Submitted:
03 July 2023
Posted:
04 July 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
1.1. Phosphorus Requirements of Fish Farmed in Open Flow Aquaculture Systems
1.2. The Environmental Impact of Aquaculture, with Emphasis on Open Flow Fish Farms
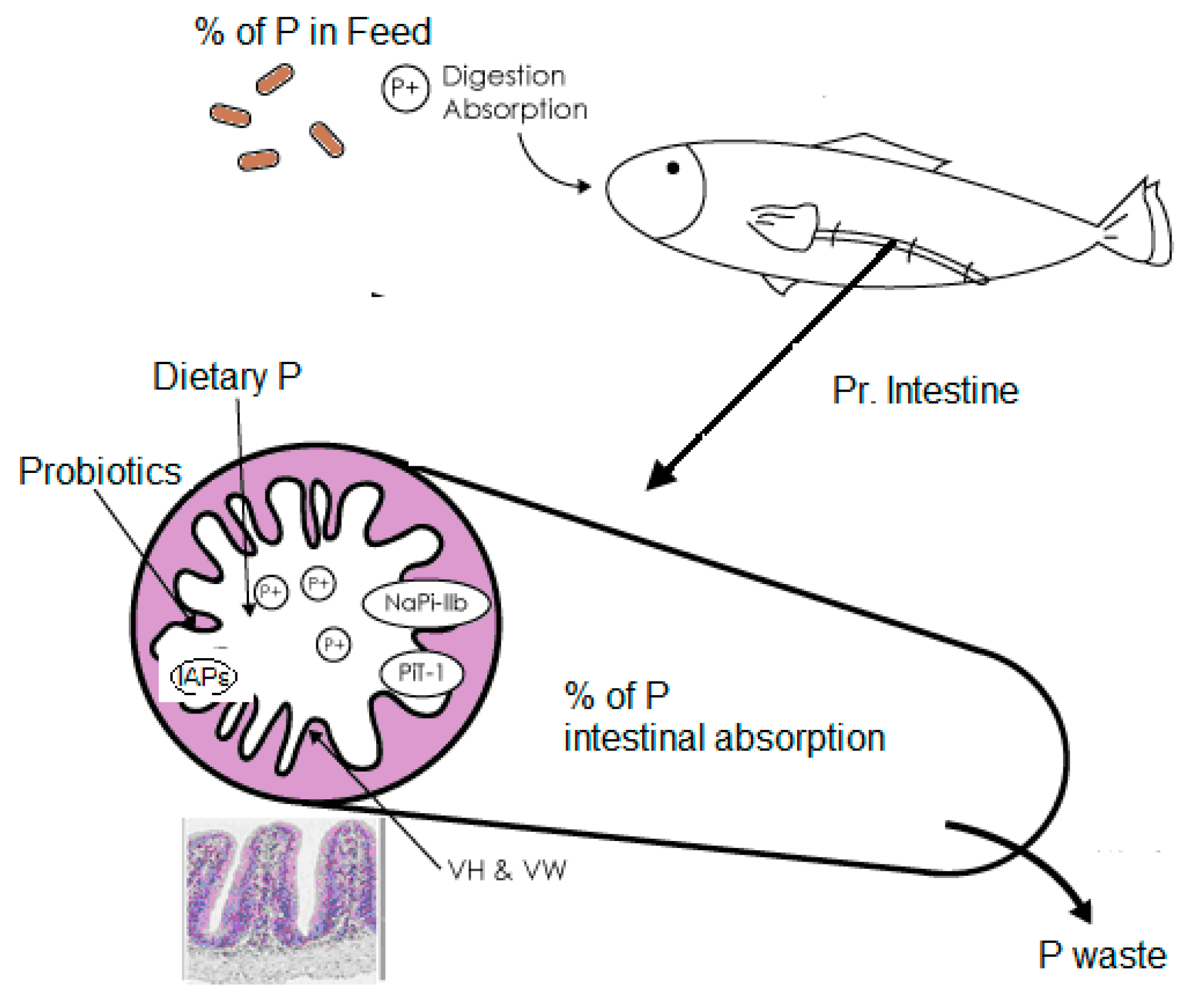
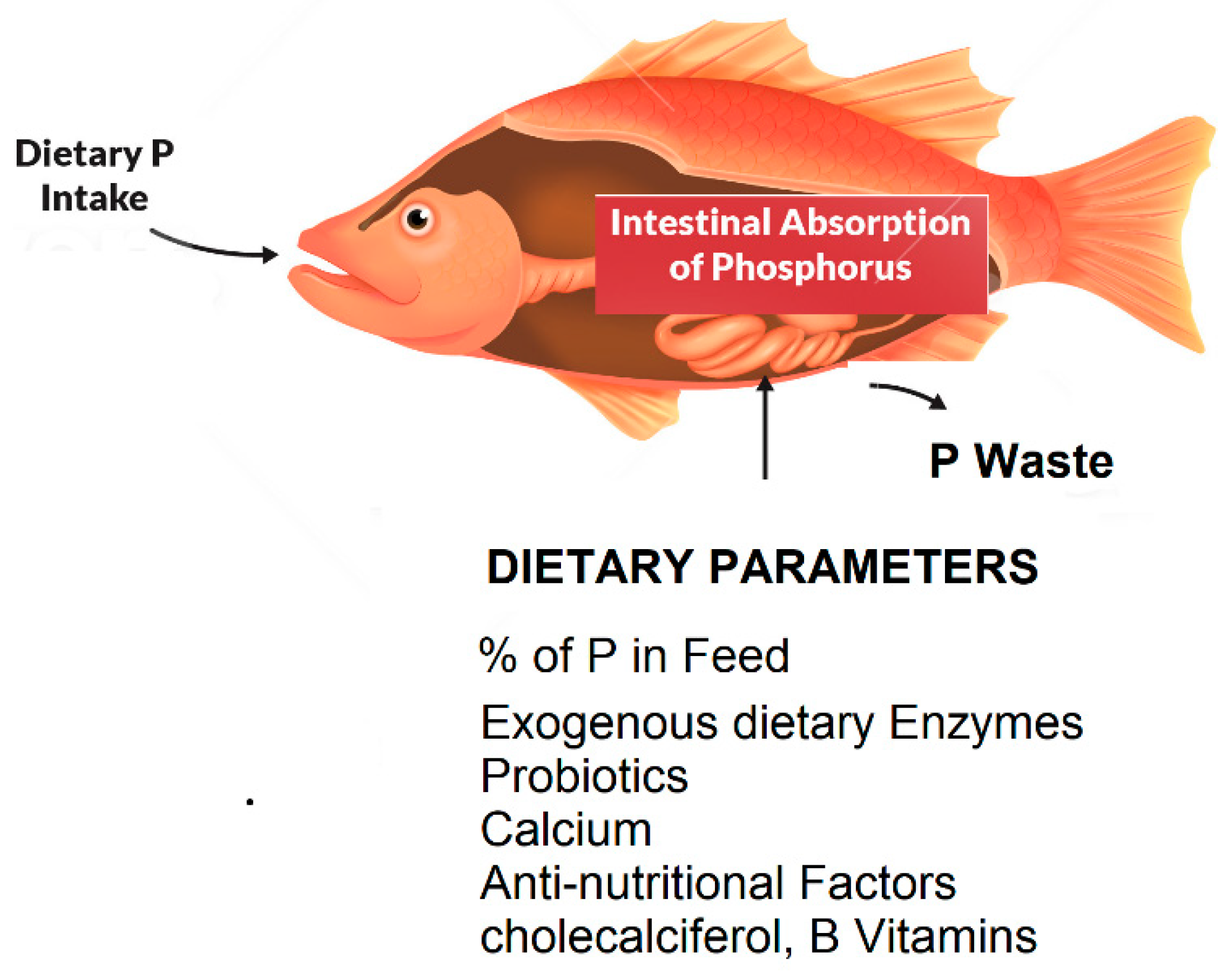
2. Morphological, Physiological, and Dietary Factors Affecting Phosphorous Absorption in Fish
2.1. Intestinal Morphology and Physiological Mechanisms
2.2. Relationship between Fish gut Structure and Feed Conversion Efficiency
2.3. The Role of Fish Feeds in Nutrient Uptake with Emphasis on Phosphorous
2.4. Effect of Probiotics on the Environmental Impact of Freshwater Fish Farms:
2.5. The Modulatory Effect of Temperature and Metabolism on Intestinal Absorption of Phosphorus in Farmed Fish
3. Current and Potential strategies for reducing phosphorus pollution of FW fish farms
3.1. Phosphorus Waste Reduction Initiatives
3.2. Management Strategies for Reducing Phosphorous Pollution from Aquacultures
3.3. The role of Probiotics
4. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Aas, T.S.; Terjesen, B.F.; Sigholt, T.; Hillestad, M.; Holm, J.; Refstie, S. ; ... & Åsgård, T. Nutritional responses in rainbow trout (Oncorhynchus mykiss) fed diets with different physical qualities at stable or variable environmental conditions. Aquaculture Nutrition 2011, 17, 657–670. [Google Scholar]
- Adeoye, A.A.; Yomla, R.; Jaramillo-Torres, A.; Rodiles, A.; Merrifield, D.L.; Davies, S.J. Combined effects of exogenous enzymes and probiotic on Nile tilapia (Oreochromis niloticus) growth, intestinal morphology and microbiome. Aquaculture 2016, 463, 61–70. [Google Scholar] [CrossRef]
- Adeshina, I.; Akpoilih, B.U.; Udom, B.F.; Adeniyi, O.V.; Abdel-Tawwab, M. Interactive effects of dietary phosphorus and microbial phytase on growth performance, intestinal morphometry, and welfare of Nile tilapia (Oreochromis niloticus) fed on low-fishmeal diets. Aquaculture 2023, 563, 738995. [Google Scholar]
