Submitted:
26 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Sample collection and isolation of S. aureus from bovine mastitis milk samples
2.2.2. Antimicrobial susceptibility testing of isolated S. aureus cultures using disc diffusion method
2.2.3. Bacterial DNA extraction, PCR amplification, and gel electrophoresis
2.2.4. Statistical Analysis
3. Results
3.1. Isolation of S. aureus from bovine mastitis milk samples in conventional and organic dairy farms
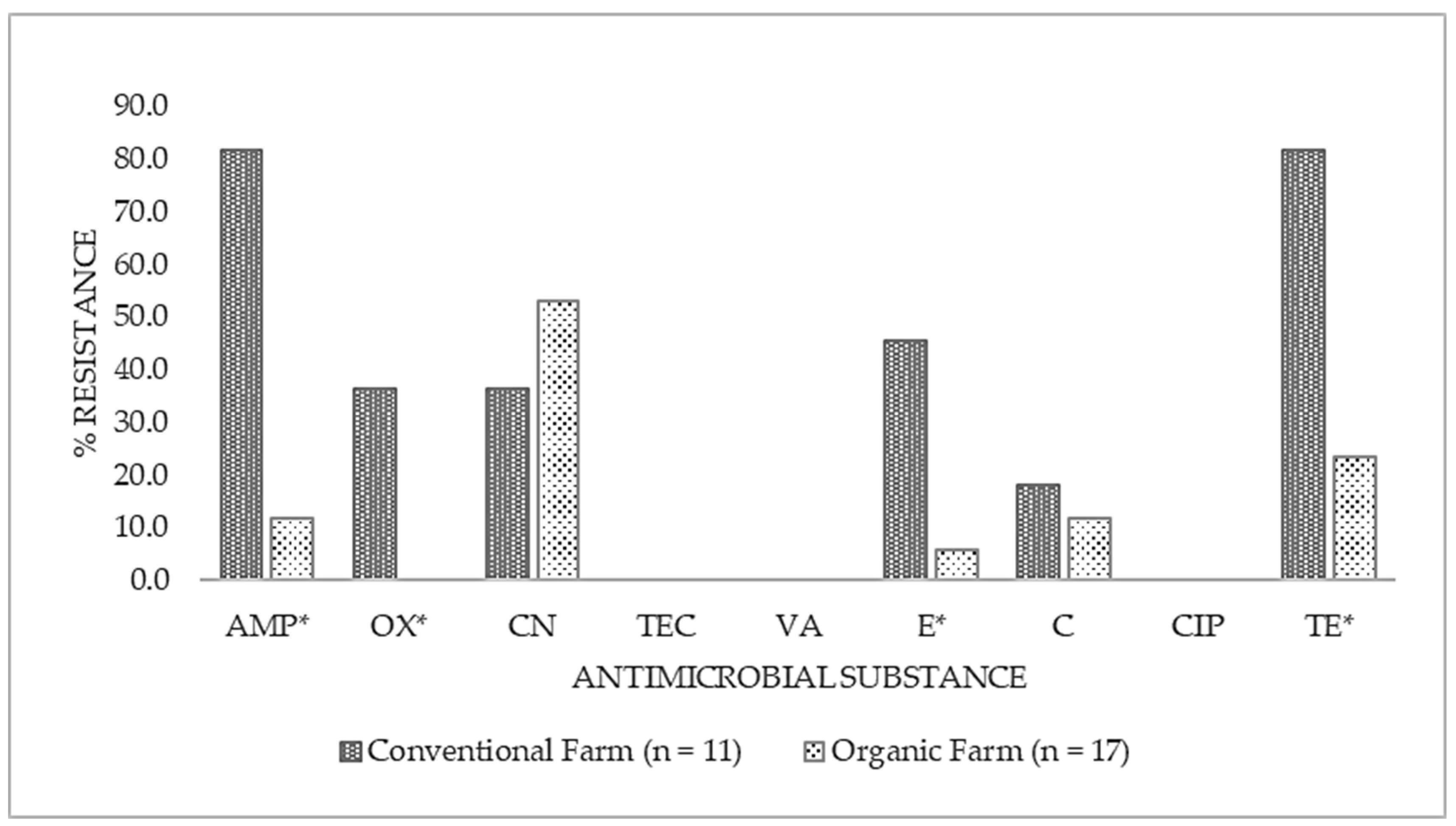
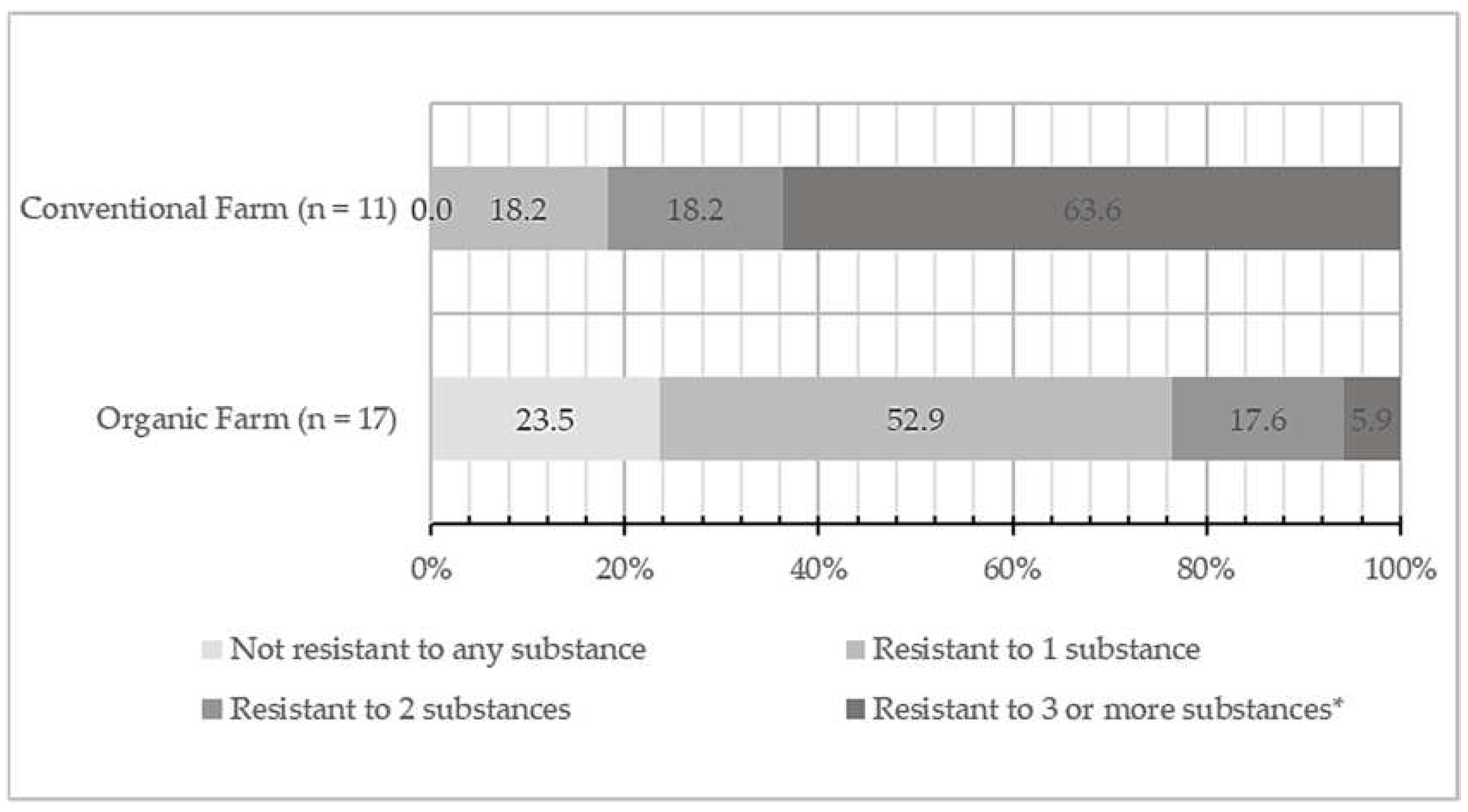
3.2. Antimicrobial susceptibility of isolated S. aureus cultures
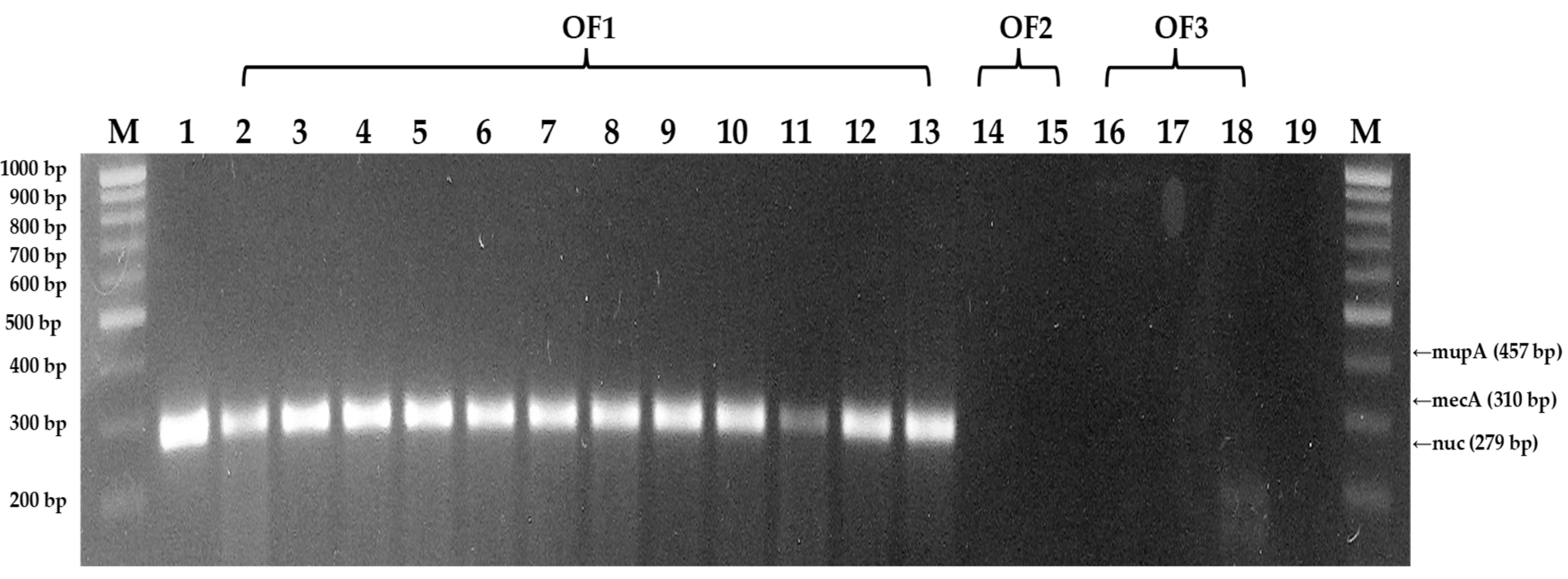
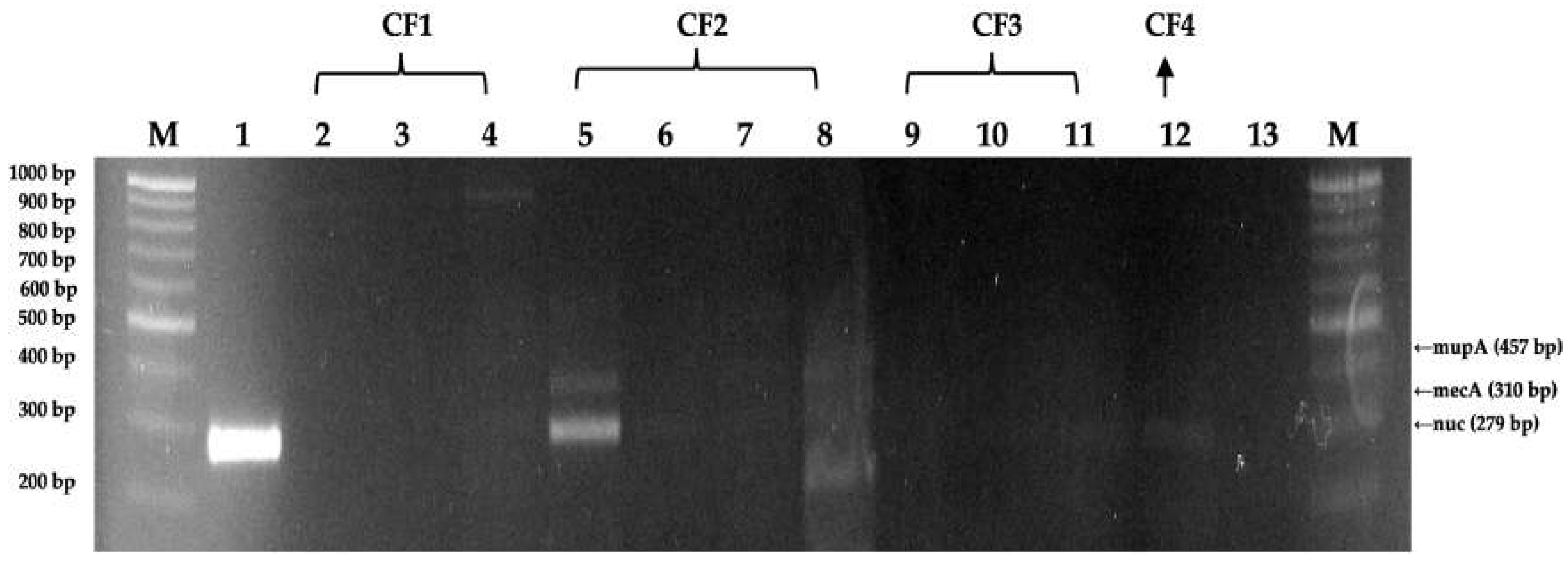
