1. Introduction
Heart transplantation (HT) is the main treatment option for patients with end-stage heart failure, providing significant improvements in survival and quality of life. However, HT patients are at risk of developing various complications during their follow-up. The common complications include early allograft failure, acute graft rejection (AGR), coronary allograft vasculopathy (CAV), renal failure, infections, and cancer. Therefore, it is crucial to establish an effective follow-up protocol for HT patients right from the early post-transplant stages.
Current guidelines recommend several examinations to monitor HT patients. Invasive procedures such as coronary angiography (ICA) and endomyocardial biopsy (EBM) are considered the gold standard for evaluating CAV and AGR, respectively. However, the role of non-invasive methods like echocardiography, stress echocardiography, computed tomography (CT), positron emission tomography (PET), and cardiac magnetic resonance imaging (CMR) in monitoring HT patients is not well-established.
The guidelines do not provide specific recommendations regarding the timing of echocardiographic evaluations, and CMR is not recommended as an alternative to serial EMB for rejection monitoring. ICA is still recommended as the gold standard, typically performed one year after surgery and subsequently every other year. It should be noted that no alternative imaging-based strategy or biomarkers have been endorsed as substitutes for EMB in graft rejection monitoring.
However, it is important to recognize that invasive procedures like ICA and EMB carry the risk of complications, including contrast-related kidney injury and procedure-related lesions. Therefore, there is a need to explore non-invasive approaches that can provide valuable information while minimizing the potential risks associated with invasive procedures. Further research and clinical evidence are required to establish the role of non-invasive imaging modalities and biomarkers in the long-term follow-up of HT patients, ensuring a comprehensive and safe monitoring strategy. Therefore, in the present paper, we will discuss all the advancements in non-invasive and contrast-saving imaging techniques which have been advocated and investigated over the past few years, to identify those that may have the potential to gate invasive procedures such as ICA and EBM, weighing their advantages and disadvantages [
1,
4].
2. Echocardiographic follow-up in transplant recipients.
The ISHLT Guidelines for Heart Transplant Recipients do not recommend echocardiography as a primary method for rejection monitoring due to certain limitations specific to HT patients [
5]. One major limitation is the increased variability of echocardiographic parameters in HT patients compared to the general population. This variability makes it challenging to establish definitive "normal" parameters for transplanted hearts and determine cut-off values for detecting allograft rejection [
7].
In the absence of established cut-off values, the comparison of echocardiographic parameters from follow-up examinations with previous measurements becomes more valuable than relying on absolute values. This approach allows for the detection of changes over time, which may indicate potential graft rejection [
7].
The ESC guidelines recommend a comprehensive echocardiographic study as a baseline evaluation six months after cardiac transplantation [
1]. Subsequent follow-up studies should be compared to the data obtained from the six-month study. Although there is no single systolic or diastolic parameter that can reliably diagnose graft rejection, a follow-up echocardiographic study that shows no change from the baseline study has a high negative predictive value for rejection [
4]. When a deterioration is observed in a specific parameter, it is important to carefully review previous studies to assess the pattern of change over time. Additionally, the presence of multiple abnormalities detected on echocardiography increases the likelihood of graft rejection [
4].
While echocardiography has limitations in detecting rejection, it still plays a role in the comprehensive evaluation of HT patients. By carefully assessing changes over time and considering multiple abnormalities, echocardiography can contribute to the overall monitoring and management of HT recipients.
In heart transplant recipients, the typical echocardiographic assessment procedure includes both two-dimensional (2D) imaging and spectral/color Doppler imaging. During these examinations, it is essential to determine the dimensions of the four cardiac chambers and major blood vessels, evaluate the functioning of the left ventricle (LV) and right ventricle (RV), examine the performance of heart valves, measure the systolic pulmonary artery pressure (sPAP), and describe the condition of the pericardium. If there are any irregularities or issues with the graft’s structure or functioning, supplementary views and data acquisitions are necessary [
1].
LV ejection fraction (EF), which is typically within the normal range after heart transplantation (HT), is not an early indicator of graft dysfunction and does not necessarily correlate with the severity of rejection detected through endomyocardial biopsy (EMB) [
8]. Additionally, LV EF is not a sensitive marker for acute graft rejection (GR) [
9].
In contrast, changes in LV diastolic function are more sensitive in detecting acute GR compared to the reduction in LV EF. Diastolic function in HT patients follows a bimodal pattern [
10]. Despite otherwise successful surgeries, HT recipients often experience a decrease in functional capacity [
11]. Diastolic dysfunction is a common occurrence in the early stages of transplantation due to factors such as hypervolemia, disparity in heart and body size between the donor and recipient, the impact of organ ischemia, and early rejection [
12]. During the initial month after HT, fluid accumulation tends to occur due to systemic inflammatory response and the administration of high doses of corticosteroids. Therefore, elevated filling pressures during this period may be indicative of volume status [
10]. Subsequently, diastolic dysfunction is primarily attributed to episodes of rejection, hypertension, and myocardial ischemia caused by cardiac allograft vasculopathy [
10].
The evaluation of diastolic function has extensively been explored by Doppler indices of mitral inflow. However, the assessment of LV filling is influenced by various factors, including preload conditions, atrial dynamics, morphology (such as dissociation between recipient and donor atrial contractions), LV compliance and contractility, end-systolic volume, and heart rate. Consequently, impaired diastolic function can stem from different causes and is not specific to rejection episodes. Additionally, the elevated heart rate commonly observed in denervated hearts further complicates the assessment of diastolic function as it frequently leads to the merging of E and A waves [
1].
Several studies have investigated the potential of tissue Doppler imaging (TDI) parameters in predicting acute GR. Constant TDI velocities, such as a 10% change in e’ compared to baseline, and high TDI velocities (e.g., e’ > 16 cm/s), appear to have good accuracy in excluding acute GR rather than detecting it, with a negative predictive value of 92%. However, these parameters still require further validation [
1].
To reliably determine elevated pulmonary capillary wedge pressure (PCWP), it is necessary to observe positive results in at least three out of the five parameters: E/A ratio, deceleration time (DT), isovolumic relaxation time (IVRT), E/e’ lateral ratio, and systolic pulmonary artery pressure (sPAP). While the individual sensitivity of these parameters is relatively low, the probability of elevated PCWP is unlikely if none of the five parameters meet the defined cutoff values [
10].
The myocardial performance index (MPI) has been suggested as an early indicator of rejection in heart transplant (HT) patients, considering that rejection affects both diastolic and systolic function simultaneously. However, the accuracy of MPI in detecting acute graft rejection remains a topic of debate. In a study conducted by Tona et al. [
13], the role of MPI as a marker for long-term allograft dysfunction was evaluated in 154 patients. The findings revealed a gradual increase in MPI over the course of long-term follow-up in HT patients with preserved left ventricular (LV) systolic function. Higher MPI values were observed in patients with multiple episodes of rejection, although no correlation was found with the development of cardiac allograft vasculopathy (CAV).