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environmental management 2019, 63, 159–172. [Google Scholar]
- Ai, F.; Wang, L.; Li, J.; Xu, Q. Effects of a-ketoglutarate (AKG) supplementation in low phosphorous diets on the growth, phosphorus metabolism and skeletal development of juvenile mirror carp (Cyprinus carpio). Aquaculture 2019, 507, 393–401. [Google Scholar]
- Ai, Q.; Mai, K.; Zhang, W.; Xu, W.; Tan, B.; Zhang, C.; Li, H. Effects of exogenous enzymes (phytase, non-starch polysaccharide enzyme) in diets on growth, feed utilization, nitrogen and phosphorus excretion of Japanese seabass, Lateolabrax japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2007, 147, 502–508. [Google Scholar]
- Alfeus, A.; Gabriel, N.N. Applications of Aquatic Plants in the Remediation of Aquaculture Wastewater: An Opportunity for African Aquaculture. In Emerging Sustainable Aquaculture Innovations in Africa (pp. 327-339). Singapore: Springer Nature Singapore.
- Amoah 2023, K.; Huang, Q.C.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y. .. & Yang, Y.Z. Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 2020, 518, 734563. [Google Scholar]
- Aslaksen, M.A.; Kraugerud, O.F.; Penn, M.; Svihus, B.; Denstadli, V.; Jørgensen, H.Y. ;... & Storebakken, T. Screening of nutrient digestibilities and intestinal pathologies in Atlantic salmon, Salmo salar, fed diets with legumes, oilseeds, or cereals. Aquaculture 2007, 272, 541–555. [Google Scholar]
- Avila, E.M.; Basantes, S.P.; Ferraris, R.P. Cholecalciferol modulates plasma phosphate but not plasma vitamin D levels and intestinal phosphate absorption in rainbow trout (Oncorhynchus mykiss). General and comparative endocrinology 1999, 114, 460–469. [Google Scholar] [PubMed]
- Avila, E.M.; Tu, H.; Basantes, S.; Ferraris, R.P. Dietary phosphorus regulates intestinal transport and plasma concentrations of phosphate in rainbow trout. Journal of Comparative Physiology B 2000, 170, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A.M.; Glover, C.; Krogdahl, Å. Feeding, digestion and absorption of nutrients. In Fish physiology (Vol. 30 2010, pp. 57-110). Academic Press.
