1. Introduction
In nanoscience, nanowhiskers are considered to be filamentous crystals with a transverse size of up to 100 nm and a length that is an order of magnitude or more greater than the transverse size. Semiconductor nanowhiskers are widely used today to create miniature elements of devices in microelectronics [
1,
2], optoelectronics [
3,
4], nanoengineering [
5,
6], solar energy [
7,
8,
9], biomedicine [
10], nanoelectromechanics [
11,
12] and gas sensing [
13,
14]. To date, there are various methods [
15,
16,
17] for obtaining nanowhiskers of a wide range of semiconductor materials, such as growth by molecular beam epitaxy, vapor deposition, laser ablation, growth catalysts, magnetron deposition, chemical epitaxy in high vacuum and others.
Carbon nanomaterials (fullerene, carbon nanotube and graphene) are becoming key components of nanotechnologies for the development of complex functional nanostructures. Light fullerenes (C
60/C
70) are a hollow sphere/ellipsoid carbon molecule less than 1 nm in diameter, with sp
2 carbon atoms located on a curved surface at the vertices of a truncated icosahedron. They have unique physical properties, in particular optical and electrical. One of the remarkable properties of fullerene molecules is their ability to self-assemble over time in pure solvents to form clusters of various shapes and sizes [
18,
19], and the nature of the solvent plays an important role in this process [
20]. Therefore, they have an excellent electron acceptor, high photosensitivity and high electron mobility [
21,
22]. The latter leads them to a range of applications, including photodetectors [
23], sensors [
24], solar cells [
25], LEDs [
26], and drug delivery [
27].
Since the discovery of C
60 fullerene nanowhiskers (C
60NWs) by the Miyazawa group in 2001 [
28,
29], they have found applications in various fields. A poor solvent is added to a saturated well-dissolved solution of C
60 and a liquid-liquid interface is formed in the middle. As a result, a supersaturated solution is formed, C
60 embryo crystals are nucleated at the liquid-liquid interface, and long C
60NWs are synthesized. Although this method was initially "static" (without external influence), later "dynamic" (ultrasound, manual mixing, etc. effects) and other modified methods were developed [
30,
31]. Similarly, C
70 fullerene nanowhiskers (C
70NWs) structures were synthesized on the basis of C
70 fullerene in the same ways [
32]. It is known that NWs formed on the basis of nanosized fullerenes are based on bottom-up technology. In this case, the regulation and control of the size and structure of the NWs is of great importance. In particular, when NWs synthesized in solution are transferred to the surface of a solid substrate, changes in their morphology occur. It should also be taken into account that the evaporation of droplets of fullerene solutions on the surface of a solid substrate leads to self-organization processes [
33,
34]. In this regard, there is a need to study the processes occurring in the volume of evaporation of droplets of fullerene solutions.
In this paper, we consider the synthesis of nanostructured C70 fullerene whiskers on the surface of a substrate by evaporating a microvolume drop of C70 solution. Experimental methods for controlling the geometric dimensions of the synthesized nanowhiskers are discussed.
