1. Obesity as a Global Health Challenge
Obesity has emerged as a significant global health challenge, affecting individuals of all ages and socioeconomic backgrounds in both developed and developing countries.1 It is a complex condition characterized by excessive body fat accumulation, which poses serious health risks and contributes to various chronic diseases. The causes of obesity are multifaceted, encompassing a combination of genetic, environmental, and behavioral factors. Sedentary lifestyles and the prevalence of highly processed, calorie-dense foods have become increasingly common in modern societies.2 Additionally, urbanization and globalization have resulted in the availability of inexpensive, high-calorie foods that are often lacking in nutritional value.2 Such obesogenic environments, coupled with the convenience of technology and decreased physical activity, contribute to weight gain and the development of obesity.
The consequences of obesity are far-reaching and impact individuals, families, and entire communities. Obese individuals face a higher risk of developing chronic diseases such as type 2 diabetes (T2D), cardiovascular diseases (CVD), and subsequently chronic kidney disease (CKD).3 The economic burden associated with obesity is significant, placing strain on healthcare systems and economies through increased healthcare costs and lost productivity.
2. MicroRNAs: An Overview of Regulatory Molecules.
MicroRNAs (miRNAs) are small non-coding RNA molecules that play a crucial role in gene regulation.4 They modulate gene expression by binding to target messenger RNAs (mRNAs), leading to mRNA degradation or translational repression. MiRNAs are involved in various biological processes and have implications in disease development and therapeutic interventions.5
Understanding the biogenesis and function of miRNAs is essential for unraveling their complex regulatory networks and their potential applications in therapeutics.
2.1. Biogenesis of miRNAs:
The biogenesis of miRNAs begins in the cell nucleus. It involves a series of steps, starting with the transcription of miRNA genes by RNA polymerase II to generate primary miRNA transcripts (pri-miRNAs). Pri-miRNAs are typically several hundred nucleotides long and contain one or more hairpin structures.4
The next step involves the processing of pri-miRNAs into precursor miRNAs (pre-miRNAs) by the microprocessor complex, which consists of the ribonuclease III enzyme Drosha and its cofactor DiGeorge syndrome critical region 8 (DGCR8). Drosha cleaves the pri-miRNA hairpin near the base, resulting in a hairpin-shaped pre-miRNA molecule approximately 70 nucleotides long.
Pre-miRNAs are then exported from the nucleus to the cytoplasm by the exportin-5 protein, in a process facilitated by the binding of the nuclear protein exportin-5 and its cofactor Ran-GTP to the pre-miRNA. In the cytoplasm, pre-miRNAs are further processed by the endonuclease Dicer, resulting in the formation of mature miRNAs.4
Dicer cleaves the pre-miRNA hairpin, releasing a double-stranded RNA molecule. One strand, known as the guide strand, is incorporated into the RNA-induced silencing complex (RISC), while the other strand, known as the passenger strand, is typically degraded. The guide strand guides the RISC complex to target messenger RNAs (mRNAs) through sequence complementarity.
2.2. miRNA Function
Once incorporated into the RISC complex, miRNAs exert their regulatory effects primarily by base-pairing with complementary sequences in the 3' untranslated regions (UTRs) of target mRNAs. This binding can result in the degradation of the target mRNA or the inhibition of its translation.6
By modulating gene expression, miRNAs fine-tune the activity of numerous target genes, allowing for the precise control of biological processes. They are involved in various cellular processes, including cell proliferation, apoptosis, and differentiation. Additionally, miRNAs have been implicated in the pathogenesis of numerous diseases.
The specificity of miRNA targeting is determined by the degree of complementarity between the miRNA and its target mRNA. Perfect or near-perfect complementarity usually leads to mRNA degradation, whereas imperfect complementarity typically leads to translational repression.
