1. Introduction
Idiopathic macular hole (IMH) is a common cause for impairment of vision in adults. The annual incidence of IMH has been shown to be 8.69 eyes per 100,000 individuals [
1]. Although it primarily occurs in adults, it may also be diagnosed in children [
2,
3]. In previous studies, IMH was described as an age- and even more as a sex-related disorder. It should be noted that females have been linked to a three-fold higher risk of developing IMH compared to males [
1], and a higher incidence is especially observed in females with advanced age [
4]. Therefore, understanding the role of female sex hormones is important to elucidate the etiology of this disease.
The retina originates from the central nervous system (CNS) and is already influenced by sex hormones in the fetal stage. It has been shown that the CNS of the male fetus is primed by high levels of testosterone [
5]. Therefore, understanding the biochemical events occurring in the brain may assist in understanding retina diseases, especially those related to gender. The majority of age-related changes in retinal gene expression are sexually divergent [
6] and sex as well as age may influence the function and structure of the retina [
7]. Moreover, the neuroprotective effect of estrogen on retinal cells has been repeatedly reported [
8,
9,
10]. While IMH has been discussed extensively in the literature before, newly obtained evidence from research on the retina suggests a revaluation of the pathogenesis of IMH from a new therapeutic perspective. Driven by the high incidence of IMH in females, this review aims to identify new possible reasons for the development of this disease. It is possible, that the etiology of IMH has an additional hormonal and neurodegenerative nature due to the biochemical effect of the estrogen hormone and the morphological anatomical structure of the fovea.
Previous studies have reported several systemic and ocular factors that are associated with the development of IMH, namely female sex, advanced age [
4], high levels of plasma fibrinogen, a history of glaucoma, chymase [
11], angiotensin-converting enzyme [
12], and a shorter axial length [
13]. In addition, IMH can also occur at a young age, although it was then associated with severe myopia [
14]. Diabetes has been shown to be protective against IMH; however, this aspect remains unclear [
4]. Chymase influence could be explained with the inhibition of Müller cell proliferation, which possesses atypical properties near the macular region and fibrosis and apoptosis through these cells [
15], or through the production and degeneration of collagen [
16]. High levels of fibrinogen may increase susceptibility to the forces of vitreous traction, perhaps by compromising the macular blood supply [
11]. The role of the vitreous during the development of IMH has been extensively studied. Eyes with macular holes demonstrated significant early stages of vitreous degeneration [
17]. IMH has been thought to be caused by focal shrinkage of the vitreous cortex in the foveal area [
18], through tangential traction in the thin and elastic premacular vitreous cortex and formation of the posterior wall of the premacular liquefied pocket [
19]. Another study suggested that dynamic vitreous traction is associated with ocular rotations due to perifoveal vitreous detachment of the posterior hyaloid from the pericentral retina, foveal dehiscence, and the exertion of anterior traction on the foveola [
20]. The hydration theory states that the development of IMH may be due to a defect in the inner retina with secondary vitreous fluid accumulation in the middle and outer retinal tissue [
21]. Subsequent studies, using mathematical and physical models to determine the evolution of IMH formation, have reported that eyes with V-shaped vitreofoveal attachment demonstrated initial structural changes in the outer foveal layers, while the eyes with U-shaped attachment showed inner morphological changes [
22]. Müller glial cells have been thought to play a role in the pathogenesis of IMH. This could be explained by the migration induction of Müller glial cells through the complement pathway and α2 macroglobulin (α2M), which then contributes to epiretinal membrane formation in IMH [
23]. Lastly, estrogen and the role of sudden changes in hormonal balance are involved in the development of IMH [
4]. The latter will be discussed in more detail in this review.
2. Biochemistry
2.1. Retina and estrogen
Studies observed the expression of estrogen receptor (OR) mRNA in the ganglion cell layer, the inner nuclear layer, and outer portion of the outer nuclear layer of the retina [
24]. It was found that mRNA of ORβ and ORα were both present in the human ocular posterior segment and that ORβ protein was localized in the ganglion cell layer and the choroid [
25]. The estrogen receptor alpha (Erα) protein was detected in the retina as well as in the retinal pigment epithelium (RPE) of young female eyes, but not in eye tissue from males and post-menopausal females [
26].
There are three important types of estrogen, namely estrone (E1), estradiol (E2), and estriol (E3) [
27]. The levels of estradiol E2 have been suggested to be a significant factor in the preservation of healthy visual function, especially in older females [
27].
2.2. Protective effect of estrogen in the retina
Estrogen has been shown to be involved in the control of gene networks via estrogenrelated receptors (ERRs; nuclear receptors). These networks are involved in all aspects of energy homeostasis from fat and glucose metabolism to mitochondrial biogenesis [
28,
29]. The protective mechanism of βE2 occurs by means of antioxidative effects that are retraced through the activation of NRF2 via two pathways: a rapid, non-genomic PI3K/AKT response and a genomic-type ER-dependent response [
30]. A study on rats showed that administration of βE2 up-regulated NRF2, triggering the expression of phase-2 antioxidant enzymes (i.e., superoxide dismutase 1 and 2, catalase, glutaredoxins 1 and 2, and thioredoxins 1 and 2), reduced production of reactive oxygen species (ROS), and ameliorated retinal damage [
30]. Estrogen has been suggested to protect the RPE from oxidative stress. ARPE-19 cells, a human RPE cell line, expressed high levels of ERα and ERβ and 17-β estradiol (17β-E2), which protected ARPE-19 cells from oxidative stress through an ERβ-dependent mechanism. It has been demonstrated that 17β-E2–mediated cytoprotection occurred through the preservation of mitochondrial function, reduction of ROS production, and induction of cellular antioxidant genes [
31]. Therefore, it is not surprising that non-feminizing estrogens have been suggested as potentially useful compounds for the neuroprotection of retinal cells [
8]. Several studies have linked estrogen to the therapeutic strategy of certain retinal diseases. Examples include the use of estrogen as prophylactic therapy for Leber’s hereditary optic neuropathy by targeting ERβ [
32]. It was also suggested as therapy for glaucoma, since it reduces retinal ganglion cell loss induced by high intraocular pressure in rats [
33,
34]. Estrogen reduced the visual system damage and rescued photoreceptor cells after ocular blast in mice [
35]. In addition, it has been considered as a therapy for proliferative vitreoretinopathy and other proliferative retinal diseases. Thereby, it appears to inhibit TGF-β2–induced collagen contraction mediated by RPE cells by inhibiting the expression of the mesenchymal markers α-SMA and fibronectin, interleukin-6 release and TGF-β2-induced Smad2 and MLC phosphorylation [
36]. Moreover, in diabetic retinopathy, it has been demonstrated that 17β-estradiolum (E2) exerts protective effects on retinal ganglion cells in a high-glucose environment [
37].
