Submitted:
04 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Surgical Procedures
2.3. Protein Extraction
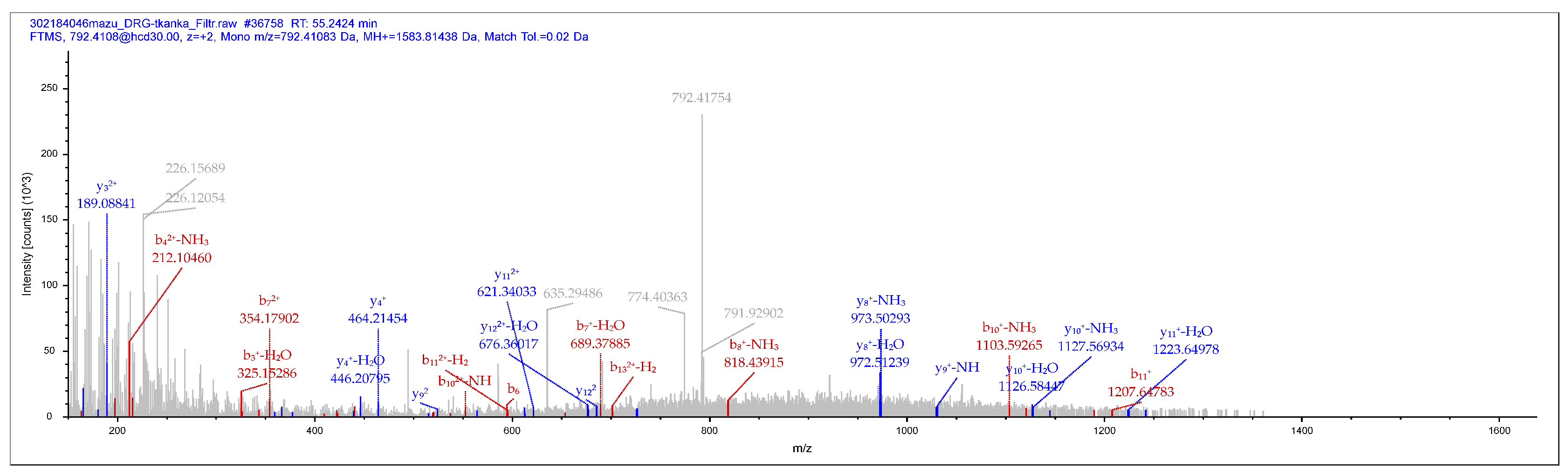
2.4. Mass Spectroscopy (MS)
2.5. MS Data Analysis
2.6. Sectioning of the Tissue Samples
2.7. Immunohistochemical Procedure and Estimation of the Total Number of PNX-containing DRG Neurons
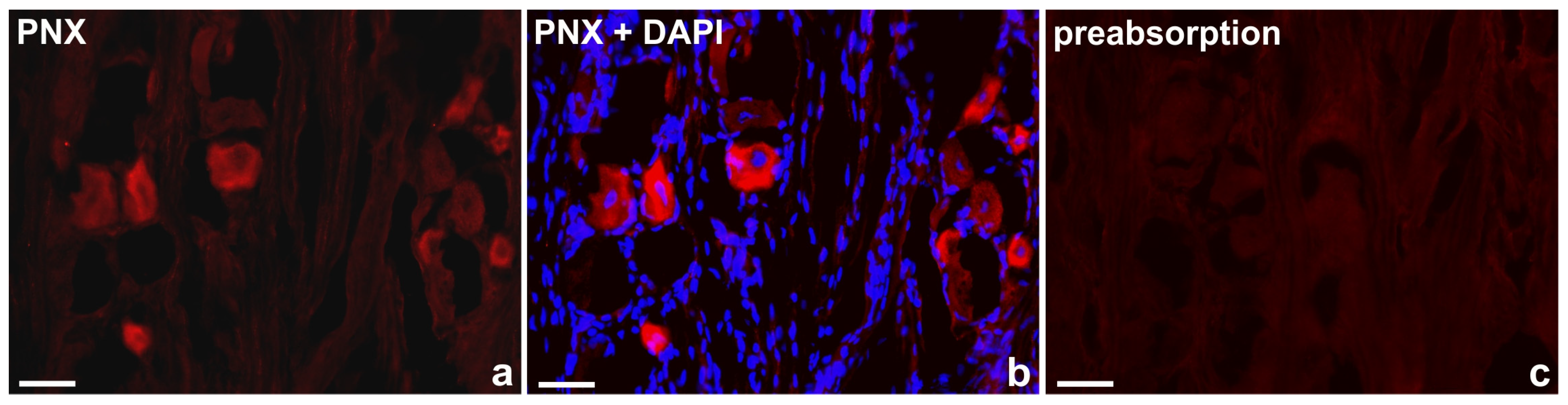
2.8. Control of Specificity of Immunohistochemical Procedures
3. Results
3.1. Mass Spectrometry Detection of PNX in Pigs DRG.
3.2. Distribution Pattern and Morphometrical Characteristics of PNX-Containing Sensory Neurons in DRG Studied
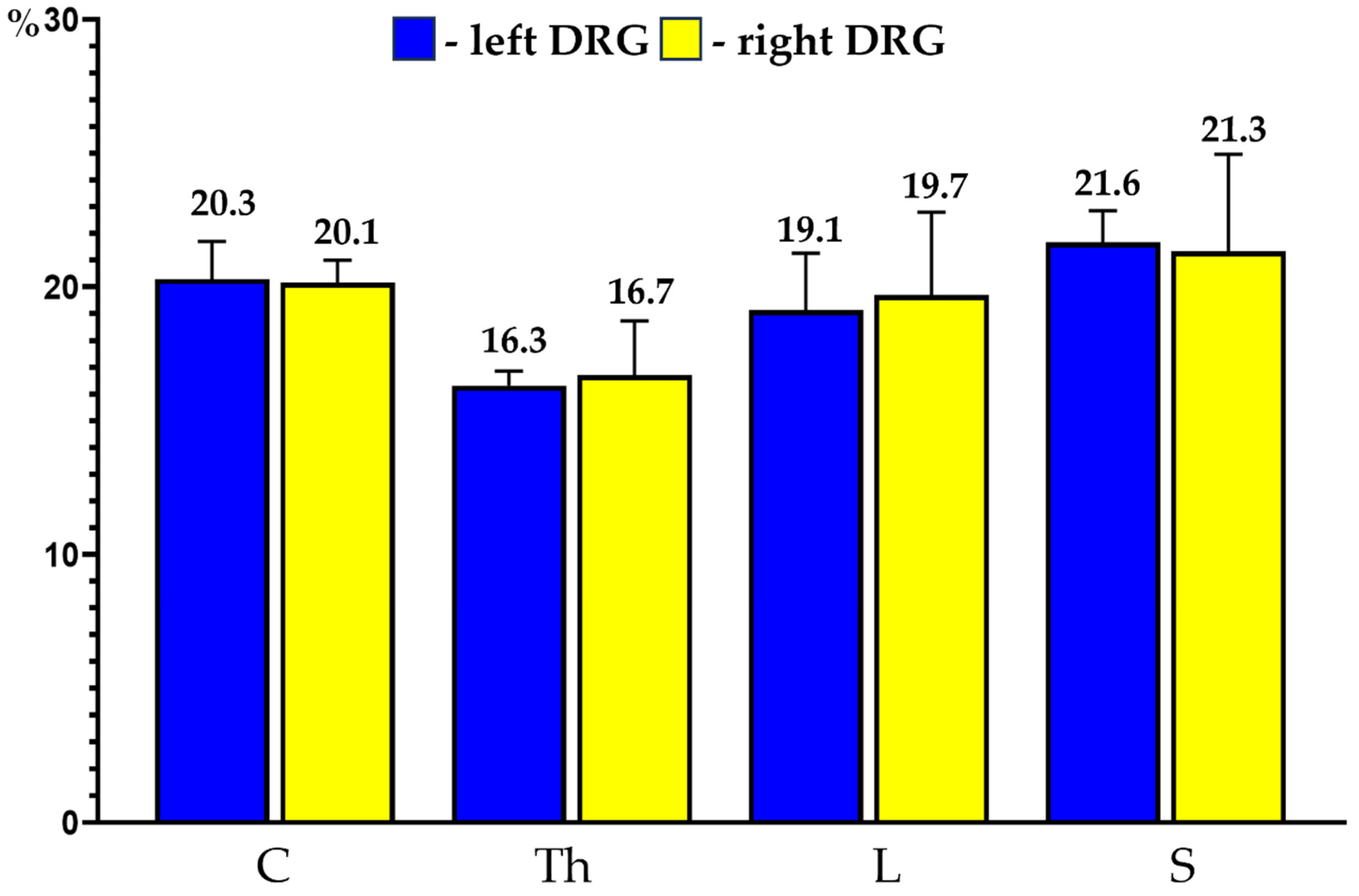
3.2.1. Distribution Pattern of PNX+ Neurons
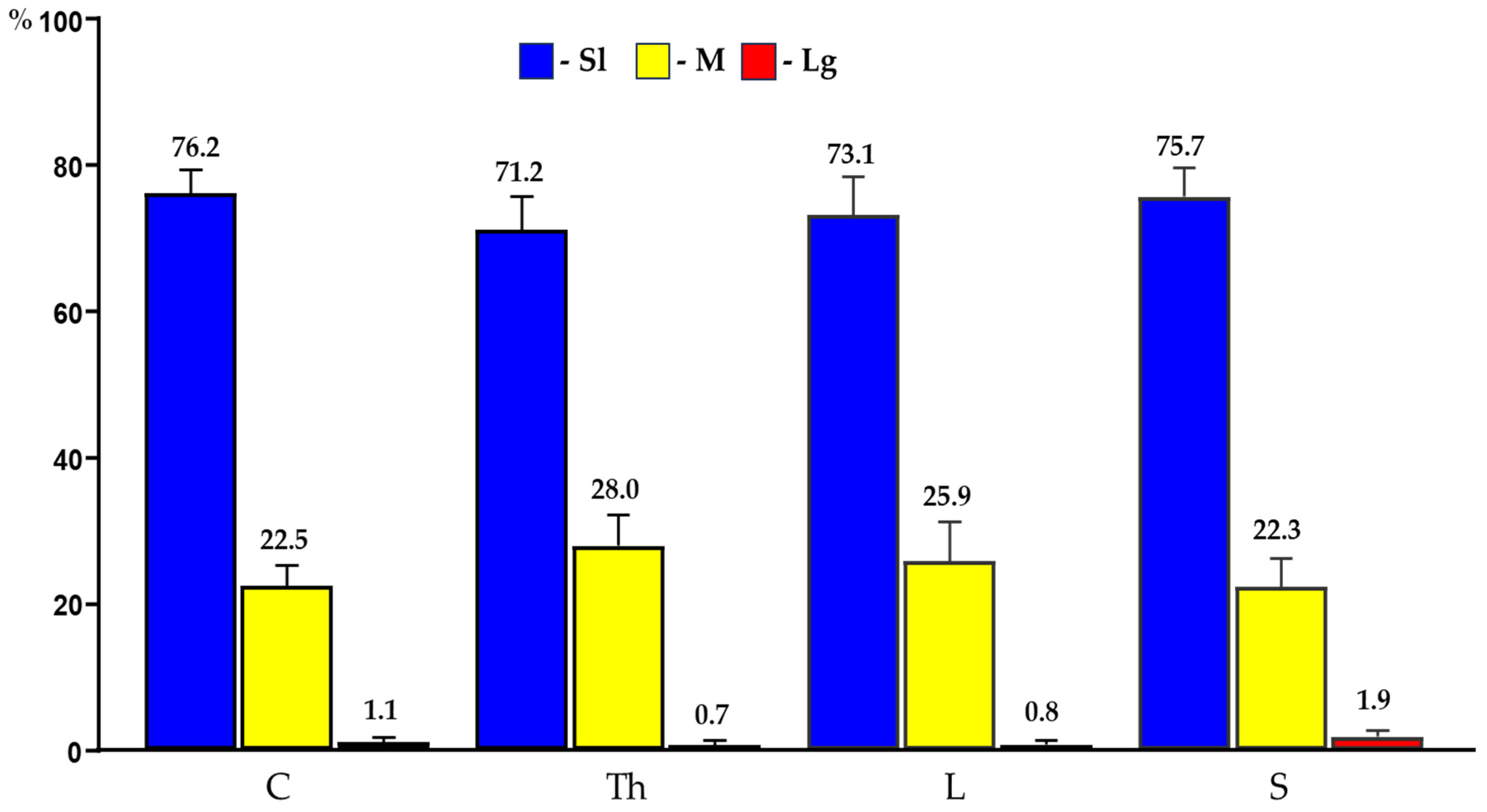
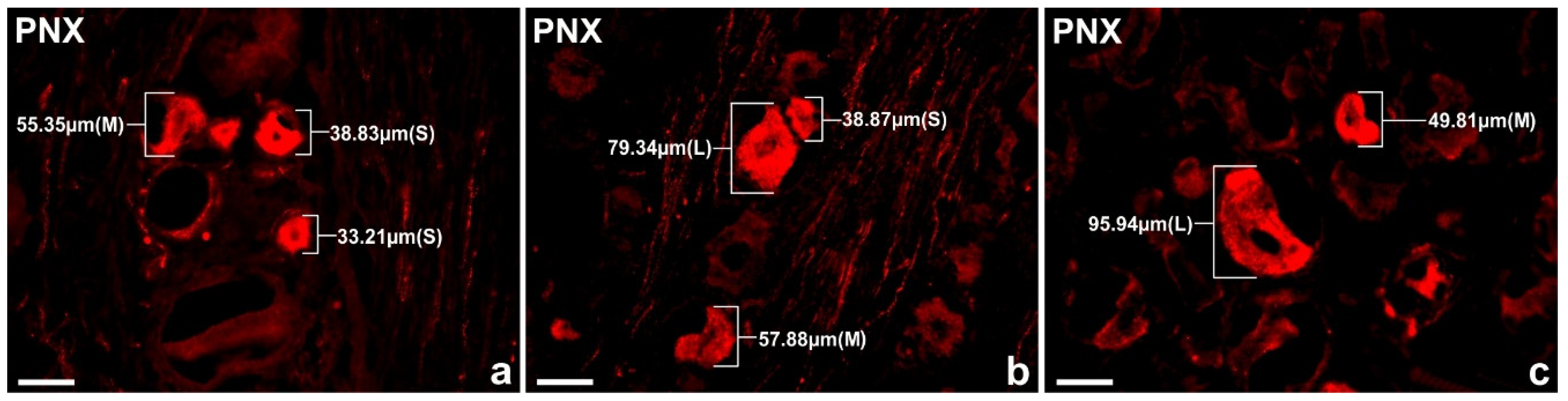
3.2.2. Morphometrical Characteristics of PNX+ Neurons
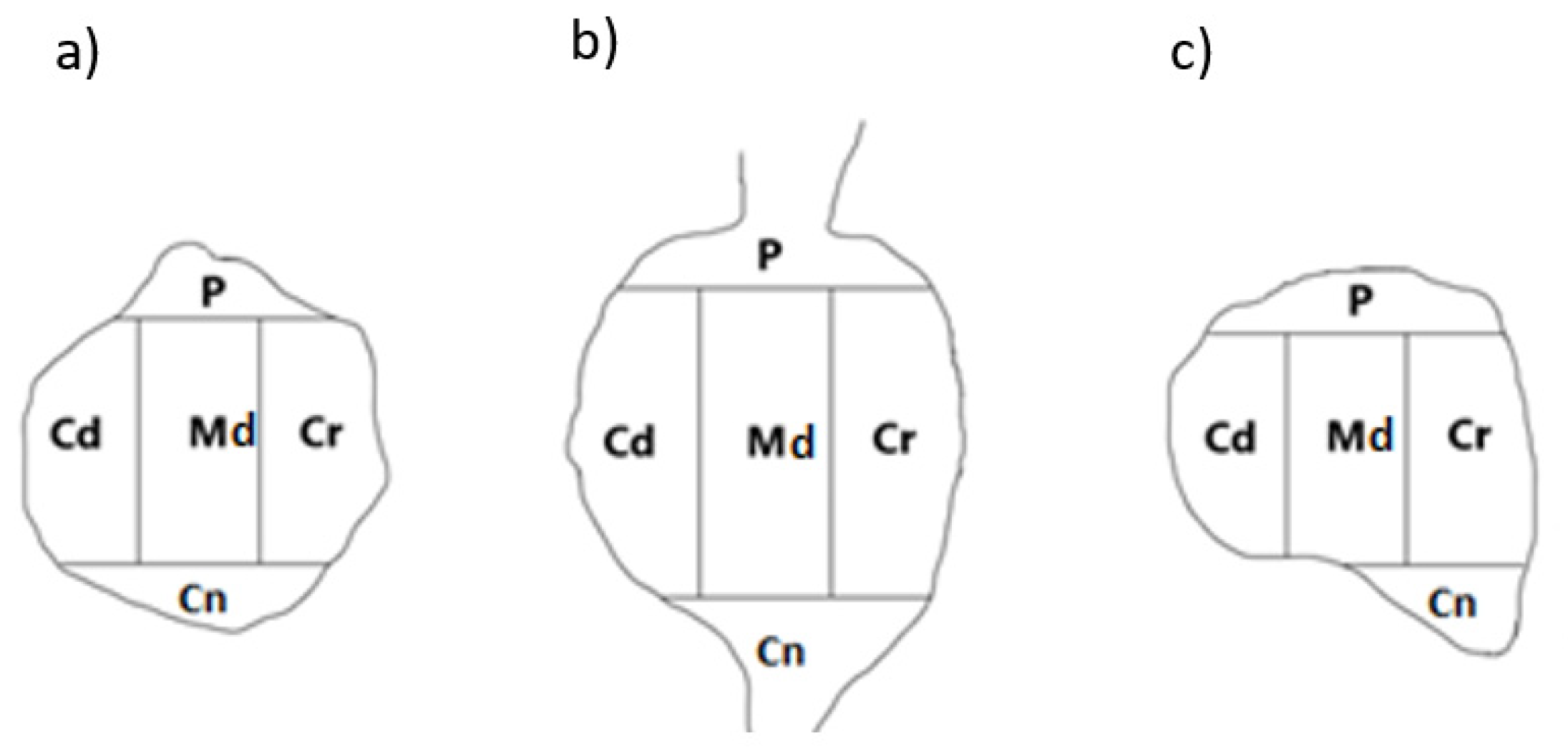
3.2.3. Intraganglionic Distribution Patterns of PNX+ Neurons.
3.2.4. Immunohistochemical Characteristic of PNX-Containing Perikarya in DRG Studied.
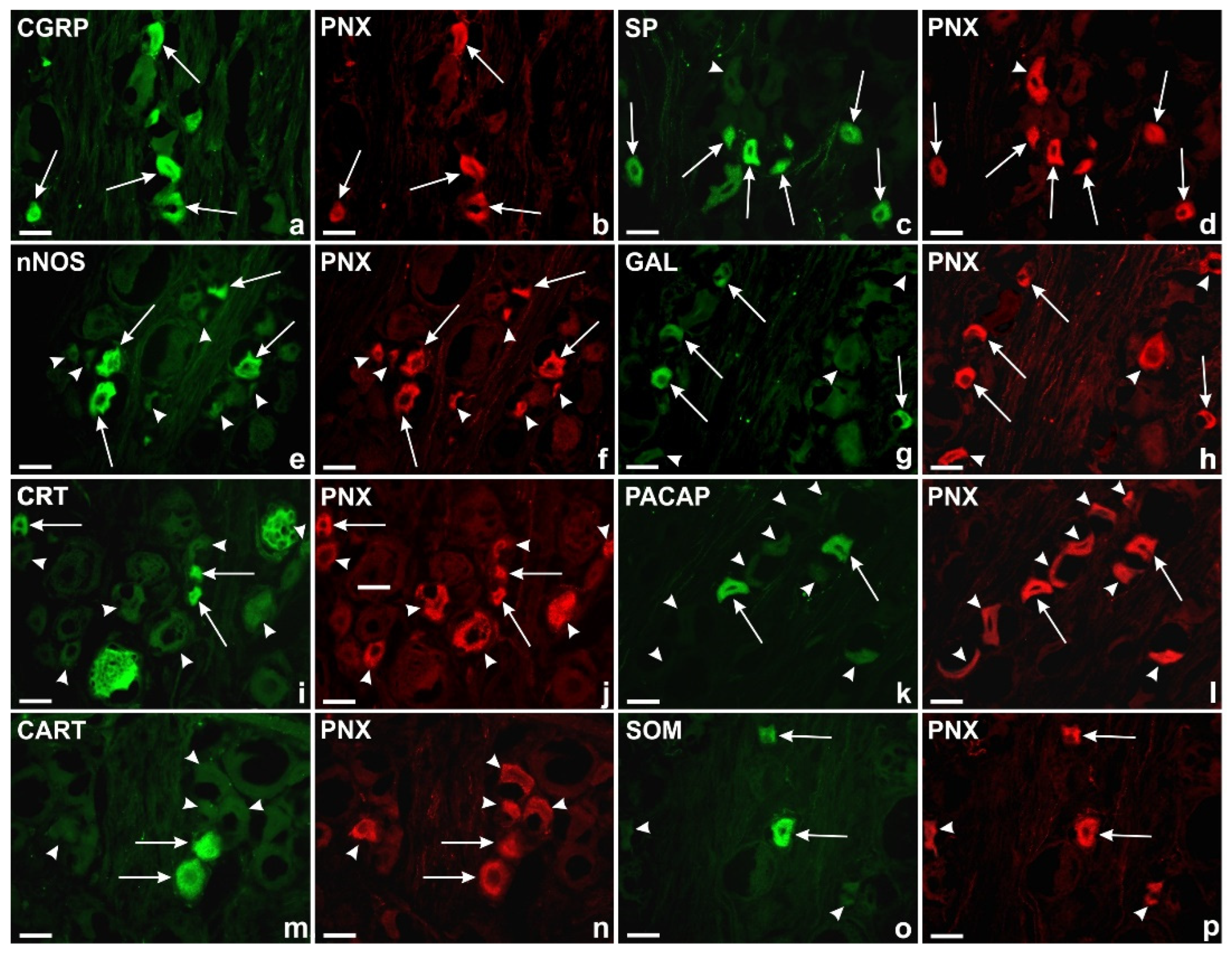
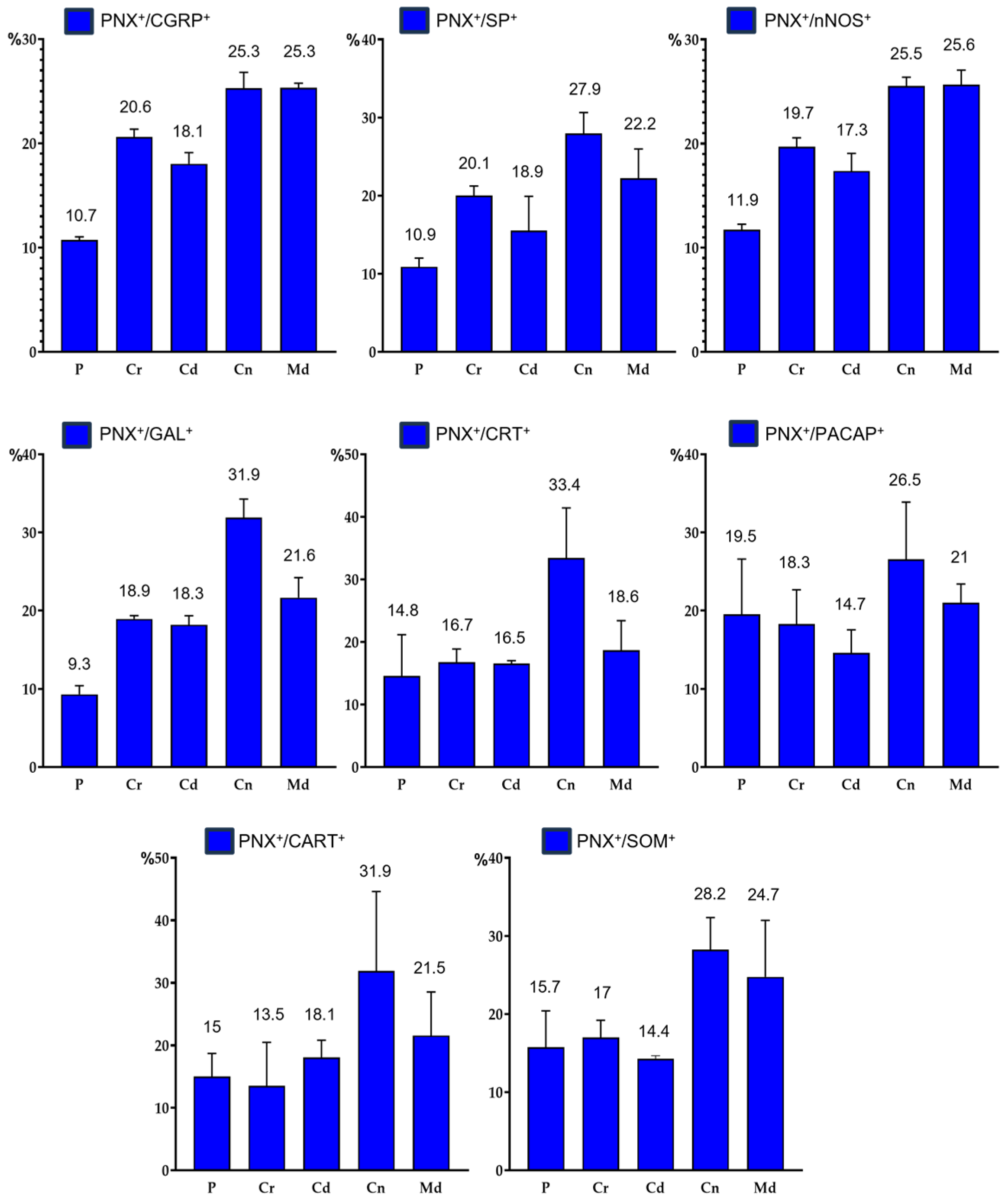
3.2.4.1. PNX+/CGRP+ Nerve Cells
3.2.4.2. PNX+/SP+ Nerve Cells
3.2.4.3. PNX+/nNOS+ Nerve Cells
3.2.4.4. PNX+/GAL+ Nerve Cells
3.2.4.5. PNX+/CRT+ Nerve Cells
3.2.4.6. PNX+/PACAP+ Nerve Cells
3.2.4.7. PNX+/CART+ Nerve Cells