3. Echocardiography: what new technologies add.
Strain echocardiography has shown greater sensitivity and accuracy compared to conventional echocardiography. This becomes particularly important in the early stages of acute cellular rejection (ACR), where standard echocardiography may not detect certain pathological changes such as pericardial effusion, wall thickening, or increased LV mass [
14]. However, strain echocardiography can identify features like myocardial edema or fibrosis, which often affect the sub-endocardial muscle fibers and lead to a decline in longitudinal graft function [
15,
16].
In the context of heart transplantation, a reduced LV global longitudinal strain (GLS) shortly after transplantation or the absence of improvement in LVGLS between 2 weeks and 3 months post-transplantation has been linked to poor outcomes [
17,
18]. Therefore, evaluating LVGLS can assist in risk stratification during the critical early period following heart transplantation, complementing traditional monitoring methods such as right-heart catheterization and endomyocardial biopsy (EMB). It’s worth noting that some heart transplant patients may exhibit reduced LVGLS despite having a normal or slightly reduced LV ejection fraction (LVEF) [
18].
The left atrium (LA) and right atrium (RA) have crucial roles in regulating ventricular filling through their reservoir, conduit, and contractile functions in cardiac physiology. In HT recipients, atrial function is influenced not only by ventricular dysfunction but also by surgical factors. In the standard technique, both atria are enlarged as they are connected in the middle portion using a combination of recipient and donor tissue. With the bicaval method, the RA is reconstructed using donor tissue only, while the LA consists of a mixture of donor and recipient tissue, with the extent of residual recipient tissue varying based on the surgical approach. In most cases, the LA roof, including the pulmonary vein ostia and the tissue in between, remains intact [
19]. Comparatively, the atria are typically smaller in size when the bicaval method is used compared to the standard technique [
20].
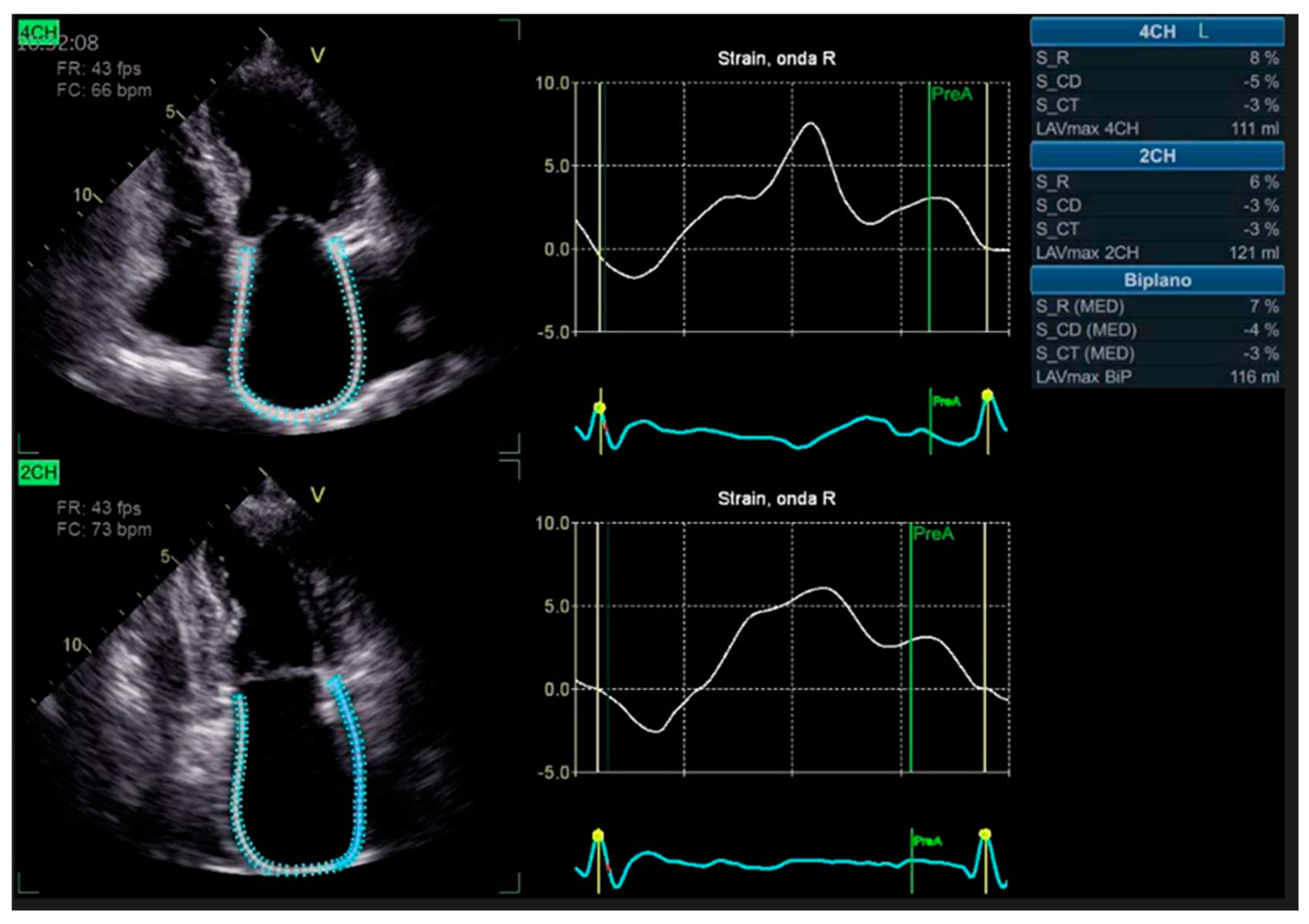
Advanced strain echocardiography can be employed to evaluate atrial function in HT recipients. Bech-Hanssen et al. [
21] demonstrated a significant reduction in atrial reservoir function, assessed using speckle tracking, in HT recipients due to elevated pulmonary capillary wedge pressure (PCWP) and LA enlargement in the LA, as well as impaired longitudinal RV function in the RA. The reduction in atrial reservoir function is most pronounced in the presence of elevated filling pressures [
22]. Zhu et al. observed alterations in LA function throughout all phases of the cardiac cycle, independent of the surgical technique employed. Peak LA strain was found to be associated with worse left ventricular systolic function, suggesting the potential importance of LA function in HT patients [
20] [
Figure 1].
4. Assessment of right ventricle and tricuspid valve.
While mitral and aortic valve abnormalities are not commonly observed in HT patients, tricuspid regurgitation (TR) is quite prevalent. In the early phases after HT, TR can be attributed to elevated pulmonary artery pressure and resistance in the recipient, and it typically resolves within a year following the surgery [
23]. However, if TR persists beyond this period, it is associated with right ventricular (RV) failure and increased mortality [
24].
