Submitted:
06 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Synthesis of Graphite-like Carbon Nitride with a High Specific Surface
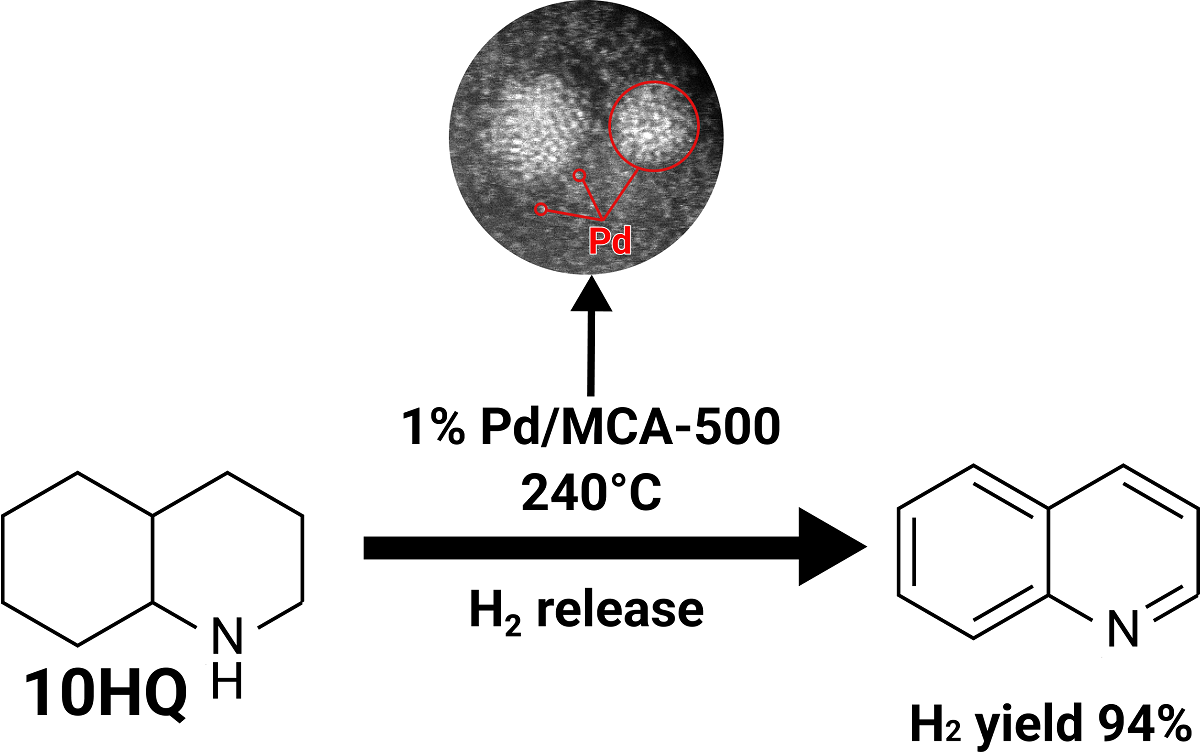
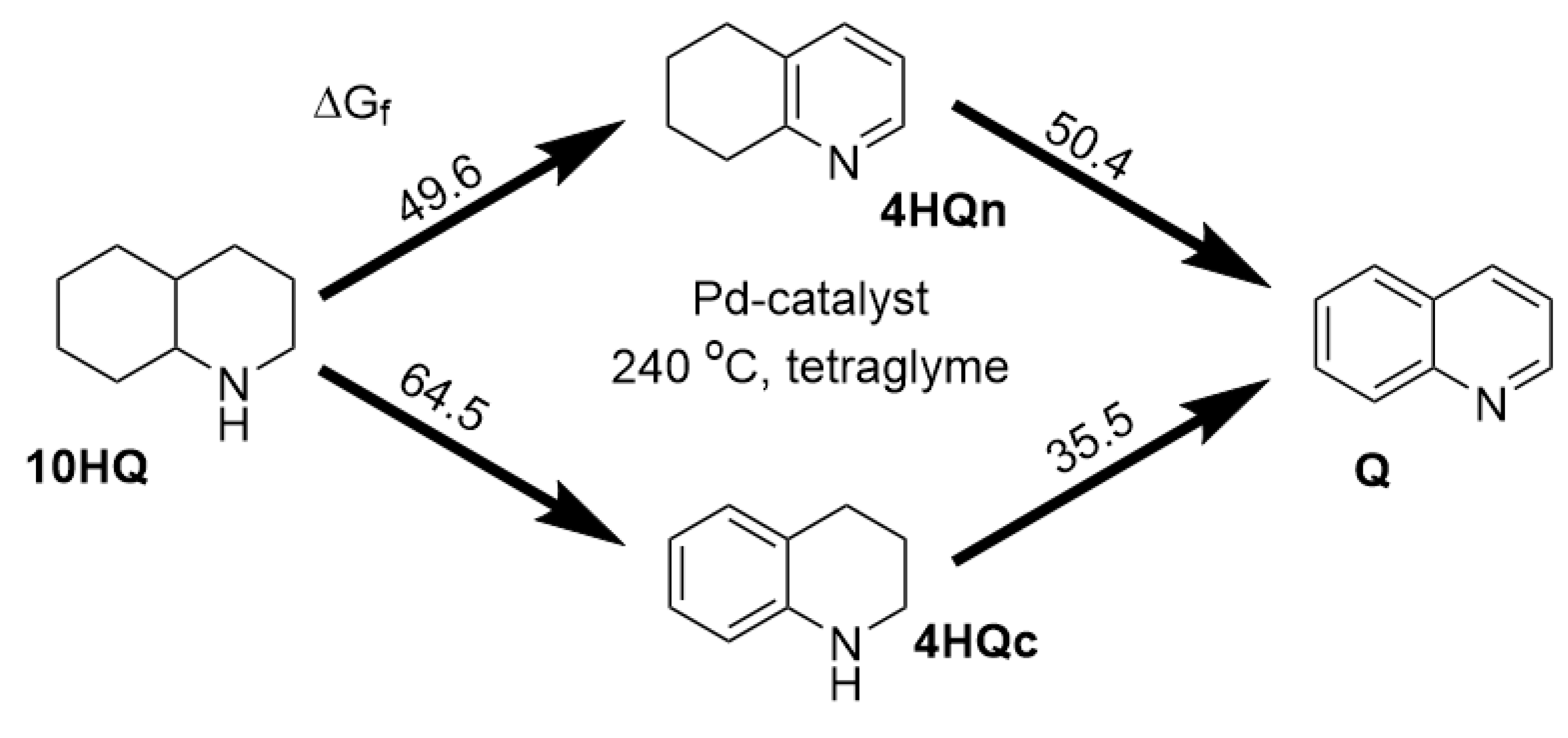
2.2. Investigation of the catalytic activity of Pd/Carbon nitride systems in the decahydroquinoline dehydrogenation
| Catalyst | Impregnation Method | M:CA Ratio (mol:mol) |
DCO, % | YH2, % |
|---|---|---|---|---|
| g-C3N4 or MCA -550 | - | - | - | 0 |
| 1%Pd/g-C3N4 | wi | 1:0 | 17 | 45 |
| 1%Pd/g-C3N4 | ai | 1:0 | 18 | 43 |
| 1%Pd/MCA -500 | wi | (1:1) | 20 | 58 |
| 1%Pd/MCA -500 | ai | (1:1) | 30 | 94 |
| 1%Pd/ MCA -400 | ai | (1:1) | -** | 12 |
| 1%PdMCA -500 | ai | (0.67:0.33) | 22 | 71 |
| 1%Pd/MCA -500 | ai | (0.9:0.1) | 20 | 50 |
| 1%Pd/C | -* | - | 41 | 78 |
3. Materials and Methods
3.1. Synthesis of the g-C3N4 and MCA-T Supports
3.2. Synthesis of Pd-Containing Catalytic Systems
3.3. Characterization Techniques
3.3.1. Dynamic Light Scattering (DLS)
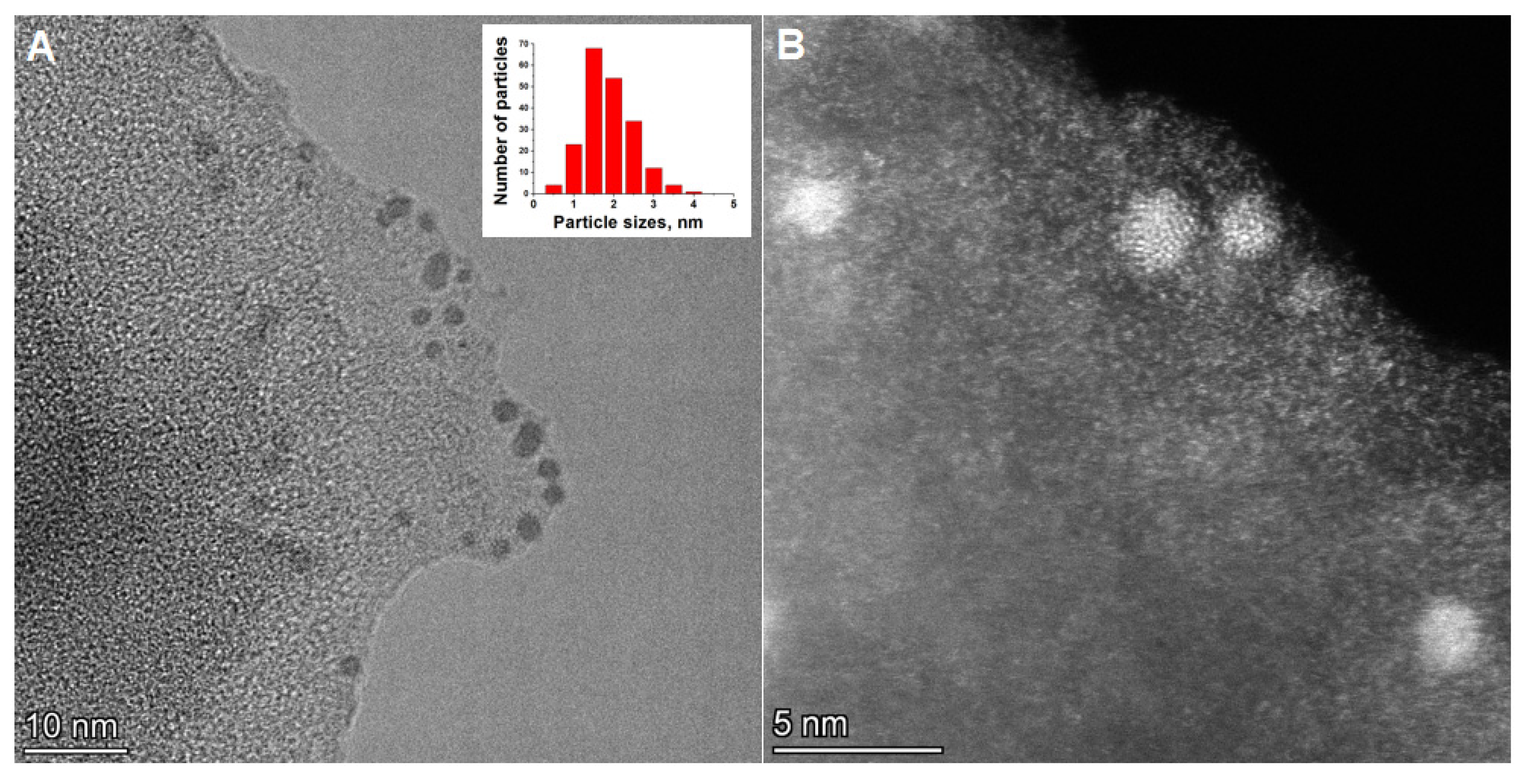
3.3.2. Electron Microscopy (SEM and TEM)
3.3.3. Adsorption Measurements (CO Chemisorption and BET)
3.3.4. X-ray Diffraction (XRD)
3.3.5. Small Angle X-ray Scattering (SAXS)
3.3.6. Elemental Analysis
3.4. Catalytic Activity Tests
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Makaryan, I.A.; Sedov, I. V.; Maksimov, A.L. Hydrogen Storage Using Liquid Organic Carriers. Russ. J. Appl. Chem. 2020, 93, 1815–1830. [Google Scholar] [CrossRef]
- Alekseeva (Bykova), M. V.; Gulyaeva, Y.K.; Bulavchenko, O.A.; Saraev, A.A.; Kremneva, A.M.; Stepanenko, S.A.; Koskin, A.P.; Kaichev, V. V.; Yakovlev, V.A. Promoting Effect of Zn in High-Loading Zn/Ni-SiO2 Catalysts for Selective Hydrogen Evolution from Methylcyclohexane. Dalt. Trans. 2022, 51, 6068–6085. [Google Scholar] [CrossRef]
- Safronov, S.P.; Vostrikov, S. V.; Samarov, A.A.; Wasserscheid, P.; Müller, K.; Verevkin, S.P. Comprehensive Thermodynamic Study of Substituted Indoles/Perhydro Indoles as Potential Liquid Organic Hydrogen Carrier System. Fuel 2023, 331, 125764. [Google Scholar] [CrossRef]
- Vostrikov, S. V.; Konnova, M.E.; Turovtzev, V. V.; Müller, K.; Verevkin, S.P. Thermodynamics of Hydrogen Storage: Equilibrium Study of the LOHC System Indole/Octahydroindole. Fuel 2023, 335, 127025. [Google Scholar] [CrossRef]
- Stepanenko, S.A.; Shivtsov, D.M.; Koskin, A.P.; Koskin, I.P.; Kukushkin, R.G.; Yeletsky, P.M.; Yakovlev, V.A. N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy. Catalysts 2022, 12. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Safronov, S.P.; Samarov, A.A.; Vostrikov, S. V. Hydrogen Storage: Thermodynamic Analysis of Alkylquinolines and Alkylpyridines as Potential Liquid Organic Hydrogen Carriers (LOHC). Appl. Sci. 2021, 11. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A Review of Hydrogen Production Processes by Photocatalytic Water Splitting – From Atomistic Catalysis Design to Optimal Reactor Engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Zheng, D.; Xue, Y.; Wang, J.; Varbanov, P.S.; Klemeš, J.J.; Yin, C. Nanocatalysts in Photocatalytic Water Splitting for Green Hydrogen Generation: Challenges and Opportunities. J. Clean. Prod. 2023, 414. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A Review on g-C3N4 for Photocatalytic Water Splitting and CO2 Reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Murugan Arunachalapandi; Selvaraj Mohana Roopan Environment Friendly g-C3N4-Based Catalysts and Their Recent Strategy in Organic Transformations. High Energy Chem. 2022, 56, 73–90. [CrossRef]
