Submitted:
28 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. A Crop with potential biomass production
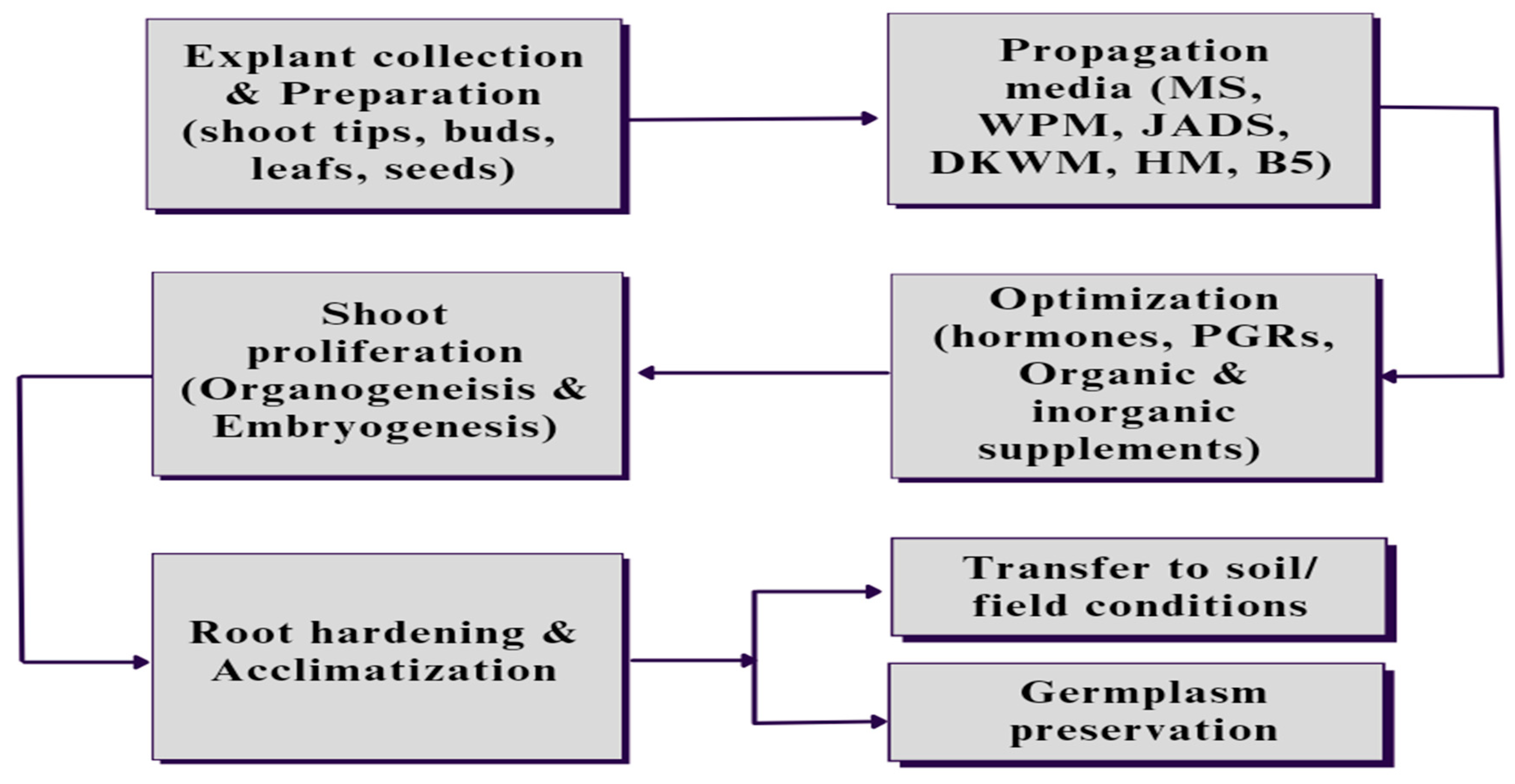
2. Micropropagation and its applications
2.1. Establishing Axenic Culture
2.2. In-vitro proliferation of shoot
2.2.1. Organogenesis
2.2.2. Somatic embryogenesis
2.3. Adventitious root formation and root hardening
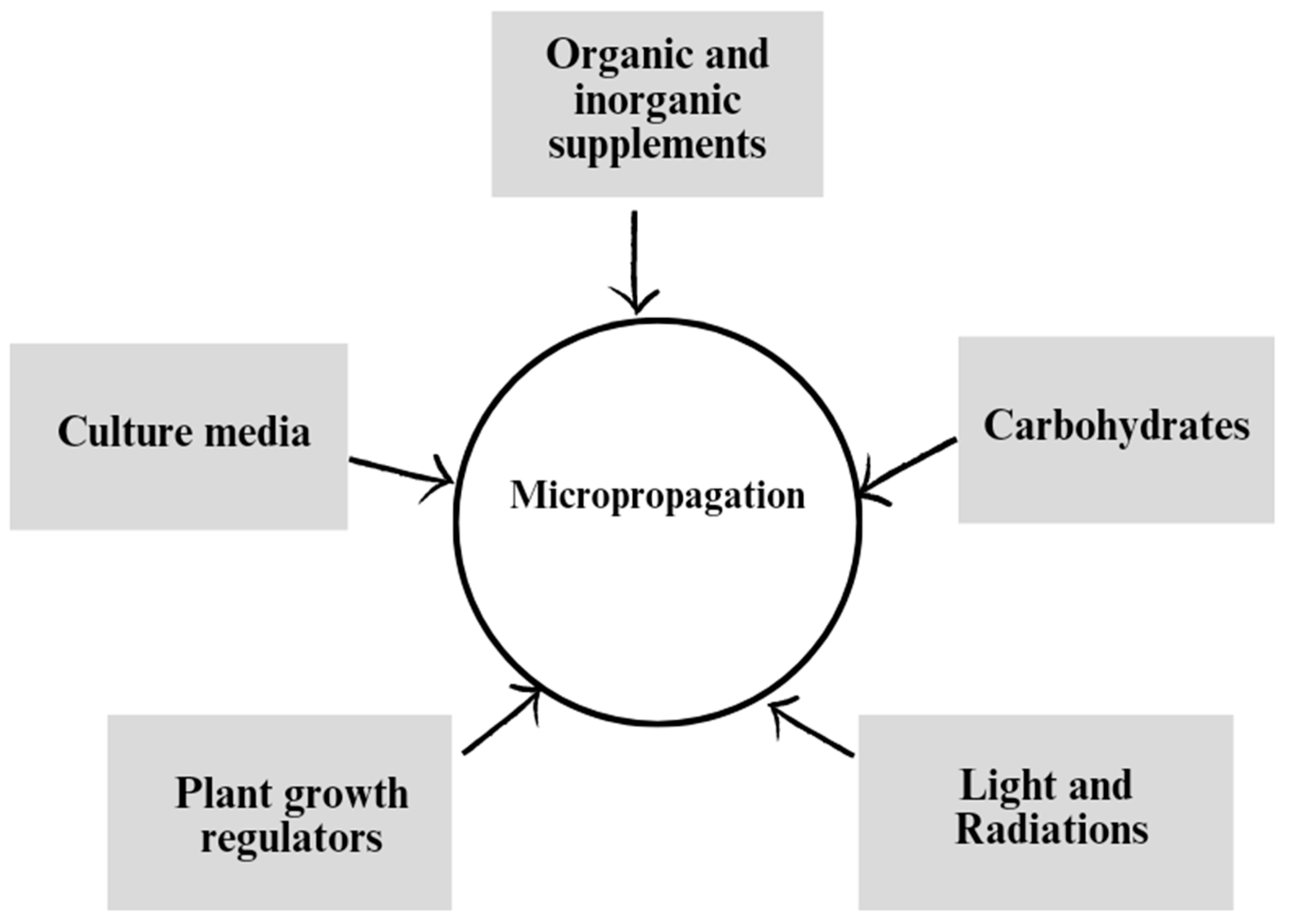
3. Factors affecting the efficiency of micropropagation
3.1. Role of Plant Growth Regulators:
3.2. Effect of culture media
3.3. Importance of organic and inorganic elements
3.4. Role of carbohydrates
3.5. Effects of radiation and light exposure
4. In-vitro germplasm preservation
4.1. Cryopreservation and cold storage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watt M., P. and Blakeway, F. C. and M. M. E. O. and J. S. M. Micropropagation of Eucalyptus. In Micropropagation of Woody Trees and Fruits; Jain S. Mohan and Ishii, K., Ed.; Springer Netherlands: Dordrecht, 2003; pp. 217–244. [Google Scholar] [CrossRef]
- Trueman, S. J.; Hung, C. D.; Wendling, I. Tissue Culture of Corymbia and Eucalyptus. Forests 2018, 9. [Google Scholar] [CrossRef]
- Nogueira, M. C. de J. A.; DE ARAUJO, V. A.; Vasconcelos, J. S.; Christoforo, A. L.; Lahr, F. A. R. Sixteen Properties of Eucalyptus Tereticornis Wood for Structural Uses. Bioscience Journal 2020, 36, 449–457. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S. M.; Duarte, V. G.; Machado, M. I. L.; Matos, F. J. A. Analgesic and Anti-Inflammatory Effects of Essential Oils of Eucalyptus. J Ethnopharmacol 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Kamal, B.; Arya, I.; Sharma, V.; Jadon, V. S. In Vitro Enhanced Multiplication and Molecular Validation of Eucalyptus F1 Hybrids. Plant Cell Biotechnol Mol Biol 2016, 17, 167–175. [Google Scholar]
- Arya, I. D.; Sharma, S.; Chauhan, S.; Arya, S. Micropropagation of Superior Eucalyptus Hybrids FRI-5 (Eucalyptus Camaldulensis Dehn x E. Tereticornis Sm) and FRI-14(Eucalyptus Torelliana F.V. Muell x E. Citriodora Hook): A Commercial Multiplication and Field Evaluation. Afr J Biotechnol 2009, 8, 5718–5726. [Google Scholar] [CrossRef]
- Kendurkar Shuchishweta Vinay and Rangaswamy, M. Genetic Transformation in Eucalyptus. In Biotechnologies of Crop Improvement, Volume 2: Transgenic Approaches; Gosal Satbir Singh and Wani, S. H., Ed.; Springer International Publishing: Cham, 2018; pp. 335–336. [Google Scholar] [CrossRef]
- El-Esawi, M. A. Micropropagation Technology and Its Applications for Crop Improvement. In Plant Tissue Culture: Propagation, Conservation and Crop Improvement; Anis Mohammad and Ahmad, N., Ed.; Springer Singapore: Singapore, 2016; pp. 523–545. [Google Scholar] [CrossRef]
- Trueman, S. J. Clonal Propagation and Storage of Subtropical Pines in Queensland, Australia. Southern African Forestry Journal 2006, 208, 49–52. [Google Scholar] [CrossRef]
- Booth, T. H. Eucalypt Plantations and Climate Change. For Ecol Manage 2013, 301, 28–34. [Google Scholar] [CrossRef]