3.3. DNA extraction and PCR amplification of isolated S. aureus isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradley, A.J. Bovine Mastitis: An Evolving Disease. The Vet. J. 2002, 164(2), 116–128. [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments - A review. Asian-Australas. J. Anim. Sci. 2020, 33(11), 1699–1713. [CrossRef]
- Barkema, H.W.; Green, M.J.; Bradley, A.J.; Zadoks, R.N. Invited review: The role of contagious disease in udder health. J. Dairy Sci. 2009, 92, 4717–4729. [CrossRef]
- Bobbo, T.; Ruegg, P.L.; Stocco, G.; Fiore, E.; Gianesella, M.; Morgante, M.; Pasotto, D.; Bittante, G.; Cecchinato, A. Associations between pathogen-specific cases of subclinical mastitis and milk yield, quality, protein composition, and cheese-making traits in dairy cows. J. Dairy Sci. 2017, 100, 4868–4883. [CrossRef]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; Mendes, T.A.; Fitzgerald, J.R.; Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: current understanding and future perspectives. BMC Vet. Res. 2022, 18,115. [CrossRef]
- Annamanedi, M.; Sheela, P.; Sundareshan, S.; Isloor, S.; Gupta, P.; Jasmeen, P.; Gargi, M.; Mallick, S.; Hegde, N. Molecular fingerprinting of bovine mastitis-associated Staphylococcus aureus isolates from India. Sci. Rep.-UK 2021, 11, 15228. [CrossRef]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New Quadriplex PCR Assay for Detection of Methicillin and Mupirocin Resistance and Simultaneous Discrimination of Staphylococcus aureus from Coagulase-Negative Staphylococci. J. Clin. Microbiol. 2004, 42(11), 4947–4955. [CrossRef]
- Seiberling, K.A.; Aruni, W.; Kim, S.; Scapa, V.I.; Fletcher, H.; Church, C.A. The effect of intraoperative mupirocin irrigation on Staphylococcus aureus within the maxillary sinus. Int. Forum Allergy Rhinol. 2012, 3, 94- 98. [CrossRef]
- Tikofsky, L.L.; Barlow, J.W.; Santisteban, C.; Schukken, Y.H.A. Comparison of Antimicrobial Susceptibility Patterns for Staphylococcus aureus in Organic and Conventional Dairy Herds. Microb. Drug Resist. 2003, 9, 39–45. http://doi.org/10.1089/107662903322541883.