3.2.4.7. PNX+/SOM+ Nerve Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yosten, G.L.C.; Lyu, R.M.; Hsueh, A.J.W.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A Novel Reproductive Peptide, Phoenixin. J Neuroendocrinol 2013, 25, 206. [Google Scholar] [CrossRef] [PubMed]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons Through the Novel Receptor GPR173. Mol Endocrinol 2016, 30, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Gołyszny, M.; Obuchowicz, E.; Zieliński, M. Neuropeptides as Regulators of the Hypothalamus-Pituitary-Gonadal (HPG) Axis Activity and Their Putative Roles in Stress-Induced Fertility Disorders. Neuropeptides 2022, 91. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Zhang, N.; Belsham, D.D. The Regulation of Phoenixin: A Fascinating Multidimensional Peptide. J Endocr Soc 2022, 6, bvab192. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Q.; Lv, S.; Ji, X. Regulation and Physiological Functions of Phoenixin. Front Mol Biosci 2022, 9, 956500. [Google Scholar] [CrossRef] [PubMed]
- Lyu, R.M.; Huang, X.F.; Zhang, Y.; Dun, S.L.; Luo, J.J.; Chang, J.K.; Dun, N.J. Phoenixin: A Novel Peptide in Rodent Sensory Ganglia. Neuroscience 2013, 250, 622. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Majewska, M.; Skowrońska, A.; Kaleczyc, J.; Majewski, M. Distribution and Chemical Coding of Phoenixin-Immunoreactive Nerve Structures in the Spinal Cord of the Pig. Ann Anat 2020, 232. [Google Scholar] [CrossRef]