2.3. Influence of estrogen on mitochondria and cone cells of the retina
Few previous studies have investigated the possible role of estrogen in cone cells. Identification of the genes expressed in cone photoreceptors has revealed that one of the isolated genes encoded ZBED4– a novel protein localized in cone photoreceptors and glial Müller cells in the human retina. Several putative ZBED4-interacting proteins have been identified, including a co-repressor of the ORα [
38]. A similar finding has been demonstrated in cone cells of zebra fish. It was noted that ES1 – a novel mitochondria-enlarging factor localized in cone mitochondria – contributes to the formation of mega-mitochondria in cone cells. Knockdown of ES1 markedly reduced mitochondrial size in cone cells. Therefore, it has been suggested that ES1 supports energy production in the zebra fish retina via mitochondrial enlargement [
39]. Theoretically, similar findings in the human retina would imply that estrogen may exert a direct effect on both types of cells (i.e., cone and Müller glial cells). Moreover, this finding suggests that cone cells are to a certain extent dependent estrogen levels for their energy production. Hence, sudden changes in the estrogen levels, such as those occurring during menopause, may exert harmful effects on cone cells, leading to their degeneration.
2.4. Role of estrogen in idiopathic macula hole
There are various findings related to the effect of estrogen in the development of IMH. Overall, a positive effect of estrogen against IMH has been reported in the literature [
11]. Indications for positive effects of estrogen against the pathogenesis of IMH were identified in RPE cells, where the female sex hormones 17β-estradiol and progesterone inhibited TGF-β2-induced collagen contraction [
36]. Another effect was found to be the induced estrogen inhibition of collagen gel contraction by glial cells [
40]. Controversially, in addition some publications reported the development of macular hole under estrogen treatment [
41,
42]. The first report evaluated the levels of estrogen in the vitreous body of patients with IMH and observed that vitreous E2 levels were higher than E1 levels. This result was attributed to the conversion of E2 from E1 by 17-β hydroxy-dehydrogenase and to the local production of estrogen based on the expression of P450 aromatase in the human eye. Other possibilities mentioned in the literature include the effect of pro-inflammatory cytokines promoting the aromatization of testosterone to E2 and glucuronidation altering E2 and E1 [
16]. Another study examined the possibility of neurosteroid production in the retina. They compared the levels of steroid hormone E2 and testosterone in the vitreous and in the serum of patients with vitreoretinal diseases (i.e., idiopathic epiretinal membrane, IMH, proliferative diabetic retinopathy, and rhegmatogenous retinal detachment). In all these vitreoretinal diseases, females had significantly higher E2 levels in the vitreous than in the serum. In contrast, males had significantly higher levels in the serum than in the vitreous body. Therefore, it has been suggested that the synthesis of E2 is locally increased only in female eyes. The investigators proposed differences between males and females in the quantity of aromatase produced in the brain and retina. They concluded that this high vitreous E2 level in females is increased due to the production of aromatase activated by reactive astrocytes because of the damage caused by these diseases. The production of E2 by astrocytes and Müller cells may be responsible for the suspected local production of E2 [
43]. In conclusion, the elevation in vitreous estrogen levels should be considered separately from the development of IMH through decreased systemic estrogen levels as observed during menopause, after hysterectomy, or after systemic anti-estrogen treatment. Yet, the protective effect of estrogen against the development of IMH is outweighing reported. This knowledge may be confirmed through anti-estrogen treatment. It should be mentioned that some studies have reported a relationship between anti-estrogen treatment (e.g., tamoxifen) and the development of IMH [
44,
45,
46]. However, the exact mechanism involved in this process remains unknown. The effect of tamoxifen on Müller cells has already been studied. Müller glial cells influence other retinal cells and play important roles in the retina, including reprogramming, regeneration, and restoration of vision [
47]. On the other hand, an influence on RPE cell has been reported. Thus, it has been found that treatment with tamoxifen and toremifene may result in retinal defects. This occurs via a dose-dependent decrease in the glutamate uptake of RPE cells, which has been noted in porcine RPE cells as well as in human RPE cell lines [
48]. Also, it has been proposed, that a toxicity of both photoreceptor and RPE cells caused by anti-estrogen drugs may be contributed to the development of anti-estrogen maculopathy [
49].
3. Anatomical and morphological effects of estrogen on the fovea
3.1. Müller glia and cone cells in the theory of Gass
The structure of the fovea regarding Müller glia and cone cells was first described by Yamada [
50]. This finding was subsequently used by Gass in forming his hypotheses about IMH formation, based on the finding that the inner half of the fovea is composed of an inverted cone-shaped zone of Müller cells (Müller cell cones). The more interesting hypothesis was the hypothesis, suggesting that Müller cell cones bind the receptor cells in the fovea together. Thus, the Müller cell cone is the primary structural support for the fovea. Gass presumed that an absence of this plug of glial cells results in formation of a macular hole [
51]. An anatomical association between cone and IMH was also reported by Ezra [
52]. He suggested that the variation in cone photoreceptor density in the operculum (40%–50% contain photoreceptors) probably reflects the amount of foveal tissue avulsed during the formation of the hole. He corroborated his hypothesis through previously obtained clinicopathological findings, showing a lower anatomical success rate following surgery in the operculum [
52].