- Meissner, K.; Sauter-Louis, C.; Heiden, S.E.; Schaufler, K.; Tomaso, H.; Conraths, F.J.; Homeier-Bachmann, T. Extended-Spectrum ß-Lactamase-Producing Escherichia coli in Conventional and Organic Pig Fattening Farms. Microorganisms 2022, 10(3), 603. [CrossRef]
- National Institute of Food and Drug Safety Evaluation. National Antibiotics Use and Resistance and Monitoring Report 2020: Animals, Livestock, and Marine Products, final version; Korea Ministry of Food and Drug Safety, joint publication with Korea Ministry of Agriculture, Food and Korea Rural Affairs, the Agriculture, Forestry and Livestock Quarantine: Seoul, South Korea, 2021; pp.3-4. Available online: https://www.mfds.go.kr/brd/m_231/view.do?seq=33051&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accesses 19 December 2022).
- USDA Global Agricultural Information Network (GAIN) 2022 Attaché report – South Korea: Dairy and Products Annual. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Dairy%20and%20Products%20Annual_Seoul%20ATO_Korea%20-%20Republic%20of_KS2022-0022.pdf Accessed December 19, 2022.
- Korean Food Standards Codex - KFS Codex, Ministry of Food and Drug Safety (in Korean). Available Online: https://foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=380 (accessed 01 December 2021).
- Clinical and Laboratory Standards Institute (CLSI). CLSI Supplement M100S. In Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI: Wayne, PA, US, 2022.
- Shortle, D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983, 22, 181–189. [CrossRef]
- Pérez-Roth, E.; Claverie-Martín, F.; Villar, J.; Méndez-Alvarez, S. Multiplex PCR for simultaneous identification of Staphylococcus aureus and detection of methicillin and mupirocin resistance. J. Clin. Microbiol. 2001, 39(11), 4037–4041.
- Roesch, M.; Perreten, V.; Doherr, M.G.; Schaeren, W., Schällibaum, M., Blum, J.W. Comparison of Antibiotic Resistance of Udder Pathogens in Dairy Cows Kept on Organic and on Conventional Farms. J. Dairy. Sci. 2006, 89(3), 989–997. [CrossRef]
- Sato, K.; Bennedsgaard, T.W.; Bartlett, P.C.; Erskine, R.J.; Kaneene, J.B. Comparison of Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Bulk Tank Milk in Organic and Conventional Dairy Herds in the Midwestern United States and Denmark. J. Food Protection 2004, 67(6), 1104–1110. [CrossRef]
- Álvarez-Fernández, E; Cancelo, A.; Díaz-Vega, C.; Capita, R.; Alonso-Calleja, C. Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: A comparison of agar disc diffusion and Sensi Test Gram-negative methods. Food Control. 2013, 30 (1), 227–234. [CrossRef]
- Tenhangen, B.A.; Alt, K.; Pfefferkorn, B.; Wiehle, L.; Kasbohrer, A.; Fetsch, A. Short Communication: Methicillin-resistant Staphylococcus aureus in conventional and organic dairy herds in Germany. J. Dairy Sci. 2018, 101(4), 3380–3386. [CrossRef]
- Ray, K.A.; Warnick, L.D.; Mitchell, R.M.; Kaneene, J.B.; Ruegg, P.L.; Wells, S.J.; Fossler, C.P.; Halbert, L.W.; May, K. Anti-microbial susceptibility of Salmonella from organic and conventional dairy farms. J. Dairy Sci. 2018, 89, 2038–2050. [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J. Antimicrob. Chemother. 2014, 69(3),827–834. [CrossRef]
- Lam, T.; Scherpenzeel, C.G.M.; den Uijl, I.E.; van Schaik, G. Dry cow therapy: Does it still deserve a blanked recommendation? In Proceedings of the National Mastitis Council 53rd Annual Meeting, Fort Worth, TX, US, 26-28 January 2014, pp. 64–72.
- Schnitt, A.; Tenhagen, B.A. Risk Factors for the Occurrence of Methicillin-Resistant Staphylococcus aureus in Dairy Herds: An Update. Foodborne Pathog. Dis. 2020, 17(10), 585–596. http://doi.org/10.1089/fpd.2019.2638.
- Hakenbeck, R.; Coyette, J. Resistant penicillin-binding proteins. Cell. Mol. Life Sci. 1998, 54, 332–340. [CrossRef]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol. Rev. 2008, 32(2), 361–385. [CrossRef]
- Tipper, D.J. Mode of action of beta-lactam antibiotics. Pharmacol. Ther. 1985, 27(1), 1–35. [CrossRef]
- Tang, S.S.; Apisarnthanarak, A.; Hsu, L.Y. Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv. Drug Deliv. Rev. 2014, 78, 3–13. http://dx.doi.org/10.1016/j.addr.2014.08.003.