Moreover, a single miRNA can regulate multiple target mRNAs, and a single mRNA can be targeted by multiple miRNAs, further expanding the regulatory potential of miRNAs. This complexity enables miRNAs to participate in intricate regulatory networks, exerting both synergistic and antagonistic effects on gene expression.7
3. MicroRNAs in Obesity
3.1. MicroRNAs and Adipose Tissue Development
MiRNAs have been identified as important regulators of adipose tissue development and function.8 Adipose tissue is a crucial energy-storing organ and plays a significant role in metabolic homeostasis. MiRNAs control adipogenesis, the process by which preadipocytes differentiate into mature adipocytes, and also influence adipocyte metabolism, insulin sensitivity, and lipid storage.8 Dysregulation of miRNAs in adipose tissue has been associated with obesity and related metabolic disorders. Understanding the specific miRNA-mediated regulatory mechanisms in adipose tissue development can provide valuable insights into the pathogenesis of obesity and potentially open up new avenues for therapeutic interventions targeting miRNAs to modulate adipose tissue function and combat metabolic diseases.
3.2. MicroRNAs in Energy Homeostasis and Metabolic Pathways
MiRNAs play a significant role in maintaining the balance between energy intake, storage, and expenditure in various tissues and organs.
MiRNAs have been implicated in the regulation of adipocyte differentiation and lipid metabolism. For example, miR-27a and miR-143 have been shown to promote adipogenesis in rat9 and pig10 by targeting key genes involved in adipocyte differentiation, such as PPARγ and adiponectin. On the other hand, miR-26a and miR-30d have been found to inhibit adipogenesis by suppressing the expression of adipogenic transcription factors.11
Furthermore, miRNAs have been identified as regulators of brown adipose tissue (BAT) and its thermogenic function. MiR-133 and miR-455 have been shown to inhibit BAT development and thermogenesis by targeting key genes involved in brown adipocyte differentiation and function.12 Conversely, miR-193b and miR-365 have been found to promote BAT activity and thermogenesis.12 A recent multiomics study identified the key nodes likely controlling non-shivering thermogenesis in adipose tissue, and identified numerous miRNAs such as miRNA-27, miRNA-34a, miRNA-106b and miRNA-125-5p.13
In addition to adipose tissue, miRNAs also play a role in the regulation of hepatic lipid metabolism. MiR-122, for instance, is highly expressed in the liver and has been shown to regulate cholesterol and fatty acid metabolism.14 Its inhibition leads to decreased hepatic lipid accumulation and improved insulin sensitivity.
Moreover, miRNAs have been implicated in the regulation of glucose metabolism and insulin signaling. For example, miR-143 have been shown to regulate insulin sensitivity by targeting key components of glycolysis and insulin signaling pathways.15
Overall, miRNAs play a crucial role in the regulation of energy homeostasis and metabolic pathways. Dysregulation of miRNA expression and function has been associated with metabolic disorders such as obesity, T2D, and dyslipidemia. Therefore, understanding the intricate miRNA-mediated regulatory networks in energy metabolism holds promise for the development of novel therapeutic approaches for metabolic diseases.
3.3. Role of MicroRNAs in Inflammation and Insulin Resistance
MicroRNAs (miRNAs) play a crucial role in the regulation of inflammation and insulin resistance, two interconnected processes that contribute to the development of various metabolic disorders, including obesity, T2D, and CVD.
Inflammation is a fundamental response of the immune system to injury or infection. However, chronic low-grade inflammation mediated by the NLRP3 inflammasome can occur in adipose tissue and other metabolic organs in the context of obesity and insulin resistance.16 MiRNAs are involved in the regulation of pro-inflammatory and anti-inflammatory pathways. For instance, miR-146a and miR-155 are key modulators of inflammation and play important roles in regulating immune responses.17 Dysregulation of these miRNAs can contribute to sustained inflammation and impair insulin signaling.