- Booth, T. H. Eucalypt Plantations and Climate Change. For Ecol Manage 2013, 301, 28–34. [Google Scholar] [CrossRef]
- Shanthi, K.; Bachpai, V. K. W.; Anisha, S.; Ganesan, M.; Anithaa, R. G.; Subashini, V.; Chakravarthi, M.; Sivakumar, V.; Yasodha, R. Micropropagation of Eucalyptuscamaldulensis for the Production of Rejuvenated Stock Plants for Microcuttings Propagation and Genetic Fidelity Assessment. New For (Dordr) 2015, 46, 357–371. [Google Scholar] [CrossRef]
- Kataria, V. and Masih, A. and C. S. and S. S. K. and K. A. and A. I. D. Clonal Propagation, a Tested Technique for Increasing Productivity: A Review of Bamboos, Eucalyptus and Chirpine. In Agricultural Biotechnology: Latest Research and Trends; Kumar Srivastava Dinesh and Kumar Thakur, A. and K. P., Ed.; Springer Nature Singapore: Singapore, 2021; pp. 37–51. [Google Scholar] [CrossRef]
- Butcher, P. A.; Skinner, A. K.; Gardiner, C. A. Increased Inbreeding and Inter-Species Gene Flow in Remnant Populations of the Rare Eucalyptus Benthamii. Conservation Genetics 2005, 6, 213–226. [Google Scholar] [CrossRef]
- Potts, B. M.; Dungey, H. S. Interspecific Hybridization of Eucalyptus: Key Issues for Breeders and Geneticists. New For (Dordr) 2004, 27, 115–138. [Google Scholar] [CrossRef]
- Jovanovic, T.; Arnold, R.; Booth, T. Determining the Climatic Suitability of Eucalyptus Dunnii for Plantations in Australia, China and Central and South America. New For (Dordr) 2000, 19, 215–226. [Google Scholar] [CrossRef]
- Singh, D.; Kaur, S.; Kumar, A. In Vitro Drought Tolerance in Selected Elite Clones of Eucalyptus Tereticornis Sm. Acta Physiol Plant 2020, 42, 17. [Google Scholar] [CrossRef]
- Venkatesh, C. S.; Sharma, V. K. Hybrid Vigour in Controlled Interspecific Crosses of Eucalyptus Tereticornis X E. Camaldulensis. Silvae Genet 1977, 26, 121–124. [Google Scholar]
- Paramathma, M.; Surendran, C.; Rai, R. S. V. STUDIES ON HETEROSIS IN SIX EUCALYPTUS SPECIES. Journal of Tropical Forest Science 1997, 9, 283–293. [Google Scholar]
- de Almeida, M. R.; de Bastiani, D.; Gaeta, M. L.; de Araújo Mariath, J. E.; de Costa, F.; Retallick, J.; Nolan, L.; Tai, H. H.; Strömvik, M. V; Fett-Neto, A. G. Comparative Transcriptional Analysis Provides New Insights into the Molecular Basis of Adventitious Rooting Recalcitrance in Eucalyptus. Plant Science 2015, 239, 155–165. [Google Scholar] [CrossRef]
- Dickinson, G. R.; Wallace, H. M.; Lee, D. J. Reciprocal and Advanced Generation Hybrids between Corymbia Citriodora and C. Torelliana: Forestry Breeding and the Risk of Gene Flow. Ann For Sci 2013, 70, 1–10. [Google Scholar] [CrossRef]
- Wendling, I.; Warburton, P. M.; Trueman, S. J. Maturation in Corymbia Torelliana × C. Citriodora Stock Plants: Effects of Pruning Height on Shoot Production, Adventitious Rooting Capacity, Stem Anatomy, and Auxin and Abscisic Acid Concentrations. Forests 2015, 6, 3763–3778. [Google Scholar] [CrossRef]
- Debergh P., C. and Read, P. E. Micropropagation. In Micropropagation: Technology and Application; Debergh P. C. and Zimmerman, R. H., Ed.; Springer Netherlands: Dordrecht, 1991. [Google Scholar] [CrossRef]