2. Results
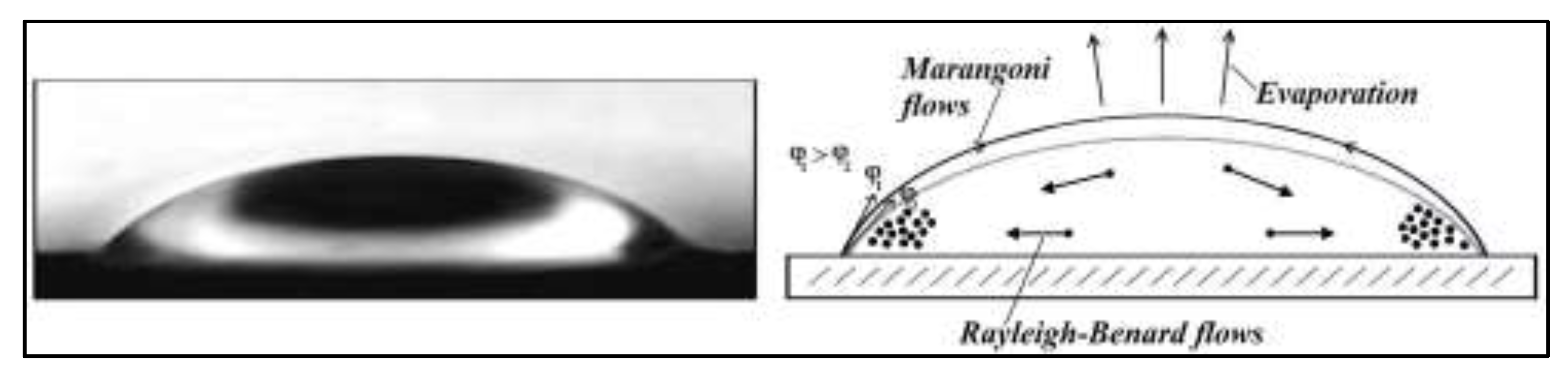
In our experiments, the shape of the initial drop of a fullerene solution with a volume of V≈40-50 μl on a wetted flat substrate is approximately described by a spherical cap (see
Figure 1, left). It can be noted that drops of a fullerene solution throughout the entire duration of thermal evaporation always retain a constant area of the base of the drop. But the contact angle (φ) of the drop gradually decreases until it disappears. The fullerene drop is protected from convective air flows until complete evaporation; the drop thermal evaporation direction is perpendicular on the surface of the spherical cap. Due to the Marangoni effect along the "droplet-air" interface and the Rayleigh-Benard effect along the evaporating droplet volume (
Figure 1, right), strong capillary flows appear and start the assembly of fullerene particles as well as the synthesis of different nanostructures based on them.
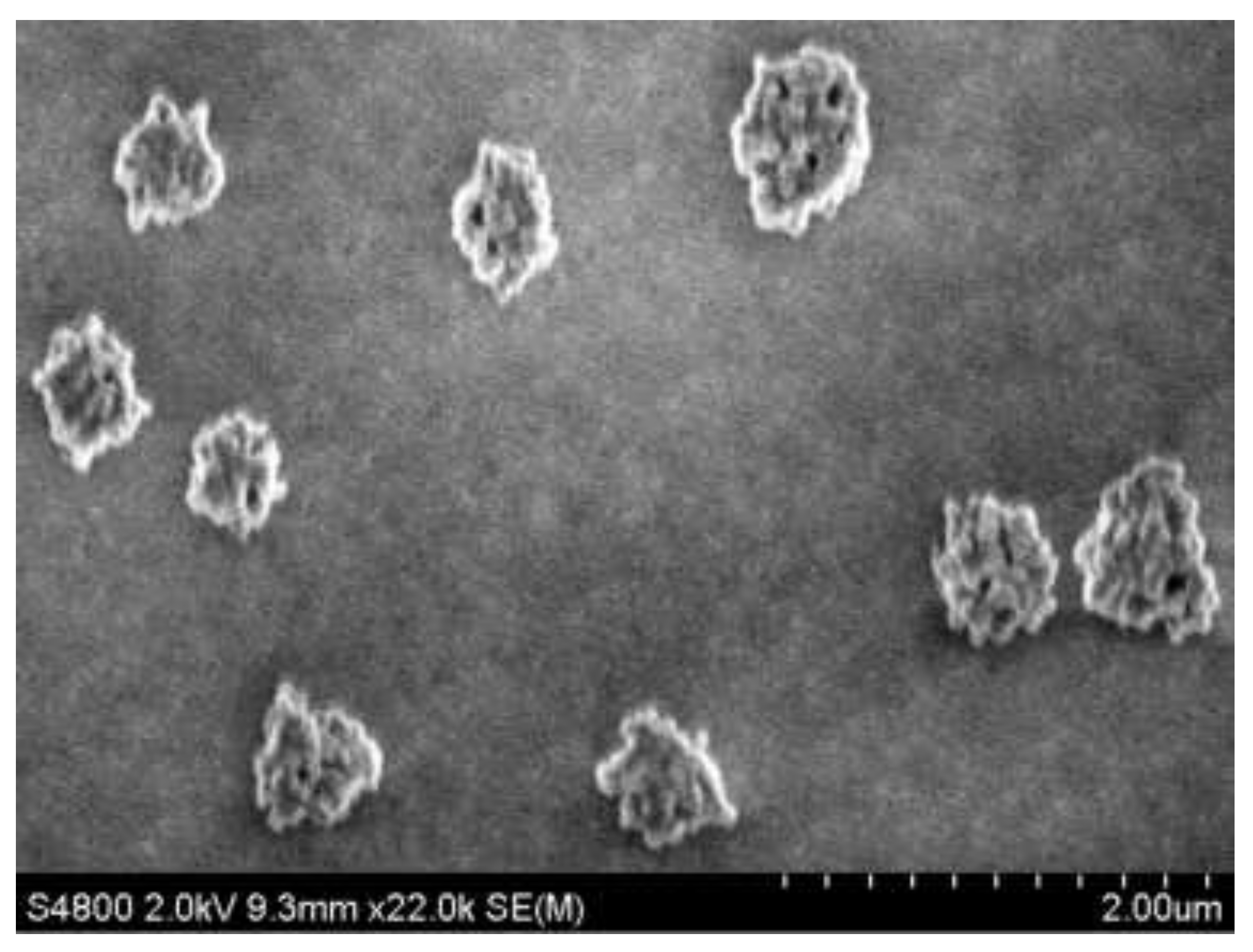
The SEM image of the structures formed during the evaporation of droplets of a C
70 solution in toluene on the substrate surface at room temperature (~24±1°С) is shown in
Figure 2. Due to the constant base area of the microdroplet, during the entire thermal evaporation of the solvent, a trace of C