Insulin resistance, a hallmark of T2D, occurs when cells become less responsive to the effects of insulin. MiRNAs are implicated in the regulation of insulin sensitivity by targeting key components of the insulin signaling pathway. For example, miR-29, miR-143, and miR-33 have been shown to modulate insulin sensitivity by regulating insulin receptor substrate-1 (IRS-1), a critical mediator of insulin signaling.18
Furthermore, miRNAs are involved in the cross-talk between inflammation and insulin resistance. They can regulate the production of inflammatory cytokines and chemokines, as well as the expression of insulin signaling molecules. MiR-155, for instance, has been shown to promote both inflammation and insulin resistance by targeting multiple molecules involved in these processes.19 More precisely, mice that display double Knock-out for both ApoE and miR-155 have high fat diet-induced obesity, adipocyte hypertrophy, non-alcoholic fatty liver disease and increased plasma leptin.19
4. MicroRNAs’ roles in the Link between Obesity and CKD
CKD is associated with an increased risk of CVD, and obesity is a well-established risk factor for both CKD and CVD.20
Several miRNAs have been identified as key regulators of renal fibrosis, inflammation, and apoptosis, which are hallmarks of CKD progression. MiR-21, for instance, is upregulated in CKD and has been shown to promote renal fibrosis by targeting anti-fibrotic factors.21 Conversely, miR-29 family members are downregulated in CKD and play a protective role against fibrosis by inhibiting extracellular matrix synthesis.21
There is a strong link between obesity and CKD, with obesity being recognized as an independent risk factor for the development and progression of CKD. MiRNAs have been implicated in mediating the connection between obesity and CKD. For instance, miR-126, which is downregulated in obesity, has been shown to promote kidney injury and fibrosis by impairing endothelial function.22 Additionally, miR-192 and miR-217 have been found to be upregulated in obesity and contribute to renal inflammation and glomerular dysfunction.23
Understanding the role of miRNAs in CKD and the link between obesity and CKD can provide insights into the underlying molecular mechanisms and potential therapeutic interventions. As discussed below (subchapter 7) , targeting specific miRNAs involved in renal pathology holds promise for the development of novel treatments to slow down or halt CKD progression.5 Furthermore, identifying circulating miRNAs as non-invasive biomarkers may enable early detection and monitoring of CKD in obese individuals, facilitating timely interventions to mitigate disease progression and improve patient outcomes.
In conclusion, miRNAs are involved in the regulation of multiple processes implicated in CKD and cardiovascular disorders, including inflammation, oxidative stress, fibrosis, and endothelial dysfunction.5 Dysregulation of specific miRNAs contributes to the progression of CKD and the development of CVD in the context of obesity.24
5. MicroRNAs in Obesity-Associated Cardiovascular Risk Factors
Obesity-associated miRNAs can directly impact cardiovascular health by affecting cardiac remodeling, endothelial function, and vascular smooth muscle cell proliferation.20
5.1. MicroRNAs in Endothelial Dysfunction and Atherosclerosis
Endothelial dysfunction is characterized by impaired endothelial cell function and is a crucial event in the development and progression of atherosclerosis, a chronic inflammatory disease of the arterial wall.25
MiRNAs play a pivotal role in endothelial dysfunction by modulating various molecular pathways involved in endothelial cell biology. They can regulate endothelial cell proliferation, migration, inflammation, and oxidative stress, which are all important factors in the development of atherosclerosis.20 Several miRNAs have been identified to be dysregulated in endothelial dysfunction and atherosclerosis, such as miR-126, miR-155, miR-21, and miR-92a.
MiR-126 is highly expressed in endothelial cells and is crucial for vascular integrity and angiogenesis.26,27 Reduced levels of miR-126 have been associated with endothelial dysfunction and increased atherosclerotic plaque vulnerability.22,28 Interestingly, exercise and diet where shown to improve obese adolescents endothelial function, and change seric miRNA-126 levels.26 MiR-155 is a pro-inflammatory miRNA that promotes endothelial cell activation and dysfunction by targeting anti-inflammatory factors.21 MiR-21 is involved in the regulation of endothelial cell apoptosis, proliferation, and migration,21 while miR-92a promotes endothelial dysfunction by inhibiting angiogenesis.29 miR-92a has been shown to be a potential biomarker of aortic valve calcification, and its use could be expanded to obese patients.24
5.2. MicroRNAs in Cardiac Remodeling and Dysfunction
In the context of obesity, altered miRNA expression profiles have been observed in both human and animal models, indicating their involvement in cardiac remodeling and dysfunction.30 Several miRNAs have been identified as key players in these processes.20 For instance, miR-133a and miR-1 are downregulated in murine obesity models and have been associated with adverse cardiac remodeling, including hypertrophy and fibrosis.31 These miRNAs target genes involved in regulating cardiac hypertrophy, such as the transcription factor GATA4 and the calcium handling protein SERCA2a.