- Cassells, A. C. Pathogen and Biological Contamination Management in Plant Tissue Culture: Phytopathogens, Vitro Pathogens, and Vitro Pests. In Plant Cell Culture Protocols; Loyola-Vargas Víctor M. and Ochoa-Alejo, N., Ed.; Humana Press: Totowa, NJ, 2012; pp. 57–80. [Google Scholar] [CrossRef]
- Wendling, I.; Brooks, P. R.; Trueman, S. J. Topophysis in Corymbia Torelliana × C. Citriodora Seedlings: Adventitious Rooting Capacity, Stem Anatomy, and Auxin and Abscisic Acid Concentrations. New For (Dordr) 2015, 46, 107–120. [Google Scholar] [CrossRef]
- Bag, N.; Chandra, S.; Palni, L. M. S.; Nandi, S. K. Micropropagation of Dev-Ringal [Thamnocalamus Spathiflorus (Trin.) Munro] — a Temperate Bamboo, and Comparison between in Vitro Propagated Plants and Seedlings. Plant Science 2000, 156, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Giri, C. C.; Shyamkumar, B.; Anjaneyulu, C. Progress in Tissue Culture, Genetic Transformation and Applications of Biotechnology to Trees: An Overview. Trees 2004, 18, 115–135. [Google Scholar] [CrossRef]
- Ansar, S.; Iqbal, M. Effect of Dietary Antioxidant on Mercuric Chloride Induced Lung Toxicity and Oxidative Stress. Toxin Rev 2015, 34, 168–172. [Google Scholar] [CrossRef]
- Keret, R.; Nakhooda, M.; Jones, N. B.; Hills, P. N. Optimisation of Micropropagation Protocols for Temperate Eucalypt Hybrids in South Africa, with a Focus on Auxin Transport Proteins. Southern Forests: a Journal of Forest Science 2021, 83, 254–263. [Google Scholar] [CrossRef]
- Trueman, S. J.; Richardson, D. M. In Vitro Propagation of Corymbia Torelliana C. Citriodora (Myrtaceae) via Cytokinin-Free Node Culture. Aust J Bot 2007, 55, 471–481. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Ramanathan, S.; Sengodagounder, S.; Senniappan, C.; Shanmuganathan, R.; Brindhadevi, K.; Kaliannan, T. Optimizing the Sterilization Methods for Initiation of the Five Different Clones of the Eucalyptus Hybrid Species. Biocatal Agric Biotechnol 2019, 22, 101361. [Google Scholar] [CrossRef]
- Kamal, B.; Arya, I. D.; Gupta, S. In-Vitro Regeneration of Interspecific F1 Hybrid (Eucalyptus Citriodora and Eucalyptus Torelliana) of Eucalyptus. Journal of Mountain Research 2022, 17. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kumar, A.; Reddy, M. S. Shoot Organogenesis in Elite Clones of Eucalyptus Tereticornis. Plant Cell, Tissue and Organ Culture (PCTOC) 2010, 102, 45–52. [Google Scholar] [CrossRef]
- Girijashankar, V. In Vitro Regeneration of Eucalyptus Camaldulensis. Physiology and Molecular Biology of Plants 2012, 18, 79–87. [Google Scholar] [CrossRef]
- Fernando, S. C.; Goodger, J. Q. D.; Gutierrez, S. S.; Johnson, A. A. T.; Woodrow, I. E. Plant Regeneration through Indirect Organogenesis and Genetic Transformation of Eucalyptus Polybractea R.T. Baker. Ind Crops Prod 2016, 86, 73–78. [Google Scholar] [CrossRef]