- Li, X.H.; Wang, X.; Antonietti, M. Mesoporous g-C3N4 Nanorods as Multifunctional Supports of Ultrafine Metal Nanoparticles: Hydrogen Generation from Water and Reduction of Nitrophenol with Tandem Catalysis in One Step. Chem. Sci. 2012, 3, 2170–2174. [Google Scholar] [CrossRef]
- Chen, H.; Shuang, H.; Lin, W.; Li, X.; Zhang, Z.; Li, J.; Fu, J. Tuning Interfacial Electronic Properties of Palladium Oxide on Vacancy-Abundant Carbon Nitride for Low-Temperature Dehydrogenation. ACS Catal. 2021, 11, 6193–6199. [Google Scholar] [CrossRef]
- Suominen, M.; Kallio, T. What We Currently Know about Carbon-Supported Metal and Metal Oxide Nanomaterials in Electrochemical CO2 Reduction. ChemElectroChem 2021, 8, 2397–2406. [Google Scholar] [CrossRef]
- Barrio, J.; Shalom, M. Rational Design of Carbon Nitride Materials by Supramolecular Preorganization of Monomers. ChemCatChem 2018, 10, 5573–5586. [Google Scholar] [CrossRef]
- Xu, J.; Long, K.Z.; Wang, Y.; Xue, B.; Li, Y.X. Fast and Facile Preparation of Metal-Doped g-C3N4 Composites for Catalytic Synthesis of Dimethyl Carbonate. Appl. Catal. A Gen. 2015, 496, 1–8. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Ma, D.; Shang, J.K.; Li, Y.X. Simple Preparation of MgO/g-C3N4 Catalyst and Its Application for Catalytic Synthesis of Dimethyl Carbonate via Transesterification. Catal. Commun. 2017, 95, 72–76. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Cheng, S.; Zhou, X.; Shang, N.; Gao, S.; Wang, C. Pd Supported on Graphene Modified G-C3N4 Hybrid: A Highly Efficient Catalyst for Hydrogenation of Nitroarenes. Appl. Organomet. Chem. 2020, 34, 1–9. [Google Scholar] [CrossRef]
- Payra, S.; Banerjee, S. Highly Efficient and Chemoselective Reduction of Nitroarenes Using Hybrid Ni@g-C3N4 as Reusable Catalyst. ChemistrySelect 2019, 4, 9556–9561. [Google Scholar] [CrossRef]
- Li, J.; Zahid, M.; Sun, W.; Tian, X.; Zhu, Y. Synthesis of Pt Supported on Mesoporous g-C3N4 Modified by Ammonium Chloride and Its Efficiently Selective Hydrogenation of Furfural to Furfuryl Alcohol. Appl. Surf. Sci. 2020, 528, 146983. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, T.G.; Bari, G.A.K.M.R.; Seo, H.W.; Park, J.W.; Hwang, H.J.; An, B.H.; Suzuki, N.; Fujishima, A.; Kim, J.H.; et al. Sulfuric Acid Treated g-CN as a Precursor to Generate High-Efficient g-CN for Hydrogen Evolution from Water under Visible Light Irradiation. Catalysts 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Feng, J.; Chen, T.; Liu, S.; Zhou, Q.; Ren, Y.; Lv, Y.; Fan, Z. Improvement of G-C3N4 Photocatalytic Properties Using the Hummers Method. J. Colloid Interface Sci. 2016, 479, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Han, H.; Dong, M.; Zhao, Q. In Situ Construction of Porous g-C3N4 Isotype Heterojunction/BiOBr Nanosheets Ternary Composite Catalyst for Highly Efficient Visible-Light Photocatalytic Activity. ChemistrySelect 2021, 6, 6212–6222. [Google Scholar] [CrossRef]