- Dalmose, A.L.; Hvistendahl, J.J.; Olsen, L.H.; Eskild-Jensen, A.; Djurhuus, J.C.; Swindle, M.M. Surgically Induced Urologic Models in Swine. J Invest Surg 2000, 13, 133–145. [Google Scholar] [CrossRef]
- Kuzmuk, K.N.; Schook, L.B. Pigs as a Model for Biomedical Sciences. The Genetics of the Pig: Second Edition 2011, 426–444. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Vet Pathol 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Gromadziński, L.; Skowrońska, A.; Holak, P.; Smoliński, M.; Lepiarczyk, E.; Żurada, A.; Majewski, M.K.; Skowroński, M.T.; Majewska, M. A New Experimental Porcine Model of Venous Thromboembolism. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, K.; Dobrzyń, K.; Kieżun, M.; Malinowska, A.; Świderska, B.; Kamiński, T.; Smolińska, N. Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Bossowska, A.; Majewski, M. Tetrodotoxin Induced Changes in the Chemical Coding of Dorsal Root Ganglion Neurons Supplying the Porcine Urinary Bladder. Pol J Vet Sci 2012, 15, 355–363. [Google Scholar] [CrossRef]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and Peripheral Expression Sites of Phoenixin-14 Immunoreactivity in Rats. Biochem Biophys Res Commun 2017, 493, 195–201. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, R.; Dickenson, A.H. Spinal Cord Mechanisms of Pain. Br J Anaesth 2008, 101, 8–16. [Google Scholar] [CrossRef]