- Bare, W.R.; Struhs, E.; Mirkouei, A.; Overturf, K.; Small, B. Engineered Biomaterials for Reducing Phosphorus and Nitrogen Levels from Downstream Water of Aquaculture Facilities. Processes 2023, 11, 1029. [Google Scholar] [CrossRef]
- Behera, B.K. Nutritional Biotechnology to Augment Aquaculture Production. In: Pandey 2021, P.K.; Parhi, J. (eds) Advances in Fisheries Biotechnology. Springer, Singapore. [CrossRef]
- Bergheim, A.; Brinker, A. Effluent treatment for flow through systems and European environmental regulations. Aquacultural Engineering 2003, 27, 61–77. [Google Scholar] [CrossRef]
- Beveridge, M.C.M.; Brummett, R.E. Aquaculture and the environment. In Freshwater Fisheries Ecology; John Wiley & Sons 2015, Ltd.: Chichester, UK, 2015; pp. 794–803. ISBN 9781118394380. [Google Scholar]
- Brownlie, W.J.; Sutton, M.A.; Reay, D.S.; Heal, K.V.; Hermann, L.; Kabbe, C.; Spears, B.M. Global actions for a sustainable phosphorus future. Nature Food 2021, 2, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Bureau, D.P.; Cho, C.Y. Phosphorus utilization by rainbow trout (Oncorhynchus mykiss): estimation of dissolved phosphorus waste output. Aquaculture 1999, 179, 127–140. [Google Scholar] [CrossRef]
- Cámara-Ruiz, M.; Balebona, M.C.; Moriñigo, M.Á.; Esteban, M.Á. Probiotic Shewanella putrefaciens (SpPdp11) as a fish health modulator: a review. Microorganisms 2020, 8, 1990. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A. Positive responses of benthic macroinvertebrates to spatial and temporal reductions in water pollution downstream from a trout farm outlet. Knowledge & Management of Aquatic Ecosystems 2019, 16. [Google Scholar]
- Cao, L.; Yang, Y.; Wang, W.M.; Yakupitiyage, A.; Yuan, D.R.; Diana, J.S. Effects of pretreatment with microbial phytase on phosphorous utilization and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture Nutrition 2008, 14, 99–109. [Google Scholar]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. The American journal of clinical nutrition 1992, 55, 299S–308S. [Google Scholar] [CrossRef]
- Chanpaisaeng, K.; Teer1apornpuntakit, J.; Wongdee, K.; Charoenphandhu, N. Emerging roles of calcium-sensing receptor in the local regulation of intestinal transport of ions and calcium. American Journal of Physiology-Cell Physiology 2021, 320, C270–C278. [Google Scholar]
- Chapman, D. (Ed.) Water quality assessments: a guide to the use of biota, sediments and water in environmental monitoring. (2nd ed). World Health Organization 1996, UNESCO & United Nations Environment Programme. London: E & FN Spon. Available online: https://apps.who.int/iris/handle/10665/41850.
- Chatvijitkul, S.; Boyd, C.E.; Davis, D.A. Nitrogen, phosphorus, and carbon concentrations in some common aquaculture feeds. Journal of the World Aquaculture Society 2018, 49, 477–483. [Google Scholar] [CrossRef]
- 26. Chen, P., Bakke et al., 2010, Q., & Wang, C. Characterizing and evaluating the expression of the type IIb sodium-dependent phosphate cotransporter (slc34a2) gene and its potential influence on phosphorus utilization efficiency in yellow catfish (Pelteobagrus fulvidraco). Fish physiology and biochemistry 2016, 42, 51–64.
- Cho, C.Y.; Bureau, D.P. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquaculture research 2001, 32, 349–360. [Google Scholar]
- Choi, Y.S.; Hong, S.W.; Kim, S.J.; Chung, I.H. Development of a biological process for livestock wastewater treatment using a technique for predominant outgrowth of Bacillus species. Water Science and Technology 2002, 45, 71–78. [Google Scholar] [PubMed]
- Coloso, R.M.; King, K.; Fletcher, J.W.; Hendrix, M.A.; Subramanyam, M.; Weis, P.; Ferraris, R.P. Phosphorus utilization in rainbow trout (Oncorhynchus mykiss) fed practical diets and its consequences on effluent phosphorus levels. Aquaculture 2003, 220, 801–820. [Google Scholar] [CrossRef]
- Dai, Y.S.; Pei, W.L.; Wang, Y.Y.; Wang, Z.; Zhuo, M.Q. Topology, tissue distribution, and transcriptional level of SLC34s in response to Pi and pH in grass carp Ctenopharyngodon idella. Fish Physiology and Biochemistry 2021, 47, 1383–1393. [Google Scholar]
- Debnath, S.; Roy, S.; Saikia, S.K. Absorption of Macronutrients in Teleost. Current Approaches in Science and Technology Research 2021, 6, 62–68. [Google Scholar]
- Denstadli, V.; Skrede, A.; Krogdahl, Å.; Sahlstrøm, S.; Storebakken, T. Feed intake, growth, feed conversion, digestibility, enzyme activities and intestinal structure in Atlantic salmon (Salmo salar L.) fed graded levels of phytic acid. Aquaculture 2006, 256, 365–376. [Google Scholar] [CrossRef]
- Dias, J.; Santigosa, E. Maximising performance and phosphorus utilisation of warm water fish through phytase supplementation. Aquaculture 2023, 569, 739360. [Google Scholar]
- Dumas, A.; De Lange, C.F.; France, J.; Bureau, D.P. Quantitative description of body composition and rates of nutrient deposition in rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 273, 165–181. [Google Scholar]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O. ;... & Eltholth, M. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquaculture nutrition 2018, 24, 1613–1622. [Google Scholar]
- Encarnação, P. Functional feed additives in aquaculture feeds. In Aquafeed formulation (pp. 217-237). Academic Press.