- Cheng, M.; Lv, P.; Zhang, X.; Xiong, R.; Guo, Z.; Wang, Z.; Zhou, Z.; Zhang, M. A New Active Species of Pd-Nx Synthesized by Hard-Template Method for Efficiently Catalytic Hydrogenation of Nitroarenes. J. Catal. 2021, 399, 182–191. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 1–35. [Google Scholar] [CrossRef]
- Jun, Y.S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Correa-Guimaraes, A.; Martín-Gil, J. Synthesis of Graphitic Carbon Nitride by Reaction of Melamine and Uric Acid. Mater. Chem. Phys. 2011, 130, 1094–1102. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, R.; Shang, L.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Template-Free Large-Scale Synthesis of g-C3N4 Microtubes for Enhanced Visible Light-Driven Photocatalytic H2 Production. Nano Res. 2018, 11, 3462–3468. [Google Scholar] [CrossRef]
- Wang, X.; Han, D.; Ding, Y.; Liu, J.; Cai, H.; Jia, L.; Cheng, X.; Wang, J.; Fan, X. A Low-Cost and High-Yield Approach for Preparing g-C3N4 with a Large Specific Surface Area and Enhanced Photocatalytic Activity by Using Formaldehyde-Treated Melamine. J. Alloys Compd. 2020, 845, 156293. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gregg, S. J.; Sing, K. S. W. Adsorption, Surface Area, & Porosity, Second Edition, 2nd ed.; Academic Press: London, 1982. [Google Scholar]
- Rashidizadeh, A.; Ghafuri, H.; Esmaili Zand, H.R.; Goodarzi, N. Graphitic Carbon Nitride Nanosheets Covalently Functionalized with Biocompatible Vitamin B1: Synthesis, Characterization, and Its Superior Performance for Synthesis of Quinoxalines. ACS Omega 2019, 4, 12544–12554. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Z.; Zeng, X.; Meng, X.; Sun, H.; Wan, Y.; Zuo, G. Self Assembly and Controlled Drug Release of a Nano-Laminated Graphite Carbon Nitride/Methotrexate Complex. J. Mater. Sci. Mater. Med. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Sánchez-Arévalo, F.M.; Huerta, L.; Bizarro, M.; Navas-Gracia, L.M.; Martín-Gil, J. Synthesis of Crumpled Nanosheets of Polymeric Carbon Nitride from Melamine Cyanurate. J. Solid State Chem. 2013, 201, 153–163. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption-Part 2: Analysis of Macropores by Mercury Porosimetry. 96. H. Giesche, Mater. Res. Soc. Symp. Proc 1991, 37, 225. [Google Scholar]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N-Pd Interaction in Nitrogen-Doped Carbon Nanotube Catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar] [CrossRef]
- Simakova, I.; Koskin, A.; Deliy, I.; Simakov, A. Nanoscaled Palladium Catalysts on Activated Carbon Support “Sibunit” for Fine Organic Synthesis. Complex Mediu. VI Light Complex. 2005, 5924, 592413. [Google Scholar] [CrossRef]
- Koskin, A.P.; Simakova, I.L.; Parmon, V.N. Reductive Debenzylation of Hexabenzylhexaazaisowurtzitane — the Key Step of the Synthesis of Polycyclic Nitramine Hexanitrohexaazaisowurtzitane. Russ. Chem. Bull. 2007, 56, 2370–2375. [Google Scholar] [CrossRef]
- Koskin, A.P.; Simakova, I.L.; Parmon, V.N. Study of Palladium Catalyst Deactivation in Synthesis of 4,10-Diformyl-2,6,8,12-Tetraacetyl-2,4,6,8,10,12-Hexaazaisowurtzitane. React. Kinet. Catal. Lett. 2007, 92, 293–302. [Google Scholar] [CrossRef]
- Available online: http://www.softscientific.com/science/WhitePapers/dynals1/dynals100.htm.