- Cowan, A.; Lyu, R.M.; Chen, Y.H.; Dun, S.L.; Chang, J.K.; Dun, N.J. Phoenixin: A Candidate Pruritogen in the Mouse. Neuroscience 2015, 310, 541–548. [Google Scholar] [CrossRef]
- Bossowska, A.; Crayton, R.; Radziszewski, P.; Kmiec, Z.; Majewski, M. Distribution and Neurochemical Characterization of Sensory Dorsal Root Ganglia Neurons Supplying Porcine Urinary Bladder. J Physiol Pharmacol 2009, 60 Suppl 4, 77–81. [Google Scholar]
- Cheng, X.; Xiao, F.; Xie, R.; Hu, H.; Wan, Y. Alternate Thermal Stimulation Ameliorates Thermal Sensitivity and Modulates Calbindin-D 28K Expression in Lamina I and II and Dorsal Root Ganglia in a Mouse Spinal Cord Contusion Injury Model. FASEB J 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F.; Iggo, A. THE SUBSTANTIA GELATINOSA OF THE SPINAL CORDA CRITICAL REVIEW. Brain 1980, 103, 717–772. [Google Scholar] [CrossRef]
- Gibson, S.J.; Polak, J.M.; Bloom, ’ S R; Sabate, $ I M; Mulderry, P.M.; Ghatei, M.A.; Mcgregor, $ G P; Morrison, J.F.B.; Kelly, J.S.; Evans, R.M.; et al. CALCITONIN GENE-RELATED PEPTIDE IMMUNOREACTIVITY IN THE SPINAL CORD OF MAN AND OF EIGHT OTHER SPECIES1. 1984, 4, 3101–3111.