- Fjelldal 2016, P.G.; Hansen, T.; Albrektsen, S. Inadequate phosphorus nutrition in juvenile Atlantic salmon has a negative effect on long-term bone health. Aquaculture 2012, 334, 117–123. [Google Scholar]
- Fontagné, S.; Silva, N.; Bazin, D.; Ramos, A.; Aguirre, P.; Surget, A.; Abrantes, A.; Kaushik, S.J.; Power, D.M. Effects of dietary phosphorus and calcium level on growth and skeletal development in rainbow trout (Oncorhynchus mykiss) fry. Aquaculture 2009, 297, 141–150. [Google Scholar]
- Fraser, T.W.; Witten, P.E.; Albrektsen, S.; Breck, O.; Fontanillas, R.; Nankervis, L. ;..& Fjelldal, P.G. Phosphorus nutrition in farmed Atlantic salmon (Salmo salar): Life stage and temperature effects on bone pathologies. Aquaculture 2019, 511, 734246. [Google Scholar]
- Glencross, B.; Fracalossi, D.M.; Hua, K.; Izquierdo, M.; Ma, K.; Øverland, M. ;... & Yakupityage, A. Harvesting the benefits of nutritional research to address global challenges in the 21st century. Journal of the World Aquaculture Society 2023. [Google Scholar] [CrossRef]
- Hassaan 2023, M.S.; Nagar, A.G.E.; Salim, H.S.; Fitzsimmons, K.; El-Haroun, E.R. Nutritional mitigation of winter thermal stress in Nile tilapia by propolis-extract: Associated indicators of nutritional status, physiological responses and transcriptional response of delta-9-desaturase gene. Aquaculture 2019, 511, 734256. [Google Scholar]
- Herath, S.S.; Satoh, S. Environmental impact of phosphorus and nitrogen from aquaculture. In Feed and feeding practices in aquaculture. D.A. Davis (Ed.) 2015, Woodhead Publishing Series in Food Science, Technology and Nutrition, Woodhead Publishing, Oxford (2015), pp. 369-386. 0001. [Google Scholar] [CrossRef]
- Hernández, A.J.; Roman, D. Phosphorus and nitrogen utilization efficiency in rainbow trout (Oncorhynchus mykiss) fed diets with lupin (Lupinus albus) or soybean (Glycine max) meals as partial replacements to fish meal. Czech Journal of Animal Science 2016, 61, 67–74. [Google Scholar] [CrossRef]
- Hernando, N.; Wagner, C.A. Mechanisms and regulation of intestinal phosphate absorption. Comprehensive Physiology 2011, 8, 1065–1090. [Google Scholar]
- Hlaváč, D.; Adámek, Z.; Hartman, P.; Másílko, J. Effects of supplementary feeding in carp ponds on discharge water quality: a review. Aquaculture international 2014, 22, 299–320. [Google Scholar] [CrossRef]
- Hlaváč, D.; Anton-Pardo, M.; Másílko, J.; Hartman, P.; Regenda, J.; Vejsada, P. . & Adámek, Z. Supplementary feeding with thermally treated cereals in common carp (Cyprinus carpio L.) pond farming and its effects on water quality, nutrient budget and zooplankton and zoobenthos assemblages. Aquaculture international 2016, 24, 1681–1697. [Google Scholar]
- Hlordzi, V.; Kuebutornye, F.K.; Afriyie, G.; Abarike, E.D.; Lu, Y.; Chi, S.; Anokyewaa, M.A. The use of Bacillus species in maintenance of water quality in aquaculture: A review. Aquaculture Reports 2020, 18, 10050. [Google Scholar]
- Huang, C.L.; Gao, B.; Xu, S.; Huang, Y.; Yan, X.; Cui, S. Changing phosphorus metabolism of a global aquaculture city. Journal of Cleaner Production 2019, 225, 1118–1133. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Obi, C.C.; Ohale, P.E.; Ahmadi, S.; Onukwuli, O.D.; Nwabanne, J.T.; Białowiec, A. Modelling and optimisation of electrocoagulation/flocculation recovery of effluent from land-based aquaculture by artificial intelligence (AI) approaches. Environmental Science and Pollution Research 2023, 30, 70897–70917. [Google Scholar] [PubMed]
- Islam, S.M.; Rohani, M.F.; Shahjahan, M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquaculture Reports 2021, 21, 100800. [Google Scholar]
- Jahan, P.; Watanabe, T.; Satoh, S.; Kiron, V. A laboratory-based assessment of phosphorus and nitrogen loading from currently available commercial carp feeds. Fisheries science 2002, 68, 579–586. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M.Á. Administration of probiotics in the water in finfish aquaculture systems: a review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- James, G.; Das, B.C.; Jose, S.; VJ, R.K. Bacillus as an aquaculture friendly microbe. Aquaculture International 2021, 29, 323–353. [Google Scholar]
- Jin, J.; Chu, Z.; Ruan, R.; Liu, W.; Chen, X.; Li, C. Phosphorus Absorption and Excretion in Hybrid Sturgeon (Huso dauricus♀ X Acipenser schrenckii♂) Intubated with Different Ca/P Ratios. Fishes 2022, 7, 138. [Google Scholar] [CrossRef]
- Jóźwiakowski, K.; Czernaś, K.; Szczurowska, A. Preliminary results of studies on the purification of water in a pond using the SCD Probiotics technology. Ecohydrology & Hydrobiology 2009, 9, 307–312. [Google Scholar]
- Khairunisa, H.; Hasan, Z.; Herawati, H.; Lili, W. Effectiveness of Water Hyacinth (Eichhornia crassipes) and Water Spinach (Ipomoea aquatica) to Reduce Nitrate and Phosphate Concentrations in Cimulu River Water, Tasikmalaya City, Indonesia. Asian Journal of Fisheries and Aquatic Research 2022, 18, 1–11. [Google Scholar] [CrossRef]
- Kraft, C.E. Estimates of phosphorus and nitrogen cycling by fish using a bioenergetics approach. Canadian Journal of Fisheries and Aquatic Sciences 1992, 49, 2596–2604. [Google Scholar] [CrossRef]
- Kumar NJP, Srideepu K, Hanuma Reddy M, Siva Reddy K V Effect of water probiotics (Pro-W) on Litopenaeus vannamei culture ponds of Nellore, Andhra Pradesh. India. International Journal of Environmental Sciences. Int J Environ Sci 2016, 6, 846–850. Available online: http://www.ipublishing.co.in/ijesarticles/fourteen/articles/volsix/EIJES6079.