- Sluis, A.; Hake, S. Organogenesis in Plants: Initiation and Elaboration of Leaves. Trends in Genetics 2015, 31, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Oberschelp, G. P. J.; Gonçalves, A. N. Assessing the Effects of Basal Media on the in Vitro Propagation and Nutritional Status of Eucalyptus Dunnii Maiden. In Vitro Cellular & Developmental Biology - Plant 2016, 52, 28–37. [Google Scholar] [CrossRef]
- Méndez-Hernández, H. A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R. N.; Juárez-Gómez, Y. L.; Skeete, A.; Avilez-Montalvo, J.; De-La-Peña, C.; Loyola-Vargas, V. M. Signaling Overview of Plant Somatic Embryogenesis. Frontiers in Plant Science. Frontiers Media S.A. February 1, 2019. 1 February. [CrossRef]
- Martínez, M. T.; San-José, M. del C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M. J.; Corredoira, E. Holm Oak Somatic Embryogenesis: Current Status and Future Perspectives. Front Plant Sci 2019, 10. [Google Scholar] [CrossRef]
- Pinto, G.; Silva, S.; Park, Y.-S.; Neves, L.; Araújo, C.; Santos, C. Factors Influencing Somatic Embryogenesis Induction in Eucalyptus Globulus Labill.: Basal Medium and Anti-Browning Agents. Plant Cell Tissue Organ Cult 2008, 95, 79–88. [Google Scholar] [CrossRef]
- Pinto, G.; Park, Y.-S.; Neves, L.; Araújo, C.; Santos, C. Genetic Control of Somatic Embryogenesis Induction in Eucalyptus Globulus Labill. Plant Cell Rep 2008, 27, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Park, Y.-S.; Silva, S.; Neves, L.; Araújo, C.; Santos, C. Factors Affecting Maintenance, Proliferation, and Germination of Secondary Somatic Embryos of Eucalyptus Globulus Labill. Plant Cell Tissue Organ Cult 2008, 95, 69–78. [Google Scholar] [CrossRef]
- Pinto, G.; Silva, S.; Neves, L.; Araújo, C.; Santos, C. Histocytological Changes and Reserve Accumulation during Somatic Embryogenesis in Eucalyptus Globulus. Trees 2010, 24, 763–769. [Google Scholar] [CrossRef]
- Thomas, T. D. The Role of Activated Charcoal in Plant Tissue Culture. Biotechnol Adv 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Jones, N. B.; van Staden, J. Micropropagation and Establishment of Eucalyptus Grandis Hybrids. South African Journal of Botany 1994, 60, 122–126. [Google Scholar] [CrossRef]
- Sapaeing, A.; Sutthinon, P.; Hilae, A.; Wattanapan, N. Effects of BA, NAA, and Activated Charcoal on Micropropagation of Nepenthes Mirabilis (Lour.) Druce; 2020. [CrossRef]
- Glocke, P.; Delaporte, K.; Collins, G.; Sedgley, M. Micropropagation of Juvenile Tissue of Eucalyptus Erythronema × Eucalyptus Stricklandii Cv. ‘Urrbrae Gem.’ In Vitro Cellular & Developmental Biology - Plant 2006, 42, 139–143. [Google Scholar] [CrossRef]
- Costa Souza, D. M. S.; Fernandes, S. B.; Avelar, M. L. M.; do Prado Frade, S. R.; Molinari, L. V.; Gonçalves, D. S.; Pereira Pinto, J. E. B.; Brondani, G. E. Light Quality in Micropropagation of Eucalyptus Grandis × Eucalyptus Urophylla. Scientia Forestalis/Forest Sciences 2020, 48. [Google Scholar] [CrossRef]
- Keret, R.; Nakhooda, M.; Jones, N.; Hills, P. Optimisation of Micropropagation Protocols for Temperate Eucalypt Hybrids in South Africa, with a Focus on Auxin Transport Proteins. Southern Forests: a Journal of Forest Science 2022, 83, 1–10. [Google Scholar] [CrossRef]
- Agrisexport.