70 nanostructures remains along the base of the drop, similar to a coffee ring. An important role is played by the temperature gradient that occurs when the surface and near-surface layers of the droplet cool sharply as a result of intense toluene evaporation. It can be seen that after the complete evaporation of toluene from a microdroplet of the C
70 solution, large quasi-spherical C
70 aggregates formed on the surface of the optical glass substrate. At the same time, the average geometric dimensions in the diameter of C
70 aggregates were ~600 nm. The resulting C
70 aggregates are porous and consist of discrete intermediate nanoaggregates with sizes up to ~40÷45 nm in diameter.
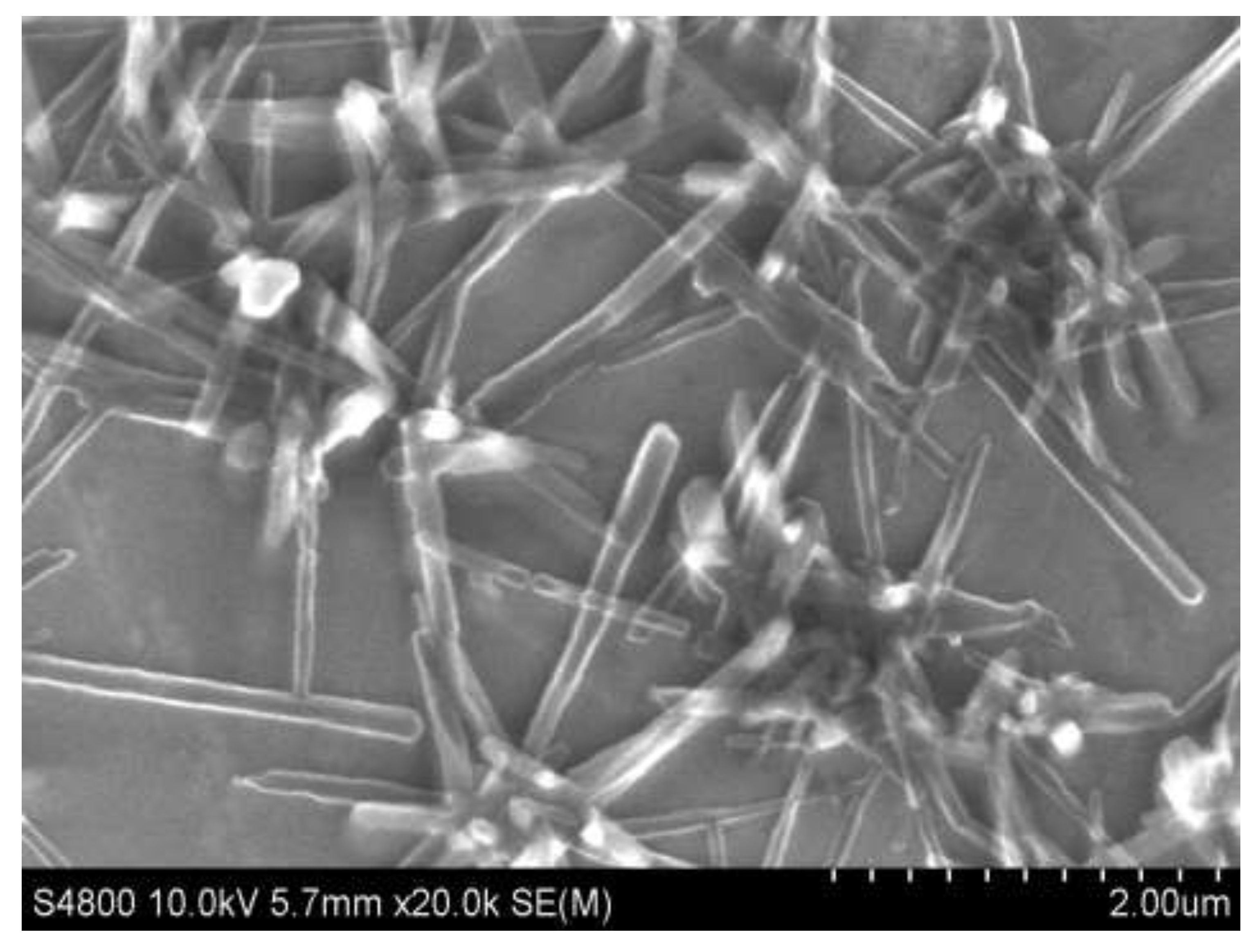
We study the process of evaporation of a C
70 solution droplet on the substrate surface at different substrate temperatures in order to synthesize one-dimensional C
70 structures. When the K-8 optical glass substrate was heated to 28°С, nanostructured filaments (nanoviskers) of C
70 fullerene of optimal shape were synthesized on the substrate surface (see
Figure 3). In this case the concentration of fullerene C
70 in the initial drop of the solution was ~1.1⋅10
-3 mol⋅L
-1.
In this case, the temperature gradient in the process of intensive evaporation of the solvent from a microdroplet at a temperature of 28°C makes it possible to overcome some of the energy difficulties in the formation of C
70NWs. We can observe X- and V-shaped C
70NWs were mainly synthesized in the volume of an evaporating drop of C
70 molecular solution on a substrate (see
Figure 3)). The average geometric dimensions of C
70NWs are ~105 nm in width and ~750 nm in length. At the same time we can observe the maximum length and width of the resulting C
70NWs reached the values ~1.7 µm and ~200 nm, respectively.
SEM-image of C
70NWs synthesized on a surface of a horizontally located glass substrate, heated to T=36°С presented in
Figure 4. In experiments with fixed concentration of C
70 (~1.1⋅10
-3 mol⋅L
-1) in a drop of the working solution, the effect of increasing the temperature of the substrate on the ongoing processes of the evaporation drop was studied. It was established that an increase in the substrate temperature not only led to a more accelerated nucleation and growth of C
70NWs, but also to a noticeable increase in the final geometric dimensions of the synthesized C
70NWs. Wherein, the distribution of C
70NWs on the substrate surface is getting denser. At the same time the average length and width of the resulting C
70NWs reached the values ~1.8 µm and ~175 nm, respectively. The presented results proved that the size of nanowhiskers can be controlled by changing the substrate temperature at a fixed concentration of C
70 in the working drop.