- Stapleton, P.D.; Taylor, P.W. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 2002, 85(1), 57–72. [CrossRef]
- Hartman, A.; Tomasz, B. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 1991, 19, 726–735. [CrossRef]
- Grobbel, M.; Hammerl, J.A.; Alt, K.; Irrgang, A.; Kaesbohrer, A.; Tenhagen, B.-A. Comparison of Antimicrobial Resistances in Escherichia coli from Conventionally and Organic Farmed Poultry from Germany. Antibiotics 2022, 11, 1282. [CrossRef]
- Velázquez-Guadarrama, N.; Olivares-Cervantes, A.; Salinas, E.; Martinez, L.; Escorcia, M.; Oropeza, R.; Rosas, I. Presence of environmental coagulase-positive staphylococci, their clonal relationship, resistance factors and ability to form biofilm. Rev. Argent. Microbiol. 2017, 49(1), 15–23. [CrossRef]
- Freney, J.; Kloos, W.E.; Hajek, V.; Webster, J.A.; Bes, M.; Brun, Y.; Vernozy-Rozand, C. Recommended minimal standards for description of new staphylococcal species. Int. J. Syst. Evol. Micr. 1999, 49(2), 489–502. [CrossRef]
- Sutherland, R.; Boon, R.J.; Griffin, K.E.; Masters, P.J.; Slocombe, B.; White, A.R. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 1985, 27, 495–498.
| Compound Class | Antibiotic Name | Code | Disc concentration (ug) | Zone diameter (mm) | ||
|---|---|---|---|---|---|---|
| R† | I† | S† | ||||
| β-lactams | Ampicillin | AMP | 10 | 15 | 16-21 | 22 |
| Oxacillin | OX | 10 | 17 | 18-24 | 25 | |
| Aminoglycosides | Gentamicin | CN | 10 | 18 | 19-27 | 28 |
| Glycopeptides | Teicoplanin | TEC | 30 | 14 | 15-21 | 22 |
| Vancomycin | VA | 30 | 16 | 17-21 | 22 | |
| Macrolides | Erythromycin | E | 15 | 21 | 22-30 | 31 |
| Phenicols | Chloramphenicol | C | 10 | 18 | 19-26 | 27 |
| Quinolones | Ciprofloxacin | CIP | 5 | 21 | 22-30 | 31 |
| Tetracyclines | Tetracycline | TE | 30 | 23 | 24-30 | 31 |
| Target gene | Primer | Oligonucleotide Sequence (5' - 3') | Amplicon Size (bp) |
|---|---|---|---|
| mecA | mecA 1 | GTAGAAATGACTGAACGTCCGATAA | 310 |
| mecA 2 | CCAATTCCACATTGTTTCGGTCTAA | ||
| nuc | nuc 1 | GCGATTGATGGTGATACGGTT | 279 |
| nuc 2 | AGCCAAGCCTTGACGAACTAAAGC | ||
| mupA | mupA 1 | TATATTATGCGATGGAAGGTTGG | 457 |
| mupA 2 | AATAAAATCAGCTGGAAAGTGTTG |
| Antibiotic Name | Code | Conventional Farm (n=11) | Organic Farm (n=17) | Total (n=28) | |||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||
| Ampicillin | AMP | 9 | 81.8 | 2 | 11.8 | 11 | 39.3 |
| Oxacillin | OX | 4 | 36.4 | 0 | 0.0 | 4 | 14.3 |
| Gentamicin | CN | 4 | 36.4 | 9 | 52.9 | 13 | 46.4 |
| Teicoplanin | TEC | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Vancomycin | VA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Erythromycin | E | 5 | 45.5 | 1 | 5.9 | 6 | 21.4 |
| Chloramphenicol | C | 2 | 18.2 | 2 | 11.8 | 4 | 14.3 |
| Ciprofloxacin | CIP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Tetracycline | TE | 9 | 81.8 | 4 | 23.5 | 13 | 46.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