- Zobayed, S. M. A.; Afreen-Zobayed, F.; Kubota, C.; Kozai, T. Mass Propagation of Eucalyptus Camaldulensis in a Scaled-up Vessel Under In Vitro Photoautotrophic Condition. Ann Bot 2000, 85, 587–592. [Google Scholar] [CrossRef]
- Tanaka, M.; Giang, D. T. T.; Murakami, A. Application of a Novel Disposable Film Culture System to Photoautotrophic Micropropagation of Eucalyptus Uro-Grandis (Urophylia x Grandis). In Vitro Cellular & Developmental Biology - Plant 2005, 41, 173–180. [Google Scholar] [CrossRef]
- Trueman, S. J.; McMahon, T. V; Bristow, M. Production of Eucalyptus Cloeziana Cuttings in Response to Stock Plant Temperature. Journal of Tropical Forest Science 2013, 25, 60–69. [Google Scholar]
- Mendonça, E. G.; Batista, T. R.; Stein, V. C.; Balieiro, F. P.; de Abreu, J. R.; Pires, M. F.; de Souza, P. A.; Paiva, L. V. In Vitro Serial Subculture to Improve Rooting of Eucalyptus Urophylla. New For (Dordr) 2020, 51, 801–816. [Google Scholar] [CrossRef]
- Leva, A.; Rinaldi, L. M. R. Recent Advances in Plant in Vitro Culture; IntechOpen: Rijeka, 2012. [Google Scholar] [CrossRef]
- Leva, A.; Rinaldi, L. M. R. Recent Advances in Plant in Vitro Culture; IntechOpen: Rijeka, 2012. [Google Scholar] [CrossRef]
- Frick, E. M.; Strader, L. C. Roles for IBA-Derived Auxin in Plant Development. J Exp Bot 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Strader, L. C.; Bartel, B. Transport and Metabolism of the Endogenous Auxin Precursor Indole-3-Butyric Acid. Molecular Plant. Oxford University Press 2011, pp 477–486. [CrossRef]
- de Oliveira, L. S.; Brondani, G. E.; Molinari, L. V.; Dias, R. Z.; Teixeira, G. L.; Gonçalves, A. N.; de Almeida, M. Optimal Cytokinin/Auxin Balance for Indirect Shoot Organogenesis of Eucalyptus Cloeziana and Production of Ex Vitro Rooted Micro-Cuttings. J For Res (Harbin) 2022, 33, 1573–1584. [Google Scholar] [CrossRef]
- Prakash, M. G.; Gurumurthi, K. Effects of Type of Explant and Age, Plant Growth Regulators and Medium Strength on Somatic Embryogenesis and Plant Regeneration in Eucalyptus Camaldulensis. Plant Cell, Tissue and Organ Culture (PCTOC) 2010, 100, 13–20. [Google Scholar] [CrossRef]
- Brondani, G. E.; Dutra, L. F.; Wendling, I.; Grossi, F.; Hansel, F. A.; Araujo, M. A. <B>Micropropagation of an <Em>Eucalyptus</Em> Hybrid (<Em>Eucalyptus Benthamii x Eucalyptus Dunnii</Em>)</B> - Doi: 10.4025/Actasciagron.V33i4.8317. Acta Sci Agron 2011, 33 (4). [CrossRef]
- Nazirah, A.; Hasnida, N.; Mohd-Saifuldullah, A. W.; Y, M.-F.; Y, A.-Z.; K, R. DEVELOPMENT OF AN EFFICIENT MICROPROPAGATION PROTOCOL FOR EUCALYPTUS HYBRID (E. UROPHYLLA × E. GRANDIS) THROUGH AXILLARY SHOOT PROLIFERATION. JOURNAL OF TROPICAL FOREST SCIENCE 2021, 33, 391–397. [Google Scholar] [CrossRef]
- Shwe, S. S.; Leung, D. W. M. Plant Regeneration from Eucalyptus Bosistoana Callus Culture. In Vitro Cellular & Developmental Biology - Plant 2020, 56, 718–725. [Google Scholar] [CrossRef]
- Faria, T.; Cezar, J.; Cézar, J.; Ribeiro-Kumara, C.; Silva Da Rocha Costa, R.; Nieri, E. M.; De Carvalho, D.; Brasil, J. E.; Pinto, P.; Rodrigues, A.; Neto, S.; Brondani, G. E. Use of Biodegradable Polyester-Based Microvessels for Micropropagation of Mature Eucalyptus Microcorys. N Z J For Sci 52, 1–13. [CrossRef]
- Skupa Petr and Opatrný, Z. and Opatrný, Z. and P. J. Auxin Biology: Applications and the Mechanisms Behind. In Applied Plant Cell Biology: Cellular Tools and Approaches for Plant Biotechnology; Nick Peter and Opatrny, Z., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014; pp. 69–102. [Google Scholar] [CrossRef]
- Teale, W. D.; Paponov, I. A.; Palme, K. Auxin in Action: Signalling, Transport and the Control of Plant Growth and Development. Nat Rev Mol Cell Biol 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Bunn, E. Development of in Vitro Methods for Ex Situ Conservation of Eucalyptus Impensa, an Endangered Mallee from Southwest Western Australia. Plant Cell Tissue Organ Cult 2005, 83, 97–102. [Google Scholar] [CrossRef]
- Kaur, S. In Vitro Regeneration of Shoots From Nodal Explants of Dendrobium Chrysotoxum Lindl. Journal of Horticultural Research 2017, 25. [Google Scholar] [CrossRef]
- WOODWARD, A. W.; BARTEL, B. Auxin: Regulation, Action, and Interaction. Ann Bot 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Chen, J.; Ziv, M. The Effect of Ancymidol on Hyperhydricity, Regeneration, Starch and Antioxidant Enzymatic Activities in Liquid-Cultured Narcissus. Plant Cell Rep 2001, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Brondani, G. E.; de Wit Ondas, H. W.; Baccarin, F. J. B.; Gonçalves, A. N.; de Almeida, M. Micropropagation of Eucalyptus Benthamii to Form a Clonal Micro-Garden. In Vitro Cellular & Developmental Biology - Plant 2012, 48, 478–487. [Google Scholar] [CrossRef]
- Gomes, F.; Canhoto, J. M. Micropropagation of Eucalyptus Nitens Maiden (Shining Gum). In Vitro Cellular & Developmental Biology - Plant 2003, 39, 316–321. [Google Scholar] [CrossRef]
- Ngomuo, M.; Mneney, E.; Ndakidemi, P. A. The <I>in Vitro</I> Propagation Techniques for Producing Banana Using Shoot Tip Cultures. Am J Plant Sci 2014, 05, 1614–1622. [Google Scholar] [CrossRef]
- Al-Aizari, A. A.; Al-Obeed, R. S.; Mohamed, M. A. H. Improving Micropropagation of Some Grape Cultivars via Boron, Calcium and Phosphate. Electronic Journal of Biotechnology 2020, 48, 95–100. [Google Scholar] [CrossRef]