- Nordlind, K.; Eriksson, L.; Seiger, Å.; Bakhiet, M. Expression of Interleukin-6 in Human Dorsal Root Ganglion Cells. Neurosci Lett 2000, 280, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ajijola, O.A.; Aliotta, E.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. PATHOLOGICAL EFFECTS OF CHRONIC MYOCARDIAL INFARCTION ON PERIPHERAL NEURONS MEDIATING CARDIAC NEUROTRANSMISSION. Auton Neurosci 2016, 197, 34. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Rehmathulla, S.; Tariq, S.; Ali, M.A.; Emerald, B.S.; Shehab, S. Co-Localization of Nociceptive Markers in the Lumbar Dorsal Root Ganglion and Spinal Cord of Dromedary Camel. Journal of Comparative Neurology 2021, 529, 3710–3725. [Google Scholar] [CrossRef] [PubMed]
- Giaid, A.; Gibson, S.J.; Ibrahim, N.B.N.; Legon, S.; Bloom, S.R.; Yanagisawa, M.; Masaki, T.; Varndell, I.M.; Polak, J.M. Endothelin 1, an Endothelium-Derived Peptide, Is Expressed in Neurons of the Human Spinal Cord and Dorsal Root Ganglia. Proc Natl Acad Sci U S A 1989, 86, 7634. [Google Scholar] [CrossRef]
- Landry, M.; Åman, K.; Dostrovsky, J.; Lozano, A.M.; Carlstedt, T.; Spenger, C.; Josephson, A.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Galanin Expression in Adult Human Dorsal Root Ganglion Neurons: Initial Observations. Neuroscience 2003, 117, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Kar, S.; Gibson, S.J.; Ghidella, S.; Gobetto, A.; Peirone, S.M.; Polak, J.M. The Immunocytochemical Distribution of Seven Peptides in the Spinal Cord and Dorsal Root Ganglia of Horse and Pig. Anat Embryol (Berl) 1990, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.N.; Kyloh, M.A.; Travis, L.; Cox, M.; Hibberd, T.J.; Spencer, N.J. Anatomical Distribution of CGRP-Containing Lumbosacral Spinal Afferent Neurons in the Mouse Uterine Horn. Front Neurosci 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Tippins, J.R.; Morris, H.R.; MacIntyre, I.; Williams, T.J. Potent Vasodilator Activity of Calcitonin Gene-Related Peptide in Human Skin. J Invest Dermatol 1986, 87, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Bunker, C.B.; Reavley, C.; Dowd, P.M.; O’Shaughnessy, D.J. Calcitonin Gene-Related Peptide in Treatment of Severe Peripheral Vascular Insufficiency in Raynaud’s Phenomenon. Lancet 1993, 342, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Mitsikostas, D.D.; Reuter, U. Calcitonin Gene-Related Peptide Monoclonal Antibodies for Migraine Prevention: Comparisons across Randomized Controlled Studies. Curr Opin Neurol 2017, 30, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Garry, M.G.; Hargreaves, K.M. Enhanced Release of Immunoreactive CGRP and Substance P from Spinal Dorsal Horn Slices Occurs during Carrageenan Inflammation. Brain Res 1992, 582, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Takasaki, K.; Saito, A.; Goto, K. Calcitonin Gene-Related Peptide Acts as a Novel Vasodilator Neurotransmitter in Mesenteric Resistance Vessels of the Rat. Nature 1988, 335, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Pintér, E.; Al-Rashed, S.; Gerard, N.; Hoult, J.R.; Brain, S.D. Neurokinin-1 Receptor Agonists Are Involved in Mediating Neutrophil Accumulation in the Inflamed, but Not Normal, Cutaneous Microvasculature: An in Vivo Study Using Neurokinin-1 Receptor Knockout Mice. J Immunol 2000, 164, 5424–5429. [Google Scholar] [CrossRef]
- Biella, G.; Panara, C.; Pecile, A.; Sotgiu, M.L. Facilitatory Role of Calcitonin Gene-Related Peptide (CGRP) on Excitation Induced by Substance P (SP) and Noxious Stimuli in Rat Spinal Dorsal Horn Neurons. An Iontophoretic Study in Vivo. Brain Res 1991, 559, 352–356. [Google Scholar] [CrossRef]
- Le Grevés, P.; Nyberg, F.; Hökfelt, T.; Terenius, L. Calcitonin Gene-Related Peptide Is Metabolized by an Endopeptidase Hydrolyzing Substance P. Regul Pept 1989, 25, 277–286. [Google Scholar] [CrossRef]
- Le Greves, P.; Nyberg, F.; Terenius, L.; Hökfelt, T. Calcitonin Gene-Related Peptide Is a Potent Inhibitor of Substance P Degradation. Eur J Pharmacol 1985, 115, 309–311. [Google Scholar] [CrossRef]
- Oku, R.; Satoh, M.; Fujii, N.; Otaka, A.; Yajima, H.; Takagi, H. Calcitonin Gene-Related Peptide Promotes Mechanical Nociception by Potentiating Release of Substance P from the Spinal Dorsal Horn in Rats. Brain Res 1987, 403, 350–354. [Google Scholar] [CrossRef]
- Kangrga, I.; Larew, J.S.A.; Randic, M. The Effects of Substance P and Calcitonin Gene-Related Peptide on the Efflux of Endogenous Glutamate and Aspartate from the Rat Spinal Dorsal Horn in Vitro. Neurosci Lett 1990, 108, 155–160. [Google Scholar] [CrossRef]
- Cooper, D.; Laidig, W.D.; Sappington, A.; MacGregor, G. A Pharmacological Review of Calcitonin Gene-Related Peptide Biologics and Future Use for Chronic Pain. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Nicoll, R.A.; Schenker, C.; Leeman, S.E. Substance P as a Transmitter Candidate. Annu Rev Neurosci 1980, 3, 227–268. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.N.; Crepps, B.A.; Perl, E.R. Relationship of Substance P to Afferent Characteristics of Dorsal Root Ganglion Neurones in Guinea-Pig. Journal of Physiology 1997, 177–191. [Google Scholar] [CrossRef]