Additionally, miR-208a and miR-499, which are encoded within the introns of myosin genes, have been implicated in obesity-related cardiac dysfunction.32 These miRNAs are upregulated in obesity and contribute to the dysregulation of calcium homeostasis and contractile function in the heart. Accordingly, they have been propsoed as potential biomarkers of acute myocardial infraction.33 Moreover, miR-21 and miR-29a have been found to promote cardiac fibrosis in the context of obesity by targeting factors involved in extracellular matrix remodeling, such as collagen synthesis and degradation enzymes.34
miRNAs may serve as diagnostic biomarkers to identify individuals at high risk for developing these conditions, enabling early intervention and personalized management approaches.
6. MicroRNAs as Biomarkers for Obesity-Related Diseases
In CKD, altered expression of miRNAs has been observed in renal tissues but also in biofluids, such as urine and blood. These miRNAs modulate key molecular pathways involved in CKD pathogenesis, including transforming growth factor-beta (TGF-β) signaling, epithelial-to-mesenchymal transition (EMT), and fibrosis. For example, miR-21 is upregulated in CKD and promotes renal fibrosis by targeting anti-fibrotic factors.21
MicroRNAs (miRNAs) have gained significant attention as potential biomarkers for obesity-related diseases due to their stability, tissue specificity, and altered expression patterns in various physiological and pathological conditions.35 The identification of reliable biomarkers is crucial for early detection, accurate diagnosis, and monitoring of obesity-related diseases. MiRNAs are thus emerging as promising candidates for fulfilling this role.
Dysregulation of miRNAs has been observed in various obesity-related diseases, suggesting their involvement in disease pathogenesis. These dysregulated miRNAs can be detected in various biological samples, such as blood, serum, plasma, urine, and adipose tissue, making them accessible for non-invasive testing and potential clinical applications.35
In T2D, several miRNAs have shown altered expression levels in insulin-producing pancreatic beta cells, liver, skeletal muscle, and adipose tissue. MiR-126, miR-375, and miR-29 have been implicated in pancreatic beta cell function and insulin secretion.28,36,37 In cardiovascular diseases, miRNAs such as miR-21, miR-126, and miR-155 have been associated with endothelial dysfunction, atherosclerosis, and cardiac remodeling.20 In NAFLD, miR-122, miR-34a, and miR-34c are dysregulated and play a role in hepatic lipid metabolism and inflammation.14
miRNAs have been identified as potential biomarkers for obesity-related cardiac abnormalities. Circulating levels of specific miRNAs, such as miR-21, miR-208a, and miR-499,7 have been found to be altered in obese individuals and correlated with the severity of cardiac dysfunction. These findings highlight the potential of miRNAs as non-invasive diagnostic tools for assessing cardiac remodeling and dysfunction in obesity.
The potential utility of miRNAs as biomarkers lies in their ability to discriminate between different disease states and provide information about disease severity, progression, and response to treatment. High-throughput technologies, such as microarray analysis and next-generation sequencing, have enabled the identification of panels of miRNAs that exhibit differential expression patterns in obesity-related diseases. These panels can serve as signatures for disease classification and prediction. Moreover, advances in quantitative polymerase chain reaction (qPCR) and sequencing technologies have facilitated the development of robust and sensitive methods for miRNA detection and quantification.
MiRNAs also offer the advantage of being able to provide information about the underlying molecular mechanisms of disease. Through their regulatory roles, miRNAs can modulate key pathways involved in disease pathogenesis, including inflammation, insulin signaling, lipid metabolism, and cell proliferation. By studying the functional implications of dysregulated miRNAs, researchers can gain insights into disease mechanisms and identify potential therapeutic targets.