3.2. Morphological changes in the retina induced through treatment with tamoxifen
The morphological changes in the retina following treatment with tamoxifen, a selective OR inhibitor, include foveal cystic changes with an outer retinal defect determined through optical coherence tomography (OCT) [
44,
53]. Also, a macular pigment absorption and incorporation in retinal tissue after longer tamoxifen use has been suggested [
54]. Gualino et al. used OCT analysis to reveal a foveolar cystoid space with focal disruption of the photoreceptor line. However, there was no evidence of macular oedema or thickening [
55]. This morphological finding due to tamoxifen treatment may be related to the direct effect of tamoxifen on the OR in the fovea cone, which is probably responsible for the disruption of the photoreceptors. This hypothesis may be confirmed using ES1 ,a novel mitochondria-enlarging factor localized in the cone mitochondria [
39]. The influence of menopause, hence a lack of estrogen, on the retina may be like that observed following treatment with tamoxifen. Furthermore, a thinning in the parafoveal regions has been observed in females compared with males [
56]. In addition, the incidence of IMH in females has been reported to increase with age. This may be simply attributed to the anatomical changes observed in the retina due to ageing. It should be noted that a morphological examination of the retina using OCT showed that the inner retinal thickness decreased by 0.5 μm per year and was 6.1 μm thinner in females compared to males [
57]. Therefore, considering the important role of Müller glial cells; the natural architecture of the macula, which showed that the inner layer of the fovea is formed by Müller cell cone [
50] and the consideration of the previously reported effects of estrogen on retina cells. All these factors together may explain the development of IMH and not elsewhere in the retina following treatment with tamoxifen or lack of estrogen.
4. Functional examination
4.1. Postive effect of estrogen on vision
It has been proposed that hormonal changes may be linked to alterations in retinal function [
58]. Studies have shown differences between the retina in males and females. Thus, it has been determined that the female retina has a better function than the male one. Using multifocal ERG, it was demonstrated that implicit time (IT) results vary inversely with the presumptive levels of estradiol in both sexes. Shorter ITs were noted in younger females compared to males, while similar ITs were detected in older females and males, and the longest ITs were seen in females with hysterectomies. The neuroretinal function in older females was clearly worse than that in younger females. In contrast, the neuroretinal function did not differ between younger and older males in all IT evaluations. A possible explanation for these results is the involvement of age-related changes in the average estradiol levels [
59]. The positive effect of estrogen on the retina has been reported by Chaychi et al. Through ERG, they showed that sex and age may influence retinal function and structure in rats. Furthermore, the ERG of premenopausal female rats was compared to that observed in menopausal female rats as well as male rats. They demonstrated that cycled female rats had better retinal function compared to menopausal female rats and suggested a beneficial effect of the estrus cycle on retinal function [
7]. A similar investigation was found, as pattern reversal evoked potentials (PRVEPs) examination has been recorded in females in four different phases of the menstrual cycle. The results showed that the PRVEP latency recorded during the ovulatory phase was statistically significantly shorter than that of other phases. The significant decrease in PRVEP latencies was attributed to the effect of estrogen on neural transmission of the visual pathways [
60].
4.2. The role of the cone cells in the development of idiopathic macula holes
Cone cells are high energy-consuming cells, they are using more ATPs for the activation of transducin and rhodopsin phosphorylation than rods [
61]. To the best of our knowledge, there are to date only two studies that examined the function of cone cells in the development of IMH.
The first study was conducted by Andréasson et al. The preoperative retinal function was measured using full-field ERG and multifocal ERG was correlated to postoperative visual acuity after surgery for the treatment of IMH. It was shown that the cone IT in the full-field 30-Hz flicker ERG, reflecting retinal function, was significantly prolonged prior to surgery compared to that of aged-matched controls. Thus, the cone IT in full-field 30-Hz flicker ERG has been suggested as a predictor of visual outcome after surgery for the treatment of IMH [
62].
The second study was performed by Ştefănescu-Dima et al. The aim of this study was to highlight the anatomical and functional changes in early stages of posterior vitreous detachment (PVD), using OCT and full-field ERG, allowing in time and correct treatment. The response of cone cells determined through full-field ERG was also proposed as a marker of retinal damage in macular pathology due to PVD. Moreover, it may be used to evaluate retinal function in macular pathology [
63]. Furthermore, a recent study demonstrated that the diameter of inner/outer segment and external limiting membrane defects is one of the important factors affecting best corrected visual acuity in IMH [
64].
5. New epidemic study
A new epidemic study examined risk factor in IMH development among a large population of about 4,500,000 individuals. According to the study, IMH occurred earlier in woman than man. Also, postmenopausal women with two or more children showed a greater risk of IMH than those who had not been pregnant (hazard ratio: 1.8) [
65]. This is a very interesting result. It is known that hormonal changes regarding the estrogen level occur during pregnancy. Estrogen concentration increases constantly during pregnancy, achieving a peak, then it decreases suddenly at the end of the pregnancy.
