Submitted:
06 July 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods and Materials
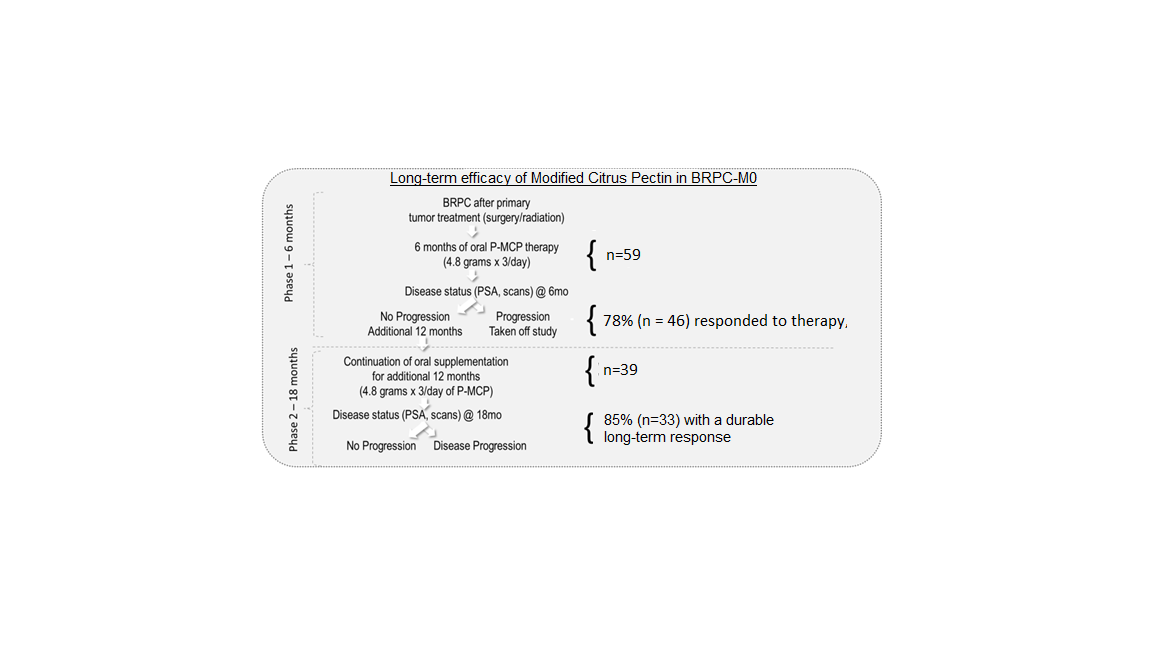
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. European Urology Oncology 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, A.N.; Usman, A.; Morgans, A.; VanderWeele, D.J.; Sosman, J.; Wu, J.D. Past, Current, and Future of Immunotherapies for Prostate Cancer. Front. Oncol. 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J Oncol 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Hao, Q.; Song, D.; Wu, Y.; et al. Incidence and Disease Burden of Prostate Cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Cancer 2020, 126, 1969–1978. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Black, L.K. The Economic Burden of Prostate Cancer: ECONOMIC BURDEN OF PROSTATE CANCER. BJU International 2011, 108, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends in Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef]
- Catalona, W.J. Prostate Cancer Screening. Medical Clinics of North America 2018, 102, 199–214. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. European Urology 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S. Management of Biochemically Recurrent Prostate Cancer after Local Therapy: Evolving Standards of Care and New Directions. Clin Adv Hematol Oncol 2013, 11, 14–23. [Google Scholar]
- Simon, N.I.; Parker, C.; Hope, T.A.; Paller, C.J. Best Approaches and Updates for Prostate Cancer Biochemical Recurrence. American Society of Clinical Oncology Educational Book 2022, 352–359. [Google Scholar] [CrossRef]
- Heijnsdijk, E.A.M.; Bangma, C.H.; Borràs, J.M.; de Carvalho, T.M.; Castells, X.; Eklund, M.; Espinàs, J.A.; Graefen, M.; Grönberg, H.; Lansdorp-Vogelaar, I.; et al. Summary Statement on Screening for Prostate Cancer in Europe: Prostate Cancer Screening in Europe. Int. J. Cancer 2018, 142, 741–746. [Google Scholar] [CrossRef]