- Hassan, P.A.; Rana, S.; Verma, G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Konarev, P. V.; Petoukhov, M. V.; Volkov, V. V.; Svergun, D.I. ATSAS 2.1, a Program Package for Small-Angle Scattering Data Analysis. J. Appl. Crystallogr. 2006, 39, 277–286. [Google Scholar] [CrossRef]
- Feigin, L.A.; Svergun, D.I. Structure Analysis by Small-Angle X-Ray and Neutron Scattering; Taylor, G.W., Ed.; Springer US: Boston, MA, 1987; ISBN 978-1-4757-6626-4. [Google Scholar]
- Shivtsov, D.M.; Koskin, A.P.; Stepanenko, S.A.; Ilyina, E.V.; Ayupov, A.B.; Bedilo, A.F.; Yakovlev, V.A. Hydrogen Production by N-Heterocycle Dehydrogenation over Pd Supported on Aerogel-Prepared Mg-Al Oxides. Catalysts 2023, 13, 10–3390. [Google Scholar] [CrossRef]
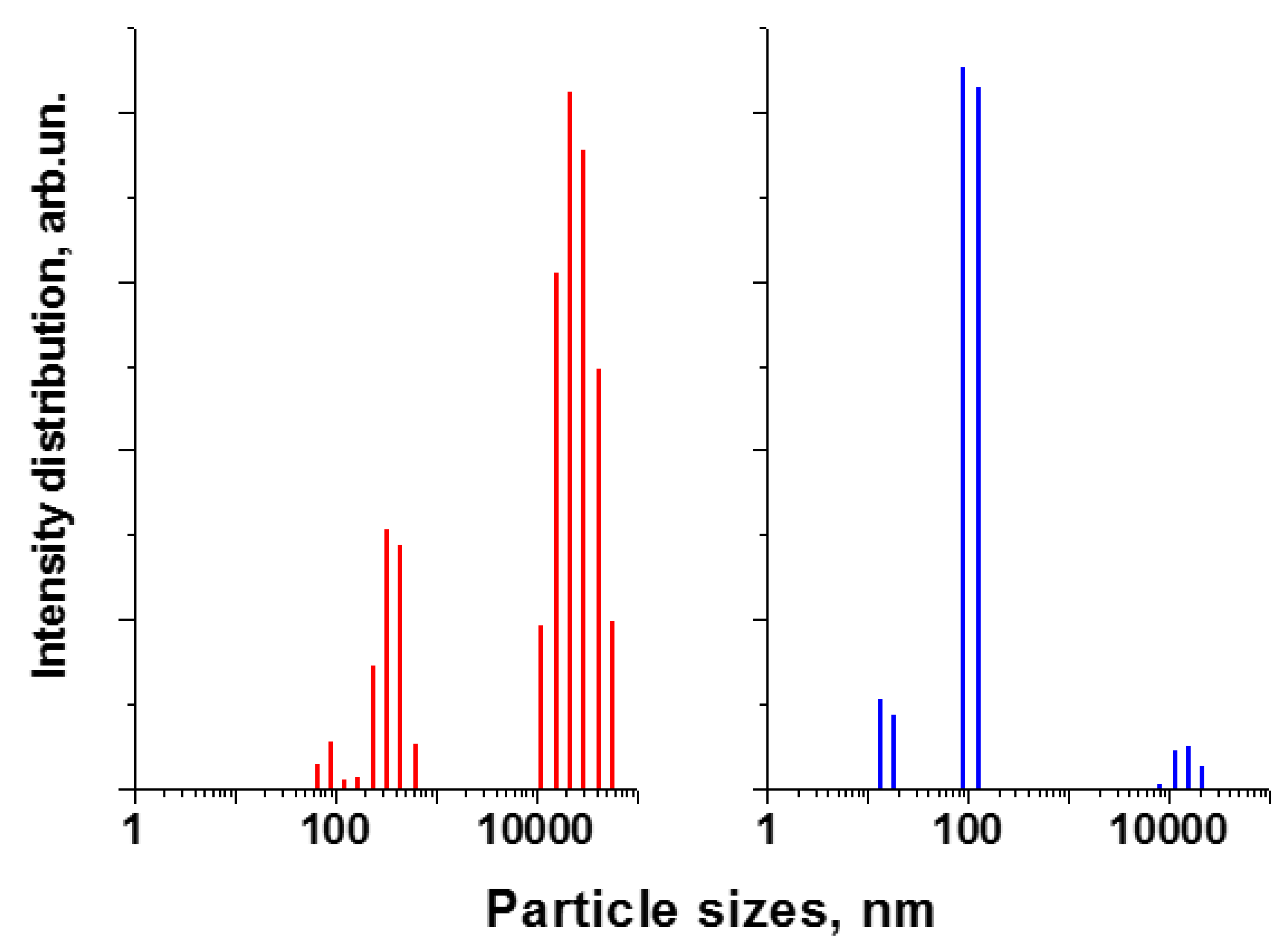
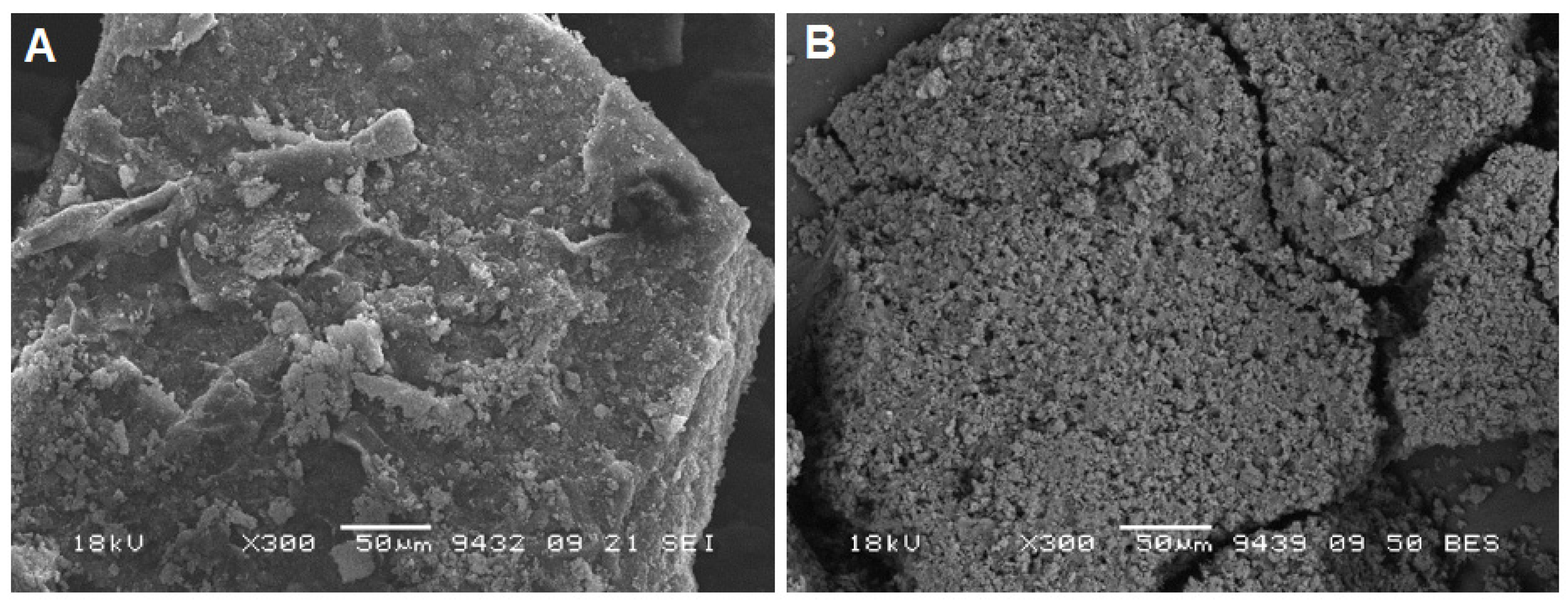