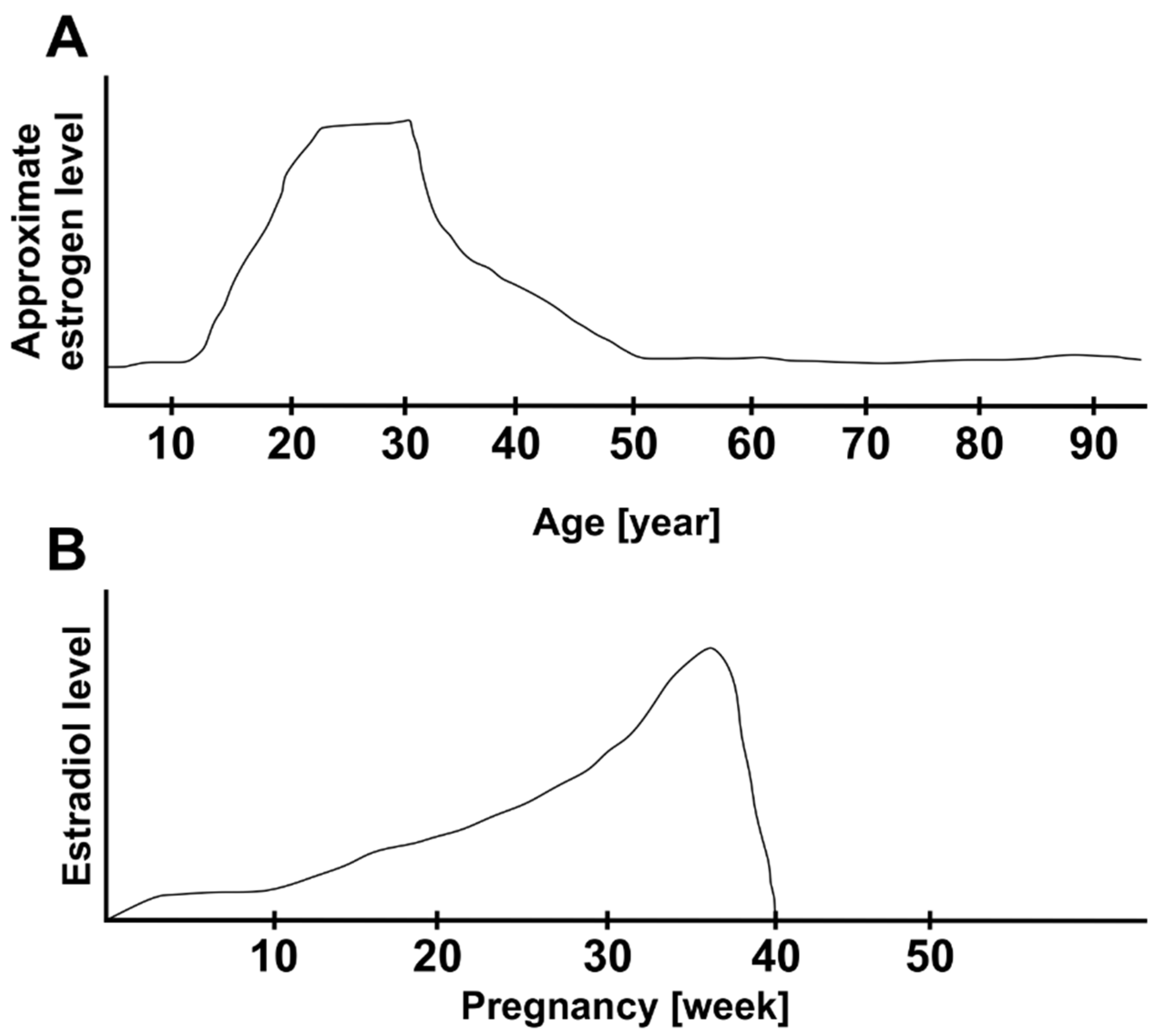
Figure 1 shows gives an overview over the estrogen level change during lifetime (A) and during pregnancy (B) []. This increase of estrogen levels during pregnancy and considerable abruptly decrease at the end may have a crucial effect the inverted cone shaped zone of Müller cells. This might, on the one hand, induce the first weakening to the fovea architecture and on the other hand effect the vitreous cortex. At a young age, these proposed effects of estrogen may be compensated after a normalization of estrogen level post pregnancy and the changes in fovea are reversible. A repeated and rapidly but irreversible decrease of estrogen levels as it occurs during menopause may weaken the cohesion of Müller cell cones irreversibly and finally destroy the plug of glial cells, which results in macular hole. This upregulation and then rapid downregulation of the estrogen level during pregnancy may have an intense effect on the fovea. This might explain the association between childbirth and an increasing risk of IMH development. Another very interesting point, which has been reported in the epidemic study of Hwang et al. is that the history of postmenopausal replacement hormone therapy (HRT) was not associated with the risk of IMH development. So, the authors concluded that this finding dost not support the estrogen theory [
67]. We assume, that the rapid change of estrogen levels is more relevant than a possible late exogen estrogen treatment, which probably occurs later in postmenopausal period. Postmenopause may be noticed late, it is often diagnosed in retrospective, usually due to the absence of menstruation. In this not diagnosable time until the supply of HRT, the sudden drop of estrogen might already be setting in and the crucial changes in cone cell und Müller cells occur leading to IMH development.
6. Discussion
As a mentioned, different studies try to explain the estrogen influence in IMH development in various ways. There are a few experimental studies, including histologic and biochemic evaluations, and some epidemic studies. A summary of relevant studies is emphasized in table 1. Despite the different results and the sometimes small study size, the majority of the studies points in the same direction as the recent epidemic study by Sunsoon Hwang and colleges [
67]. Yet, all these studies are unable to explain the certain molecular and biochemical mechanisms underlying IMH development especially regarding the cone Müller cells.
Retrospectively, Gass built a gold standard in regard to explaining IMH formation with his hypothesis [
51]. At his time, he was unable to confirm his hypothesis at the molecular level. Since Gass work, enormous technological advantages in retina imaging, functional measurements, and biochemical and molecular evulations happend.
This review connected the hypothesis of Gass and the molecular and the hormonal effect of estrogen on cone and Müller glial cells. It is remarkable, that Gass came up with this great hypothesis even without detailed knowledge about molecular events in cone cells and Müller glial cells under estrogen influence. Due to all these findings regarding the positive effect of estrogen against IMH development, we assume IMH has a neurodegenerative nature. It needs to be discussed, how to use this positive effect of estrogen against IMH. But a systemic supply of estrogen can provide undesirable side effects, for example high risk of systemic thrombosis, tumor development, and others. A creation molecule similar to estrogen similar and specific, which can adopt an estrogen receptor specific on cone cell, but without systemic adverse events. A local supply of estrogen eye drops may be revolutionary key word for future treatment. On the other hand, it is very important to identify the source of vitreous estrogen. Perhaps it possible to make a specific programming and intelligent activation of vitreous Astrocyte targeting locally estrogen production in fovea area. This idea needs more research in the future.