- Janikiewicz, P.; Wasilewska, B.; Mazur, U.; Franke-Radowiecka, A.; Majewski, M.; Bossowska, A. The Influence of an Adrenergic Antagonist Guanethidine (GUA) on the Distribution Pattern and Chemical Coding of Dorsal Root Ganglia (DRG) Neurons Supplying the Porcine Urinary Bladder. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Han, D.S.; Lee, C.H.; Shieh, Y.D.; Chang, C.T.; Li, M.H.; Chu, Y.C.; Wang, J.L.; Chang, K.V.; Lin, S.H.; Chen, C.C. A Role for Substance P and Acid-Sensing Ion Channel 1a in Prolotherapy with Dextrose-Mediated Analgesia in a Mouse Model of Chronic Muscle Pain. Pain 2022, 163, E622–E633. [Google Scholar] [CrossRef]
- Kozłowska, A.; Mikołajczyk, A.; Adamiak, Z.; Majewski, M. Distribution and Chemical Coding of Sensory Neurons Innervating the Skin of the Porcine Hindlimb. Neuropeptides 2017, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.W.; Lawson, S.N. Cell Type and Conduction Velocity of Rat Primary Sensory Neurons with Substance P-like Immunoreactivity. Neuroscience 1989, 28, 745–753. [Google Scholar] [CrossRef]
- Simões, A.L.B.; Silva, G.A.R.; Giorgetto, C.; de Cassia do Carmo-Campos, E.; Dias, F.J.; Fazan, V.P.S. Substance P in Dorsal Root Ganglion Neurons in Young and Adult Rats, after Nociceptive Stimulation during the Neonatal Period. Anatomical Record 2018, 301, 849–861. [Google Scholar] [CrossRef]
- Kourosh-Arami, M.; Hosseini, N.; Mohsenzadegan, M.; Komaki, A.; Joghataei, M.T. Neurophysiologic Implications of Neuronal Nitric Oxide Synthase. Rev Neurosci 2020, 31, 617–636. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, D.Y. Neuronal Nitric Oxide Synthase: Structure, Subcellular Localization, Regulation, and Clinical Implications. Nitric Oxide 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Thippeswamy, T.; Morris, R. The Roles of Nitric Oxide in Dorsal Root Ganglion Neurons. Ann N Y Acad Sci 2002, 962, 103–110. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, X.; Green, D.P.; Dong, X. Peripheral Mechanisms of Chronic Pain. Medical review (Berlin, Germany) 2022, 2, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.A.; Ferreira, A.F.B.; Da Silva, J.T.; Alves, A.S.; Martins, D.O.; Britto, L.R.G.; Chacur, M. Effects of Selective Inhibition of NNOS and INOS on Neuropathic Pain in Rats. Mol Cell Neurosci 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.; Rajpoot, S.; Bezsonov, E.E.; Orekhov, A.N.; Saluja, R.; Wary, A.; Axen, C.; Wary, K.; Baig, M.S. The Expanding Roles of Neuronal Nitric Oxide Synthase (NOS1). PeerJ 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Terenghi, G.; Riveros-Moreno, V.; Hudson, L.D.; Ibrahim, N.B.N.; Polak, J.M. Immunohistochemistry of Nitric Oxide Synthase Demonstrates Immunoreactive Neurons in Spinal Cord and Dorsal Root Ganglia of Man and Rat. J Neurol Sci 1993, 118, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Bornstein, J.C.; Anderson, C.R. Distinct Chemical Classes of Medium-Sized Transient Receptor Potential Channel Vanilloid 1-Immunoreactive Dorsal Root Ganglion Neurons Innervate the Adult Mouse Jejunum and Colon. Neuroscience 2008, 156, 334–343. [Google Scholar] [CrossRef]
- Russo, D.; Clavenzani, P.; Mazzoni, M.; Chiocchetti, R.; Guardo, G. Di; Lalatta-Costerbosa, G. Immunohistochemical Characterization of TH13-L2 Spinal Ganglia Neurons in Sheep (Ovis Aries). Microsc Res Tech 2010, 73, 128–139. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z.; Xu, X.J. Galanin in Somatosensory Function. Ann N Y Acad Sci 1998, 863, 383–389. [Google Scholar] [CrossRef]
- Hökfelt, T.; Wiesenfeld-Hallin, Z.; Villar, M.; Melander, T. Increase of Galanin-like Immunoreactivity in Rat Dorsal Root Ganglion Cells after Peripheral Axotomy. Neurosci Lett 1987, 83, 217–220. [Google Scholar] [CrossRef]
- Hao, J.-X.; Shi, T.-J.; Xu, I.S.; Kaupilla, T.; Xu, X.-J.; Hökfelt, T.; Bartfai, T.; Wiesenfeld-Hallin, Z. Intrathecal Galanin Alleviates Allodynia-like Behaviour in Rats after Partial Peripheral Nerve Injury. European Journal of Neuroscience 1999, 11, 427–432. [Google Scholar] [CrossRef]
- Metcalf, C.S.; Klein, B.D.; McDougle, D.R.; Zhang, L.; Smith, M.D.; Bulaj, G.; White, H.S. Analgesic Properties of a Peripherally Acting and GalR2 Receptor-Preferring Galanin Analog in Inflammatory, Neuropathic, and Acute Pain Models. J Pharmacol Exp Ther 2015, 352, 185–193. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, Signaling, and Pharmacology of Galanin Peptides and Receptors: Three Decades of Emerging Diversity. Pharmacol Rev 2015, 67, 118–175. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.; Zhang, Y.Z.; Håkanson, R.; Luts, A.; Sjölund, B.; Uddman, R.; Sundler, F. Pituitary Adenylate Cyclase Activating Peptide Is a Sensory Neuropeptide: Immunocytochemical and Immunochemical Evidence. Neuroscience 1993, 57, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, T.J.; Ji, R.R.; Zhang, Y. then; Sundler, F.; Hannibal, J.; Fahrenkrug, J.; Hökfelt, T. Expression of Pituitary Adenylate Cyclase-Activating Polypeptide in Dorsal Root Ganglia Following Axotomy: Time Course and Coexistence. Brain Res 1995, 705, 149–158. [Google Scholar] [CrossRef]