Early RV dilation is frequently observed in HT patients but tends to resolve within a few weeks [
25]. However, persistent RV failure is a well-recognized cause of mortality. Therefore, even when RV systolic function appears to be within the normal range, more subtle RV dysfunction can be detected through RV strain analysis, providing insights into the overall RV performance [
26].
5. Stress echocardiography.
Stress echocardiography is a commonly used functional imaging test in which the most frequently used stressor is Dobutamine [
21]. However, due to the diminished heart rate response to exercise resulting from the cardiac denervation state in HT patients, exercise protocols have limited sensitivity (15-33%) [
21]. Several factors support the role of stress echocardiography in the diagnosis of inducible ischemia in HT patients. Coronary artery disease in these patients is often diffuse, making the myocardium more vulnerable to demand ischemia. Additionally, there is impaired coronary collateral circulation. From a technical perspective, patients with cardiac denervation may exhibit an exaggerated chronotropic response to Dobutamine infusion [
22].
Numerous studies have evaluated the ability of dobutamine stress echocardiography (DSE) to detect inducible ischemia compared to ICA. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of DSE in diagnosing cardiac allograft vasculopathy (CAV) range from 63% to 95%, 55% to 95%, 40% to 92%, and 62% to 92%, respectively. These values vary depending on the angiographic definitions of CAV used, which can range from any angiographic abnormalities to 50% stenosis [
22,
27]. Data regarding the sensitivity and NPV of DSE to detect any stage of CAV are inconsistent, with a study by Eroglu et al. showing a relatively modest sensitivity of 63% [
27]. These discrepancies may be explained by the timing of DSE after HT. DSE is not recommended for routine surveillance of CAV beyond 5 years post-HT due to the high prevalence of the disease and the low NPV of DSE [
28]. Furthermore, DSE results do not provide additional prognostic utility in HT patients.
Integration of novel echocardiographic parameters, such as strain imaging, with DSE has shown to be more accurate than visual assessment alone in diagnosing coronary artery disease in non-transplant populations [
28,
29]. Further studies utilizing these techniques may potentially enhance the sensitivity of DSE in detecting early-stage CAV and provide incremental prognostic value. Evaluation of coronary flow reserve (CFR) to assess the presence of microvascular disease is not routinely performed in HT patients, despite encouraging evidence from previous studies [
30,
31]. Reduced CFR has also been shown to be associated with major cardiovascular events in HT patients [
30]. In fact, HT patients with normal systolic function and no evidence of CAV may still exhibit coronary microvascular impairment due to structural remodeling and loss of vasodilatory capacity in the microvasculature [
31].
6. The role of computed tomography angiography in the follow up of heart transplant recipients.
Cardiac computed tomography angiography (CCTA) is emerging as a non-invasive diagnostic tool in the follow-up of heart transplant (HT) recipients, offering high sensitivity and negative predictive values in detecting coronary artery disease (CAD) [
32]. Several studies have demonstrated that CCTA has lower costs, shorter examination time, reduced radiation exposure, and lower risk of vascular complications compared to ICA [
33]. Additionally, last generation CCTA machines has good accuracy in analyzing more distal coronary segments compared to coronary angiography [
33]. However, achieving a low heart rate is necessary to obtain optimal diagnostic accuracy with CCTA. Guidelines recommend a resting heart rate of less than 60 bpm for ideal image quality [
5]. HT patients often have an elevated resting heart rate due to cardiac denervation, resulting in the loss of vagal rate regulation. Dual-source CT (DSCT) or multisegment reconstruction (MSR) techniques enable good image quality at low radiation dosages, even at higher heart rates (>75 bpm) [
34]. Interestingly, one study showed that CT images acquired in HT patients at an average heart rate of 74 bpm were still superior to those obtained in non-HT patients at 73 bpm. This is attributed to the denervation of the transplanted heart, which leads to an absence of heart rate variation. Further studies have confirmed that a regular heart rate is associated with better image quality [
36].
Advancements in CCTA imaging, such as fractional flow reserve derived from CT (FFR-CT), have been studied for the HT population. CT-derived FFR can identify early CAV and analyze all coronary artery branches [
37]. FFR-CT evaluations have demonstrated the ability to predict mortality and the risk of re-transplantation [
38]. The use of CT in the evaluation of CAV has been included in the guidelines for the management of HT patients as a Class IIb recommendation (Level of Evidence: C) [
39]. However, limited data are available on the role of CCTA in the follow-up of HT patients. One study conducted by Rohnean et al. reported slow progression of CAV over a 5-year follow-up period as detected by qualitative CCTA assessment [
40]. The time to significant stenosis was longer than 3 years among the 62 HT patients evaluated, leading to a recommendation of a 2-year interval between follow-up studies in patients with a normal baseline CT. The anatomical and histopathological characteristics of CAV differ from those of atherosclerotic disease. CAV typically affects small, distal vessels, while luminal narrowing of larger epicardial coronaries occurs only in advanced stages. Unlike the eccentric and focal lesions observed in atherosclerotic disease, CAV manifests as concentric intimal hyperplasia without positive wall remodeling, making it easily overlooked by an inexperienced examiner [
41].