Table 1.
Summary of relevant studies.
Table 1.
Summary of relevant studies.
| Study |
Species |
Relevant results |
Category |
| Zhu C et al. 2015 |
Rats |
Protective mechanism of βE2 through antioxidative effects through NRF2 activation |
Positive effect of estrogen on retina |
| Giddabasappa A et al. 2010 |
Human |
17β-E2–mediated cytoprotection occurred through the preservation of mitochondrial function, reduction of ROS production, induction of cellular antioxidant genes |
| Honig MG et al. 2021 |
Mice |
Estrogen reduced the visual system damage and rescued photoreceptor cells after ocular blast in mice |
| Farber DB et al. 2010 |
Human |
ZBED4 a novel protein localized in cone cell and glial Müller cells, several putative ZBED4 interacting proteins including a co-repressor of the ORα |
| Masuda T et al. 2016 |
Zebra fish |
ES1, a novel mitochondria-enlarging factor localized in cone mitochondria, contributes to the formation of mega-mitochondria in cone cells |
| The Eye Disease Case-Control Study Group 1994 |
Human |
Positive effect of estrogen against IMH |
Epidemic study |
| Sungsoon H et al.2022 |
Human |
IMH occurs earlier in woman than man, postmenopausal women with two or more childern showed a greater risk for IMH than those who had not been pregnant |
| Kimura K et al. 2014 |
Human |
Positive effect of estrogen in RPE cells, estrogen appears to inhibit TGF-β2–induced collagen contraction |
Collagen
contraction study |
| Qiu QH et al. 2012 |
Human |
Estrogen can inhibit collagen gel contraction, which caused by cultured human retinal glial cells |
| James M et al. 1980 |
Human |
Development of macular hole under estrogen treatment |
Controverse study |
| McDonnell PJ et al. 1982 |
Human |
Development of macular hole under estrogen treatment |
| Inokuchi N 2015 |
Human |
Vitreous E2 level is higher than the E1 level in IMH patients |
| Nishikawa Y 2017 |
Human |
Production of E2 by astrocytes and Müller cells may be responsible for the suspected local production of E2 |
| Chung SE et al. 2010 |
Human |
Relationship between antiestrogen treatment and IMH development |
Antiestrogen study |
| Cronin BG et al. 2005 |
Human |
Relationship between antiestrogen treatment and IMH development |
| Torrell Belzach N et al. 2020 |
Human |
Relationship between antiestrogen treatment and IMH development |
| Mäenpää H et al. 2002 |
Human |
Tamoxifen: dose-dependent decrease in the glutamate uptake of RPE cells, which has been noted in porcine RPE cells as well as in human RPE cell lines |
| Yamada E et al. 1969 |
Human |
Structure of fovea in regard to Müller glia and cone cells |
Fovea structure |
| Gass JD et al. 1999 |
Human |
Important hypothesis, suggesting that Müller cell cones bind the receptor cells in the fovea together |
| Ezra E 2001 |
Human |
Anatomical association between cones and IMH |
| Yilmaz H et al. 1998 |
Human |
Pattern reversal evoked potentials (PRVEP) latency during the ovulatory phase was significantly shorter than that of other phases |
Retina function |
| Andréasson S et al. 2014 |
Human |
Cone IT in full-field 30-Hz flicker ERG has been suggested as a predictor of visual outcome after surgery for IMH treatment |
| Ştefănescu-Dima AŞ 2016 |
Human |
Response of cone cells determined through full-field ERG was proposed as a marker of retinal damage in macular pathology |
7. Summary
Gass reported about the opportunity of the Müller cell cone for the binding of the fovea structure [
51]. It is possible that morphological changes in the fovea, such as those observed in IMH, are partially related to changes in cone cells. This is to be interpreted by the abundant presence of cone cells in fovea region. As previously mentioned, the estrogen hormone influences Müller glia and cone cells [
38,
39]. Currently, our understanding of the precise effects of estrogen on cone cells is limited. The hormonal effect of estrogen and its biochemical involvement in the neuroretina is very complex. Therefore, even a daily regulation of ERRβ, a transcriptional regulator of energy metabolism protecting rod photoreceptors from dystrophy, in photoreceptor cells of rats has contributed to their adaptation to 24-hour changes in metabolic demands [