Despite the potential of miRNAs as biomarkers, there are challenges that need to be addressed.38 Standardization of sample collection, processing, and analysis methods is essential to ensure reproducibility and comparability of results. Furthermore, the identification of disease-specific miRNA signatures requires large-scale validation studies involving diverse populations.39 Additionally, the development of miRNA-based diagnostic tests and their integration into clinical practice requires regulatory approval and validation in prospective clinical trials.
In conclusion, miRNAs hold great promise as biomarkers for obesity-related diseases. Their altered expression patterns in various biological samples make them attractive candidates for non-invasive testing and monitoring. The ability of miRNAs to provide insights into disease mechanisms further enhances their potential as diagnostic and prognostic tools. Continued research and validation efforts are needed to fully exploit the clinical utility of miRNAs in the management of obesity-related diseases, with the ultimate goal of improving patient outcomes and personalized medicine.
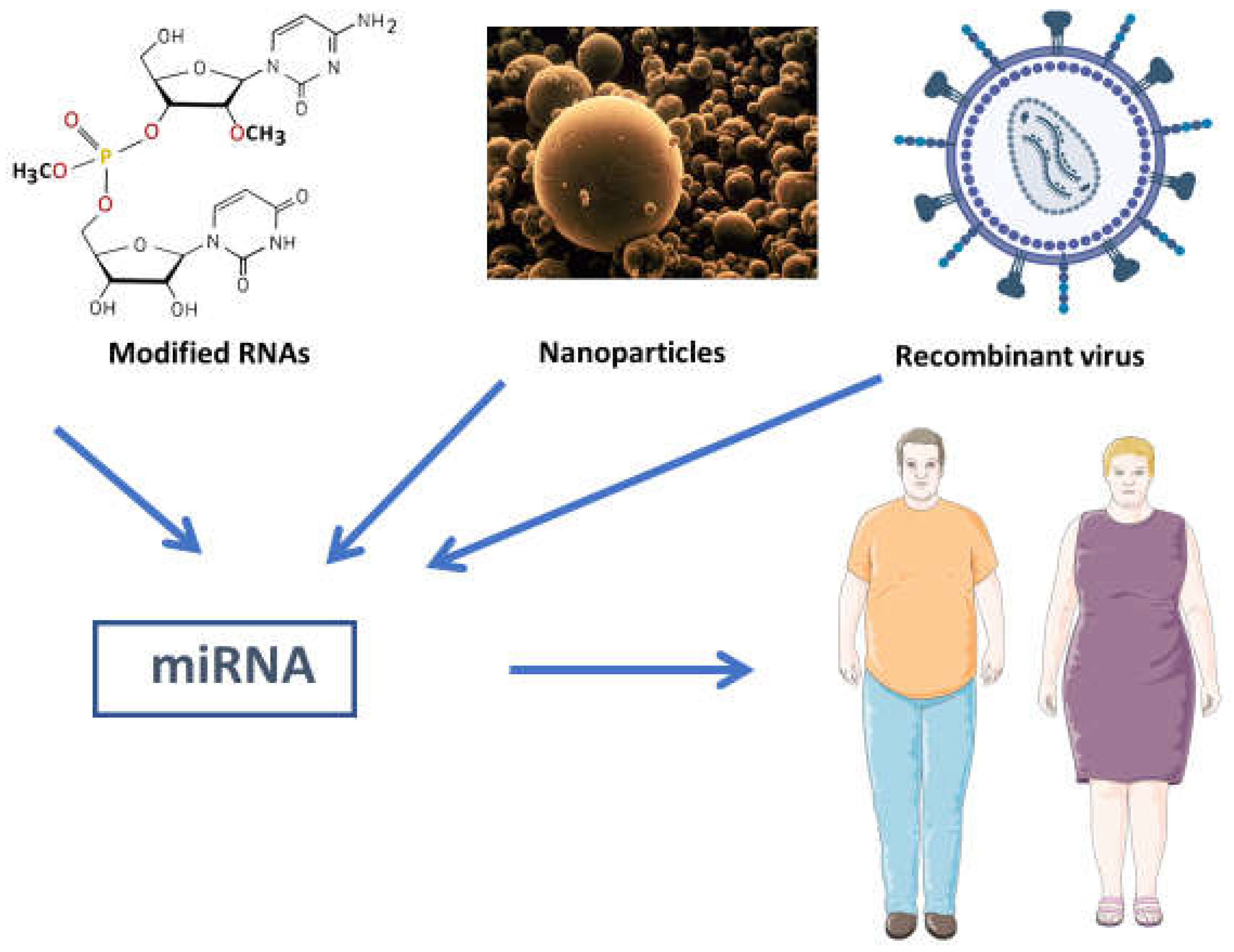
7. Targeting MicroRNAs for Therapeutic Interventions. Challenges and Future Perspectives.
Understanding the role of miRNAs in CKD and the connection between obesity and cardiovascular disorders can provide valuable insights into the underlying mechanisms and potential therapeutic targets. Targeting specific miRNAs involved in renal and cardiovascular pathology may offer novel strategies for the prevention and treatment of CKD and CVD in the context of obesity.
Targeting miRNAs holds therapeutic potential for mitigating obesity-related cardiac remodeling and dysfunction (
Figure 1). Preclinical studies have demonstrated that manipulating miRNA expression levels can ameliorate pathological cardiac changes. For instance, miR-133a replacement therapy has shown beneficial effects in reducing cardiac hypertrophy and improving contractile function in obese animal models.
40,41
By modulating the expression or function of specific miRNAs, it may be possible to influence adipogenesis, adipocyte metabolism, insulin sensitivity, and other key pathways involved in obesity. Various approaches, such as antisense oligonucleotides, miRNA mimics, and small molecule inhibitors, are being explored to manipulate miRNA activity.42 However, more research is needed to fully understand the safety, efficacy, and delivery challenges associated with miRNA-based therapies in the context of obesity.
8. Conclusions
In conclusion, miRNAs play pivotal roles in the pathogenesis of obesity, chronic kidney disease, and cardiovascular disorders, offering exciting opportunities for clinical applications. Further research into miRNA biology and their interactions with metabolic pathways and tissue-specific targets will likely provide valuable insights for developing innovative strategies to combat these global health challenges.
References