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021 2021, 11, 2711. [Google Scholar]
- Lallès, J.P. Intestinal alkaline phosphatase in the gastrointestinal tract of fish: biology, ontogeny, and environmental and nutritional modulation. Reviews in Aquaculture 2020, 12, 555–581. [Google Scholar]
- Liebert, F.; Portz, L. Nutrient utilization of Nile tilapia Oreochromis niloticus fed plant based low phosphorus diets supplemented with graded levels of different sources of microbial phytase. Aquaculture 2005, 248, 111–119. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Z.; Wang, C.; Li, D.; Bureau, D.P. A web-based combined nutritional model to precisely predict growth, feed requirement and waste output of gibel carp (Carassius auratus gibelio) in aquaculture operations. Aquaculture 2018, 492, 335–348. [Google Scholar]
- Liu, Q.; Wan, F.; Liu, Y.; Liu, R.; et al. , 2010, Q.; Wang, A.; Ye, C. Effects of dietary inorganic phosphorus levels on growth, liver and intestinal morphology and digestive enzymes in fingerling obscure puffer (Takifugu obscurus). Aquaculture 2021, 545, 737124. [Google Scholar] [CrossRef]
- Lock, E.J.; Waagbø, R.; Wendelaar Bonga, S.; Flik, G. The significance of vitamin D for fish: a review. Aquaculture nutrition 2010, 16, 100–116. [Google Scholar]
- Lu, H.; Adamek, M.; Jung, S.J.; Lee, K.J.; Kim, J.D. Effects of phytase supplementation on phosphorus utilization, growth performance, and bone mineralization in growing sturgeon (Acipenser schrenckii). Aquaculture 2013, 396-399, 16–22. [Google Scholar]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Reviews in Aquaculture 2020, 12, 640–663. [Google Scholar]
- Luo, G. Review of waste phosphorus from aquaculture: Source, removal and recovery. Rev Aquac 2023, 15, 1058–1082. [Google Scholar] [CrossRef]
- Maas, R.M.; Verdegem, M.C.; Debnath, S.; Marchal, L.; Schrama, J.W. Effect of enzymes (phytase and xylanase), probiotics (B. amyloliquefaciens) and their combination on growth performance and nutrient utilisation in Nile tilapia. Aquaculture 2021, 533, 736226. [Google Scholar]
- MacMillan, J.R.; Huddleston, T.; Woolley, M.; Fothergill, K. Best management practice development to minimize environmental impact from large flow-through trout farms. Aquaculture 2003, 226, 91–99. [Google Scholar] [CrossRef]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. International Scholarly Research Notices, 2012. Ibáñez, A.L.; OA, M.H.; HC, R.S. Use of probiotics in aquaculture. ISRN Microbiology 2012, 2012, 916845–916845. [Google Scholar] [PubMed]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.; Heinsbroek, L.T.; Schneider, O.; Blancheton, J.P. ;... & Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquacultural engineering 2010, 43, 83–93. [Google Scholar]
- Mavraganis, T.; Constantina, C.; Kolygas, M.; Vidalis, K.; Nathanailides, C. Environmental issues of Aquaculture development. Egyptian Journal of Aquatic Biology and Fisheries 2020, 24, 441–450. [Google Scholar] [CrossRef]
- Mavraganis, T.; Thorarensen, H.; Tsoumani, M.; Nathanailides, C. On the environmental impact of freshwater fish farms in Greece and in Iceland. Annual Research & Review in Biology 2017, 1–7. [Google Scholar]
- Mavraganis, T.; Tsoumani, M.; Kolygas, M.; Chatziefstathiou, M.; Nathanailides, C. Using seasonal variability of water quality parameters to assess the risk of aquatic pollution from rainbow trout fish farms in Greece. International Journal of Energy and Water Resources 2021, 5, 379–389. [Google Scholar] [CrossRef]
- Médale, F.; Boujard, T.; Vallée, F.; Blanc, D.; Mambrini, M.; Roem, A.; Kaushik, S.J. Voluntary feed intake, nitrogen and phosphorus losses in rainbow trout (Oncorhynchus mykiss) fed increasing dietary levels of soy protein concentrate. Aquatic living resources 1998, 11, 239–246. [Google Scholar] [CrossRef]
- Medeiros, L.; Nornberg, B.; Azevedo, R.; Cardoso, A.; Rosas, V.T.; Tesser, M.B. ; ... & Marins, L.F. Dietary addition of recombinant Bacillus subtilis expressing a fungal phytase increases phosphorus fixation in muscle of Pacific white shrimp Litopenaeus vannamei. Aquaculture International 2023, 1–14. [Google Scholar]
- Milián-Sorribes, M.C.; Tomás-Vidal, A.; Peñaranda, D.S.; Carpintero, L.; Mesa, J.S.; Dupuy, J. ;... & Martínez-Llorens, S. Estimation of phosphorus and nitrogen waste in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) diets including different inorganic phosphorus sources. Animals 2021, 11, 1700. [Google Scholar]
- Moraes, M.A.B.; Carmo, C.F.; Tabata, Y.A.; Vaz-dos-Santos, A.M.; Mercante, C.T.J. Environmental indicators in effluent assessment of rainbow trout (Oncorhynchus mykiss) reared in raceway system through phosphorus and nitrogen. Brazilian Journal of Biology 2016, 76, 1021–1028. [Google Scholar]
- Morales, G.A.; Azcuy, R.L.; Casaretto, M.E.; Márquez, L.; Hernández, A.J.; Gómez, F. ;... & Mereu, A. Effect of different inorganic phosphorus sources on growth performance, digestibility, retention efficiency and discharge of nutrients in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 568–574. [Google Scholar]