The integration of intravascular ultrasound (IVUS) with coronary angiography reveals approximately 19% more cases of CAV, making it the most sensitive test available for monitoring CAV progression in the clinical setting [
42]. However, CCTA has been proposed as an alternative to IVUS for routine follow-up of HT patients due to its superior spatial resolution [
43]. In a recent meta-analysis, the sensitivity, specificity, positive predictive value, and negative predictive value of CT for detecting CAV were reported as 97%, 81%, 78%, and 97%, respectively [
44]. Nevertheless, there is currently no standard method available for the assessment of CAV on coronary CTA images, and distinguishing CAV from atherosclerotic lesions can be challenging [
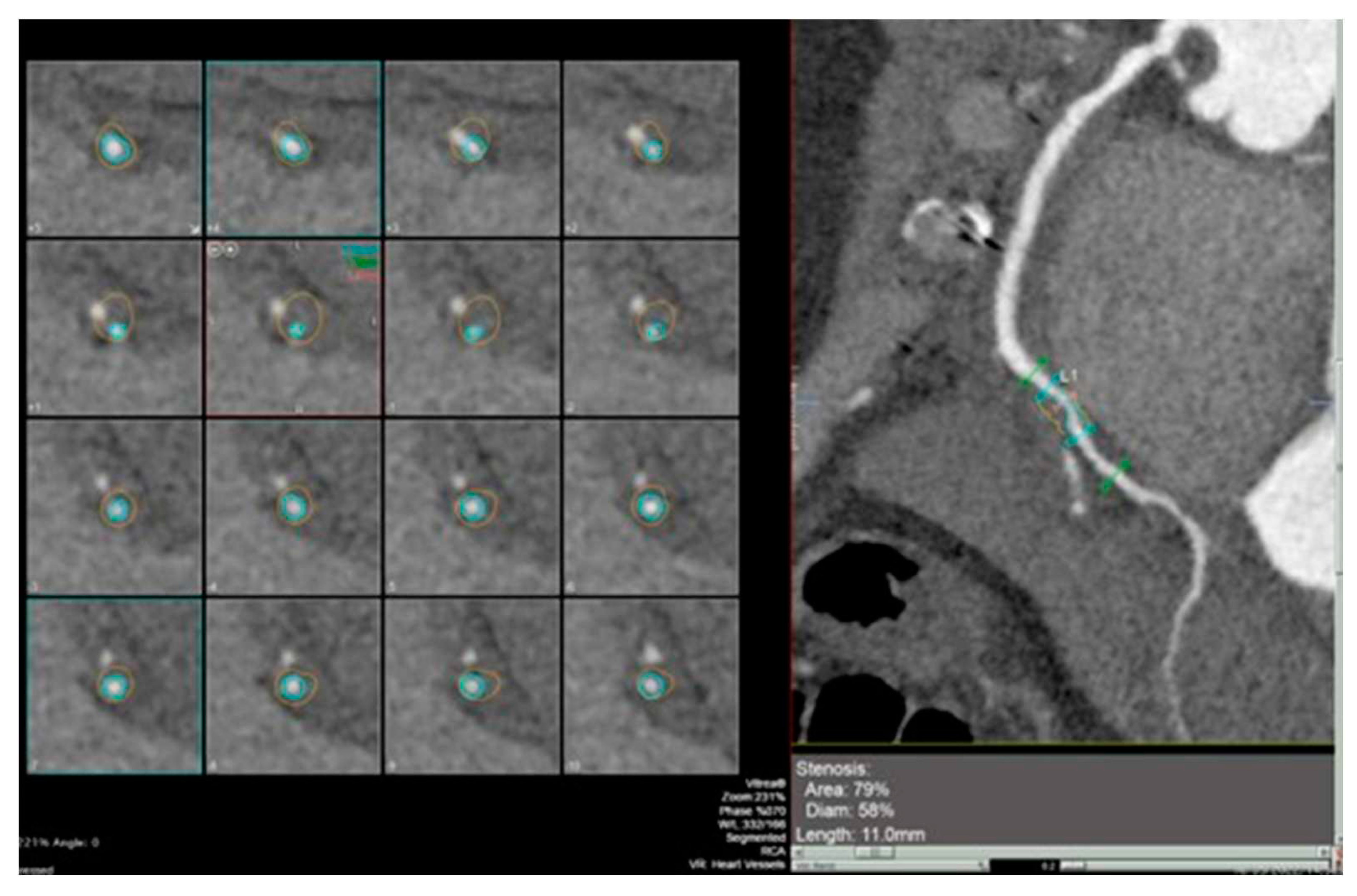
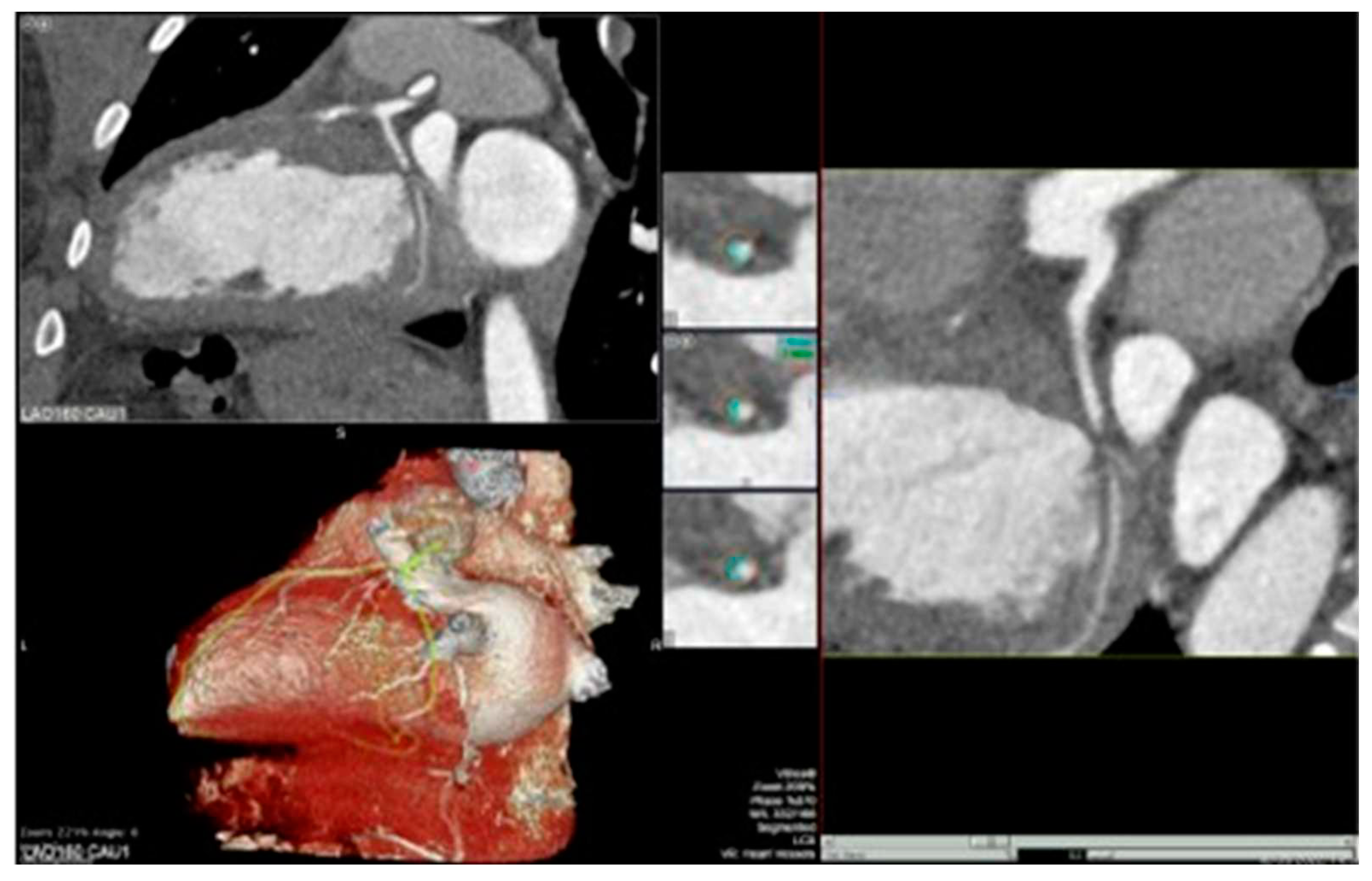
Figure 2 and
Figure 3].