Under the same conditions, we studied the effect of the initial concentration on the size of the synthesized nanoparticles.
Figure 5 presents SEM-image of nanostructured whiskers of C
70 fullerene synthesized on the smooth surface of a substrate heated to T≈36°C. An increase in the fullerene concentration (up to ~1.5⋅10
-3 mol⋅L
-1) in the initial droplet led to a noticeable increase in the final C
70NW size. It is easy to observe that the longest C
70NWs has a size of ~28 micrometers in length, ~2 micrometers in width, as well as the shortest length and width are ~6 micrometers and ~200÷250 nm, respectively (
Figure 5). So it was shown that the geometric dimensions of the C
70NWs can be controlled by changing the initial concentration of the fullerene solution.
The experimental results reflecting the change in the geometric dimensions of the synthesized C
70NWs at fixed concentration of C
70 fullerene with different substrate temperatures presented in
Table 1.
3. Discussion
We presented an experimental method for the synthesis of cost-effective and compatible C70NWs in the volume of an evaporating droplet on a substrate. Our electron microscopic measurements confirm the formation of one dimensional C70NWs during the evaporation of a drop on the surface of a substrate heated from 28°C. It was found that changing both the concentration of fullerene in the initial drop and the substrate temperature provides an opportunity to tune the geometric dimensions of C70NWs to the desired value.
At a fixed concentration of C70 (~1.1⋅10-3 mol⋅L-1) in an initial drop, change in the substrate temperature from T1=28°C to T2=36°C led to a noticeable increase in the final geometric dimensions of the synthesized C70NWs. In this case, the ratio of average length (~1.35 µm) to width (~152 nm) of the synthesized C70NWs is about 9:1. At a fixed substrate temperature (T=36°C) with a relatively high concentration of fullerene (~1.5⋅10-3 mol⋅L-1) C70NWs with the largest length and width of ~28 μm and ~2 μm, respectively, were synthesized. It was shown that the method used is effective for the synthesis of micro- and nano-sized whiskers, which can be used for various purposes of the "bottom-up" technology.
4. Materials and Methods
In our experiments we used the high purity (~99.8%) powders of fullerene C70 (Sigma-Aldrich, USA) as well as organic solvent – toluene (C6H5CH3, Sigma-Aldrich, USA). The mixture of “toluene+C70 powders”, located in a hermetically sealed glass flask, was dissolved by continuous mechanical stirring at a frequency of ~1.5 Hz for 1.5 hours using a programmable laboratory magnetic stirrer of the MS-11H brand, WIGO, (Poland). Thereafter, the C70 solution was sonicated for 15 min using an ultrasonic bath brand DC-120H. Further, dosed drops of the C70 molecular solution were taken using a VITLAB dosing pipette (VITLAB GmbH, Germany).
Standard K-8 optical glass with a surface roughness of ≤7 nm was used as a substrate. Before each experiment, the surface of the used glass substrate was plasma cleaned at a nano level using a Plasma Cleaner device (Harrick Plasma, «PDC-002», USA).
We used a high-resolution scanning electron microscope (hereinafter SEM) brand JSM-IT200 (Joel, Japan) to establish the morphological features and determine the exact geometrical sizes of one-dimensional C70NWs.
5. Conclusions
For the first time an evaporating drop method for synthesis of nanostructured C70NWs based on the self-organization of C70 molecules during thermal evaporation of toluene from C70 droplets located on the surface of a flat glass substrate has been proposed and implemented. The optimal substrate temperature for the start of the synthesis of C70 fullerene nanowhiskers in the volume of droplet evaporation was experimentally established. It was shown that the geometric dimensions of the synthesized C70NWs can be controlled both by changing the C70 concentration in the initial droplet and by changing the temperature of the substrate used. A selective synthesis of fullerene nanowhiskers was carried out. The results of this work can be used to predict and control the geometric dimensions of nanostructured whiskers of various kinds, which will have great potential in applications such as nano- and microelectronics, solar cells, nonlinear optics, sensors, and electromechanics.