- George Edwin, F. and Hall, M. A. and K. G.-J. De. The Components of Plant Tissue Culture Media I: Macro- and Micro-Nutrients. In Plant Propagation by Tissue Culture: Volume 1. The Background; George Edwin F. and Hall, M. A. and K. G.-J. De, Ed.; Springer Netherlands: Dordrecht, 2008; pp. 65–113. [Google Scholar] [CrossRef]
- Pérez-Tornero, O.; Burgos, L. Different Media Requirements for Micropropagation of Apricot Cultivars. Plant Cell Tissue Organ Cult 2000, 63, 133–141. [Google Scholar] [CrossRef]
- WHITE, P. J.; BROADLEY, M. R. Calcium in Plants. Ann Bot 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Al-Mayahi, A. M. W. Effect of Calcium and Boron on Growth and Development of Callus and Shoot Regeneration of Date Palm Barhee. Canadian Journal of Plant Science 2020, 100, 357–364. [Google Scholar] [CrossRef]
- Brunoni, F.; Rolli, E.; Dramis, L.; Incerti, M.; Abarca, D.; Pizarro, A.; Diaz-Sala, C.; Ricci, A. Adventitious Rooting Adjuvant Activity of 1,3-Di(Benzo[d]Oxazol-5-Yl)Urea and 1,3-Di(Benzo[d]Oxazol-6-Yl)Urea: New Insights and Perspectives. Plant Cell, Tissue and Organ Culture (PCTOC) 2014, 118, 111–124. [Google Scholar] [CrossRef]
- Lopes da Silva, A. L.; Gollo, A.; Brondani, G.; Horbach, M.; Oliveira, L.; Machado, M.; LIMA, K.; Costa, J. Micropropagation of Eucalyptus Saligna Sm. from Cotyledonary Nodes. Pak J Bot 2015, 47, 311–318. [Google Scholar]
- Sharma, S.; Ramamurthy, V. Micropropagation of 4-Year-Old Elite Eucalyptus Tereticornis Trees. Plant Cell Rep 2000, 19, 511–518. [Google Scholar] [CrossRef]
- Gago, D.; Vilavert, S.; Bernal, M. Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The Effect of Sucrose Supplementation on the Micropropagation of Salix Viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors. Forests 2021, 12. [Google Scholar] [CrossRef]
- Joshi, I.; Bisht, P.; Sharma, V. K.; Uniyal, D. P. In Vitro Clonal Propagation of Mature Eucalyptus F1 Hybrid (Eucalyptus Tereticornis Sm. x E. Grandis Hill Ex. Maiden). Silvae Genet 2003, 52, 110–113. [Google Scholar]
- Trueman, S. J.; Richardson, D. M. In Vitro Propagation of Corymbia Torelliana C. Citriodora (Myrtaceae) via Cytokinin-Free Node Culture. Aust J Bot 2007, 55, 471–481. [Google Scholar] [CrossRef]
- Sandhu, M.; Wani, S. H.; Jiménez, V. M. In Vitro Propagation of Bamboo Species through Axillary Shoot Proliferation: A Review. Plant Cell, Tissue and Organ Culture (PCTOC) 2018, 132, 27–53. [Google Scholar] [CrossRef]
- Nadgauda, R. S.; Parasharami, V. A.; Mascarenhas, A. F. Precocious Flowering and Seeding Behaviour in Tissue-Cultured Bamboos. Nature 1990, 344, 335–336. [Google Scholar] [CrossRef]
- Shen, G.; Tan, S.; Sun, X.; Chen, Y.; Li, B. Experimental Evidence for the Importance of Light on Understory Grass Communities in a Subtropical Forest. Front Plant Sci 2020, 11. [Google Scholar] [CrossRef]
- Mankessi, F.; Saya, A.; Baptiste, C.; Nourissier, S.; Monteuuis, O. In Vitro Rooting of Genetically Related Eucalyptus Urophylla × Eucalyptus Grandis Clones in Relation to the Time Spent in Culture. Trees 2009, 23, 931–940. [Google Scholar] [CrossRef]
- Fett-Neto, A. G.; Fett, J. P.; Goulart, L. W. V.; Pasquali, G.; Termignoni, R. R.; Ferreira, A. G. Distinct Effects of Auxin and Light on Adventitious Root Development in Eucalyptus Saligna and Eucalyptus Globulus. Tree Physiol 2001, 21, 457–464. [Google Scholar] [CrossRef]
- Fogaça, C. M.; Fett-Neto, A. G. Role of Auxin and Its Modulators in the Adventitious Rooting of Eucalyptus Species Differing in Recalcitrance. Plant Growth Regul 2005, 45, 1–10. [Google Scholar] [CrossRef]
- Watt, M. P.; Thokoane, N. L.; Mycock, D.; Blakeway, F. In Vitro Storage of Eucalyptus Grandis Germplasm under Minimal Growth Conditions. Plant Cell Tissue Organ Cult 2000, 61, 161–164. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and Application of Photoautotrophic Micropropagation Plant System. Plant Cell, Tissue and Organ Culture (PCTOC) 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Miranda, N. A.; Xavier, A.; Otoni, W. C.; Gallo, R.; Gatti, K. C.; de Moura, L. C.; Souza, D. M. S. C.; Maggioni, J. H.; Santos, S. S. de O. Quality and Intensity of Light in the In Vitro Development of Microstumps of Eucalyptus Urophylla in a Photoautotrophic System. Forest Science 2020, 66, 754–760. [Google Scholar] [CrossRef]
- Frade, S. R. do P.; Souza, D. M. S. C.; Fernandes, S. B.; Avelar, M. L. M.; Molinari, L. V.; Gonçalves, D. S.; Magalhães, T. A.; Brondani, G. E. Spectral Quality Influence on in Vitro Morphophysiological Responses of Eucalyptus Dunnii Maiden and Eucalyptus Grandis w.Hill Ex Maiden x E. Urophylla s.t.Blake. N Z J For Sci 2023, 53. [Google Scholar] [CrossRef]
- Souza, D. M. S. C.; Avelar, M. L. M.; Fernandes, S. B.; Silva, E. O.; Duarte, V. P.; Molinari, L. V.; Brondani, G. E. Spectral Quality and Temporary Immersion Bioreactor for in Vitro Multiplication of Eucalytpus Grandis × Eucalyptus Urophylla. 3 Biotech 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Matheus, D.; Souza, S. C.; Martins, A. R.; Fernandes, S. B.; Lopes Martins Avelar, M.; Vaz Molinari, L.; Santos Gonçalves, D.; Brondani, G. E. In Vitro Multiplication of Eucalyptus Pilularis and Eucalyptus Grandis x E. Urophylla (Urograndis Eucalypt): Effect of Light Quality in Temporary Immersion Bioreactor; 2022; Vol. 20.