The visual assessment of coronary lesions on CCTA can be subjective and dependent on the reader’s experience [
45]. To address this limitation, quantitative software tools have been introduced for the assessment of stenosis severity on CCTA. These tools provide more objective measurements and help in evaluating the progression of coronary vessel wall volume over time.
A study by Karolyi et al. demonstrated a progression of coronary vessel wall volume within the first two years after HT using quantitative CCTA analysis [
46]. This progression was primarily due to non-calcified lesions that caused mild luminal narrowing. When assessing the characteristics of these lesions, the main components of non-calcified lesions showed high attenuation (131-350 HU), corresponding to fibrous tissue. A smaller percentage of the lesions exhibited intermediate attenuation (75-130 HU), consistent with fibro-fatty tissue, and a low-attenuation (<75 HU) component, indicative of lipid-rich content [
47]. These findings are characteristic of CAV, where coronary lesions tend to be diffuse, and significant focal luminal narrowing develops in only a small number of patients.
Further studies with longer follow-up periods are needed to determine the prognostic value of subclinical CAV progression detected through quantitative CCTA analysis. These studies will help define the implications of early changes in coronary vessel wall volume on the long-term outcomes of heart transplant recipients.
7. Diagnostic challenges and potential benefits of CMR in the follow-up of heart transplant recipients
CMR is indeed considered the gold standard imaging modality for assessing cardiac morphology, ventricular volumes, systolic function, and myocardial mass [
48]. It offers comprehensive evaluation of the heart, allowing for the assessment of various parameters and functions.
In the context of HT recipients, CMR has shown utility in assessing the activity of inflammatory changes in the myocardium. This includes the detection of myocardial edema, hyperemia, capillary leak, and irreversible injury using a combination of non-contrast techniques such as T2-weighted imaging, as well as parametric mapping techniques such as T1 and T2 mapping. These techniques provide valuable information about the presence and extent of inflammatory processes within the transplanted heart [
49].
The study by Vermes et al. [
50] supports the use of a multi-parametric sequential approach using CMR to diagnose acute rejection in heart transplant recipients. They found that combining basal T2 mapping with basal extracellular volume (ECV) measurement yielded the best diagnostic accuracy. This approach has the potential to reduce the need for invasive endomyocardial biopsies (EMBs) by more than 50%. Interestingly, the study also found that T1 mapping values before and after contrast injection did not show significant increases in the presence of rejection. This highlights the limitations of measuring absolute myocardial T1 values, as they can be influenced by various factors such as the acquisition scheme, magnetization transfer, flow, and T2 effects [
51].
Overall, CMR with its multi-parametric approach offers valuable insights into the inflammatory processes occurring in heart transplant recipients and can help guide clinical decision-making and reduce the need for invasive procedures like EMBs.
The presence of an unexplained fall in LVEF combined with a non-diagnostic EMB poses a diagnostic dilemma in patients several years post-cardiac transplantation. The limitations of EMB in this group include hindrance by endomyocardial fibrosis from prior biopsy sites and difficulties in central venous access due to multiple prior cannulations [
52]. Additionally, diffuse myocardial fibrosis, which is common in long-term heart transplant recipients, can make it difficult to obtain adequate myocardial samples for biopsy [
47]. Therefore, the development of a non-invasive test for acute rejection is particularly valuable in these patients.
CMR offers high spatial resolution and accurate assessment of ventricular volume and function through cine imaging. While nuclear cardiac gated blood pool imaging can also provide LVEF measurements, CMR’s advantage lies in the absence of ionizing radiation, which is beneficial for patients on long-term immunosuppressive therapy [
52]. Furthermore, CMR allows for the calculation of GLS, which may serve as an early integrated biomarker of pathologies affecting the subendocardium, such as CAV and allograft failure [
54]. Moreover, abnormal GLS has been associated with an increased risk of adverse cardiac events in HT patients [
54].
Studies, such as the randomized trial conducted by Anthony et al., have demonstrated the feasibility of CMR-based surveillance for acute rejection (AR), reducing the potential complications associated with EMB-based surveillance [
55]. In the pediatric population, where signs of allograft rejection may be more difficult to appreciate, CMR can play an important role in cardiac rejection surveillance. EMB in children often requires general anesthesia, adding to the procedural risk and invasiveness [
57,
58]. CMR offers non-invasive myocardial tissue characterization and can be informative in the pediatric setting, providing a surveillance protocol that requires no intravenous cannulation, gadolinium administration, or breath holding during T2 mapping sequences [
55].
The use of CMR-derived GLS, combined with data from cine imaging, perfusion imaging, late gadolinium enhancement (LGE), T1 mapping, and T2 mapping, holds promise in improving the prognostic assessment of cardiac allograft rejection [
54]. However, prospective studies are needed to determine whether a GLS-guided strategy is associated with improved long-term outcomes. Additionally, in HT recipients with chronic kidney disease, CMR-FT GLS can provide prognostic information without the need for contrast administration, addressing concerns about the risk of nephrogenic systemic fibrosis associated with gadolinium-based contrast agents [
54].
Overall, CMR offers valuable non-invasive imaging capabilities in the evaluation and surveillance of heart transplant recipients, with the potential to improve diagnostic accuracy, reduce invasive procedures, and provide prognostic information.
8. Conclusion
New non-invasive techniques have been proposed and tested to reduce the need for invasive examinations in the follow-up of HT patients. A multimodality imaging approach, incorporating advanced echocardiography, CMR, and CCTA can provide valuable information in detecting post-transplant complications.
Advanced echocardiography techniques, such as strain imaging, three-dimensional echocardiography, and tissue Doppler imaging, offer enhanced assessment of cardiac function and can help identify abnormalities in HT patients. GLS, in particular, has shown promise as an early marker of cardiac allograft rejection and other subclinical pathologies affecting the myocardium [
54]. These techniques provide valuable information about ventricular function, wall motion abnormalities, and graft vasculopathy.
CMR is considered the gold standard imaging modality for assessing cardiac morphology, ventricular volumes, systolic function, and myocardial mass in HT patients [
48]. CMR enables accurate assessment of ventricular volume and function through cine imaging, and it allows for the evaluation of myocardial tissue characteristics using various sequences, such as T1 and T2 mapping, LGE, and parametric mapping techniques [
49]. These techniques can help detect inflammation, myocardial edema, fibrosis, and irreversible injury, providing valuable insights into graft health and the presence of complications.
CCTA, on the other hand, is particularly useful in evaluating coronary artery disease and CAV in HT recipients. It allows for non-invasive assessment of coronary artery stenosis, plaque burden, and morphology. Quantitative software tools have been developed to assess stenosis severity on CCTA, aiding in the detection of CAV progression and potentially reducing the need for ICA [
46].
