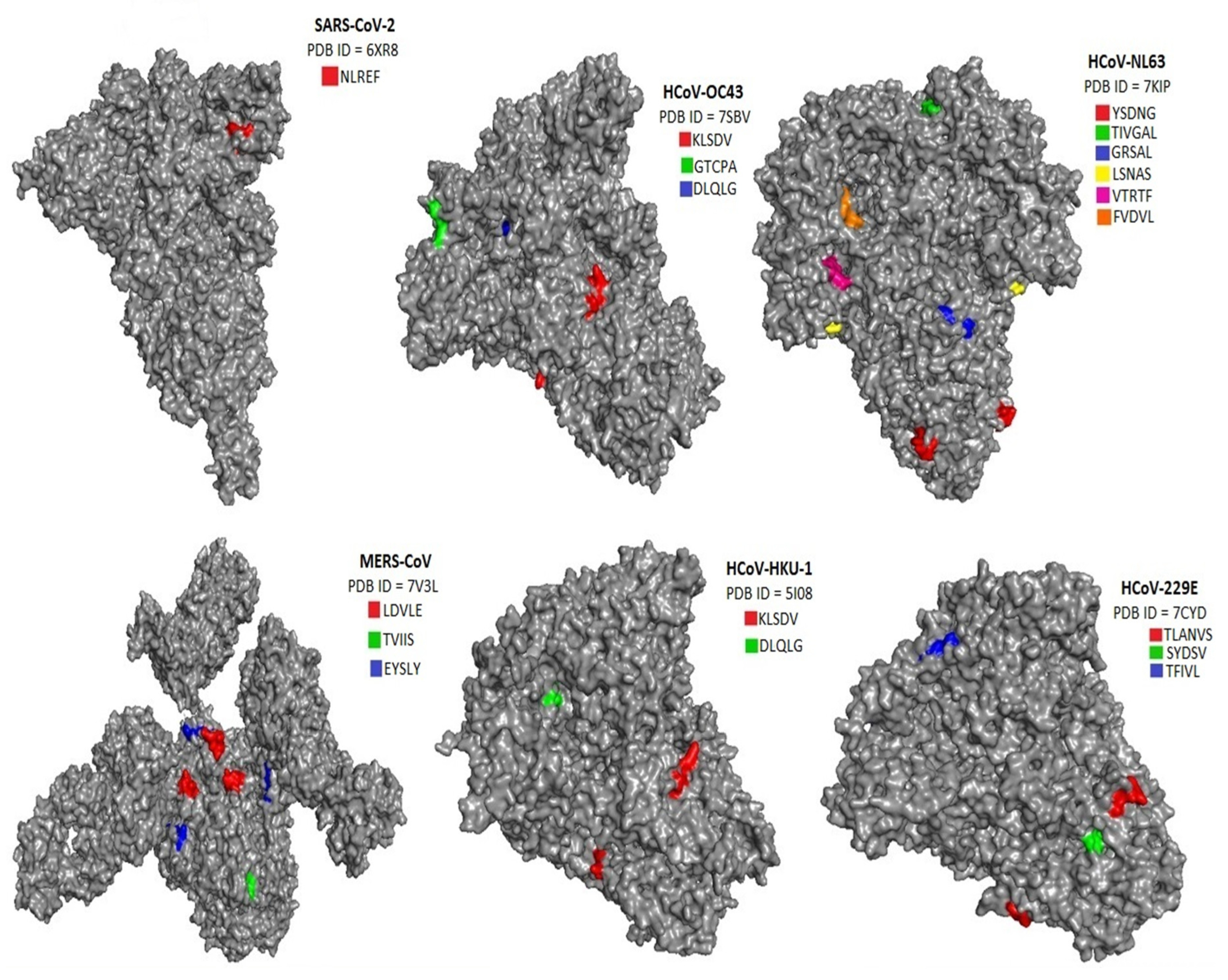
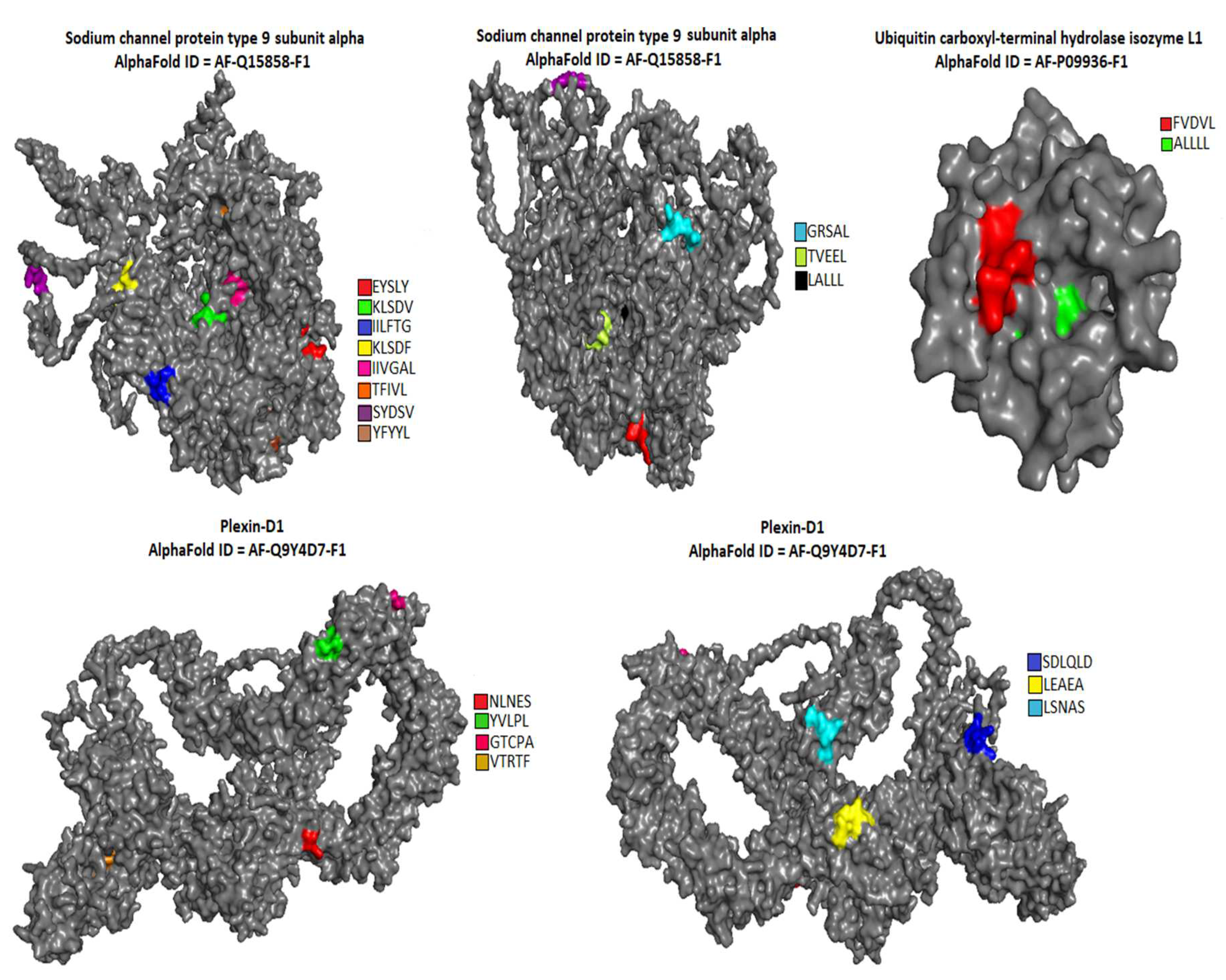
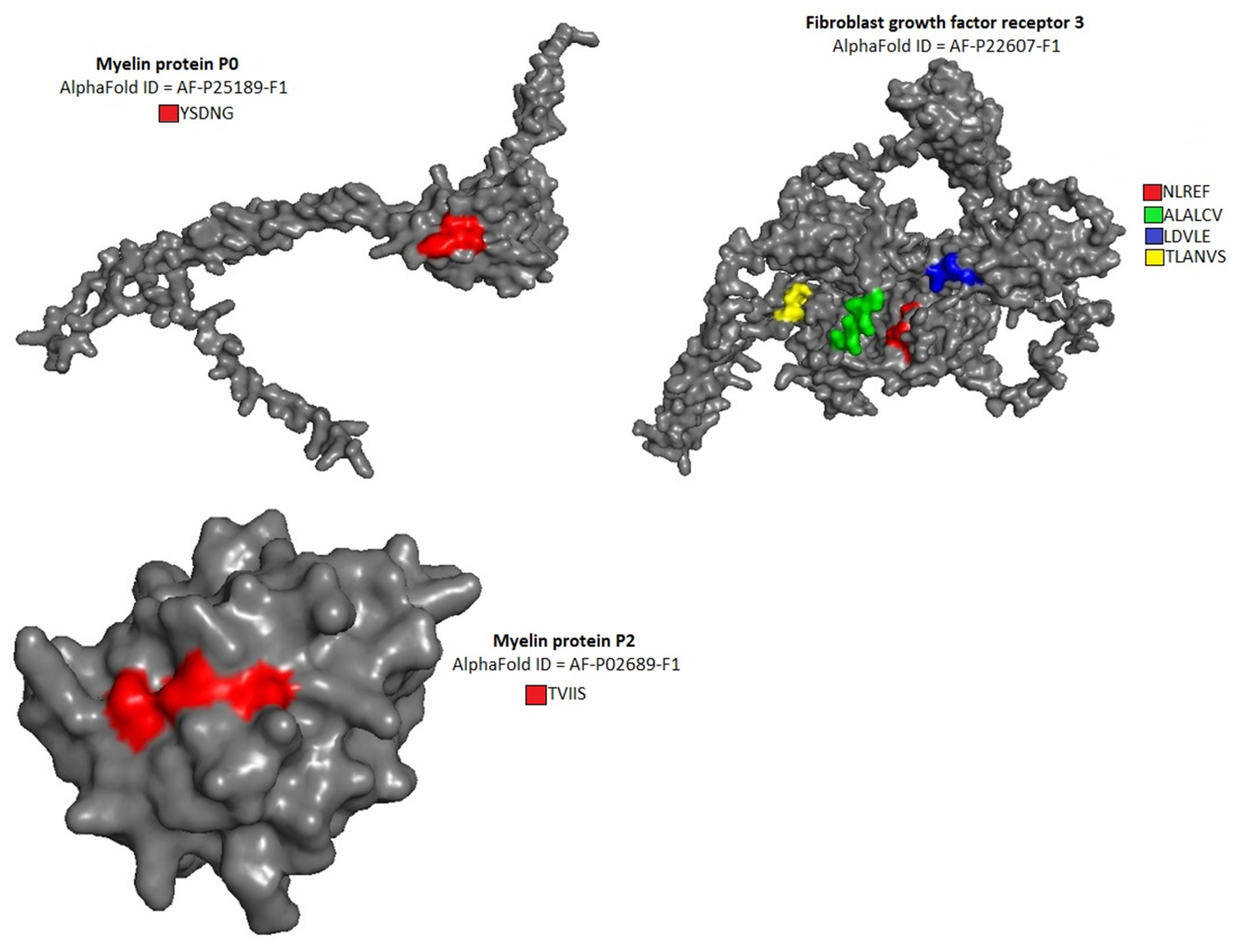
The location of pentapeptides in the spike proteins of human coronaviruses and in the autoantigens of the human nervous system were shown in
Figure 2,
Figure 3 and
Figure 4. Most of the similar pentapeptides are located on the surface of the proteins.
4. Discussion
The aim of the study was to tackle the possibility of the molecular mimicry between human coronaviruses and autoantigens of the human peripheral nervous system. Both seasonal and highly pathogenic coronaviruses were analyzed with an assessment of possible pentapeptide candidates among their spike, membrane and nucleocapsid proteins. The literature search was initialized to find out potential protein candidates from the peripheral nervous system, that may cross-react with coronaviruses with the development of autoimmune reactions. 