- Roque-Borda, C. A.; Kulus, D.; de Souza, A.; Kaviani, B.; Vicente, E. F. Cryopreservation of Agronomic Plant Germplasm Using Vitrification-Based Methods: An Overview of Selected Case Studies. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Padayachee, K.; Watt, M. P.; Edwards, N.; Mycock, D. J. Cryopreservation as a Tool for the Conservation of Eucalyptus Genetic Variability: Concepts and Challenges. Southern Forests: a Journal of Forest Science 2009, 71, 165–170. [Google Scholar] [CrossRef]
- Blakesley, D.; Kiernan, R. Cryopreservation of Axillary Buds of a Eucalyptus Grandis x Eucalyptus Camaldulensis Hybrid. Cryo Letters 2001, 22, 13–18. [Google Scholar] [PubMed]
- Hung, C. D.; Trueman, S. J. Preservation of Encapsulated Shoot Tips and Nodes of the Tropical Hardwoods Corymbia Torelliana × C. Citriodora and Khaya Senegalensis. Plant Cell, Tissue and Organ Culture (PCTOC) 2012, 109, 341–352. [Google Scholar] [CrossRef]
- Costa Souza, D. M. S.; Fernandes, S. B.; Avelar, M. L. M.; do Prado Frade, S. R.; Molinari, L. V.; Gonçalves, D. S.; Pereira Pinto, J. E. B.; Brondani, G. E. Light Quality in Micropropagation of Eucalyptus Grandis × Eucalyptus Urophylla. Scientia Forestalis/Forest Sciences 2020, 48. [Google Scholar] [CrossRef]
| Explant-source/type | Culture media/PGRs/additives | Experimental outcomes/remarks/productivity/root hardening etc. | References |
|---|---|---|---|
| Nodal segments | MS media, WPM, 0.5 mgL-1 IBA, 1.0mgL-1 BAP, B5, NAA, Kinetin | Full strength MS media supplemented with 1.0mgL-1 BAP showed best shoot elongation; ½ MS media supplemented with 0.5 mgL-1 IBA showed best rooting; the resulting hybrid produced yields 3-5 times more wood than parental species | [5] |
| Nodal segments (30–32-year trees) | MS media, WPM, B5 medium, SH medium, IBA, BAP, NAA, | Best in-vitro studies were obtained from the explants collected during the period of January to February and August to September; ½ MS media + BAP resulted in best rooting (92%); Up to 98% plants survived acclimatization | [6] |
| Stem cuttings and axillary buds | MS Media, 2.22 µm BA, 1.16µm Kinetin, 0.029µm gibberellic acid, 400mgL-1 PVP, 30gL-1 sucrose | Micropropagation and micro cutting showed higher adventitious rooting (24.8-100% and 43-95% respectively) than stem cuttings (9.3-75.5%) | [12] |
| Maintained Elite clones (KE8, CE2, T1, and Y8) | Basal MS media, 2.5 µM BA, 0.5 µM NAA, D-mannitol (0, 250, 500, 750, and 1000 mM) | Culture Growth Index of all clones was reduced significantly because of drought stress | [17] |
| - | Full and ½ strength MS media | Concentrations of IAA, IBA as well the stem anatomy has no effect on the rooting potential of shoots | [22] |
| Nodal cuttings | IAA, IBA (0, 1, 3, 8gKg-1) | The position from which explant is harvested can affect the rooting potential and seed vigor. Explants obtained from 7/8 and 9/10 apical position showed enhanced rooting and shooting | [25] |
| Cuttings from 6-month-old parental plants | MS media, IAA, meta-topolin, kinetin, BAP, vit. B5, Biotin, Sucrose | 0.5mgL-1 meta-topolin and 1mgL-1 IAA enhanced shoot elongation as well bud proliferation while 0.5mgL-1 IAA resulted in the most consistent rooting percentages. Moreover, equal expression of AUX1 and PIN1 transporter genes increased responsiveness towards PGRs | [29] |
| Nodal segments | MS media, 58mM sucrose, 0.5 μM NAA, 2.5 μM BA, | Media supplemented with 1.0 μM 2,4-D, 5.0 μM BA and 500 mgL-1 cefotaxime showed maximum (44.6%) shoot bud organogenesis | [33] |
| Nodal segments | MS media, 2mgL-1 BAP, 0.1mgL-1 NAA | Media supplemented with 0.5mgL-1 showed best shoot elongation, 1/2MS + 1mgL-1 IAA Showed best root induction and elongation, and direct regeneration was observed in MS + 20:1 BAP: NAA | [34] |
| Young shoot segments | WPM, MS media, 2iP, NAA, BAP, sucrose | Media suppelmented with BA resulted in 99% shoot proliferation, media supplemented with 2iP resulted in 93% shoot regeneration and IBA promoted rooting in 60% of the clones. | [35] |
| Nodal segments | EDM basal media (a novel basal media for E. dunnii) supplemented with 20gL-1 sucrose and without PGRs | Higher Fe, Cu, Zn and Mn concentrations in EDMm media increased rooting. Moreover, high S and K concentrations in EDMm increased growth rate and multiplication. Also no Fe chlorosis / oxidation was observed in shoots cultured on EDMm | [37] |
| Zygotic embryo | One of the following media and growth regulators: ½ or full-strength MS media/ WPM/ B5/ DKW/ JADS media/ 3mgL-1 NAA/ 10mL-1 silver nitrate/ 0.5mgL-1 DTT/ 100mgL-1 ascorbic acid/ 0.5mgL-1 DTE/ 1%m/v PVP/ 1% m/v PVPP/ 0.01% w/v activated charcoal | The best media for somatic embryogenesis was B5 and MS. Moreover, Silver nitrate, activated charcoal and DTE reduced browning of explants | [41] |
| Somatic embryos | MS media supplemented with 3mgL-1 NAA | MS media scarce of PGRs is highly efficient to promote cotyledonary embryo proliferation and germination | [42] |
| Zygotic embryo cotyledons | Hormone free MS media | Reserve accumulation of mature zygotic embryos was analyzed. Cotyledonary somatic embryos possess low density of starch and no lipids/ proteins. | [43] |