By combining information from advanced echocardiography, CMR, and CCTA, clinicians can obtain a comprehensive evaluation of HT patients, identifying complications such as rejection, graft vasculopathy, and CAD. This multimodality approach offers the potential to optimize patient care by providing detailed information without the need for invasive procedures, reducing patient discomfort and potential complications.
It is important to note that while these non-invasive techniques show great promise, their clinical utility and long-term prognostic value in the follow-up of HT patients are still being investigated. Further research and prospective studies are needed to establish their effectiveness, refine their protocols, and determine their impact on patient outcomes.
9. Future directions
Highlighting the evolving landscape of follow-up care for HT patients, with advancements in non-invasive and contrast-sparing diagnostic tools is crucial. HT remains the gold standard treatment for patients with advanced heart failure, and the use of these tools can greatly impact the management and outcomes of these patients.
Chronic inflammation plays a significant role in the development of complications after HT, including graft rejection and CAV. The impact of chronic inflammation on epicardial and pericoronary fat, which can serve as a marker of inflammation, is an area that requires further investigation with the use of CCTA. Also CMR multiparametric mapping techniques, such as T1 and T2 mapping, offer the potential to assess inflammation and tissue characterization in a non-invasive manner. These mapping techniques may become a first-line strategy in the surveillance of rejection, providing valuable information about the myocardium and aiding in the early detection of rejection episodes.
In addition to rejection, microvascular disease can contribute to cardiac dysfunction in HT patients. Stress CMR and CCTA with regadenoson, can be useful in detecting microvascular disease even in the absence of coronary artery stenosis. By inducing stress and assessing myocardial perfusion, stress CMR/CCTA can provide insights into the function of the microvasculature and help identify patients who may benefit from targeted therapies.
To optimize the care of HT patients, it is crucial to have specialized centers with dedicated HT teams and expertise in multimodality imaging. Large HT centers with experience in various imaging modalities, including echocardiography, CMR, and CCTA, can provide comprehensive evaluation and management. This multidisciplinary approach ensures that patients receive the best possible care, incorporating the advantages of non-invasive imaging techniques and tailoring treatment strategies based on individual patient needs.
While advancements in non-invasive imaging techniques have shown great promise, further studies are necessary to strengthen the evidence base and establish their role in routine clinical practice. These studies will help provide more substantial evidence regarding the benefits of these tools in terms of cost-effectiveness, time efficiency, and reduction in complications. By incorporating these tools into the follow-up care of HT patients, the healthcare system can potentially benefit from improved patient outcomes and resource allocation.
Author Contributions
Conceptualization, V.P., and A.I.G..; methodology, G.M., G.D.C. and A.C..; validation, R.M. and S.I.; investigation, E.C.. and M.T.S..; writing—original draft preparation, E.C. and G.M..; writing—review and editing, V.P. and M.P.M.; visualization, C.T. and N.P..; supervision, F.T. and G.G..; project administration, F.A. and C.M.D.. All authors have read and agreed to the published version of the manuscript.”
Institutional Review Board Statement
Ethical review and approval were waived for this study due to study type (review).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Braga, F.G.M.; Binder, T.; et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur. Hear. J. - Cardiovasc. Imaging 2015, 16, 919–948. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. J. Hear. Lung Transplant. 2010, 29, 717–727. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; Ghionzoli, N.; Mandoli, G.; Sisti, N.; D’ascenzi, F.; Focardi, M.; Bernazzali, S.; Vergaro, G.; Emdin, M.; Valente, S.; et al. The role of non-invasive imaging modalities in cardiac allograft vasculopathy: an updated focus on current evidences. Hear. Fail. Rev. 2021, 27, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Velleca, A.; A Shullo, M.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) Guidelines for the Care of Heart Transplant Recipients. J. Hear. Lung Transplant. 2022. [Google Scholar] [CrossRef]
- Valantine, H.A.; Hatle, L.K.; Appleton, C.P.; Gibbons, R.; Popp, R.L. Variability of Doppler Echocardiographic Indexes of Left Ventricular Filling in Transplant Recipients and in Normal Subjects. J. Am. Soc. Echocardiogr. 1990, 3, 276–284. [Google Scholar] [CrossRef]
- Gorcsan J 3rd, Snow FR, Paulsen W, Arrowood JA, Thompson JA NJ. Echocardiographic profile of the transplanted human heart in clinically well recipients. J Hear Lung Transplant. 1992;Jan-Feb:11(1 Pt 1).
- Clemmensen, T.S.; Løgstrup, B.B.; Eiskjær, H.; Poulsen, S.H. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: Relation to coronary allograft vasculopathy. J. Hear. Lung Transplant. 2015, 34, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sade, L.; Sezgin, A.; Uluçam, M.; Taymaz, S.; Şimşek, V.; Tayfun, E.; Tokel, K.; Aşlamaci, S.; Müderrisoğlu, H. Evaluation of the Potential Role of Echocardiography in the Detection of Allograft Rejection in Heart Transplant Recipients. Transplant. Proc. 2006, 38, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Bech-Hanssen, O.; Al-Habeeb, W.; Ahmed, W.; Di Salvo, G.; Pergola, V.; Al-Admawi, M.; Al-Amri, M.; Al-Shahid, M.; Al-Buraiki, J.; Fadel, B.M. Echocardiography Detects Elevated Left Ventricular Filling Pressures in Heart Transplant Recipients. Echocardiography 2015, 32, 411–419. [Google Scholar] [CrossRef]