68]. It is possible that fovea cones as hard worker cells with high consumed energy [
61] through its sensitivity to estrogen hormone, which in turn notably is important for cell protective and in energy metabolism involved hormone [
28,
29,
30], react with degeneration of sudden decrease in estrogen level in menopause, which results in macular holes. This interpretation may be confirmed by the objectifiable measurement of the function of cone cells in patients with IMH. Another important finding is the difference between the systemic and local vitreous estrogen levels in IMH. However, this remains controversial with currently limited available evidence. Therefore, future studies focusing on vitreous estrogen levels in patients with retinal disease are needed. A summary illustration in
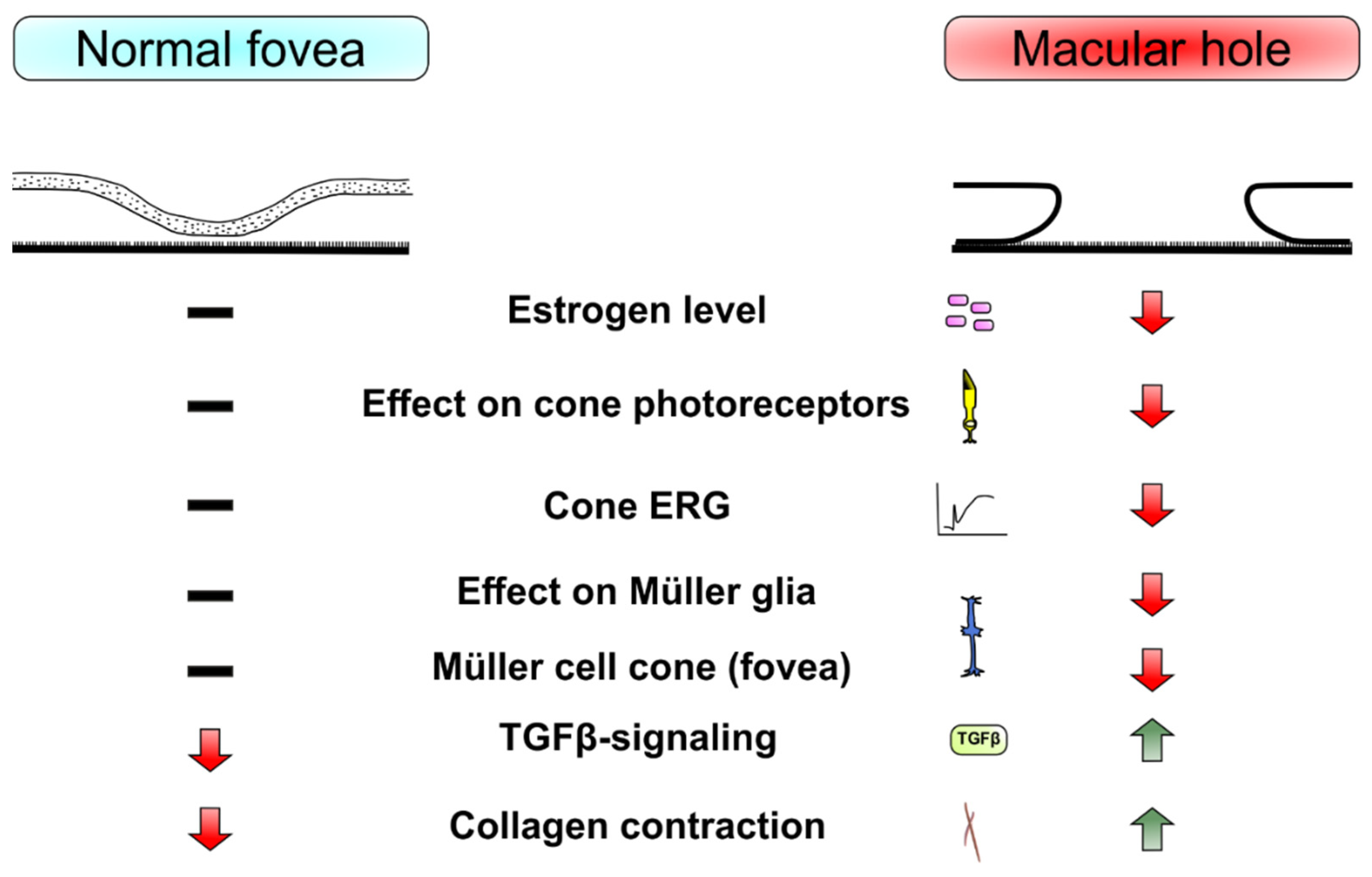
Figure 2 show a basic view of mentioned mean player in the development of IMH regarding to estrogen influence.
8. Conclusions
Understanding the effect of hormones is essential to better comprehend vitreoretinal disease and determine the most appropriate treatment. However, this research area is at an early stage. Fovea cone energy production probably depends on estrogen. Since the presence of OR in cone mitochondria, it may be possible, that the fovea cones through their sensibility to estrogen and through highly energy consumption are very vulnerable to damage through a sudden change in the systemic levels of estrogen in females. This may be key in improving the current strategies for the management of IMH. Finally, strategies aimed at exploiting the effects of estrogen on cone cells may play an important role in prophylactic therapy for IMH in the future.
Author Contributions
Conceptualization, N.W., H.B.D., S.C.J.; methodology, N.W, H.B.D., S.C.J.; software, N.W.; validation, N.W.; formal analysis, N.W.; investigation, N.W.; resources, N.W., H.B.D., S.C.J. and T.T.; data curation, N.W.; writing—original draft preparation, N.W.; writing—review and editing, N.W., H.B.D., S.C.J.; visualization, N.W., T.T.; supervision, N.W., T.T., S.C.J.; project administration, N.W.; funding acquisition, N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all co-workers involved in the conduct of the study for their commitment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology. 2009;116:1366-9. [CrossRef]
- Park JC, Frimpong-Ansah KN. Idiopathic macular hole in a child. Eye. 2012;26:620-1. [CrossRef]
- Lim LS, Fernandez-Sanz G, Levasseur S, Grigg JR, Hunyor AP. Idiopathic full thickness macular hole in a 10-year-old girl. Int J Retina Vitreous. 2018;4:25. [CrossRef]
- Evans JR, Schwartz SD, McHugh JD, Thamby-Rajah Y, Hodgson SA, Wormald RP et al. Systemic risk factors for idiopathic macular holes: a case-control study. Eye (Lond). 1998;12:256-9. [CrossRef]
- Weisz J, Ward IL. Plasma testosterone and progesterone titres of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306-16. [CrossRef]
- Du M, Mangold CA, Bixler GV, Brucklacher RM, Masser DR, Stout MB et al. Retinal gene expression responses to aging are sexually divergent. Mol Vis. 2017;23:707-17.
- Chaychi S, Polosa A, Lachapelle P. Differences in retinal structure and function between aging male and female Sprague-Dawley rats are strongly influenced by the Estrus cycle. PLoS One. eCollection 2015. 2015;10:e0136056. [CrossRef]
- Nixon E, Simpkins JW. Neuroprotective effects of nonfeminizing oestrogens in retinal photoreceptor neurons. Invest Ophthalmol Vis Sci. 2012;53:4739-47. [CrossRef]
- Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM et al. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci. 2003;44:3155-62. [CrossRef]
- Nakazawa T, Takahashi H, Shimura M. Estrogen has a neuroprotective effect on axotomized RGCs through ERK signal transduction pathway. Brain Res. 2006;1093:141-9. [CrossRef]
- Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am J Ophthalmol. 1994;118:754-61.