| Axillary shoots | ½ MS, 4.4μM, 1μM NAA, 1gL-1 sucrose | WPM and QL media supplemented with Gibberlic acids showed enhanced shoot proliferation, ½ WPM supplemented with 20 μM IBA showed enhanced rooting, 67% Plantlet hardening was achieved | [47] |
| Shoot segments | MS media, 0.02mgL-1 IBA | Vitron vessel placed in Low Photon Flux density at 3000ppm CO2 for 24 hours/ day yielded best growth and quality of plantlets | [52] |
| Seedlings raised from seeds | - | Low temperatures 18°C/13°C to 23°C/18°C (day/night) reduced the number of harvested cuttings however did not affect the percentage of roots proliferated from cuttings. While, increasing temperature 33°C/28°C resulted in an increased number of cutting per stock plant. | [53] |
| Hypocotyl segments and cotyledonary leaves | MS media supplemented with different concentrations of NAA and TDZ, 0.8gL-1 PVP, 0.1gL-1 biotin, 0.1gL-1 calcium pantothenate, 30gL-1 sucrose | 0.44 µM BAP increased regeneration of adventitious buds | [59] |
| Zygotic embryos and cotyledons | MS media supplemented with 3gL-1 sucrose and different concentrations of NAA, 2,4-D, BA, ABA | 1mgL-1 NAA resulted in maximum callus induction, the frequency of callus proliferation depends on the age of explant with 10-year-old explants showing maximum proliferation, highest frequency of somatic embryogenesis was observed in callus from mature zygotic embryos, low ABA concentrations increased number of somatic embryos | [60] |
| Nodal segments | 1/2 MS supplemented with different concentrations of BAP, NAA and GA3 | 0.050 mgL-1 BAP achieved optimal bud proliferation + 0.50 mgL-1 NAA while ½ MS media supplemented with 0.2-1 and 0.10 mgL-1 GA3 + 0.10 mgL-1 BAP showed highest shoot elongation | [61] |
| Nodal segments | MS media without PGRs | Media free from GA3 + BAP resulted in best shoot elongation, WPM + 0.05mgL-1 NAA + 0.5mgL-1 BAP resulted in maximum axillary bud proliferation | [71] |
| Nodal segments | ½ MS media, De-Fossard Medium, 0.9 µgL-1 BA, 0.5 µM NAA | Best multiplication rate (2.25) was achieved, 93% of the plants survived acclimatization | [72] |
| Nodal segments | MS media supplemented with 0.05 μM NAA, 0.4 μM BA, 1mgL-1 nicotinic acid, 1mgL-1 pyridoxine-HCl, 1mgL-1 thiamine, 2mgL-1 glycine, 50mgL-1 myo-inositol, 30gL-1 sucrose | Endogenous rhythms cause time related fluctuations resulting in rooting variations among closely related genotypes | [88] |
| Epicotyl segments | ½ MS supplemented with 1/6x CaCl2, 2% (w/v) sucrose | Auxin reduced mean rooting time, light conditions did not affect the rooting efficiency, with increased age decreased rooting capability was observed | [89] |
| Axillary buds | ½ MS supplemented with 1gL-1 ABA | Encapsulation by calcium alginate and storing under low light intensities resulted in the preservation of cultures for up to 3 months without affecting its viability | [91] |
| Apical shoots | MS media supplemented with 0.04mgL-1 BA, 1% Sucrose, with/ without charcoal | 38%-85% survival was observed with plants exposed to PSV2 for 30 min in liquid nitrogen | [99] |
| Nodal segments | MS media supplemented with 30gL-1 sucrose | Best in-vitro establishment, multiplication, shooting, and rooting was achieved by Red-Blue LEDs and Fluorescent lamps | [101] |
| Species | Explant | Sterilant | Media | PGR (if any) | Area studied | Scope of work | References |
|---|---|---|---|---|---|---|---|
| E. camaldulensis × E. tereticornis and E. torelliana × E. citriodora | Nodal segments from mature trees (30-32 yrs) | 0.15% HgCl2 |
MS medium, WPM, SH medium, B5 medium | BAP, NAA | Hybridisation of Eucalypt species | Two hybrids developed that showed suprior performance than parental genotypes | [17] |
| E. grandis × E. nitens | Axial buds | 10g l-1 CaOCl | MS media | BAP, IAA, metatopolin, kinetin | Individual evalualtion of each stage of micropropagation | Auxins are principal components of media and expression of different auxin transporters might be used as markers to identify Eucalypt spp amenable for micropropagation | [29] |
| E. erythronema × E. stricklandii | Seedlings germinated in vitro | 3% NaOCl | MS media supplemented with sucrose 30gL-1 | IBA, NAA, Gibberlic acids | First micropropagation report of ornamemtal Eucalypt spp. | Successful micorpropagation from juvenile seedlings acheived | [47] |
| E.benthamii × E. dunni | Nodal segments from 1 year old plants | NaOCl | ½ strength MS media | PVP40, NAA, BAP | Optimization of chlorine concentration for explant sterlisation and optimum ratio of PGRs for shoot elongation | 0.5% NaOCl is suggested for nodal segments ; 0.50 mg L-1 BAP + 0.05 mg L-1 NAA provides highest number of bud proliferation | [61] |
| E. erythronema × E. stricklandii | Nodal segments | 1% NaOCl | MS media supplemented with sucrose 30gL-1 | 0.05μM NAA and 2.22μM BAP | Effect of different light intensities on micropropagation efficiency | Red/ blue LEDs and Florescent light results in higher vigor, High photosynthesis, increased shoot and root proliferation | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