- LeJemtel, T.H. Review of a controlled trial of exercise rehabilitation after heart transplantation. Curr. Cardiol. Rep. 1999, 1, 32–32. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, E.W.; Epstein, M.; Ota, D.; Hoagland, P.M.; Gordon, J.B.; Adamson, R.M.; McDaniel, M.; Peterson, K.L.; Smith, S.C.; E Jaski, B. Right and left ventricular function after cardiac transplantation. Changes during and after rejection. Circulation 1991, 84, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Tona, F.; Caforio, A.L.P.; Piaserico, S.; Bontorin, M.; De Simone, G.; Leone, M.G.; Fortina, A.B.; Gambino, A.; Feltrin, G.; Calzolari, D.; et al. Abnormal total ejection isovolume index as early noninvasive marker of chronic rejection in heart transplantation*. Transpl. Int. 2005, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Antończyk, K.; Niklewski, T.; Antończyk, R.; Zakliczyński, M.; Zembala, M.; Kukulski, T. Speckle-Tracking Echocardiography for Monitoring Acute Rejection in Transplanted Heart. Transplant. Proc. 2018, 50, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Caracciolo, G.; Cho, E.J.; Scott, R.L.; Steidley, D.E.; Wilansky, S.; Arabia, F.A.; Khandheria, B.K.; Sengupta, P.P. Natural History of Left Ventricular Mechanics in Transplanted Hearts: Relationships With Clinical Variables and Genetic Expression Profiles of Allograft Rejection. JACC: Cardiovasc. Imaging 2010, 3, 989–1000. [Google Scholar] [CrossRef]
- Saleh, H.K.; Villarraga, H.R.; Kane, G.C.; Pereira, N.L.; Raichlin, E.; Yu, Y.; Koshino, Y.; Kushwaha, S.S.; Miller, F.A.; Oh, J.K.; et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J. Hear. Lung Transplant. 2011, 30, 652–658. [Google Scholar] [CrossRef]
- Sarvari, S.I.; Gjesdal, O.; Gude, E.; Arora, S.; Andreassen, A.K.; Gullestad, L.; Geiran, O.; Edvardsen, T. Early Postoperative Left Ventricular Function by Echocardiographic Strain is a Predictor of 1-Year Mortality in Heart Transplant Recipients. J. Am. Soc. Echocardiogr. 2012, 25, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, A.M.; Mastrobuoni, S.; Bastarrika, G.; Praschker, B.L.; Agüero, P.A.; Castaño, S.; Herreros, J.; Rabago, G. Bicaval versus standard technique in orthotopic heart transplant: assessment of atrial performance at magnetic resonance and transthoracic echocardiography. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 457–462. [Google Scholar] [CrossRef]
- Bech-Hanssen, O.; Pergola, V.; Al-Admawi, M.; Fadel, B.M.; Di Salvo, G. Atrial function in heart transplant recipients operated with the bicaval technique. Scand. Cardiovasc. J. 2016, 50, 42–51. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, Y.; Qiao, W.; Tian, F.; Sun, W.; Wang, Y.; Wu, C.; Li, H.; Yi, L.; Zhong, Y.; et al. Impaired left atrial function in clinically well heart transplant patients. Int. J. Cardiovasc. Imaging 2021, 37, 1937–1945. [Google Scholar] [CrossRef]
- Derumeaux, G.; Redonnet, M.; Mouton-Schleifer, D.; Bessou, J.P.; Cribier, A.; Saoudi, N.; Koning, R.; Soyer, R.; Letac, B. Dobutamine stress echocardiography in orthotopic heart transplant recipients. J. Am. Coll. Cardiol. 1995, 25, 1665–1672. [Google Scholar] [CrossRef]
- Akosah, K.O.; Mohanty, P.K. Role of dobutamine stress echocardiography in heart transplant patients. Chest 1998, 113, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, H.J.; Lee, S.E.; Jung, S.-H.; Yun, T.-J.; Kim, J.J.; Lee, J.W. Prevalence and Risk Factors of Post–heart Transplant Tricuspid Regurgitation. Transplantation 2022, 106, e297–e303. [Google Scholar] [CrossRef] [PubMed]
- Wartig, M.; Tesan, S.; Gäbel, J.; Jeppsson, A.; Selimovic, N.; Holmberg, E.; Dellgren, G. Tricuspid regurgitation influences outcome after heart transplantation. J. Hear. Lung Transplant. 2014, 33, 829–835. [Google Scholar] [CrossRef]
- Bhatia, S.J.; Kirshenbaum, J.M.; Shemin, R.J.; Cohn, L.H.; Collins, J.J.; Di Sesa, V.J.; Young, P.J.; Mudge, G.H.; Sutton, M.G.; A, M.; et al. Time course of resolution of pulmonary hypertension and right ventricular remodeling after orthotopic cardiac transplantation. Circulation 1987, 76, 819–826. [Google Scholar] [CrossRef]
- Chamberlain, R.; Edwards, N.F.A.; Scalia, G.M.; Chan, J. Novel left and right ventricular strain analysis to detect subclinical myocardial dysfunction in cardiac allograft rejection. Int. J. Cardiovasc. Imaging 2022, 38, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; D’Hooge, J.; Sutherland, G.R.; Marciniak, A.; Thijs, D.; Droogne, W.; Herbots, L.; Van Cleemput, J.; Claus, P.; Bijnens, B.; et al. Quantitative dobutamine stress echocardiography for the early detection of cardiac allograft vasculopathy in heart transplant recipients. Hear. 2008, 94, e3–e3. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Exner, B.; Schmiedehausen, K.; Huchzermeyer, C.; Reulbach, U.; Nixdorff, U.; Platsch, G.; Kuwert, T.; Daniel, W.G.; Flachskampf, F.A.; et al. Strain-Rate Imaging During Dobutamine Stress Echocardiography Provides Objective Evidence of Inducible Ischemia. Circulation 2003, 107, 2120–2126. [Google Scholar] [CrossRef]
- Ingul, C.B.; Stoylen, A.; Slordahl, S.A.; Wiseth, R.; Burgess, M.; Marwick, T.H. Automated Analysis of Myocardial Deformation at Dobutamine Stress Echocardiography: An Angiographic Validation. J. Am. Coll. Cardiol. 2007, 49, 1651–1659. [Google Scholar] [CrossRef]
- Tona, F.; Caforio, A.L.; Montisci, R.; Gambino, A.; Angelini, A.; Ruscazio, M.; Toscano, G.; Feltrin, G.; Ramondo, A.; Gerosa, G.; et al. Coronary Flow Velocity Pattern and Coronary Flow Reserve by Contrast-Enhanced Transthoracic Echocardiography Predict Long-Term Outcome in Heart Transplantation. Circulation 2006, 114, I–49. [Google Scholar] [CrossRef]
- Cecere, A.; Kerkhof, P.L.M.; Civieri, G.; Angelini, A.; Gambino, A.; Fraiese, A.; Bottio, T.; Osto, E.; Famoso, G.; Fedrigo, M.; et al. Coronary Flow Evaluation in Heart Transplant Patients Compared to Healthy Controls Documents the Superiority of Coronary Flow Velocity Reserve Companion as Diagnostic and Prognostic Tool. Front. Cardiovasc. Med. 2022, 9, 887370. [Google Scholar] [CrossRef]