Author Contributions
Conceptualization, U.K.M.; methodology, U.K.M.; investigation, U.K.M. and B.A.A.; writing—original draft preparation, U.K.M.; writing—review and editing, S.A.B. and U.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the Fund for Basic Research of the Academy of Sciences of Uzbekistan: "Investigation of the physical regularities of the self-organization processes of organic nanoscale materials in liquid systems".
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ogawa, K.; Kato, T.; Ikegami, A.; Tsuji, H.; Aoki, N.; Ochiai, Y.; Bird, J.P. Electrical properties of field-effect transistors based on C60 nanowhiskers. Appl. Phys. Lett. 2006, 88, 1–3. [Google Scholar] [CrossRef]
- Larsen, C.; Barzegar, H.R.; Nitze, F.; Wagberg, T.; Edman, L. On the fabrication of crystalline C60 nanorod transistors from solution. J. Nanotechnol. 2012, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joyce, H.J.; Gao, Q.; Tan, H.H.; Jagadish, C.; Kim, Y.; Zou, J.; Smith, L.M.; Jackson, H.E.; Yarrison-Rice, J.M.; Parkinson, P.; Johnston, M.B. III–V semiconductor nanowires for optoelectronic device applications. Prog. Quantum. Electron. 2011, 35, 23–75. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H.; Bocchetta, P. Polymer/Fullerene Nanocomposite for Optoelectronics-Moving toward Green Technology. J. Compos. Sci. 2011, 6, 1–15. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis and properties of fullerene nanowhiskers and fullerene nanotubes. J. Nanosci. Nanotechnol. 2009, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Salhi, B.; Hossain, M.; Mukhaimer, A.; Al-Sulaiman, F.A. Nanowires: a new pathway to nanotechnology-based applications. J. Electroceram. 2016, 37, 34–49. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef]
- Zhang, G.; Finefrock, S.; Liang, D.; Yadav, G.G.; Yang, H.; Fang, H.; Wu, Y. Semiconductor nanostructure-based photovoltaic solar cells. Nanoscale 2011, 3, 2430–2443. [Google Scholar] [CrossRef]
- Sun, K.; Kargar, A.; Park, N.; Madsen, K.N.; Naughton, P.W.; Bright, T.; Jing, Y.; Wang, D. Compound Semiconductor Nanowire Solar Cells. IEEE J. Sel. Top. Quantum Electron 2011, 17, 1033–1049. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Nudejima, Sh.; Takaku, S.; Kanehira, K.; Sonezaki, Sh.; Taniguchi, A. Effects of fullerene nanowhiskers on cytotoxicity and gene expression. Health 2010, 2, 1456–1459. [Google Scholar] [CrossRef]
- Akiyama, T. Development of Fullerene Thin-Film Assemblies and Fullerene-Diamine Adducts towards Practical Nanocarbon-Based Electronic Materials. Bull. Chem. Soc. Jpn. 2019, 92, 1181–1199. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties. Sci. Technol. Adv. Mater. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Polymeric nanocomposites reinforced with nanowhiskers: Design, development, and emerging applications. J. Plast. Film Sheeting. 2020, 36, 1–22. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, W.H. Carbon Nanotubes as Active Components for Gas Sensors. J. Sens. 2009, 2009, 1–16. [Google Scholar] [CrossRef]
- Naumova, O.V.; Nastaushev, Yu.V.; Svitasheva, S.N.; Sokolov, L.V.; Zakharov, N.D.; Werner, P.; Gavrilova, T.A.; Dultsev, F.N.; Aseev, A.L. Molecular-beam epitaxy-grown Si whisker structures: morphological, optical and electrical properties. Nanotechnol. 2008, 19, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Dubrovskii, V.G.; Sibirev, N.V.; Cirlin, G.E.; Sartel, C.; Tchernycheva, M.; Harmand, J.C.; Glas, F. Growth of Inclined GaAs Nanowires by Molecular Beam Epitaxy: Theory and Experiment. Nanoscale Res. Lett. 2010, 5, 1692–1697. [Google Scholar] [CrossRef]