- Muhammetoglu, A.; Kocer, M.A.T.; Durmaz, S. Evaluation of different management scenarios for trout farm effluents using dynamic water quality modeling. Environmental Monitoring and Assessment 2022, 194, 312. [Google Scholar]
- Nakajima, M.; Sugiura, S. Effects of dietary NaCl on the in vivo apparent absorption of dietary nutrients determined in rainbow trout (Oncorhynchus mykiss). Aquaculture 2016, 460, 1–7. [Google Scholar] [CrossRef]
- Nathanailides, C.; Kolygas, M.; Choremi, K.; Mavraganis, T.; Gouva, E.; Vidalis, K.; Athanassopoulou, F. Probiotics have the potential to significantly mitigate the environmental impact of freshwater fish farms. Fishes 2021, 6, 76. [Google Scholar]
- Ndiaye, W.N.; Deschamps, M.H.; Comeau, Y.; Chowdhury, K.; Bunod, J.D.; Letourneau-Montminy, M.P.; Vandenberg, G. In situ chelation of phosphorus using microencapsulated aluminum and iron sulfate to bind intestinal phosphorus in rainbow trout (Oncorhynchus mykiss). Animal Feed Science and Technology 2020, 269, 114675. [Google Scholar] [CrossRef]
- Nimalan, N.; Sørensen, S.L.; Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Gancarčíková, S. ;... & Sørensen, M. Supplementation of lactic acid bacteria has positive effects on the mucosal health of Atlantic salmon (Salmo salar) fed soybean meal. Aquaculture Reports 2023, 28, 101461. [Google Scholar]
- Omar, S.S.; Anwar, A.Y.; El-Haroun, E.R.; Davies, S.J. Evaluation of protein enriched co-products originating from wheat fermentation in diets of common carp Cyprinus carpio to examine effects on growth response, mineral retention, haematological status and intestinal integrity. Aquaculture Nutrition 2021, 27, 1336–1351. [Google Scholar] [CrossRef]
- Perera, E.; Yúfera, M. Soybean meal and soy protein concentrate in early diet elicit different nutritional programming effects on juvenile zebrafish. Zebrafish 2016, 13, 61–69. [Google Scholar] [CrossRef]
- Preston, D.L.; Lamb, R.W. Effects of trout aquaculture on water chemistry of tropical montane streams in Ecuador. River Research and Applications 2021, 37, 1562–1566. [Google Scholar]
- Priya Virmani, I.; Pragya, Goswami, R.K.; Singh, B.; Sharma, J.G.; Giri, B. Role of microbial phytases in improving fish health. Reviews in Aquaculture 2023. [Google Scholar] [CrossRef]
- Rao 2023, V.A. Bioremediation technology to maintain healthy ecology in aquaculture ponds. Fishing Chimes. September 2002, 22, 39–42. [Google Scholar]
- Reda, R.M.; Selim, K.M. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquaculture International 2015, 23, 203–217. [Google Scholar] [CrossRef]
- Rico, A.; Vighi, M.; Van den Brink, P.J.; ter Horst, M.; Macken, A.; Lillicrap, A. ;... & Telfer, T.C. Use of models for the environmental risk assessment of veterinary medicines in European aquaculture: current situation and future perspectives. Reviews in Aquaculture 2019, 11, 969–988. [Google Scholar]
- Rimoldi, S.; Bossi, E.; Harpaz, S.; Cattaneo, A.G.; Bernardini, G.; Saroglia, M.; Terova, G. Intestinal B0AT1 (SLC6A19) and PEPT1 (SLC15A1) mRNA levels in European sea bass (Dicentrarchus labrax) reared in fresh water and fed fish and plant protein sources. Journal of nutritional science 2015, 4, e21. [Google Scholar] [CrossRef]
- Ringø, E.; Olsen, R.E.; Gifstad, T.Ø.; Dalmo, R.A.; Amlund, H.; Hemre, G.I.; Bakke, A.M. Prebiotics in aquaculture: a review. Aquaculture Nutrition 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Rosenthal, H. Fish farm effluents and their control in EC countries: Summary of a workshop. Journal of Applied Ichthyology. 1994, 10, 215–224. [Google Scholar] [CrossRef]
- Rossi Jr, W.; Allen, K.M.; Habte-Tsion, H.M.; Meesala, K.M. Supplementation of glycine, prebiotic, and nucleotides in soybean meal-based diets for largemouth bass (Micropterus salmoides): Effects on production performance, whole-body nutrient composition and retention, and intestinal histopathology. Aquaculture 2021, 532, 736031. [Google Scholar] [CrossRef]
- Sambraus, F.; Hansen, T.; Daae, B.S.; Thorsen, A.; Sandvik, R.; Stien, L.H. ;... & Fjelldal, P.G. Triploid Atlantic salmon Salmo salar have a higher dietary phosphorus requirement for bone mineralization during early development. Journal of Fish Biology 2020, 97, 137–147. [Google Scholar]
- Santigosa, E.; García-Meilán, I.; Valentin, J.M.; Pérez-Sánchez, J.; Médale, F.; Kaushik, S.; Gallardo, M.A. Modifications of intestinal nutrient absorption in response to dietary fish meal replacement by plant protein sources in sea bream (Sparus aurata) and rainbow trout (Onchorynchus mykiss). Aquaculture 2011, 317, 146–154. [Google Scholar] [CrossRef]
- Sarker, P.K. Microorganisms in Fish Feeds, Technological Innovations, and Key Strategies for Sustainable Aquaculture. Microorganisms 2023, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Sindilariu, P.D.; Schulz, C.; Reiter, R. Treatment of flow-through trout aquaculture effluents in a constructed wetland. Aquaculture 2007, 270, 92–104. [Google Scholar] [CrossRef]