- Arlen, P.M.; Bianco, F.; Dahut, W.L.; D’Amico, A.; Figg, W.D.; Freedland, S.J.; Gulley, J.L.; Kantoff, P.W.; Kattan, M.W.; Lee, A.; et al. Prostate Specific Antigen Working Group Guidelines on Prostate Specific Antigen Doubling Time. Journal of Urology 2008, 179, 2181–2186. [Google Scholar] [CrossRef]
- Pound, C.R. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA 1999, 281, 1591. [Google Scholar] [CrossRef]
- Aggarwal, R.; Heller, G.; Hillman, D.; Xiao, H.; Picus, J.; Wang, J.; Taplin, M.E.; Dorff, T.; Appleman, L.J.; Weckstein, D.; et al. LBA63 PRESTO: A Phase III, Open-Label Study of Androgen Annihilation in Patients (Pts) with High-Risk Biochemically Relapsed Prostate Cancer (AFT-19). Annals of Oncology 2022, 33, S1428. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of Prostate Cancer–Specific Mortality Following Biochemical Recurrence After Radical Prostatectomy. JAMA 2005, 294, 433. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, M.-H.; McLeod, D.; Carroll, P.R.; Moul, J.W.; D’Amico, A.V. Predictors of Prostate Cancer–Specific Mortality After Radical Prostatectomy or Radiation Therapy. JCO 2005, 23, 6992–6998. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the Definition of Biochemical Recurrence in Patients Treated for Localized Prostate Cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel Report and Recommendations for a Standard in the Reporting of Surgical Outcomes. Journal of Urology 2007, 177, 540–545. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; Cumberbatch, M.; De Santis, M.; Tilki, D.; Fanti, S.; et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. European Urology 2019, 75, 967–987. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Feng, Z.; Trock, B.J.; Humphreys, E.B.; Carducci, M.A.; Partin, A.W.; Walsh, P.C.; Eisenberger, M.A. The Natural History of Metastatic Progression in Men with Prostate-Specific Antigen Recurrence after Radical Prostatectomy: Long-Term Follow-up: METASTATIC PROGRESSION IN PSA-RECURRENT PROSTATE CANCER. BJU International 2012, 109, 32–39. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A. Management of Patients with Biochemical Recurrence After Local Therapy for Prostate Cancer. Hematology/Oncology Clinics of North America 2013, 27, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining Biochemical Failure Following Radiotherapy with or without Hormonal Therapy in Men with Clinically Localized Prostate Cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. International Journal of Radiation Oncology*Biology*Physics 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and Cardiovascular Disease During Androgen Deprivation Therapy for Prostate Cancer. JCO 2006, 24, 4448–4456. [Google Scholar] [CrossRef]
- Keizman, D.; Zahurak, M.; Sinibaldi, V.; Carducci, M.; Denmeade, S.; Drake, C.; Pili, R.; Antonarakis, E.S.; Hudock, S.; Eisenberger, M. Lenalidomide in Nonmetastatic Biochemically Relapsed Prostate Cancer: Results of a Phase I/II Double-Blinded, Randomized Study. Clinical Cancer Research 2010, 16, 5269–5276. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Ben-Porat, L.; Scher, H.I.; Chan, H.M.; Fearn, P.A.; Fuks, Z.Y.; Leibel, S.A.; Venkatraman, E.S. Outcome Predictors for the Increasing PSA State After Definitive External-Beam Radiotherapy for Prostate Cancer. JCO 2005, 23, 826–831. [Google Scholar] [CrossRef]
- Suzman, D.L.; Zhou, X.C.; Zahurak, M.L.; Lin, J.; Antonarakis, E.S. Change in PSA Velocity Is a Predictor of Overall Survival in Men with Biochemically-Recurrent Prostate Cancer Treated with Nonhormonal Agents: Combined Analysis of Four Phase-2 Trials. Prostate Cancer Prostatic Dis 2015, 18, 49–55. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Moul, J.W.; Carroll, P.R.; Sun, L.; Lubeck, D.; Chen, M.-H. Surrogate End Point for Prostate Cancer-Specific Mortality After Radical Prostatectomy or Radiation Therapy. JNCI Journal of the National Cancer Institute 2003, 95, 1376–1383. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. The Veterans Administration Cooperative Urological Research Group Members of this group areS0022534717598894-57c401330b4195dbd1713587ed540477 Lino J. Arduino Veterans Administration Center (VAC), Des Moines, Iowa S0022534717598894-61e5a9195ff74e58ab72b9f6a7b48125 John C. Bailar Veterans Administration Central Office (VACO), Washington, D. C. S0022534717598894-1936883d21db441268a90e6e79675523 Leslie E. Becker VAC, Leavenworth, Kansas S0022534717598894-3619ae2ed4ad7e7734f8222e5502f779 Henry I. Berman Veteran Prediction of Prognosis for Prostatic Adenocarcinoma by Combined Histological Grading and Clinical Staging. Journal of Urology 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Zahurak, M.; Sinibaldi, V.; Carducci, M.A.; Pili, R.; Laufer, M.; DeWeese, T.L.; Eisenberger, M.A. Marimastat in the Treatment of Patients with Biochemically Relapsed Prostate Cancer: A Prospective Randomized, Double-Blind, Phase I/II Trial. Clinical Cancer Research 2005, 11, 4437–4443. [Google Scholar] [CrossRef]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A Randomized Phase II Study of Pomegranate Extract for Men with Rising PSA Following Initial Therapy for Localized Prostate Cancer. Prostate Cancer Prostatic Dis 2013, 16, 50–55. [Google Scholar] [CrossRef]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-Risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. European Urology 2023, 83, 267–293. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef]
- Bilgin, S.; Erden Tayhan, S.; Yıldırım, A.; Koç, E. Investigation of the Effects of Isoeugenol-Based Phenolic Compounds on Migration and Proliferation of HT29 Colon Cancer Cells at Cellular and Molecular Level. Bioorganic Chemistry 2023, 130, 106230. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients 2021, 13, 4295. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Leppert, J.T.; Zomorodian, N.; Aronson, W.; Hong, J.; Barnard, R.J.; Seeram, N.; Liker, H.; Wang, H.; Elashoff, R.; et al. Phase II Study of Pomegranate Juice for Men with Rising Prostate-Specific Antigen Following Surgery or Radiation for Prostate Cancer. Clinical Cancer Research 2006, 12, 4018–4026. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb Perspect Med 2017, 7, a030452. [Google Scholar] [CrossRef]
- Salehi; Fokou; Yamthe; Tali; Adetunji; Rahavian; Mudau; Martorell; Setzer; Rodrigues; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11, 1483. [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Wu, S.; Xu, X.-R.; Chen, F. Antiproliferative Activity of Peels, Pulps and Seeds of 61 Fruits. Journal of Functional Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol Inhibits TGF-Β1-Induced Epithelial-to-Mesenchymal Transition and Suppresses Lung Cancer Invasion and Metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Letters 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mariño, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting Galectin-Driven Regulatory Circuits in Cancer and Fibrosis. Nat Rev Drug Discov 2023. [Google Scholar] [CrossRef] [PubMed]
- Jun Yan; Katz, A. PectaSol-C Modified Citrus Pectin Induces Apoptosis and Inhibition of Proliferation in Human and Mouse Androgen-Dependent and- Independent Prostate Cancer Cells. Integr Cancer Ther 2010, 9, 197–203. [CrossRef]
- Demotte, N.; Wieërs, G.; Van Der Smissen, P.; Moser, M.; Schmidt, C.; Thielemans, K.; Squifflet, J.-L.; Weynand, B.; Carrasco, J.; Lurquin, C.; et al. A Galectin-3 Ligand Corrects the Impaired Function of Human CD4 and CD8 Tumor-Infiltrating Lymphocytes and Favors Tumor Rejection in Mice. Cancer Research 2010, 70, 7476–7488. [Google Scholar] [CrossRef] [PubMed]