- Mulder, H.; Uddman, R.; Moller, K.; Zhang, Y.Z.; Ekblad, E.; Alumets, J.; Sundler, F. Pituitary Adenylate Cyclase Activating Polypeptide Expression in Sensory Neurons. Neuroscience 1994, 63, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Corness, J.; Shi, T.J.; Xu, Z.Q.; Brulet, P.; Hökfelt, T. Influence of Leukemia Inhibitory Factor on Galanin/GMAP and Neuropeptide Y Expression in Mouse Primary Sensory Neurons after Axotomy. Exp Brain Res 1996, 112, 79–88. [Google Scholar] [CrossRef]
- Lioudyno, M.; Skoglösa, Y.; Takei, N.; Lindholm, D. Rapid Communication Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Protects Dorsal Root Ganglion Neurons From Death and Induces Calcitonin Gene-Related Peptide (CGRP) Immunoreactivity In Vitro. 1998, 51, 243–256. [Google Scholar]
- Xu, X.; Yang, X.; Zhang, P.; Chen, X.; Liu, H.; Li, Z. Effects of Exogenous Galanin on Neuropathic Pain State and Change of Galanin and Its Receptors in DRG and SDH after Sciatic Nerve-Pinch Injury in Rat. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Zacharko-Siembida, A.; Kulik, P.; Szalak, R.; Lalak, R.; Arciszewski, M.B. Co-Expression Patterns of Cocaine- and Amphetamine-Regulated Transcript (CART) with Neuropeptides in Dorsal Root Ganglia of the Pig. Acta Histochem 2014, 116, 390–398. [Google Scholar] [CrossRef]
- Kozsurek, M.; Lukácsi, E.; Fekete, C.; Wittmann, G.; Réthelyi, M.; Puskár, Z. Cocaine- and Amphetamine-Regulated Transcript Peptide (CART) Is Present in Peptidergic C Primary Afferents and Axons of Excitatory Interneurons with a Possible Role in Nociception in the Superficial Laminae of the Rat Spinal Cord. Eur J Neurosci 2007, 26, 1624–1631. [Google Scholar] [CrossRef]
- Hökfelt, T.; Elde, R.; Johansson, O.; Luft, R.; Nilsson, G.; Arimura, A. Immunohistochemical Evidence for Separate Populations of Somatostatin-Containing and Substance P-Containing Primary Afferent Neurons in the Rat. Neuroscience 1976, 1. [Google Scholar] [CrossRef]
- Kardon, A.P.; Polgár, E.; Hachisuka, J.; Snyder, L.M.; Cameron, D.; Savage, S.; Cai, X.; Karnup, S.; Fan, C.R.; Hemenway, G.M.; et al. Dynorphin Acts as a Neuromodulator to Inhibit Itch in the Dorsal Horn of the Spinal Cord. Neuron 2014, 82, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Stantcheva, K.K.; Iovino, L.; Dhandapani, R.; Martinez, C.; Castaldi, L.; Nocchi, L.; Perlas, E.; Portulano, C.; Pesaresi, M.; Shirlekar, K.S.; et al. A Subpopulation of Itch-Sensing Neurons Marked by Ret and Somatostatin Expression. EMBO Rep 2016, 17, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Stengel, A. Current State of Phoenixin-the Implications of the Pleiotropic Peptide in Stress and Its Potential as a Therapeutic Target. Front Pharmacol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.; Polgár, E.; Chalmers, J.; Minson, J.B.; Llewellyn-Smith, I.; Heizmann, C.W.; Somogyi, P. Different Populations of Parvalbumin- and Calbindin-D28k-Immunoreactive Neurons Contain GABA and Accumulate 3H-D-Aspartate in the Dorsal Horn of the Rat Spinal Cord. J Comp Neurol 1991, 314, 114–124. [Google Scholar] [CrossRef]
- Egea, J.; Malmierca, E.; Rosa, A.O.; Del Barrio, L.; Negredo, P.; Nuñez, A.; López, M.G. Participation of Calbindin-D28K in Nociception: Results from Calbindin-D28K Knockout Mice. Pflugers Arch 2012, 463, 449–458. [Google Scholar] [CrossRef]
- Ren, K.; Ruda, M.A.; Jacobowitz, D.M. Immunohistochemical Localization of Calretinin in the Dorsal Root Ganglion and Spinal Cord of the Rat. Brain Res Bull 1993, 31, 13–22. [Google Scholar] [CrossRef]
- Stebbing, M.J.; Balasubramanyan, S.; Smith, P.A. Calbindin-D-28K like Immunoreactivity in Superficial Dorsal Horn Neurons and Effects of Sciatic Chronic Constriction Injury. Neuroscience 2016, 324, 330–343. [Google Scholar] [CrossRef]
- Ichikawa, H.; Jacobowitz, D.M.; Sugimoto, T. Calretinin-Immunoreactive Neurons in the Trigeminal and Dorsal Root Ganglia of the Rat. Brain Res 1993, 617, 96–102. [Google Scholar] [CrossRef]
| Antigen | Code | Dilution | Host | Supplier |
|---|---|---|---|---|
| Primary Antibodies | ||||
| CART | MAB 163 | 1:1000 | Mouse | R&D Systems, Germany |
| CGRP | T-5027 | 1:800 | Guinea pig | Peninsula Laboratories, San Carlos, CA, USA |
| CRT | 6B3 | 1:2000 | Mouse | SWANT, Switzerland |
| GAL | T-5036 | 1:1500 | Guinea pig | Peninsula Laboratories, San Carlos, CA, USA |
| nNOS | N2280 | 1:200 | Mouse | Sigma-Aldrich, St. Louis, MO, USA |
| PACAP | T-5039 | 1:1000 | Guinea pig | Peninsula Laboratories, San Carlos, CA, USA |
| PGP 9.5 | 7863-2004 | 1:5000 | Mouse | Biogenesis, United Kingdom |
| PNX | H-079-01 | 1:7000 | Rabbit | Phoenix Pharmaceuticals Inc, Burlingame, CA, USA, |
| SOM | MAB 354 | 1:50 | Rat | Merck Millipore, Temecula, CA, USA |
| SP | 8450-0004 | 1:200 | Rat | Bio-Rad, Kidlington, UK |
| Secondary reagents | ||||
| CY3-conjugated anti-rabbit | 711-166-152 | 1:700 | Donkey | Jackson I.R.; USA, Baltimore Pike |