- Shah, N.R.; Blankstein, R.; Villines, T.; Imran, H.; Morrison, A.R.; Cheezum, M.K. Coronary CTA for Surveillance of Cardiac Allograft Vasculopathy. Curr. Cardiovasc. Imaging Rep. 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nous, F.M.A.; Roest, S.; Dijkman, E.D.; Attrach, M.; Caliskan, K.; Brugts, J.J.; Nieman, K.; Hirsch, A.; Constantinescu, A.A.; Manintveld, O.C.; et al. Clinical implementation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients. Transpl. Int. 2021, 34, 1886–1894. [Google Scholar] [CrossRef]
- Günther, A.; Aaberge, L.; Abildgaard, A.; Ragnarsson, A.; Edvardsen, T.; Jakobsen, J.; Andersen, R. Coronary computed tomography in heart transplant patients: detection of significant stenosis and cardiac allograft vasculopathy, image quality, and radiation dose. Acta Radiol. 2018, 59, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Bartykowszki, A.; Kolossváry, M.; Jermendy. L.; Karády, J.; Szilveszter, B.; Károlyi, M.; Balogh, O.; Sax, B.; Merkely, B.; Maurovich-Horvat, P. Image Quality of Prospectively ECG-Triggered Coronary CT Angiography in Heart Transplant Recipients. Am. J. Roentgenol. 2018, 210, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Stolzmann, P.; Leschka, S.; Scheffel, H.; Krauss, T.; Desbiolles, L.; Plass, A.; Genoni, M.; Flohr, T.G.; Wildermuth, S.; Marincek, B.; et al. Dual-Source CT in Step-and-Shoot Mode: Noninvasive Coronary Angiography with Low Radiation Dose1. Radiology 2008, 249, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Budde, R.P.J.; Nous, F.M.A.; Roest, S.; Constantinescu, A.A.; Nieman, K.; Brugts, J.J.; Koweek, L.M.; Hirsch, A.; Leipsic, J.; Manintveld, O.C. CT-derived fractional flow reserve (FFRct) for functional coronary artery evaluation in the follow-up of patients after heart transplantation. Eur. Radiol. 2022, 32, 1843–1852. [Google Scholar] [CrossRef]
- Yang, H.-M.; Khush, K.; Luikart, H.; Okada, K.; Lim, H.-S.; Kobayashi, Y.; Honda, Y.; Yeung, A.C.; Valantine, H.; Fearon, W.F. Invasive Assessment of Coronary Physiology Predicts Late Mortality After Heart Transplantation. Circulation 2016, 133, 1945–1950. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef]
- Rohnean, A.; Houyel, L.; Sigal-Cinqualbre, A.; To, N.-T.; Elfassy, E.; Paul, J.-F. Heart Transplant Patient Outcomes: 5-Year Mean Follow-Up by Coronary Computed Tomography Angiography. Transplantation 2011, 91, 583–588. [Google Scholar] [CrossRef]
- Bogot NR, Durst R, Shaham D, Admon D. Cardiac CT of the Transplanted Heart: Indications, Technique, Appearance, and Complications 1 LEARNING OBJECTIVES FOR TEST 2 CME FEATURE. RadioGraphics. 2007;27:1297-1309.
- Pflugfelder, P.W.; Boughner, D.R.; Rudas, L.; Kostuk, W.J. Enhanced detection of cardiac allograft arterial disease with intracoronary ultrasonographic imaging. Am. Hear. J. 1993, 125, 1583–1591. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative Evaluation of Left and Right Ventricular Endomyocardial Biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Hoe, J. Quantification of Coronary Arterial Stenoses by Multidetector CT Angiography in Comparison With Conventional Angiography: Methods, Caveats, and Implications. JACC: Cardiovasc. Imaging 2011, 4, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Károlyi, M.; Kolossváry, M.; Bartykowszki, A.; Kocsmár, I.; Szilveszter, B.; Karády, J.; Merkely, B.; Maurovich-Horvat, P. Quantitative CT assessment identifies more heart transplanted patients with progressive coronary wall thickening than standard clinical read. J. Cardiovasc. Comput. Tomogr. 2019, 13, 128–133. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.A.; Broersen, A.; Kitslaar, P.H.; Roos, C.J.; Dijkstra, J.; Lelieveldt, B.P.F.; Jukema, J.W.; Schalij, M.J.; Delgado, V.; Bax, J.J.; et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int. J. Cardiovasc. Imaging 2013, 29, 1177–1190. [Google Scholar] [CrossRef]
- Bellenger, N.; Burgess, M.; Ray, S.; Lahiri, A.; Coats, A.; Cleland, J.; Pennell, D. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur. Hear. J. 2000, 21, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13–13. [Google Scholar] [CrossRef]
- Vermes, E.; Pantaléon, C.; Auvet, A.; Cazeneuve, N.; Machet, M.C.; Delhommais, A.; Bourguignon, T.; Aupart, M.; Brunereau, L. Cardiovascular magnetic resonance in heart transplant patients: diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J. Cardiovasc. Magn. Reson. 2018, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; E Arai, A.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Taylor, A.J.; Vaddadi, G.; Pfluger, H.; Butler, M.; Bergin, P.; Leet, A.; Richardson, M.; Cherayath, J.; Iles, L.; Kaye, D.M. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur. J. Hear. Fail. 2010, 12, 45–51. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Cao, J.; Li, X.; Lin, L.; Chen, W.; Wang, Y.-N.; Jin, Z.-Y. Cardiovascular Magnetic Resonance Mapping and Strain Assessment for the Diagnosis of Cardiac Involvement in Idiopathic Inflammatory Myopathy Patients With Preserved Left Ventricular Ejection Fraction. J. Thorac. Imaging 2021, 36, 254–261. [Google Scholar] [CrossRef]
- Shenoy, C.; Romano, S.; Hughes, A.; Okasha, O.; Nijjar, P.S.; Velangi, P.; Martin, C.M.; Akçakaya, M.; Farzaneh-Far, A. Cardiac Magnetic Resonance Feature Tracking Global Longitudinal Strain and Prognosis After Heart Transplantation. JACC: Cardiovasc. Imaging 2020, 13, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Anthony, C.; Imran, M.; Pouliopoulos, J.; Emmanuel, S.; Iliff, J.; Liu, Z.; Moffat, K.; Qiu, M.R.; McLean, C.A.; Stehning, C.; et al. Cardiovascular Magnetic Resonance for Rejection Surveillance After Cardiac Transplantation. Circulation 2022, 145, 1811–1824. [Google Scholar] [CrossRef]
- Wagner, K.; Oliver, M.C.; Boyle, G.J.; Miller, S.A.; Law, Y.M.; Pigula, F.; Webber, S.A. Endomyocardial biopsy in pediatric heart transplant recipients: A useful exercise? (Analysis of 1169 biopsies). Pediatr. Transplant. 2000, 4, 186–192. [Google Scholar] [CrossRef] [PubMed]
- A Braunlin, E.; Shumway, S.J.; Bolman, R.M.; McDonald, K.M.; Ring, W.S.; Olivari, M.T.; E Nakhleh, R. Usefulness of surveillance endomyocardial biopsy after pediatric cardiac transplantation. Clin. Transplant. 1998, 12. [Google Scholar] [CrossRef]
- Cornicelli, M.D.; Rigsby, C.K.; Rychlik, K.; Pahl, E.; Robinson, J.D. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 1–9. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).