- Guess, B.W.; Scholz, M.C.; Strum, S.B.; Lam, R.Y.; Johnson, H.J.; Jennrich, R.I. Modified Citrus Pectin (MCP) Increases the Prostate-Specific Antigen Doubling Time in Men with Prostate Cancer: A Phase II Pilot Study. Prostate Cancer Prostatic Dis 2003, 6, 301–304. [Google Scholar] [CrossRef]
- Pienta, K.J.; Nailk, H.; Akhtar, A.; Yamazaki, K.; Replogle, T.S.; Lehr, J.; Donat, T.L.; Tait, L.; Hogan, V.; Raz, A. Inhibition of Spontaneous Metastasis in a Rat Prostate Cancer Model by Oral Administration of Modified Citrus Pectin. JNCI Journal of the National Cancer Institute 1995, 87, 348–353. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Sud, S.; Mossine, V.V.; Mawhinney, T.P.; Anthony, D.C.; Glinsky, G.V.; Pienta, K.J.; Glinsky, V.V. Inhibition of Prostate Cancer Bone Metastasis by Synthetic TF Antigen Mimic/Galectin-3 Inhibitor Lactulose-l-Leucine. Neoplasia 2012, 14, 65–73. [Google Scholar] [CrossRef]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-Binding Site by Pectins and Galactomannans. Journal of Biological Chemistry 2016, 291, 13318–13334. [Google Scholar] [CrossRef]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the Hallmarks of Cancer: Galectins as Multifunctional Mediators of Tumor Progression. Journal of Experimental Medicine 2020, 217, e20182041. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, Function and Therapeutic Potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Méndez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging Regulatory Checkpoints Linking Tumor Immunity and Angiogenesis. Current Opinion in Immunology 2017, 45, 8–15. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a Novel Biomarker for Disease Diagnosis and a Target for Therapy (Review). Int J Mol Med 2017. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of Human Cancer Cell Growth and Metastasis in Nude Mice by Oral Intake of Modified Citrus Pectin. JNCI Journal of the National Cancer Institute 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Changes in Cell Growth, Cyclin/Kinase, Endogenous Phosphoproteins and Nm23 Gene Expression in Human Prostatic JCA-1 Cells Treated with Modified Citrus Pectin. Biochem Mol Biol Int 1995, 37, 833–841. [Google Scholar] [PubMed]