| FITC-conjugated anti-mouse IgG | 715-096-151 | 1:800 | Donkey | Jackson I.R.; USA, Baltimore Pike |
| FITC-conjugated anti-guinea pig IgG | 706-095-148 | 1:800 | Donkey | Jackson I.R.; USA, Baltimore Pike |
| FITC-conjugated anti-rat IgG | 712-095-153 | 1:400 | Donkey | Jackson I.R.; USA, Baltimore Pike |
| PRIMARY ANTIBODY | PRIMARY ANTYBODY | SECONDARY ANTIBODY |
|---|---|---|
| PNX (rabbit) | CART (mouse) | CY3 (rabbit), FITC (mouse) |
| PNX (rabbit) | CGRP (guinea pig) | CY3 (rabbit), FITC (guinea pig) |
| PNX (rabbit) | CRT (mouse) | CY3 (rabbit), FITC (mouse) |
| PNX (rabbit) | GAL (guinea pig) | CY3 (rabbit), FITC (guinea pig) |
| PNX (rabbit) | nNOS (mouse) | CY3 (rabbit), FITC (mouse) |
| PNX (rabbit) | PACAP (guinea pig) | CY3 (rabbit), FITC (guinea pig) |
| PNX (rabbit) | PGP (mouse) | CY3 (rabbit), FITC (mouse) |
| PNX (rabbit) | SOM (rat) | CY3 (rabbit), FITC (rat) |
| PNX (rabbit) | SP (rat) | CY3 (rabbit), FITC (rat) |
| Antigen | Code | Dilution | Supplier |
|---|---|---|---|
| CART | MAB0041 | 1:1000 | R&D Systems, Germany |
| CGRP | T-4030 | 1:800 | Peninsula Laboratories, San Carlos, CA, USA |
| CRT | 6-His human calretinin (recombinant) Lot No.: 22 |
1:2000 |
SWANT, Switzerland |
| GAL | T-4862 | 1:1500 | Peninsula Laboratories, San Carlos, CA, USA |
| nNOS | N3033 | 1:200 | Sigma, St. Louis, MO, USA |
| PACAP | A9808 | 1:1000 | Sigma, St. Louis, MO, USA |
| PNX | 079-01 | 1:7000 | Phoenix Pharmaceuticals Inc; Burlingame; Kalifornia; USA |
| SOM | S9129 | 1:50 | Sigma-Aldrich, St. Louis, MO, USA |
| SP | S6883 | 1:200 | Sigma-Aldrich, St. Louis, MO, USA |
| Segments of the Spinal Cord (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DRG | C1 | C4 | C7 | Th1 | Th7 | Th15 | L1 | L3 | L6 | S1 | S3 |
| left | 21.5 ± 3.9 |
17.5 ± 3.1 |
21.9 ± 1.7 |
16.2 ± 2.0 |
17.3 ± 0.7 |
15.4 2.3 |
17.8 ± 2.4 |
16.3 ± 0.8 |
23.3 ± 3.6 |
21.6 ± 2.0 |
13.3 ± 4.6 |
| right | 18.5 ± 7.2 |
21.1 ± 6.6 |
20.9 ± 1.0 |
18.1 ± 1.9 |
21.7 ± 0.6 |
19.3 ± 3.7 |
15.7 ± 4.1 |
17.6 ± 2.0 |
25.8 ± 1.2 |
24.6 ± 0.9 |
16.7 ± 7.6 |
| Segments of the Spinal Cord (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Th | L | S | |||||||||
| DRG | Sl | M | Lg | Sl | M | Lg | Sl | M | Lg | Sl | M | Lg |
| left | 74.7 ± 3.1 |
23.9 ± 2.3 |
1.4 ± 0.9 |
70.6 ± 3.1 |
28.5 ± 2.9 |
0.9 ± 0.3 |
75.4 ± 5.9 |
23.8 ± 5.8 |
0.8 ± 0.6 |
76.3 ± 1.0 |
21.9 ± 0.8 |
1.8 ± 0.2 |
| right | 76.7 ± 3.1 |
22.1 ± 2.9 |
1.2 ± 0.6 |
71.4 ± 4.9 |
27.8 ± 4.7 |
0.8 ± 0.7 |
72.4 ± 5.2 |
26.6 ± 5.2 |
1.0 ± 0.6 |
75.4 ± 4.8 |
22.5 ± 4.8 |
2.1 ± 0.9 |
| Segments of Spinal Cord (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| DRG Subdomains |
C | Th | L | S | ||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| P | 15.1 ± 2.7 |
19.4 ± 2.1 |
12.1 ± 2.3 |
11.2 ± 1.8 |
10.7 ± 0.7 |
11.3 ± 2.0 |
11.3 ± 2.1 |
10.6 ± 1.3 |
| Cr | 20.9 ± 1.7 |
18.7 ± 1.2 |
20.4 ± 1.7 |
22.1 ± 1.6 |
20.1 ± 1.4 |
18.5 ± 3.8 |
20.4 ± 1.9 |
21.3 ± 4.2 |
| Cd | 17.3 ± 3.2 |
16.6 ± 1.5 |
17.4 ± 1.9 |
19.1 ± 2.4 |
19.2 ± 3.5 |
19.5 ± 1.9 |
18.1 ± 1.4 |
19.4 ± 4.1 |
| Cn | 24.2 ± 5.0 |
25.1 ± 3.9 |
26.1 ± 4.5 |
26.3 ± 1.8 |
27.9 ± 5.7 |
28.8 ± 4.5 |
31.1 ± 6.0 |
26.1 ± 6.7 |
| Md | 22.5 ± 1.5 |
20.2 ± 2.1 |
24.0 ± 1.3 |
21.3 ± 0.9 |
22.1 ± 5.8 |
21.9 ± 2.1 |
19.1 ± 1.9 |
22.6 ± 4.4 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 57.1 ± 21.4 | 71.5 ± 4.9 | 73.3 ± 11.4 | 75.1 ± 6.7 |
| M | 42.10 ± 21.7 | 27.0 ± 4.9 | 26.1 ± 11.9 | 21.3 ± 3.2 |
| Lg | 0.8 ± 0.7 | 1.5 ± 0.6 | 0.6 ± 0.6 | 3.6 ± 3.6 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 53.4 ± 25.1 | 59.8 ± 11.2 | 67.3 ± 13.4 | 67.7 ± 11.7 |
| M | 45.9 ± 25.3 | 39.4 ± 11.8 | 31.2 ± 12.7 | 31.0 ± 11.0 |
| Lg | 0.7 ± 0.6 | 0.8 ± 0.6 | 1.5 ± 0.9 | 1.3 ± 0.7 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 38.9 ± 15.2 | 43.4 ± 9.3 | 51.0 ± 3.4 | 50.9 ± 4.8 |
| M | 58.6 ± 15.1 | 56.1 ± 9.0 | 47.1 ± 4.6 | 47.9 ± 5.0 |
| Lg | 2.5 ± 3.3 | 0.5 ± 0.3 | 1.9 ± 1.2 | 1.2 ± 1.1 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 99.3 ± 1.0 | 97.3 ± 3.3 | 99.7 ± 0.5 | 100.0 |
| M | 0.7 ± 1.0 | 2.7 ± 3.3 | 0.3 ± 0.5 | 0.0 |
| Lg | 0.0 | 0.0 | 0.0 | 0.0 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 80.3 ± 2.6 | 98.1 ± 3.3 | 98.9 ± 1.9 | 99.5 ± 0.8 |
| M | 17.2 ± 2.5 | 1.8 ± 3.1 | 1.0 ± 1.7 | 0.5 ± 0.8 |
| Lg | 2.5 ± 4.1 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.0 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
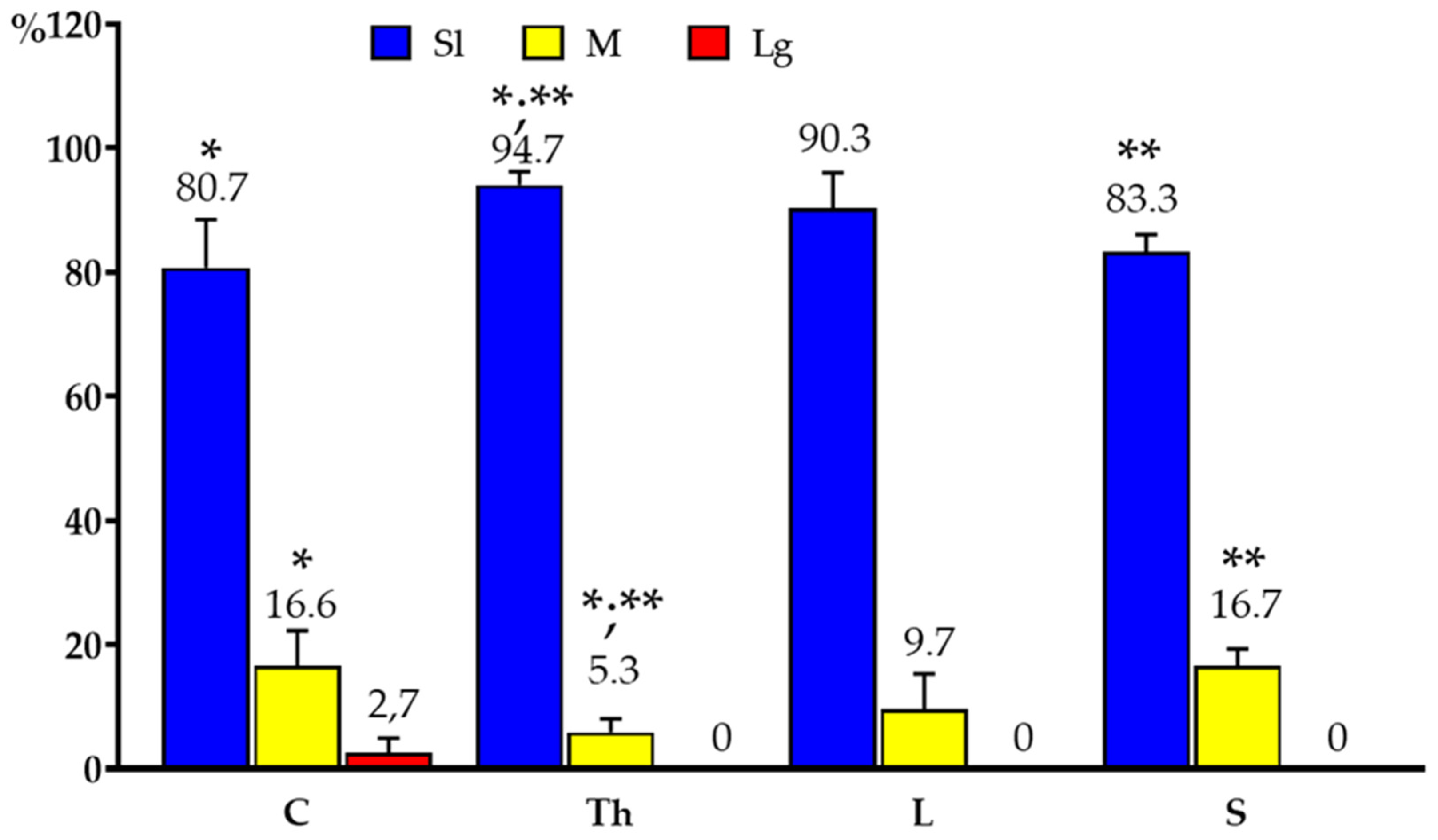
| Sl | 80.7 ± 7.7 * |
94.7 ± 2.1 *;** |
90.3 ± 5.6 |
83.3 ± 2.7 ** |
| M | 16.6 ± 5.6 * |
5.3 ± 2.1 *;** |
9.7 ± 5.6 | 16.7 ± 2.7 ** |
| Lg | 2.7 ± 2.3 | 0.0 | 0.0 | 0.0 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 20.4 ± 6.2 | 66.6 ± 2.8 | 41.1 ± 5.5 | 49.2 ± 2.7 |
| M | 79.6 ± 6.2 | 33.4 ± 2.8 | 56.4 ± 1.0 | 50.8 ± 2.7 |
| Lg | 0.0 | 0.0 | 2.5 ± 1.4 | 0.0 |
| Segments of the Spinal Cord (%) | ||||
|---|---|---|---|---|
| Size of DRG Cells | C | Th | L | S |
| Sl | 82.5 ± 2.1 | 81.9 ± 2.3 | 86.9 ± 4.5 | 89.6 ± 9.0 |
| M | 17.5 ± 2.1 | 17.7 ± 1.7 | 12.8 ± 2.0 | 10.4 ± 9.0 |
| Lg | 0.0 | 0.4 ± 0.5 | 0.3 ± 1.8 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