- Maruichi M, Oku H, Takai S, Muramatsu M, Sugiyama T, Imamura Y et al. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: a possible involvement of chymase in the pathogenesis of macular hole patients Curr Eye Res. 2004;29:321-5. [CrossRef]
- Shah SP, Bunce C, Johnston RL, Laidlaw DA. Are biometric parameters a risk factor for idiopathic macular hole formation? Results of a matched case-control series. Br J Ophthalmol.2006;90:117-8. [CrossRef]
- Kobayashi H, Kobayashi K, Okinami S. Macular hole and myopic refraction. Br J Ophthalmol. 2002;86:1269-73. [CrossRef]
- Sugiyama T, Katsumura K, Nakamura K, Kobayashi M, Muramatsu M, Maruichi M et al. Effects of chymase on the macular region in monkeys and porcine Muller cells: probable involvement of chymase in the onset of idiopathic macular holes Ophthalmic Res. 2006;38:201-8. Epub May 4 2006. [CrossRef]
- Inokuchi N, Ikeda T, Nakamura K, Morishita S, Fukumoto M, Kida T, et al. Vitreous estrogen levels in patients with an idiopathic macular hole. Clin Ophthalmol. 2015;9:549-52. [CrossRef]
- Ghadiali Q, Zahid S, Dolz-Marco R, Tan A, Engelbert M. An assessment of vitreous degeneration in eyes with vitreomacular traction and macular holes. J Ophthalmol. 2017;2017:6834692. [CrossRef]
- Gass JD. Idiopathic senile macular Hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629-39. [CrossRef]
- Kishi S, Hagimura N, Shimizu K. The role of the premacular liquefied pocket and premacular vitreous cortex in idiopathic macular hole development Am J Ophthalmol. 1996;122:622-8. [CrossRef]
- Johnson MW, Van Newkirk MR, Meyer KA. Perifoveal vitreous detachment is the primary pathogenic event in idiopathic macular hole formation. Arch Ophthalmol. 2001;119:215-22.
- Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23:421-4. [CrossRef]
- Theodossiadis G, Petrou P, Eleftheriadou M, Moustakas AL, Datseris I, Theodossiadis P. Focal vitreomacular traction: a prospective study of the evolution to macular hole: the mathematical approach. Eye (Lond). 2014;28:1452-60. [CrossRef]
- Zhang P, Zhu M, Zhao Y, Qian J, Dufresne C, Turner R et al. A proteomic approach to understanding the pathogenesis of idiopathic macular hole formation. Clin Proteom. eCollection 2017. 2017;14:37. [CrossRef]
- Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. First published: 24 December 2001. [CrossRef]
- Munaut C, Lambert V, Noël A, Frankenne F, Deprez M, Foidart JM et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001;85:877-82. [CrossRef]
- Ogueta SB, Schwartz SD, Yamashita CK, Farber DB. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Ophthalmol Vis Sci. 1999;40:1906-11.
- Hutchinson CV, Walker JA, Davidson C. Oestrogen, ocular function and low-level vision: a review. J Endocrinol. 2014;223:R9-18. [CrossRef]
- Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677-96. [CrossRef]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M et al. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101:6472-7. [CrossRef]
- Zhu C, Wang S, Wang B, Du F, Hu C, Li H, et al. 17β-Estradiol up-regulates Nrf2 via PI3K/AKT and estrogen receptor signaling pathways to suppress light-induced degeneration in rat retina. Neuroscience. 2015 Sep 24;304:328-39. [CrossRef]
- Giddabasappa A, Bauler M, Yepuru M, Chaum E, Dalton JT, Eswaraka J. 17-β estradiol protects ARPE-19 cells from oxidative stress through oestrogen receptor-β. Invest Ophthalmol Vis Sci. 2010;51:5278-87. [CrossRef]
- Pisano A, Preziuso C, Iommarini L, Perli E, Grazioli P, Campese AF et al. Targeting estrogen receptor β as preventive therapeutic strategy for Leber’s hereditary optic neuropathy.Hum Mol Genet. 2015;24:6921-31. [CrossRef]
- Russo R, Cavaliere F, Watanabe C, Nucci C, Bagetta G, Corasaniti MT et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008;173:583-90. [CrossRef]
- Prokai-Tatrai K, Xin H, Nguyen V, Szarka S, Blazics B, Prokai L, Koulen P. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm. 2013 Aug 5;10(8):3253-61. [CrossRef]
- Honig MG, Del Mar NA, Henderson DL, O'Neal D, Doty JB, Cox R, Li C, Perry AM, Moore BM, Reiner A. Raloxifene Modulates Microglia and Rescues Visual Deficits and Pathology After Impact Traumatic Brain Injury. Front Neurosci. 2021 Oct 29;15:701317. [CrossRef]
- Kimura K, Orita T, Fujitsu Y, Liu Y, Wakuta M, Morishige N, et al. Inhibition by female sex hormones of collagen gel contraction mediated by retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2014;55:2621-30. [CrossRef]
- Hao M, Li Y, Lin W, Xu Q, Shao N, Zhang Y et al. Estrogen prevents high-glucose-induced damage of retinal ganglion cells via mitochondrial pathway. Graefes Arch Clin Exp Ophthalmol. 2015;253:83-90. [CrossRef]
- Farber DB, Theendakara VP, Akhmedov NB, Saghizadeh M. ZBED4, a novel retinal protein expressed in cones and Müller cells. Adv Exp Med Biol. 2010;664:79-87. [CrossRef]
- Masuda T, Wada Y, Kawamura S. ES1 is a mitochondrial enlarging factor contributing to form megamitochondria in zebrafish cones Sci Rep. 2016 Mar 1;6:22360. [CrossRef]
- Qiu QH, Chen ZY, Yin LL, Zheng Z, Wu XW. Effects of estrogen on collagen gel contraction by human retinal glial cells. Chin Med J (Engl). 2012;125:4098-103.