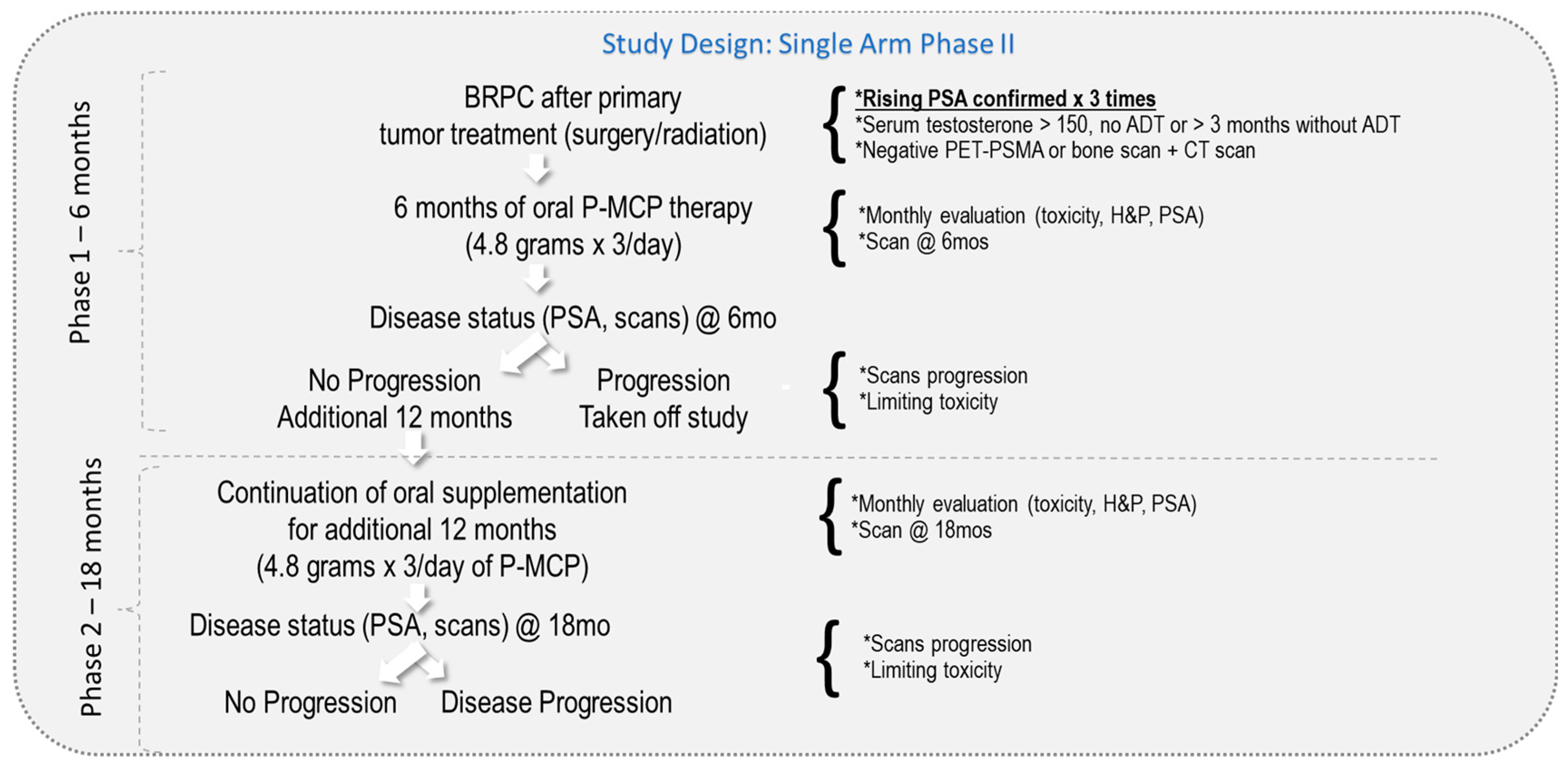
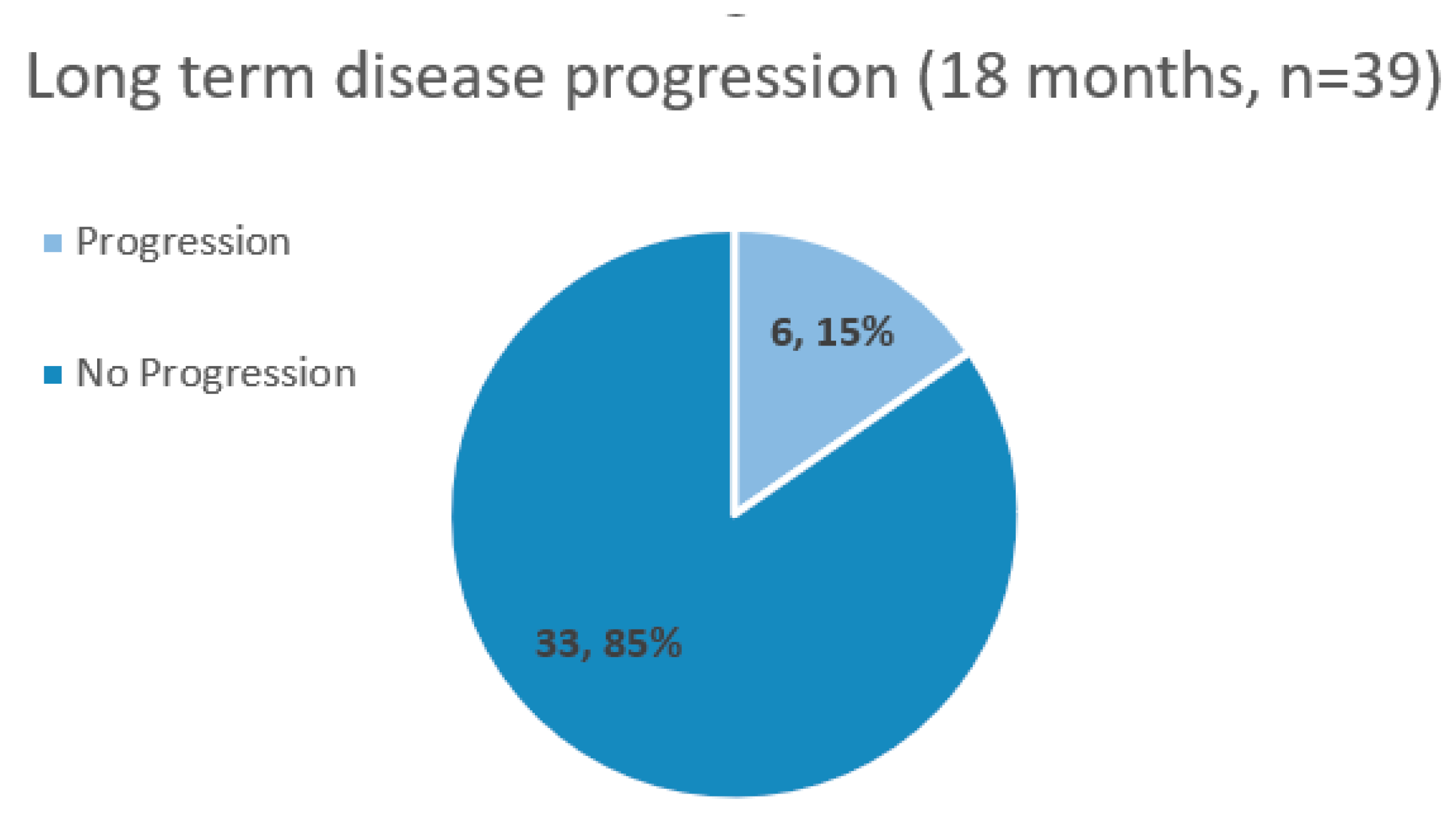
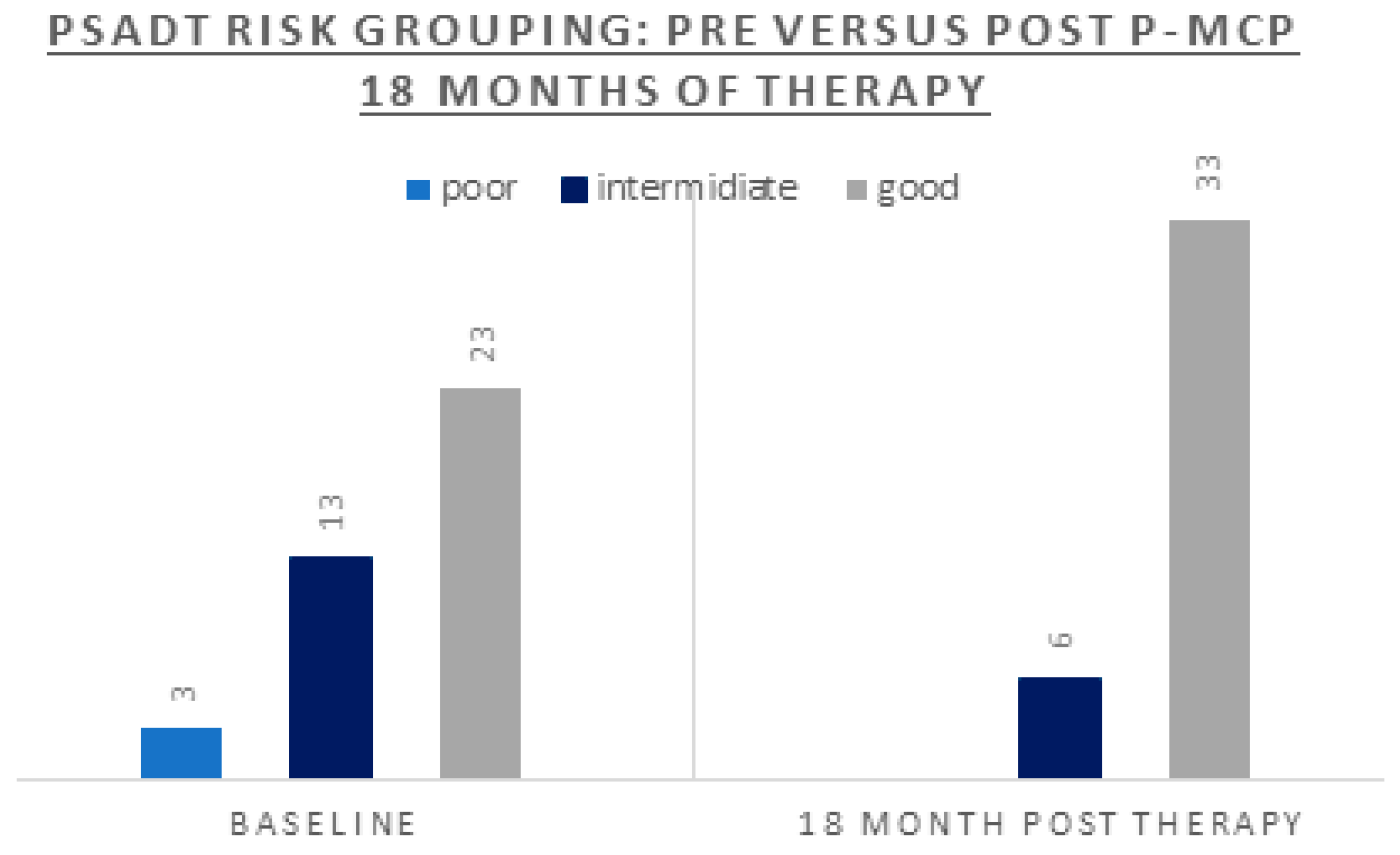
| Parameter | n = 39 |
|---|---|
| Age (years): Median (range) | 75 (52 - 88) |
| Gleason: % (n) 6 7 8-10 |
41% (n=16) 38% (n=15) 21% (n=8) |
| Local therapy: % (n) Radical prostatectomy Radiation therapy Surgery+RT |
18% (n=7) 54% (n=21) 28% (n=11) |
| Prior ADT | 38% (n=15) |
| PSA (ng/ml): Median (range) | 4.1 (0.28-30) |
| PSADT (months) risk grouping: % (n) Poor <3 Intermediate 3-8.99 Good ≥9 |
8% (n=3) 33% (n=13) 59% (n=23) |
| PSADT (months): Median (range) Whole cohort Poor PSADT risk Intermediate risk Good risk |
10.3 (1.4 – 55) 1.6 (1.4 – 1.8) 5.12 (3.5 – 8.2) 14.74 (9.10 – 54.6) |
| Parameter | Whole cohort (n=39) |
According to Pre-study PSADT (months) risk grouping |
||
|---|---|---|---|---|
| Poor < 3.00 (n=3) |
Intermediate 3.00-8.99 (n=13) |
Good ≥ 9.00 (n=23) |
||
| Overall response to therapy (decrease or stabilization of PSA, and/or lengthening of PSADT, with negative scans) |
85% (n=33) |
66% (n=2) |
77% (n=10) |
91% (n=21) |
| PSA response Stable/decreased Progression |
54% (n=21) 46% (n=18) |
67% (n=2) 33% (n=1) |
23% (n=3) 77% (n=10) |
70% (n=16) 30% (n=7) |
| PSADT (months): Median (range) | 43.5 (3.5-981) | 9.8 (6-200) | 18.3 (6.7-500) | 47.7 (3.5-981) |
| PSADT (months) risk grouping: % (n) | 0% (n=0) | 13% (n=5) | 87% (n=34 | |
| PSADT lengthening | 90% (n=35) | 100% (n=3) | 92% (n=12) | 87% (n=20) |
| Change to a better PSADT risk grouping | 36% (n=14) | 100% (n=3) | 85% (n=11) | not applicable |
| Radiologic response Negative scans Disease progression |
90% (n=35) 10% (n = 4) |
67% (n=2) 33% (n=1) |
75% (n=11) 15% (n=2) |
96% (n=22) 4% (n=1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