- Standen, B.T.; Rawling, M.D.; Davies, S.J.; Castex, M.; Foey, A.; Gioacchini, G. ;... & Merrifield, D.L. Probiotic Pediococcus acidilactici modulates both localised intestinal-and peripheral-immunity in tilapia (Oreochromis niloticus). Fish & shellfish immunology 2013, 35, 1097–1104. [Google Scholar]
- Stevens, E.D.; Devlin, R.H. Gut size in GH-transgenic coho salmon is enhanced by both the GHtransgene and increased food intake. Journal of Fish Biology 2005, 66, 1633–1648. [Google Scholar] [CrossRef]
- Stojanović, K.; Živić, M.; Marković, Z.; Đorđević, J.; Jovanović, J.; Živić, I. How changes in water quality under the influence of land-based trout farms shape chemism of the recipient streams—case study from Serbia. Aquaculture International 2019, 27, 1625–1641. [Google Scholar] [CrossRef]
- Sugiura, S. Identification of intestinal phosphate transporters in fishes and shellfishes. Fisheries Science 2009, 75, 99–108. [Google Scholar] [CrossRef]
- Sugiura, S. Effects of dietary phosphorus restriction on phosphorus balance in rainbow trout Oncorhynchus mykiss. Aquaculture Science 2015, 63, 245–253. [Google Scholar]
- Sugiura, S.H. Phosphorus, aquaculture, and the environment. Reviews in Fisheries Science & Aquaculture 2018, 26, 515–521. [Google Scholar]
- Sugiura, S.H.; McDaniel, N.K.; Ferraris, R.P. In vivo fractional Pi absorption and NaPi-II mRNA expression in rainbow trout are upregulated by dietary P restriction. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 2003, 285, R770–R78. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Marchant, D.D.; Kelsey, K.; Wiggins, T.; Ferraris, R.P. Effluent profile of commercially used low-phosphorus fish feeds. Environmental pollution 2006, 140, 95–101. [Google Scholar] [CrossRef]
- Sugiura, S.H.; McDaniel, N.K.; Ferraris, R.P. In vivo fractional Pi absorption and NaPi-II mRNA expression in rainbow trout are upregulated by dietary P restriction. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 2003, 285, R770–R781. [Google Scholar] [CrossRef]
- Sunitha, K.; Padmavathi, P. Influence of Probiotics on Water Quality and Fish Yield in Fish Ponds. International Journal of Pure & Applied Sciences & Technology 2013, 19, 48–60. [Google Scholar]
- Tacon, A.G.; Forster, I.P. Aquafeeds and the environment: policy implications. Aquaculture 2003, 226, 181–189. [Google Scholar] [CrossRef]
- Tahar, A.; Kennedy, A.M.; Fitzgerald, R.D.; Clifford, E.; Rowan, N. Longitudinal evaluation of the impact of traditional rainbow trout farming on receiving water quality in Ireland. PeerJ 2018, 6, e5281. [Google Scholar] [CrossRef]
- Terova, G.; Robaina, L.; Izquierdo, M.; Cattaneo, A.; Molinari, S.; Bernardini, G.; Saroglia, M. PepT1 mRNA expression levels in sea bream (Sparus aurata) fed different plant protein sources. SpringerPlus 2013, 2, 1–14. [Google Scholar] [CrossRef]
- Thaib, A.; Handayani, L.; Hanum, A.; Nurhayati, N.; Syahputra, F. Evaluating the addition of starry triggerfish (Abalistes stellaris) bone charcoal as a feed supplement to the growth performance and intestinal villi length of nile tilapia (Oreochromis niloticus). Depik 2021, 10, 194–200. [Google Scholar] [CrossRef]
- Thurlow, C.M.; Williams, M.A.; Carrias, A.; Ran, C.; Newman, M.; Tweedie, J. ;... & Liles, M.R. Bacillus velezensis AP193 exerts probiotic effects in channel catfish (Ictalurus punctatus) and reduces aquaculture pond eutrophication. Aquaculture 2019, 503, 347–356. [Google Scholar]
- True, B.; Johnson, W.; Chen, S. Reducing phosphorus discharge from flow-through aquaculture I: facility and effluent characterization. Aquacultural Engineering 2004, 32, 129–144. [Google Scholar] [CrossRef]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: theory and applications. Aquacultural engineering 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Varol, M.; Balcı, M. Characteristics of effluents from trout farms and their impact on water quality and benthic algal assemblages of the receiving stream. Environmental Pollution 2020, 266, 115101. [Google Scholar] [CrossRef]
- Vélez-Calabria, G.; Peñaranda, D.S.; Jover-Cerdá, M.; Llorens, S.M.; Tomás-Vidal, A. Successful inclusion of high vegetable protein sources in feed for rainbow trout without decrement in intestinal health. Animals 2021, 11, 3577. [Google Scholar] [CrossRef]
- Venold, F.F.; Penn, M.H.; Krogdahl, Å.; Overturf, K. Severity of soybean meal induced distal intestinal inflammation, enterocyte proliferation rate, and fatty acid binding protein (Fabp2) level differ between strains of rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 364, 281–292. [Google Scholar] [CrossRef]
- Venold, F.F.; Penn, M.H.; Thorsen, J.; Gu, J.; Kortner, T.M.; Krogdahl, Å.; Bakke, A.M. Intestinal fatty acid binding protein (fabp2) in Atlantic salmon (Salmo salar): Localization and alteration of expression during development of diet induced enteritis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2013, 164, 229–240. [Google Scholar]
- Vidakovic, A.; Huyben, D.; Sundh, H.; Nyman, A.; Vielma, J.; Passoth, V. ;... & Lundh, T. Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquaculture Nutrition 2020, 26, 275–286. [Google Scholar]