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J Obes 2020, 2020, 6134362. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Gai, Z.; et al. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Metzinger-Le Meuth, V.; Metzinger, L. miR-223 and other miRNA’s evaluation in chronic kidney disease: Innovative biomarkers and therapeutic tools. Noncoding RNA Res 2019, 4, 30–35. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Metzinger-Le Meuth, V.; Burtey, S.; Maitrias, P.; Massy, Z.A.; Metzinger, L. microRNAs in the pathophysiology of CKD-MBD: Biomarkers and innovative drugs. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat Rev Mol Cell Biol 2021, 22, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; et al. Influence of L-carnitine on the Expression Level of Adipose Tissue miRNAs Related to Weight Changes in Obese Rats. Pak J Biol Sci 2016, 19, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; et al. MicroRNAs miR-27a and miR-143 regulate porcine adipocyte lipid metabolism. Int J Mol Sci 2011, 12, 7950–7959. [Google Scholar] [CrossRef]
- Arias, N.; et al. MicroRNAs involved in the browning process of adipocytes. J Physiol Biochem 2016, 72, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Goody, D.; Pfeifer, A. MicroRNAs in brown and beige fat. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rodó, J.; et al. Integrated gene expression profiles reveal a transcriptomic network underlying the thermogenic response in adipose tissue. Sci Rep 2023, 13, 7266. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.P.; Jopling, C.L. Regulation and biological function of the liver-specific miR-122. Biochem Soc Trans 2010, 38, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-H.; Liu, B.; Wu, J.-D.; Yan, Y.-Y.; Wang, J.-N. miR-143 is involved in endothelial cell dysfunction through suppression of glycolysis and correlated with atherosclerotic plaques formation. Eur Rev Med Pharmacol Sci 2016, 20, 4063–4071. [Google Scholar]
- Vandanmagsar, B.; et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Duan, Q.; et al. Super enhancers at the miR-146a and miR-155 genes contribute to self-regulation of inflammation. Biochim Biophys Acta 2016, 1859, 564–571. [Google Scholar] [CrossRef]
- Chakraborty, C.; Doss CG, P.; Bandyopadhyay, S.; Agoramoorthy, G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA 2014, 5, 697–712. [Google Scholar] [CrossRef]
- Virtue, A.; et al. MicroRNA-155 Deficiency Leads to Decreased Atherosclerosis, Increased White Adipose Tissue Obesity, and Non-alcoholic Fatty Liver Disease: A NOVEL MOUSE MODEL OF OBESITY PARADOX. J Biol Chem 2017, 292, 1267–1287. [Google Scholar] [CrossRef]
- Maitrias, P.; et al. The Involvement of miRNA in Carotid-Related Stroke. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1608–1617. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Floege, J.; Biessen, E.A.L.; Jankowski, J.; van der Vorst, E.P.C. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. IJMS 2020, 21, 6547. [Google Scholar] [CrossRef] [PubMed]