- Xia, M.; Guo, H.Y.; Hussain, M.I. Controllable Combustion Synthesis of SiC Nanowhiskers in a Si-C-N System: The Role of the Catalyst. Appl. Sci. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.; Hill, J.P.; Ariga, K. Solvent Engineering for Shape-Shifter Pure Fullerene (C60). J. Am. Chem. Soc. 2009, 131, 6372–6373. [Google Scholar] [CrossRef]
- Makhmanov, U.K.; Kokhkharov, A.M.; Bakhramov, S.A.; Erts, D. The formation of self-assembled structures of C60 in solution and in the volume of an evaporating drop of a colloidal solution. Lith. J. Phys. 2020, 60, 194–204. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Fullerenes in molecular liquids. Solutions in “good” solvents: Another view. J. Mol. Liq. 2011; 161, 1–12. [Google Scholar]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Itaka, K.; Yamashiro, M.; Yamaguchi, J.; Haemori, M.; Yaginuma, S.; Matsumoto, Y.; Kondo, M.; Koinuma, H. High-Mobility C60 Field-Effect Transistors Fabricated on Molecular-Wetting Controlled Substrates. Adv. Mater. 2006, 18, 1713–1716. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, G.; Jiang, B.; Ji, D.; Kong, H.; Riehemann, K.; Ji, Q.; Fuchs, H. Self-assembled fullerene (C60)-pentacene superstructures for photodetectors. SmartMat. 2021, 2, 109–118. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Hierarchically Structured Fullerene C70 Cube for Sensing Volatile Aromatic Solvent Vapors. ACS Nano. 2016, 10, 6631–6637. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.K.; Kar, S.; Leszczynski, J. “Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells. Mater. 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grebinyka, A.; Grebinyk, S.; Prylutska, S.; Rittere, U.; Matyshevska, O.; Dandekar, T.; Frohm, M. C60 fullerene accumulation in human leukemic cells and perspectives of LED-mediated photodynamic therapy. Free Radic. Biol. Med. 2018, 124, 319–327. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today. 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Miyazawa, K.I.; Obayashi, A.; Kuwabara, M. C60 nanowhiskers in a mixture of lead zirconate titanate sol–C60 toluene solution. J. Am. Ceram. Soc. 2001, 84, 3037–3039. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties. Sci. Technol. Adv. Mater. 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Hotta, K.; Miyazawa, K. Synthesis and growth investigation of C60 fullerene nanowhiskers. J. Phys. Conf. Ser. 2009, 159, 1–5. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tachibana, M.; Kojima, K. Photo-assisted growth of C60 nanowhiskers from solution. J. Cryst. Growth 2005, 274, 617–621. [Google Scholar] [CrossRef]
- Miyazawa, K. C70 Nanowhiskers Fabricated by Forming Liquid/Liquid Interfaces in the Systems of Toluene Solution of C70 and Isopropyl Alcohol. J. Am. Ceram. Soc. 2002, 85, 1297–1299. [Google Scholar] [CrossRef]
- Bakhramov, S.A.; Makhmanov, U.K.; Kokhkharov, A.M. Synthesis of nanoscale fullerene C60 filaments in the volume of an evaporating drop of a molecular solution and preparation of thin nanostructured coatings on their basis. Appl. Sol. Energy 2019, 55, 309–314. [Google Scholar] [CrossRef]
- Makhmanov, U.K.; Kokhkharov, A.M.; Bakhramov, S.A.; Esanov, S.A. Synthesis of fullerene C60 nanotubes in the volume of an evaporating drop of colloidal solution. Rom. J. Phys. 2022, 67, 601–609. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).