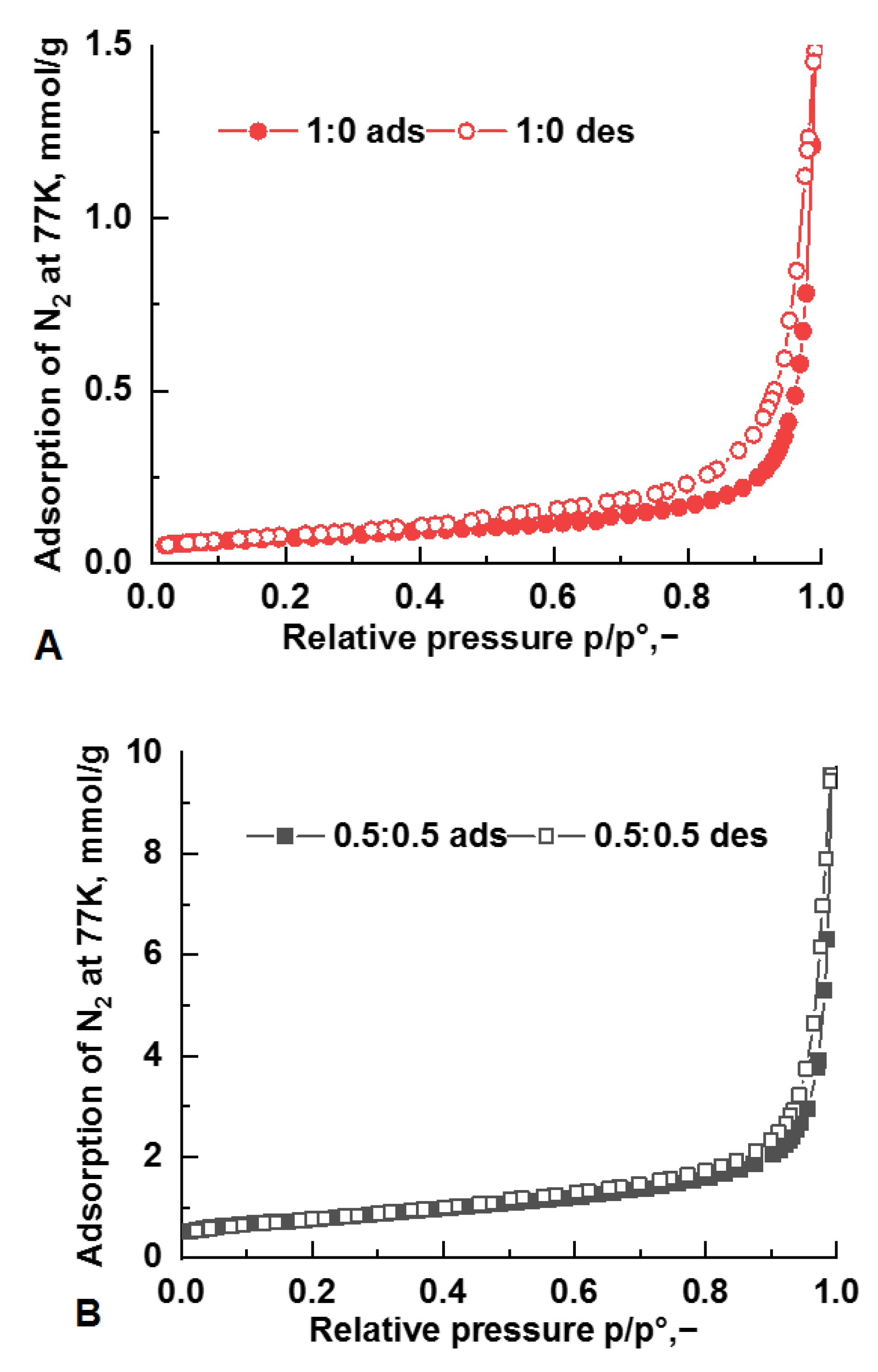
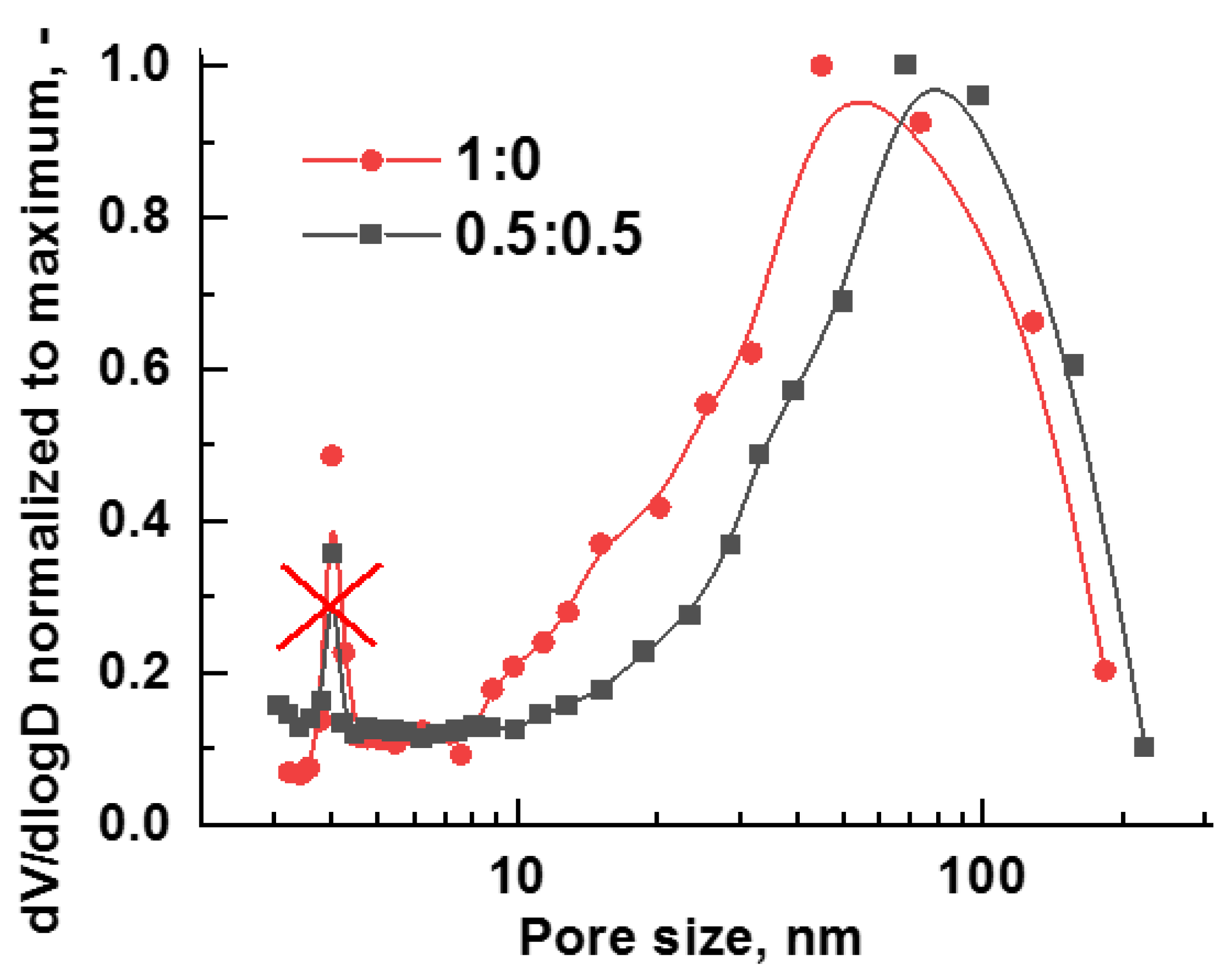
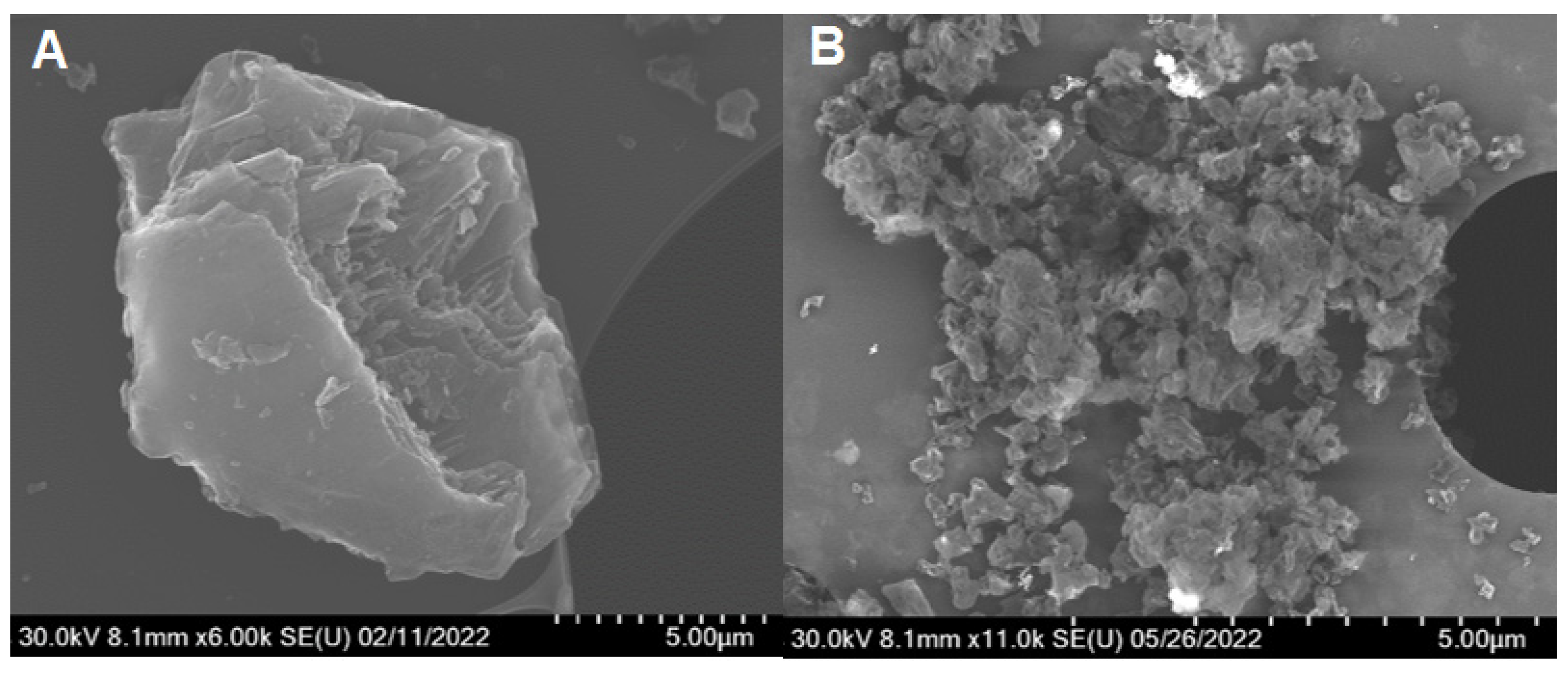
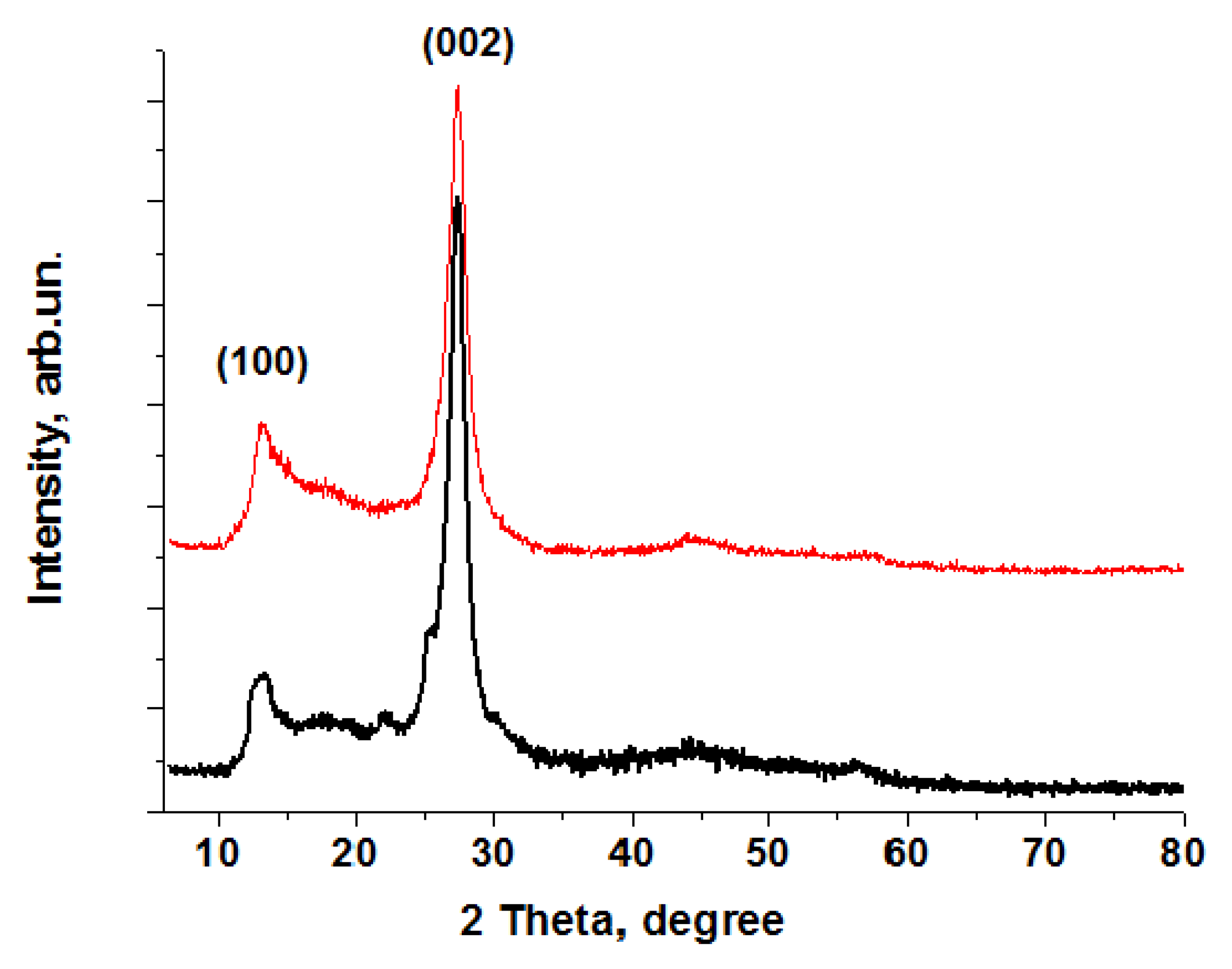
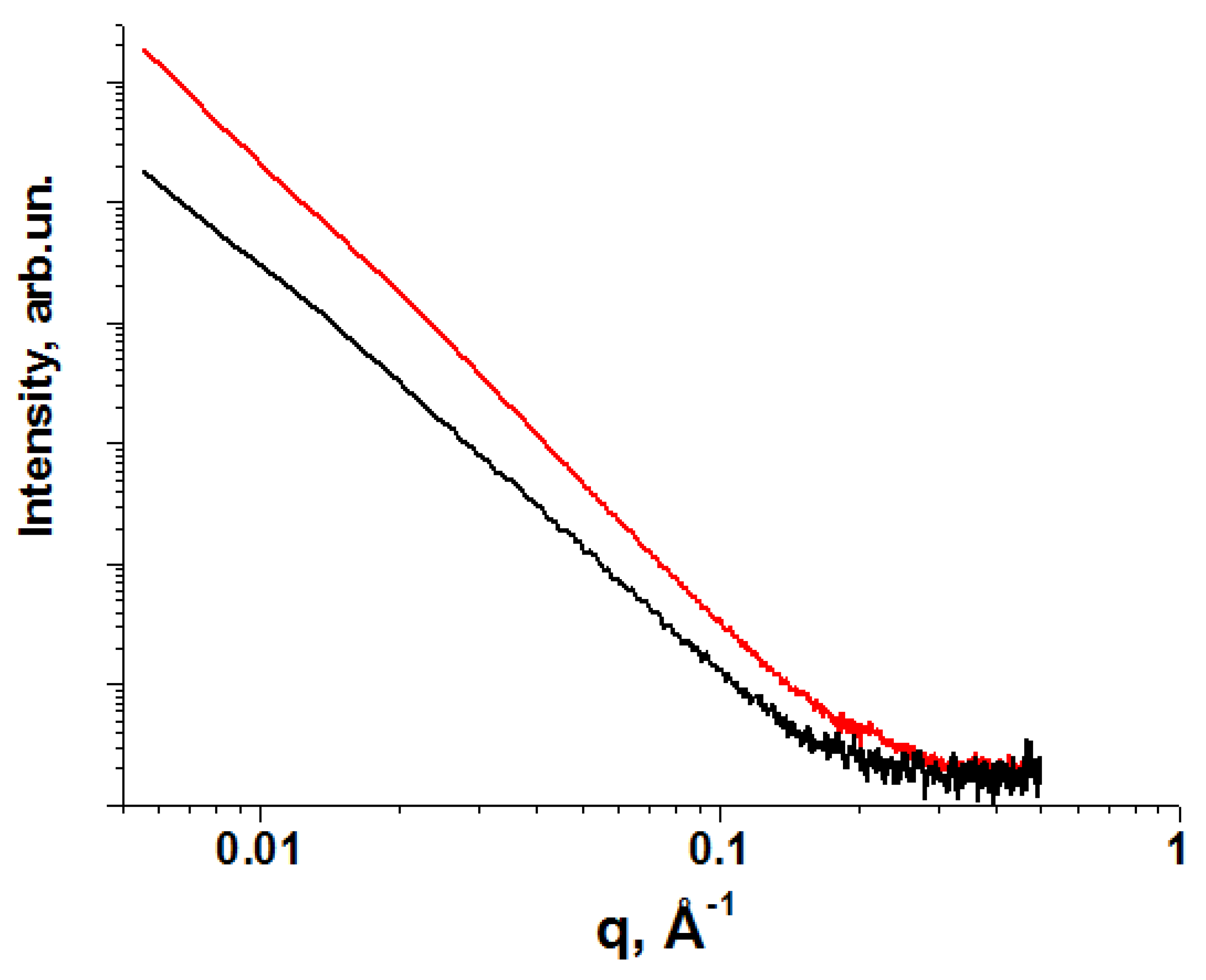
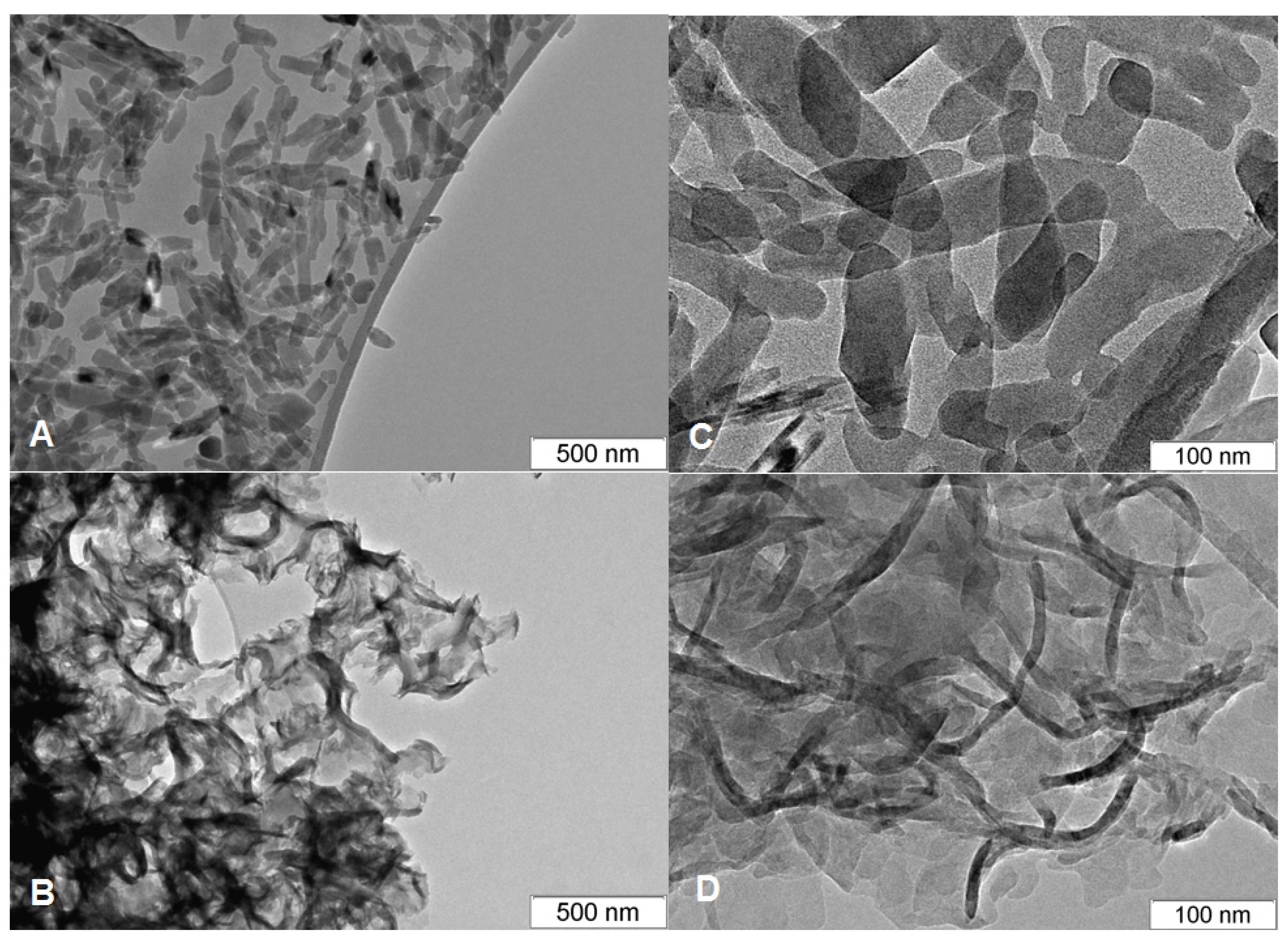
| Method | Solutions Used for Precipitation (Solvent, Concentration, Temperature) |
<D> in MCA-Solvent Suspension*, nm | SSA** of Calcined (450 °C) Sample, m2/g | |
|---|---|---|---|---|