- Fourdinier, O.; et al. Syndecan-1 and Free Indoxyl Sulfate Levels Are Associated with miR-126 in Chronic Kidney Disease. IJMS 2021, 22, 10549. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; et al. Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol 2011, 225, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.; Metzinger, L.; Maitrias, P.; Caus, T.; Metzinger-Le Meuth, V. Aortic valve calcification in the era of non-coding RNAs: The revolution to come in aortic stenosis management? Noncoding RNA Res 2020, 5, 41–47. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Donghui, T.; et al. Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Microvasc Res 2019, 123, 86–91. [Google Scholar] [CrossRef]
- Bassand, K.; et al. miR-126-3p is essential for CXCL12-induced angiogenesis. J Cell Mol Med 2021. [CrossRef]
- Fourdinier, O.; et al. Serum levels of miR-126 and miR-223 and outcomes in chronic kidney disease patients. Sci Rep 2019, 9, 4477. [Google Scholar] [CrossRef]
- Nader, J.; et al. miR-92a: A Novel Potential Biomarker of Rapid Aortic Valve Calcification. J Heart Valve Dis 2017, 26, 327–333. [Google Scholar]
- de Franciscis, S.; Metzinger, L.; Serra, R. Roles and Clinical Applications of Biomarkers in Cardiovascular Disease. Biomed Res Int 2016, 2016, 8982796. [Google Scholar] [CrossRef]
- Frias, F.d.T.; et al. MyomiRs as Markers of Insulin Resistance and Decreased Myogenesis in Skeletal Muscle of Diet-Induced Obese Mice. Front Endocrinol (Lausanne) 2016, 7, 76. [Google Scholar] [CrossRef]
- Dorn, G.W.; Matkovich, S.J.; Eschenbacher, W.H.; Zhang, Y. A human 3’ miR-499 mutation alters cardiac mRNA targeting and function. Circ Res 2012, 110, 958–967. [Google Scholar] [CrossRef]
- Xiao, J.; et al. Serum microRNA-499 and microRNA-208a as biomarkers of acute myocardial infarction. Int J Clin Exp Med 2014, 7, 136–141. [Google Scholar] [PubMed]
- Scolari, F.L.; Faganello, L.S.; Garbin, H.I.; Piva EMattos, B.; Biolo, A. A systematic review of microRNAs in patients with hypertrophic cardiomyopathy. Int J Cardiol 2021, 327, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Coenen-Stass, A.M.L.; Wood, M.J.A. Assessment of RT-qPCR normalization strategies for accurate quantification of extracellular microRNAs in murine serum. PLoS ONE 2014, 9, e89237. [Google Scholar] [CrossRef]
- Lv, L.-L.; et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Renal Physiol. 2013, 305, F1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; et al. Upregulated long noncoding RNA LOC105375913 induces tubulointerstitial fibrosis in focal segmental glomerulosclerosis. Sci Rep 2019, 9, 716. [Google Scholar] [CrossRef]
- Zhou, S.-S.; et al. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 2019, 16, 661–674. [Google Scholar] [CrossRef]
- Torella, D.; et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ. Res. 2011, 109, 880–893. [Google Scholar] [CrossRef]
- Matkovich, S.J.; et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 2010, 106, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat Rev Genet 2022, 23, 265–280. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).