50 pentapeptides similarities between human autoantigens of small nerve fibers and antigens of human coronaviruses were evaluated, where 60% of the matches were with spike proteins, and 20% each are membrane and nucleocapsid proteins of coronaviruses.
Anticipating the main points of the discussion of the results obtained, attention should be paid to the fact that shared pentapeptides can be located both within immunogenic epitopes and outside them.
Three variants of shared pentapeptides arrangements are possible. The first variant: the TVIIS pentapeptide, shared for two autoantigens of the myelin P2 protein and 12 immunoreactive epitopes of the MERS-CoV virus, is located in the immunoreactive epitopes of both the virus and the autoantigen of nervous system. The second variant: the LALCV pentapeptide, shared for the autoantigen of the fibroblast growth factor receptor 3 and MERS-CoV virus, is located outside the immunoreactive epitopes in both the virus and the autoantigen of nervous system. The third variant: the NLNES pentapeptide, shared for the autoantigen of nervous system plexin-D1 and 32 immunoreactive epitopes of SARS-CoV-2, is located within the immunoreactive epitopes of the virus, but outside the immunoreactive epitopes of the autoantigen of the nervous system. Theoretically possible forth variant, when the shared pentapeptide is located within immunoreactive epitopes of the nervous system, but outside of the immunoreactive epitopes of the virus, we have not encountered in this paper. The considered statements are clearly visible on the example of the search for shared pentapeptides for autoantigens of the nervous system and for the spike protein of human coronaviruses (
Table 1).