| Melamine (M) | Cyanuric Acid (CA) | |||
| m1 | H2O, ~0.2% 25 °C |
H2O, ~0.2% 25 °C |
194 | 61 |
| m2 | H2O, ~2.5%, 90 °C |
H2O, ~2.5%, 90 °C |
366 | 24 |
| m3 | DMSO, ~2.5%, 25 °C |
DMSO, ~2.5%, 25 °C |
130 | 72 |
| m4 | DMSO, ~7.5%, 25 °C |
DMSO, ~7.5%, 25 °C |
288 | 34 |
| m5 | HCl-H2O (1M), ~2%, 25 °C |
NH4OH-H2O (1M), ~2%, 25 °C |
201 | 59 |
| Carbonization Temperature, °C | Yield of Carbonization Product, % | BET SSA*, m2/g |
|---|---|---|
| 300 | 78 | 4 |
| 400 | 47 | 3 |
| 450 | 35 | 59 |
| 500 | 21 | 71 |
| 550 | 11 | 121 |
| M:CA (mol:mol) | Temperature, °C | Yield of Carbonization Product, % | BET SSA*, m2/g |
|---|---|---|---|
| 1:0 | 550 | 34 | 3 |
| 0.9:0.1 | 500 | 29 | 12 |
| 0.67:0.33 | 500 | 24 | 41 |
| 0.5:0.5 | 500 | 21 | 71 |
| 0.6:0.4 | 500 | 9 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