- Vidakovic, A.; Huyben, D.; Sundh, H.; Nyman, A.; Vielma, J.; Passoth, V.; et al. Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquac. Nutr. 26 (2) 2020, 275–286. [Google Scholar] [CrossRef]
- Vidakovic, A.; Langeland, M.; Sundh, H.; Sundell, K.; Olstorpe, M.; Vielma, J.; et al. Evaluation of growth performance and intestinal barrier function in Arctic Charr (Salvelinus alpinus) fed yeast (Saccharomyces cerevisiae), fungi (Rhizopus oryzae) and blue mussel (Mytilus edulis). Aquac. Nutr. 22 (6) 2016, 1348–1360. [Google Scholar] [CrossRef]
- Vielma, J.; Lall, S.; Koskela, J.; Mattila, P. Influence of low dietary cholecalciferol intake on phosphorus and trace element metabolism by rainbow trout (Oncorhynchus mykiss, Walbaum). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 1999, 122, 117–125. [Google Scholar]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol 2006, 127, 283–292. [Google Scholar] [CrossRef]
- Wang, C.; Xu, S.; Jiang, C.; Peng, X.; Zhou, X.; Sun, Q. ;... & Zhuang, X. Improvement of the growth performance, intestinal health, and water quality in juvenile crucian carp (Carassius auratus gibelio) biofortified system with the bacteria-microalgae association. Aquaculture 2023, 562, 738848. [Google Scholar]
- Wang, L.; Fan, Z.; Wu, D.; Li, J.; Xu, Q.; Miao, L. ;... & Zheng, X. Effects of dietary α-ketoglutarate on the growth performance, digestive enzymes, tor signaling pathway and intestinal microbiota of juvenile mirror carp (Cyprinus carpio) fed low phosphorus diets. Aquaculture 2023, 739736. [Google Scholar]
- Wang, M.; Yi, M.; Lu, M.; Gao, F.; Liu, Z.; Huang, Q. ;... & Zhu, D. Effects of probiotics Bacillus cereus NY5 and Alcaligenes faecalis Y311 used as water additives on the microbiota and immune enzyme activities in three mucosal tissues in Nile tilapia Oreochromis niloticus reared in outdoor tanks. Aquaculture Reports 2020, 17, 100309. [Google Scholar]
- Wang, Y.; He, Z. Effect of probiotics on alkaline phosphatase activity and nutrient level in sediment of shrimp, Penaeus vannamei, ponds. Aquaculture 2009, 287, 94–97. [Google Scholar] [CrossRef]
- Webb, J.A. Effects of trout farms on stream macroinvertebrates: linking farm-scale disturbance to ecological impact. Aquaculture Environment Interactions 2012, 3, 23–32. [Google Scholar] [CrossRef]
- Wilfart, A.; Garcia-Launay, F.; Terrier, F.; Soudé, E.; Aguirre, P.; Skiba-Cassy, S. A step towards sustainable aquaculture: Multiobjective feed formulation reduces environmental impacts at feed and farm levels for rainbow trout. Aquaculture 2023, 562, 738826. [Google Scholar] [CrossRef]
- Aas, T.S.; Terjesen, B.F.; Sigholt, T.; Hillestad, M.; Holm, J.; Refstie, S. . & Åsgård, T. Nutritional responses in rainbow trout (Oncorhynchus mykiss) fed diets with different physical qualities at stable or variable environmental conditions. Aquaculture Nutrition 2011, 17, 657–670. [Google Scholar]
- Wu, P.; Xie, L.; Wu, X.; Wang, Y.; Wu, Y.; Li, N. ;... & Chen, Z. Effect of Rhodopseudomonas sphaeroides–Treated wastewater on yield, digestive enzymes, antioxidants, nonspecific immunity, and intestinal microbiota of common carp. North American Journal of Aquaculture 2019, 81, 385–398. [Google Scholar]
- Yang, Y.-H.; Wang, Y.-Y.; Lu, Y.; Li, Q.-Z. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion on rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2011, 19, 405–419. [Google Scholar] [CrossRef]
- Ye, C.X.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Yang, H.J.; Niu, J. Effect of dietary calcium and phosphorus on growth, feed efficiency, mineral content and body composition of juvenile grouper, Epinephelus coioides. Aquaculture 2006, 255, 263–271. [Google Scholar] [CrossRef]
- Yeganeh Rastekenari, H.; Kazami, R.; Shenavar Masouleh, A.; Banavreh, A.; Najjar Lashgari, S.; Sayed Hassani, M.H. ;... & Hallajian, ssA. Autochthonous probiotics Lactococcus lactis and Weissella confusa in the diet of fingerlings great sturgeon, Huso huso: effects on growth performance, feed efficiency, haematological parameters, immune status and intestinal morphology. Aquaculture Research 2021, 52, 3687–3695. [Google Scholar]
- Yi, M.; Wang, C.; Wang, H.; Zhu, X.; Liu, Z.; Gao, F.; Ke, X.; Cao, J.; Wang, M.; Liu, Y.; Lu, M. The in situ remediation of aquaculture water and sediment by commercial probiotics immobilized on different carriers. Water Reuse 2021, 11, 572–585. [Google Scholar] [CrossRef]
- Yigit, N.O.; Bahadir Koca, S.; Didinen, B.I.; Diler, I. Effect of protease and phytase supplementation on growth performance and nutrient digestibility of rainbow trout (Oncorhynchus mykiss, Walbaum) fed soybean meal-based diets. Journal of Applied Animal Research 2018, 46, 29–32. [Google Scholar] [CrossRef]
- Zheng, C.C.; Wu, J.W.; Jin, Z.H.; Ye, Z.F.; Yang, S.; Sun, Y.Q.; Fei, H. Exogenous enzymes as functional additives in finfish aquaculture. Aquaculture Nutrition 2020, 26, 213–224. [Google Scholar] [CrossRef]
- Zhou, Y.L.; He, G.L.; Jin, T.; Chen, Y.J.; Dai, F.Y.; Luo, L.; Lin, S.M. High dietary starch impairs intestinal health and microbiota of largemouth bass, Micropterus salmoides. Aquaculture 2021, 534, 736261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