- James M, Feman SS. Macular holes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215:59-63.
- McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol. 1982;93:777-86. [CrossRef]
- Nishikawa Y, Morishita S, Horie T, Fukumoto M, Sato T, Kida T et al. A comparison of sex steroid concentration levels in the vitreous and serum of patients with vitreoretinal diseases. PLOS ONE. 2017;12:e0180933. [CrossRef]
- Chung SE, Kim SW, Chung HW, Kang SW. Estrogen antagonist and development of macular hole. Korean J Ophthalmol. 2010;24:306-9. [CrossRef]
- Cronin BG, Lekich CK, Bourke RD. Tamoxifen therapy conveys increased risk of developing a macular hole. Int Ophthalmol. 2005;26:101-5. Epub September 15 2006. [CrossRef]
- Torrell Belzach N, Vela Segarra JI, Crespí Vilimelis J, Alhayek M. Case. Bilateral Macular Hole Related to Tamoxifen Low-Dose Toxicity. Rep Ophthalmol. 2020 Oct 13;11(3):528-533. [CrossRef]
- Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431-42. [CrossRef]
- Mäenpää H, Mannerström M, Toimela T, Salminen L, Saransaari P, Tähti H. Glutamate uptake is inhibited by tamoxifen and toremifene in cultured retinal pigment epithelial cells Pharmacol Toxicol. 2002;91:116-22. [CrossRef]
- Shinkai A, Saito W, Hashimoto Y, Ishida S, Improvements in visual acuity and macular morphology following cessation of anti-estrogen drugs in a patient with anti-estrogen maculopathy resembling macular telangiectasia type 2: a pathogenic hypothesis. BMC Ophthalmol. 2019 Dec 30;19(1):267. [CrossRef]
- Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol. 1969;82:151-9. [CrossRef]
- Gass JD. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol. 1999;821-3. [CrossRef]
- Ezra E. Idiopathic full thickness macular hole: natural history and pathogenesis. Br J Ophthalmol. 2001;85:102-8. [CrossRef]
- Baget-Bernaldiz M, Soler Lluis N, Romero-Aroca P, Traveset-Maeso A. Optical coherence tomography study in tamoxifen maculopathy. Arch Soc Esp Oftalmol. 2008;83:615-8.
- Bicer T, Imamoglu GI, Dogan AS, Avarisli NA, Kabatas N, Bicer BK, Gurdal C. The effects of adjuvant hormonotherapy on tear functions in patients with breast cancer. Int Ophthalmol. 2020. Aug;40(8):2077-2083. [CrossRef]
- Gualino V, Cohen SY, Delyfer MN, Sahel JA, Gaudric A. Optical coherence tomography findings in tamoxifen retinopathy. Am J Ophthalmol. 2005;140:757-8. [CrossRef]
- Wagner-Schuman M, Dubis AM, Nordgren RN, Lei Y, Odell D, Chiao H et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52:625-34. [CrossRef]
- Bafiq R, Mathew R, Pearce E, Abdel-Hey A, Richardson M, Bailey T et al. Age, sex, and ethnic variations in inner and outer retinal and choroidal thickness on spectral-domain optical coherence tomography. Am J Ophthalmol. 2015;160:1034-1043.e1. [CrossRef]
- Eisner A, Burke SN, Toomey MD. Visual sensitivity across the menstrual cycle. Vis Neurosci. 2004;21:513-31. [CrossRef]
- Ozawa GY, Bearse MA Jr, Harrison WW, Bronson-Castain KW, Schneck ME, Barez S et al. Differences in neuroretinal function between adult males and females. Optom Vis Sci. Bronson: Harrison WW. 2014;91:602-7. [CrossRef]
- Yilmaz H, Erkin EF, Mavioğlu H, Sungurtekin U. Changes in pattern reversal evoked potentials during menstrual cycle. Int Ophthalmol. 1998;22:27-30. [CrossRef]
- Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917-21. [CrossRef]
- Andréasson S, Ghosh F. Cone implicit time as a predictor of visual outcome in macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2014;252:1903-9. [CrossRef]
- Ştefănescu-Dima AŞ, Corîci CA, Mănescu MR, Sas TN, Iancău M, Mocanu CL. Posterior vitreous detachment and macular anatomical changes - a tomographic-electroretinographic study. Rom J Morphol Embryol. 2016;57:751-8.
- Karatepe AS, Menteş J, Erakgün ET, Afrashi F, Nalçacı S, Akkın C et al. Vitreoretinal interface characteristics in eyes with idiopathic macular holes: qualitative and quantitative analysis. Turk J Ophthalmol. 2018;48:70-4. [CrossRef]
- Gynäkologische Endokrinologie, Band I, 2 Auflage, Karl-Heinrich Wulf und Heinrich Schmidt-Matthiesen. Urban&Schwarzenberg, München-Wien-Baltimore 1987.
- Schwangerschaft I, Band 4, 3 Auflage, Karl-Heinrich Wulf und Heinrich Schmidt-Matthiesen, München-Wien-Baltimore 1992.
- Hwang S, Kang SW, Kim SJ, Choi J, Son KY, Lim DH, Shin DW, Choi D, Chang Y, Ryu S, Cho J. Risk factors for the development of idiopathic macular hole: a nationwide population-based cohort study. Sci Rep. 2022 Dec 16;12(1):21778. [CrossRef]
- Kunst S, Wolloscheck T, Grether M, Trunsch P, Wolfrum U, Spessert R. Photoreceptor cells display a daily rhythm in the orphan receptor Esrrβ. Mol Vis. eCollection 2015;21:173-84.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).