Analysis of the search for shared pentapeptides for autoantigens of the nervous system and the membrane protein of human coronaviruses (
Table 2) and for autoantigens the nervous system and nucleocapsid protein of human coronaviruses (
Table 3) showed, that shared pentapeptides are located within immunoreactive epitopes of the virus, but outside of immunoreactive autoantigens of the nervous system (variant three).
The presented considerations are the rationale for formulating the following questions in relation to the phenomenon of molecular mimicry per se:
1. What is the need for the realization of the molecular mimicry phenomenon of location of shared pentapeptides exactly within autoreactive epitopes?
2. Is it possible to realize the molecular mimicry phenomenon when one of the shared pentapeptides is located outside the autoreactive epitope?
3. Is it possible to realize the molecular mimicry phenomenon when the shared pentapeptides in both the virus and the studied autoantigens are located outside the autoreactive epitopes?
4. What is the contribution to the realization of the molecular mimicry phenomenon of shared pentapeptides of various viral proteins (spike glycoprotein, membrane protein, nucleoprotein)?
5. To what extent are the questions posed directly related to post-viral pathology associated with the activation of autoantigens of the nervous system?
Considering these questions, 2 main interpretations may be given to the obtained results. The first is to consider all available pentapeptide similarities as potential candidates for the development of autoimmune processes. The second is to take into account only those of them that are located in immunoreactive epitopes.
Discussing the results based on the first interpretation, 27 common pentapeptides were identified among spike proteins of coronaviruses and autoantigens of the human nervous system. Seasonal coronaviruses had more such potential sites for cross-reactivity than highly pathogenic ones, and plexin D1 and sodium channel protein type 9 subunit alpha had the largest number of common with viruses pentapeptides. Among the membrane proteins, 4 such pentapeptides were identified, while almost all of them had similarities with the sodium channel protein. Among the nucleocapsid proteins, 11 potential candidates for cross-reactivity were identified, which were almost uniformly represented in both highly pathogenic and seasonal coronaviruses.
Considering the second interpretation, where only peptides located in immunologic epitopes are essential for the cross-reactivity, the number of potential candidates for autoimmune processes is narrowing. Such pentapeptides have been identified only in spike proteins of coronaviruses. For highly pathogenic coronaviruses, only one similarity was revealed - between the MERS–CoV virus and fibroblast growth factor receptor 3. For seasonal coronaviruses, five similarities were revealed. For HCoV-HKU-1 virus – with sodium channel protein type 9 subunit alpha and plexin-D1. For HCoV-OC43 virus – with plexin-D1. For HCoV-NL63 virus – with plexin-D1 and ubiquitin carboxyl-terminal hydrolase isozyme L1.
Fibroblast growth factor receptor 3 is promising primarily due to the role of growth factors in the pathophysiology of viral infection. Their increased concentrations were detected in patients suffering from COVID-19 [
22,
23]. The induction of growth factors possibly occurs due to endothelial dysfunction and may counter the adverse effects of cytokine storms and several other complications. Antifibroblast growth factor receptor 3 may be detected in patients with the idiopathic or autoimmune small fiber neuropathy pain, including corneal pain in the fibromyalgia-type post-COVID-19 syndrome [
24,
25].
The similar assumption is true for the plexin-D1 and seasonal coronaviruses, where the human protein is another promising target for the autoimmune aggression, especially in the face-related pain syndromes [
26]. Plexin was primarily discovered in neurodevelopment, as a part of axonal guidance, but recently its role was described in cardiovascular development and diseases, endotheliocyte migration, angiogenesis and synapse formation [
27]. Its autoantibodies were described in several cases of idiopathic pain, linking together endothelial dysfunction and autoimmune processes in the nervous system [
28].
Seasonal coronaviruses share numerous amino acid sequences with the sodium channel protein type 9 subunit alfa, which disfunction can be responsible for neuropathic pain in humans [
29]. It is infamous for being involved in pain pathophysiology both in hereditary and autoimmune disease, including small finer neuropathy, erythromelalgia and paroxysmal extreme pain disorder [
30]. The main mechanism involves the electric conduction dysfunction, cased either by autoantibodies to this protein or impairment of its structure [
31].
Ubiquitin carboxyl-terminal hydrolase isozyme L1, that may be a target for the molecular mimicry in coronaviruses, is known for its multiple roles in the inflammation, angiogenesis, neuronal injury and neurodegeneration [
21], Hence, its alteration can be important in the development small fiber neuropathy and microvascular complications in the post-COVID-19 conditions.